To the editor,
During the coronavirus disease 2019 (COVID‐19) pandemic, venovenous extracorporeal membrane oxygenation (V‐V ECMO) has confirmed its role as a life‐saving support option for patients with very severe respiratory failure. 1 However, during the course of the pandemic from different cohorts declining survival rates were reported, an observation that could not yet be explained conclusively. 1 , 2 , 3 In our tertiary ECMO referral center, 90‐day survival of all 59 COVID‐19 patients supported with ECMO throughout the first three waves decreased from 47% (7/15 patients) during the first wave (March 2020–July 2020), to 38% (8/21 patients) during the second wave (October 2020–February 2021) down to 17% (4/23 patients) during the third wave (March 2021–June 2021). 4 In the course of the most recent fourth wave of the pandemic (August 2021–May 2022), we supported additional 29 patients with V‐V ECMO. Here, we present survival data of these patients in the context of the results from the preceding waves.
The data come from a non‐interventional retrospective single‐center registry, as described previously. 4 We included all COVID‐19 patients supported with V‐V ECMO from the beginning of the COVID‐19 pandemic in our center in March 2020 until May 15, 2022. Indication for V‐V ECMO, standard treatment procedures and criteria for discontinuation of ECMO in our center followed established criteria and were applied unchanged throughout the COVID‐19 pandemic. 5 The duration of ECMO support was not limited by predefined time limits. For statistical analyses Prism (version 9; GraphPad Software Inc., San Diego, CA, USA) was used. Baseline and outcome parameters were compared between the patients treated during the first three waves of the pandemic and those treated thereafter. Continuous variables were compared using the Mann–Whitney test, categorical variables were evaluated using Fisher's exact test. Survival time was visualized using Kaplan–Meier plots and statistical differences between the groups were calculated using the log‐rank (Mantel‐Cox) test. Patients were grouped according to the waves of the pandemic in our center (1st–3rd wave vs. 4th wave). In all evaluations, a p‐value at or below 0.05 was considered statistically significant.
59/88 patients (67%) were treated during the first three waves, and 29/88 (33%) received ECMO during the fourth wave of the pandemic. Median age (IQR) of all patients was 55 (47–62) years, and 28/88 patients (32%) were female. A detailed analysis of the patients treated during the first three waves was published recently. 4 Comparing the patients treated during the first three waves with those treated during the fourth wave, the latter were younger (median age (IQR) wave 1–3: 59 (53–63) years; wave 4: 48 (42–56) years; p < 0.001), and they were treated more frequently with tocilizumab (wave 1–3: 9/59 patients (15%), wave 4: 19/29 patients (66%); p < 0.001) and methylprednisolone (wave 1–3: 46/59 patients (78%), wave 4: 29/29 patients (100%); p = 0.004; Table 1). Blood purification was used only during the first and the second wave, and then its use was stopped due to the alarm signals found in the CYCOV trial. 6 Before initiation of ECMO, the ratio of partial pressure of arterial oxygen and the fraction of inspired oxygen (PaO2/FiO2 ratio) was lower in patients supported during the fourth wave (median PaO2/FiO2 ratio (IQR) wave 1–3: 67.6 (51.9–84.2) mm Hg; wave 4: 56.6 (48.2–72.3) mm Hg; p = 0.048, Table 1).
TABLE 1.
Patient baseline characteristics
| All patients: March 2020–May 2022 (n = 88) | First–third wave: March 2020–June 2021 (n = 59) | Fourth wave: August 2021–May 2022 (n = 29) | p‐value | |
|---|---|---|---|---|
| Age (years) | 55 (47–62) | 59 (53–63) | 48 (42–56) | <0.001 |
| Sex | 0.225 | |||
| Male | 60 (68%) | 43 (73%) | 17 (59%) | |
| Female | 28 (32%) | 16 (27%) | 12 (41%) | |
| Body mass index (kg/m2) | 30.86 (28–36) | 29.39 (27–35) | 33.24 (29–38) | 0.108 |
| Comorbidities | ||||
| Hypertension | 30 (34%) | 24 (41%) | 6 (21%) | 0.093 |
| Diabetes | 18 (20%) | 15 (25%) | 3 (10%) | 0.159 |
| Coronary heart disease | 8 (9%) | 7 (12%) | 1 (3%) | 0.263 |
| Hematological malignancy | 1 (1%) | 1 (2%) | 0 | >0.999 |
| Solid organ malignancy | 3 (3%) | 2 (3%) | 1 (3%) | >0.999 |
| Immunosuppressive therapy | 3 (3%) | 3 (5%) | 0 | 0.548 |
| Scores | ||||
| SOFA | 8 (7–10) | 9 (7–10) | 8 (7–9) | 0.099 |
| RESP | 1 (0–3) | 1 (0–2) | 2 (−0.5–4) | 0.258 |
| PRESERVE | 4 (2–6) | 4 (3–6) | 3 (0.5–4) | <0.001 |
| Pre‐ECMO patient conditions | ||||
| Days of in‐hospital treatment before ECMO | 8.7 (5.6–14.0) | 8.7 (5.6–14.7) | 8.2 (5.2–13.9) | 0.596 |
| Days of ICU‐treatment before ECMO | 7.6 (4.6–12.7) | 7.8 (4.6–13.7) | 6.8 (4.7–12.4) | 0.846 |
| Duration of mechanical ventilation before ECMO (days) | 7.6 (4.6–12.7) | 7.8 (4–12.7) | 6.8 (4.7–12.4) | 0.817 |
| Prone positioning | 77 (88%) | 49 (83%) | 28 (97%) | 0.093 |
| Pre‐ECMO ventilation parameters | ||||
| FiO2 (%) | 1.0 (1.0–1.0) | 1.0 (0.9–1.0) | 1.0 (1.0–1.0) | 0.037 |
| Positive end–expiratory pressure (mbar) | 15 (14–16) | 15 (14–16) | 15 (15–17) | 0.078 |
| Peak pressure (mbar) | 34 (31–36) | 34 (31–36) | 33 (31–36) | 0.708 |
| Dynamic driving pressure (mbar) | 18 (16–21) | 18 (16–21) | 18 (15.5–20) | 0.485 |
| Tidal volume (ml) | 429 (341–520) | 431 (346–517) | 410 (333–585) | 0.855 |
| Breathing rate (1/min) | 27 (22–30) | 27 (22–32) | 27 (24–30) | 0.772 |
| Pre–ECMO arterial blood gas analysis | ||||
| pH | 7.31 (7.18–7.39) | 7.31 (7.21–7.40) | 7.30 (7.14–7.39) | 0.535 |
| PaO2 (mm Hg) | 60.5 (50.4–74) | 64.2 (50.9–75.4) | 56.6 (48.2–69.8) | 0.137 |
| PaCO2 (mm Hg) | 61.2 (48.0–73.9) | 60 (46.9–71.7) | 64.2 (51.5–76) | 0.385 |
| PaO2/FiO2 (mm Hg) | 61.7 (51.9–79.9) | 67.6 (51.9–84.2) | 56.6 (48.2–72.3) | 0.047 |
| Bicarbonate (mmol/L) | 24.5 (22.1–28.4) | 24.1 (22.5–28.2) | 25.3 (21.1–29.4) | 0.746 |
| Lactate (mmol/L) | 1.6 (1.2–2.1) | 1.6 (1.2–2.0) | 1.8 (1.2–2.6) | 0.214 |
| Medical treatment | ||||
| Hydroxychloroquin | 11 (13%) | 11 (19%) | 0 | 0.014 |
| Lopinavir–ritonavir | 6 (7%) | 6 (10%) | 0 | 0.172 |
| Tocilizumab | 28 (32%) | 9 (15%) | 19 (66%) | <0.001 |
| Remdesivir | 9 (10%) | 8 (14%) | 1 (3%) | 0.261 |
| Methylprednisolon | 75 (85%) | 46 (78%) | 29 (100%) | 0.004 |
Note: Data are median (IQR) or n (%). Continuous variables were compared using the Mann–Whitney test. Categorical variables were evaluated using Fisher's exact test.
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; PRESERVE, predicting death for severe ARDS on venovenous ECMO; RESP, respiratory extracorporeal membrane oxygenation survival prediction; SOFA, sequential organ failure assessment.
Over the course of the pandemic, the virus mutated and different variants prevailed at different times. In our cohort, not for all patients, virus genome sequencing data was available. Data for waves 1–3 was reported previously. 4 During the fourth wave, in 10/29 patients (34%) the delta variant was detected, and only in one patient (3%) the omicron variant. For 18/29 patients (62%), no sequencing data was available.
In the entire cohort, 55/88 patients (63%) survived until day 30, 48/88 patients (55%) survived until day 60 and 44/88 patients (50%) survived until day 90 (Table 2, Figure 1). Of those patients treated during the fourth wave of the pandemic, 25/29 (86%) survived until day 90 (Figure 1). Survival at all time points was significantly higher during the fourth wave as compared with the preceding waves (Table 2, Figure 1). Duration of ECMO support was significantly longer during the fourth wave compared with the earlier waves (30 vs. 15 days, p = 0.012). As mentioned above, we did not change criteria for discontinuation of ECMO throughout the pandemic, yet, we cannot exclude that greater acceptance of longer durations of therapy influenced survival time during the fourth wave.
TABLE 2.
ECMO support and outcome
| All patients: March 2020–May 2022 (n = 88) | First–Third wave: March 2020–June 2021 (n = 59) | Fourth wave: August 2021–May 2022 (n = 29) | p‐value | |
|---|---|---|---|---|
| ECMO—cannulation strategy | ||||
| Dual–lumen, jugular | 70 (80%) | 43 (73%) | 27 (93%) | 0.046 |
| Femoral–femoral | 14 (16%) | 12 (20%) | 2 (7%) | 0.130 |
| Femoral–jugular | 4 (5%) | 4 (7%) | 0 | 0.298 |
| ECMO support duration (days) | 21.5 (10.9–39.3) | 14.9 (9.5–32.6) | 29.8 (18.6–49.1) | 0.012 |
| Blood purification (CytoSorb®) | 12 (14%) | 12 (20%) | 0 (0%) | 0.007 |
| Causes of death | ||||
| Intracranial hemorrhage | 12 (14%) | 9 (15%) | 3 (10%) | 0.744 |
| Other major bleeding | 3 (3%) | 3 (5%) | 0 | 0.548 |
| Respiratory failure | 10 (11%) | 10 (17%) | 0 | 0.027 |
| Septic shock | 11 (13%) | 11 (19%) | 0 | 0.014 |
| Multiorgan failure | 8 (9%) | 7 (12%) | 1 (3%) | 0.263 |
| Death on ECMO | 40 (45%) | 36 (61%) | 4 (14%) | <0.001 |
| Survival rate after ECMO initiation | ||||
| 30 days | 55 (63%) | 30 (51%) | 25 (86%) | 0.008 a |
| 60 days | 48 (55%) | 23 (39%) | 25 (86%) | <0.001 a |
| 90 days | 44 (50%) | 19 (32%) | 25 (86%) | <0.001 a |
| To hospital discharge | 42 (48%) | 20 (34%) | 22 (76%) | <0.001 |
Note: Data are median (IQR) or n (%). Continuous variables were compared using the Mann–Whitney test. Categorical variables were evaluated using Fisher's exact test.
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
p‐values are derived from log–rank (Mantel–Cox) tests.
FIGURE 1.
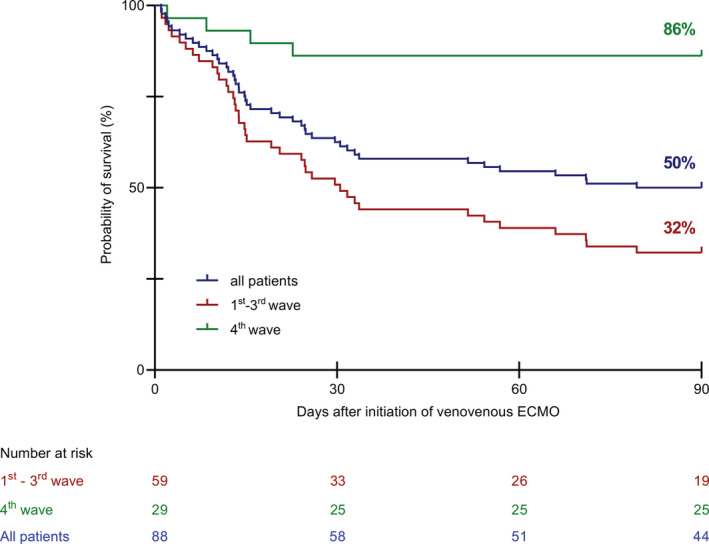
Kaplan–Meier curves for survival time until day 90 during four waves of the COVID‐19 pandemic. ECMO, extracorporeal membrane oxygenation.
We report treatment and survival data from 88 patients with COVID‐19 supported with V‐V ECMO in our center during the first more than 2 years of the pandemic between March 2020 and May 2022. Overall survival until day 90 after initiation of V‐V ECMO was 50%. This survival rate was similar to the results from the first year of the pandemic reported from the Extracorporeal Life Support Organization (ELSO) registry. 1 Interestingly, during the fourth wave of the pandemic survival was significantly higher compared with the preceding waves.
Between the cohorts from the first three waves and the fourth wave the lower age of the patients treated during the fourth wave stands out (48 vs. 59 years).The extent to which this difference or other factors affect the observed differences in survival during the different waves in our center cannot be determined with certainty. However, patient age is a known risk factor for increased mortality in patients with COVID‐19 requiring ECMO. 1 , 7 The duration of mechanical ventilation before ECMO, another parameter that has previously been described as relevant for the survival of the patients, was shorter during the fourth wave; however, this difference did not reach statistical significance. 8 These differences might at least in part explain the higher survival rate. However, the PaO2/FiO2 ratio before ECMO was lower during the fourth wave, suggesting higher disease severity before ECMO initiation. Beyond that, during later waves, patients did not receive hydroxychloroquine and more patients received tocilizumab and methylprednisolone. One may speculate that better targeted therapies resulted in improved outcomes.
Data from this single‐center cohort illustrate varying survival rates during different waves of the pandemic. The reasons for this observation may be manifold including differences in baseline and in treatment characteristics, and some of the observations may also be explained by an effect of chance in this rather small single‐center cohort. However, overall 90‐day survival of 50% in COVID‐19 patients supported with V‐V ECMO in our center since the beginning of the pandemic is in line with the results reported from the large ELSO registry and with observations from pre‐pandemic non‐COVID‐19 cohorts. 1 , 9 These results are considerably better than observations from a large nationwide cohort of COVID‐19 patients supported with ECMO in Germany from January 2020 to the end of September 2021, describing overall survival of only 34%. 10 , 11 Careful selection of patients that most likely benefit from ECMO and would die without this invasive and highly resource‐dependent support option is key for responsible planning and ECMO program management, a balance previously described as “ECMO sweet spot”. 12 Considering this approach, our findings confirm a role for ECMO in the treatment of selected patients with severe COVID‐19 associated respiratory failure.
AUTHOR CONTRIBUTIONS
Concept/design: Eugen Widmeier and Alexander Supady, Data analysis/interpretation: Eugen Widmeier and Alexander Supady, Drafting article: Alexander Supady, Critical revision of article: Eugen Widmeier, Tobias Wengenmayer, Sven Maier, Christoph Benk, Viviane Zotzmann, Dawid L. Staudacher, Alexander Supady, Approval of article: Eugen Widmeier, Tobias Wengenmayer, Sven Maier, Christoph Benk, Viviane Zotzmann, Dawid L. Staudacher, Alexander Supady, Statistics: Eugen Widmeier and Alexander Supady, Data collection: Eugen Widmeier, Tobias Wengenmayer, Sven Maier, Christoph Benk, Viviane Zotzmann, Dawid L. Staudacher, Alexander Supady.
FUNDING INFORMATION
This work was financed by internal funds from the Department of Interdisciplinary Medical Intensive Care at the University of Freiburg Medical Center.
CONFLICTS OF INTEREST
All authors have completed the ICMJE form (available upon request from the corresponding author). AS reports research grants and lecture fees from CytoSorbents and lecture fees from Abiomed, both outside the submitted work.
ETHICS STATEMENT
Data collection was approved by the institutional ethics committee of the University of Freiburg (EK 151/14), due to the retrospective and observational nature of the study the need for informed consent was waived.
DATA AVAILABILITY STATEMENT
All data will be available from the corresponding author on reasonable request.
REFERENCES
- 1. Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for COVID‐19: evolving outcomes from the International Extracorporeal Life Support Organization Registry. Lancet. 2021;398:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broman LM, Eksborg S, Lo Coco V, De Piero ME, Belohlavek J, Lorusso R, et al. Extracorporeal membrane oxygenation for COVID‐19 during first and second waves. Lancet Respir Med. 2021;9:e80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt M, Langouet E, Hajage D, James SA, Chommeloux J, Brechot N, et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID‐19 ARDS in Sorbonne hospitals. Paris Crit Care. 2021;25:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Widmeier E, Wengenmayer T, Maier S, Benk C, Zotzmann V, Staudacher DL, et al. Extracorporeal membrane oxygenation during the first three waves of the coronavirus disease 2019 pandemic—a retrospective single‐center registry study. Artif Organs. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badulak J, Antonini MV, Stead CM, Shekerdemian L, Raman L, Paden ML, et al. Extracorporeal membrane oxygenation for COVID‐19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 2021;67:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Supady A, Weber E, Rieder M, Lother A, Niklaus T, Zahn T, et al. Cytokine adsorption in patients with severe COVID‐19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open‐label, randomised, controlled trial. Lancet Respir Med. 2021;9:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID‐19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karagiannidis C, Strassmann S, Merten M, Bein T, Windisch W, Meybohm P, et al. High in‐hospital mortality rate in patients with COVID‐19 receiving extracorporeal membrane oxygenation in germany: a critical analysis. Am J Respir Crit Care Med. 2021;204:991–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedrichson B, Mutlak H, Zacharowski K, Piekarski F. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care. 2021;25:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedrichson B, Kloka JA, Neef V, Mutlak H, Old O, Zacharowski K, et al. Extracorporeal membrane oxygenation in coronavirus disease 2019: a nationwide cohort analysis of 4279 runs from Germany. Eur J Anaesthesiol. 2022;39:445–51. [DOI] [PubMed] [Google Scholar]
- 11. Karagiannidis C, Slutsky AS, Bein T, Windisch W, Weber‐Carstens S, Brodie D. Complete countrywide mortality in COVID patients receiving ECMO in Germany throughout the first three waves of the pandemic. Crit Care. 2021;25:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Supady A, Biever PM, Staudacher DL, Wengenmayer T. Choosing the right reference cohort for assessing outcome of venovenous ECMO. Crit Care. 2022;26:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available from the corresponding author on reasonable request.


