Abstract
Background and aims:
The influence of nonalcoholic fatty liver disease (NAFLD) on the long-term risk of cirrhosis and hepatocellular carcinoma (HCC) in Asian populations has not been widely investigated.
Methods:
We enrolled 129 374 adults aged ≥30 years, all of whom participated in a health screening program from 2008 through 2013, were seronegative for HBsAg and anti-HCV antibodies, and had limited daily alcohol consumption (<20 g/day for men and <10 g/day for women). Abdominal ultrasonography was performed to determine the presence of NAFLD. The participants were divided into NAFLD with elevated or normal, and non-NAFLD with normal liver enzymes groups. The incidences of cirrhosis and HCC were determined through computerized data linkage with nationwide registries. Cox’s proportional hazard models were used to estimate the hazard ratios (HRs) of NAFLD on the risks of cirrhosis and HCC.
Results:
The incidence rates of cirrhosis and HCC increased by the groups of non-NAFLD with normal liver enzyme (n=66 801, 51%), NAFLD with normal liver enzymes (n=41 461, 32%), and NAFLD with elevated liver enzymes (n=21 112, 16%). In the NAFLD with elevated liver enzymes and NAFLD with normal liver enzymes groups, the corresponding multivariate-adjusted HRs for cirrhosis were 3.51 (2.36–5.22) and 0.73 (0.46–1.16), and those for HCC were 1.91 (1.08–3.38) and 0.57 (0.31–1.04), respectively, compared with the non-NAFLD group (P for trend < .001). The findings were consistent after restricting the analysis to nonobese individuals (BMI < 25 kg/m2) and nonobese without diabetes (P < .05).
Conclusions:
Individuals with NAFLD and elevated liver enzyme levels exhibited significantly higher risks for cirrhosis and HCC and should be monitored.
Keywords: nonobese, long-term risk, metabolic disease, prospective study
Introduction
With a global prevalence of 25% in the adult population and substantial associated health-care expenses, the public health burden of nonalcoholic fatty liver disease (NAFLD) is considerable1. The spectrum of NAFLD ranges from steatosis to a more progressive nonalcoholic steatohepatitis (NASH) that can lead to fibrosis and cirrhosis. NAFLD has also become the second leading indicator of the need for liver transplantation and the third leading cause of hepatocellular carcinoma (HCC)2, 3 Because liver conditions attributable to chronic hepatitis viral infection have been substantially reduced by hepatitis B vaccination programs and effective direct antiviral agents for hepatitis C, NAFLD is likely to become a key health concern in the future.
The high prevalence of NAFLD renders current HCC surveillance challenging. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are surrogates for hepatocellular injury. Elevated concentrations of these markers are noninvasive indicators of NAFLD4, 5. Nonetheless, some patients who have received biopsy-based NAFLD diagnoses persistently exhibit normal ALT levels6, 7. Incorporating imaging in addition to these 2 seromarkers might provide more accurate noninvasive NAFLD diagnosis. Claims database studies have suggested that patients with NAFLD and normal ALT may be at lower risk of cirrhosis or HCC8, 9. However, because of a lack of personal lab tests9 and the predominant enrolling of participants with Caucasian men8, these findings are vulnerable to substantial misclassification and poor generalizability.
Obesity is generally associated with NAFLD. However, not all people with obesity develop NAFLD, and NAFLD can develop in the nonobese population10. Approximately 40% of individuals with NAFLD worldwide are classified as nonobese, and nearly 20% are considered lean11. Nonobese patients with NAFLD are increasingly being recognized as developing end-stage liver diseases even more rapidly than obese individuals12. Notable differences in lifestyles, genetics, and body composition make Asians more likely to have nonobese NAFLD compared with non-Asian populations13. In particular, NAFLD in Asian populations is more likely to be associated with lobular inflammation and higher grades of ballooning compared with non-Asian populations14. Large-scale follow-up studies to establish cirrhosis or HCC risks associated with NAFLD, particularly among nonobese Asian individuals, are essential for thoroughly understanding the disease.
This study investigated the long-term impacts of NAFLD, diagnosed on the basis of ultrasound imaging with or without elevated ALT and AST levels, on the associated risk of cirrhosis and HCC. We prospectively examined healthy adults who underwent medical screening, and we analyzed 2 subgroups: nonobese individuals and nonobese individuals without diabetes.
Methods
Study Population and Data Collection
The study participants were healthy individuals aged ≥30 years who participated in a health screening program run by a private health-care institution in Taiwan (Supplementary). All participants were followed from 2008 through 2013. In brief, the participants underwent a series of blood, urine, and anthropometric tests and physical examinations upon study enrolment15. Every participant provided signed informed consent for the use of their data generated from medical screenings for biomedical investigations. The study protocol was approved by the Institutional Review Board of the National Yang Ming Chiao Tung University, Taipei, Taiwan.
The initial cohort consisted of 186 943 adults. We excluded the participants that (1) had received results of hepatitis B surface antigen or antibodies against hepatitis C virus seropositivity (n = 29 045), (2) had inadequate information on alcohol quantity or excessive daily alcohol consumption (n = 19 139; >20 g/day for men and >10 g/day for women, according to Asia-Pacific Guidelines)16, (3) had missing data on ultrasonography or testing of ALT or AST (n = 2827); and 4) had prevalent cirrhosis or HCC or died less than 6 months after study entry (n = 478). Finally, 135 454 healthy individuals free of underlying liver diseases were included in this study. The participants with body mass index (BMI) < 25 kg/m2 were considered nonobese17 (n = 89 423), and a subset of this group, those considered nonobese without diabetes (n = 86 260) was analyzed separately.
Definition of NAFLD
All of the study participants were examined by board-certified gastroenterologists using high-resolution real-time abdominal ultrasonography. The presence of fatty liver within the hepatic parenchyma was assessed according to parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, and bright vessel walls18. We categorized individuals with NAFLD by using liver enzyme levels as proxy markers for NASH. An elevated liver enzyme level was defined as a serum concentration of either AST ≥37 U/L in men or ≥31 U/L in women or ALT ≥40 U/L in men or ≥31 U/L in women8, 19. We classified the participants into 3 groups: non-NAFLD with normal liver enzymes, NAFLD with normal liver enzyme, and NAFLD with elevated liver enzyme levels. Individuals without NAFLD but with elevated liver enzyme levels were excluded from this analysis (n = 6080).
Follow-Up and Cirrhosis and HCC Diagnoses
We applied computerized data linkage, namely the National Health Insurance database, the National Cancer Registration Profiles, and the National Death Certification system, to follow-up the occurrence of diseases and vital status of the study participants from January 1, 2008, to December 31, 2015. These nationwide registries are universally compulsory and they cover nearly 100% of the total population. The administrative and claims data stored in these registries are complete, accurate, and up-to-date20. The incident events of cirrhosis and HCC were identified by the International Classification of Diseases codes as described in the Supplementary.
Statistical Methods
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) and the details were described in the Supplementary.
Results
Baseline Characteristics of Study Cohort
This prospective study enrolled 41 461 (32%) individuals with NAFLD and normal liver enzyme levels, 21 112 (16%) individuals with NAFLD and elevated liver enzyme levels, and 66 801 (51%) individuals without NAFLD and with normal liver enzyme levels (Figure 1). Among the 129 374 participants in the study cohort, the mean age was 44.3 ± 11.6 years. At baseline, 89 423 (69%) participants had a BMI of <25 kg/m2 and 86 260 (67%) had not received a diabetes diagnosis. Compared with the participants with NAFLD and normal liver enzyme levels, those with NAFLD and elevated liver enzyme levels were younger, more likely to be male, cigarette smokers, and exhibited higher alpha-fetoprotein, triglyceride, and cholesterol levels, and presence of diabetes (P < .05) (Table 1).
Figure 1. Flowchart of Study Participant Enrollment.
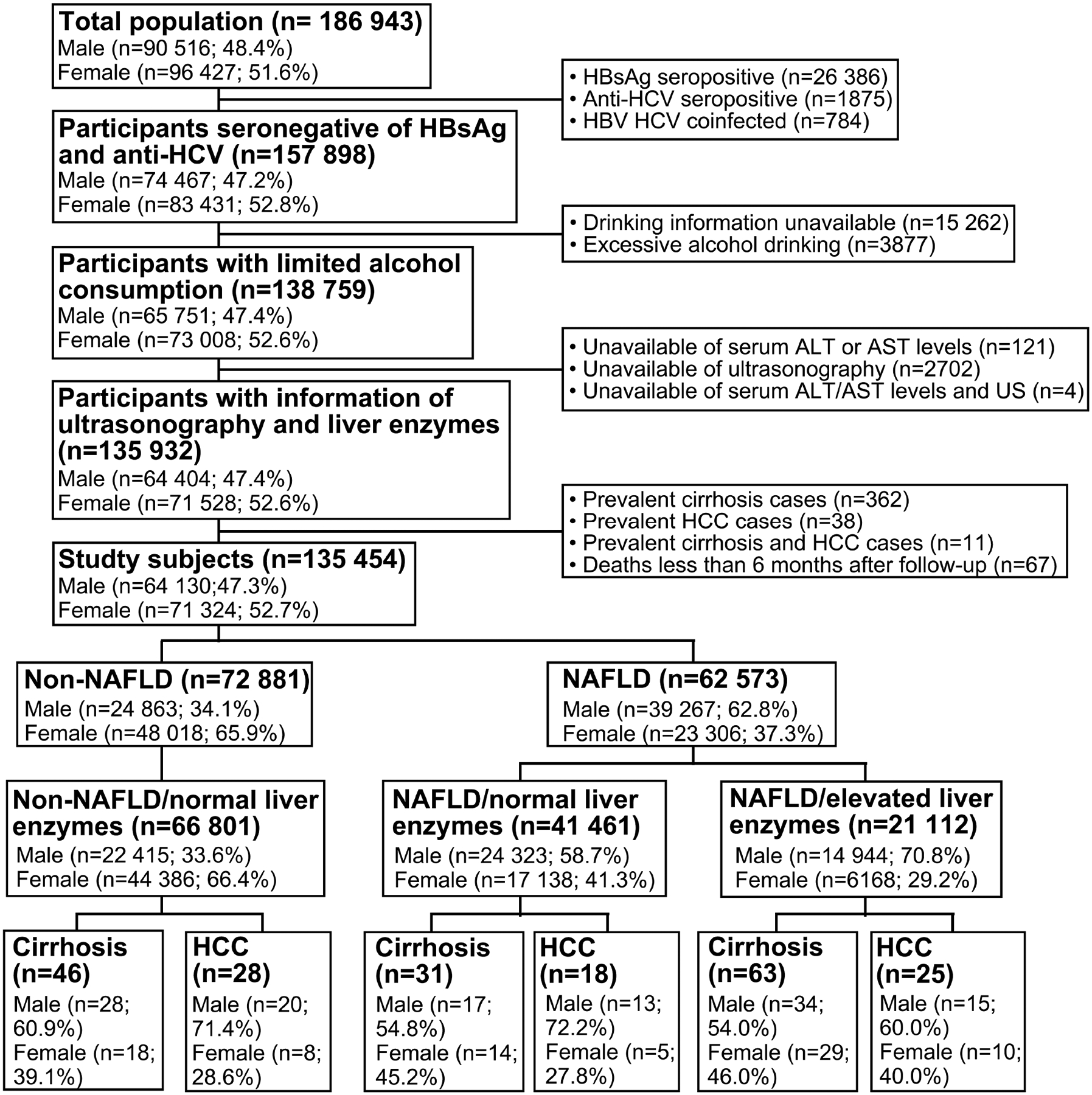
Abbreviations: HBsAg: hepatitis B surface antigen; HBV: hepatitis B virus; HCV: hepatitis C virus; ALT: alanine aminotransferase; AST: aspartate transaminase; US: ultrasonography; HCC: hepatocellular carcinoma; NAFLD: nonalcoholic fatty liver disease.
Table 1.
Baseline Characteristics of Study Population
| Non-NAFLD (n = 66,801) | NAFLD with normal liver enzyme levelsa (n = 41,461) | NAFLD with increased liver enzyme levels (n = 21,112) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | n | (%) | n | (%) | n | (%) | P valueb | P valuec |
| Age, y | ||||||||
| <50 | 52,099 | 78.0 | 25,171 | 60.7 | 14,937 | 70.8 | <.0001 | <.0001 |
| 50–59 | 8414 | 12.6 | 9006 | 21.7 | 3746 | 17.7 | ||
| 60–69 | 4284 | 6.4 | 5277 | 12.7 | 1853 | 8.8 | ||
| ≥70 | 2004 | 3.0 | 2007 | 4.8 | 576 | 2.7 | ||
| Means ± SD | 42.36 ± 11.11 | 47.32 ± 11.98 | 44.38 ± 11.11 | <.0001 | <.0001 | |||
| Sex | ||||||||
| Female | 44,386 | 66.4 | 17,138 | 41.3 | 6168 | 29.2 | <.0001 | <.0001 |
| Male | 22,415 | 33.6 | 24,323 | 58.7 | 14,944 | 70.8 | ||
| Cigarette smoking | ||||||||
| Never | 54,120 | 81.9 | 29,446 | 72.0 | 13,897 | 66.7 | <.0001 | <.0001 |
| Ever | 11,968 | 18.1 | 11,445 | 28.0 | 6942 | 33.3 | ||
| Missing | 713 | 570 | 273 | |||||
| Platelet count, 103/μL | ||||||||
| ≥200 | 56,056 | 83.9 | 35,757 | 86.3 | 18,514 | 87.7 | <.0001 | <.0001 |
| <200 | 10,744 | 16.1 | 5702 | 13.8 | 2597 | 12.3 | ||
| Missing | 1 | 2 | 1 | |||||
| AFP level, ng/mL | ||||||||
| <5 | 60,496 | 90.9 | 37,276 | 90.1 | 18,772 | 89.1 | <.0001 | .0001 |
| ≥5 | 6043 | 9.1 | 4093 | 9.9 | 2290 | 10.9 | ||
| Missing | 262 | 92 | 50 | |||||
| Triglyceride level, mg/dL | ||||||||
| <150 | 61,748 | 92.4 | 27,820 | 67.10 | 9930 | 47.0 | <.0001 | <.0001 |
| ≥150 | 5053 | 7.6 | 13,641 | 32.90 | 11,182 | 53.0 | ||
| Total cholesterol level, mg/dL | ||||||||
| <200 | 42,744 | 64.0 | 20,540 | 49.5 | 8715 | 41.3 | <.0001 | <.0001 |
| ≥200 | 24,057 | 36.0 | 20,920 | 50.5 | 12,396 | 58.7 | ||
| Missing | 0 | 1 | 1 | |||||
| Diabetesd | ||||||||
| No | 65,635 | 98.3 | 37,675 | 90.9 | 18,372 | 87.0 | <.0001 | <.0001 |
| Yes | 1164 | 1.7 | 3779 | 9.1 | 2738 | 13.0 | ||
| Missing | 2 | 7 | 2 | |||||
NOTE. N = 129,374.
AFP, α-fetoprotein; NAFLD, nonalcoholic fatty liver disease.
Normal liver enzyme levels were defined as an ALT level <40 IU/L and an AST level <37 IU/L for men and an ALT level <31 IU/L and an AST level <31 IU/L for women.
Comparisons of the non-NAFLD and NAFLD study participants with normal liver enzyme levels.
Comparisons between normal and increased liver enzyme levels among NAFLD participants.
Diabetes was defined as a fasting blood glucose level of ≥126 mg/dL or self-reported on the use of diabetes medications.
Incidence of Cirrhosis and HCC in the Entire Cohort
After 753 581 person-years of follow-up, 140 newly developed cirrhosis cases were identified, yielding an incidence rate of 18.6 per 100 000 person-years. Individuals who had NAFLD together with elevated liver enzyme levels had higher cirrhosis incidence rates than patients with NAFLD and normal liver enzyme levels and patients without NAFLD and with normal liver enzyme levels. The group incidences were 52.1, 12.8, and 11.8, per 100 000 person-years, respectively (Table 2). The cumulative incidence of cirrhosis with a median follow-up of 6 years was significantly higher in the participants with NAFLD and elevated liver enzyme levels (0.43%) compared with the participants with NAFLD and normal liver enzyme levels (0.14%, P < .001; Figure 2A).
Table 2.
Incidence Rates of Liver Cirrhosis or Hepatocellular Carcinoma
| Cirrhosis | Hepatocellular carcinoma | ||||||
|---|---|---|---|---|---|---|---|
| NAFLD status | No. | Events | Person-years | Incidence rate† | Events | Person-years | Incidence rate† |
| Total population | |||||||
| Non-NAFLD | 66 801 | 46 | 390 900 | 11.8 | 28 | 390 947 | 7.2 |
| NAFLD/normal liver enzymes‡ | 41 461 | 31 | 241 741 | 12.8 | 18 | 241 766 | 7.5 |
| NAFLD/elevated liver enzymes | 21 112 | 63 | 120 940 | 52.1 | 25 | 121 040 | 20.7 |
| Overall | 129 374 | 140 | 753 581 | 18.6 | 71 | 753 753 | 9.4 |
| Nonobese (BMI <25 kg/m 2 ) | |||||||
| Non-NAFLD | 61 053 | 35 | 357 147 | 9.8 | 22 | 357 194 | 6.2 |
| NAFLD/normal liver enzymes‡ | 21 684 | 16 | 127 419 | 12.6 | 9 | 127 429 | 7.1 |
| NAFLD/elevated liver enzymes | 6686 | 27 | 39 070 | 69.1 | 8 | 39 118 | 20.5 |
| Overall | 89 423 | 78 | 523 636 | 14.9 | 39 | 523 741 | 7.5 |
| Nonobese and without diabetes | |||||||
| Non-NAFLD | 60 133 | 30 | 351 684 | 8.5 | 21 | 409 526 | 5.1 |
| NAFLD/normal liver enzymes‡ | 20 048 | 11 | 117 755 | 9.3 | 5 | 117 761 | 4.3 |
| NAFLD/elevated liver enzymes | 6079 | 20 | 35 538 | 56.3 | 5 | 35 572 | 14.1 |
| Overall | 86 260 | 61 | 504 977 | 12.1 | 31 | 562 859 | 5.5 |
Abbreviation: NAFLD: nonalcoholic fatty liver disease.
per 100,000 person-years
Normal liver enzyme levels defined by ALT < 40 and AST < 37 IU/L for male and ALT < 31 and AST < 31 IU/L for female participants.
Figure 2.
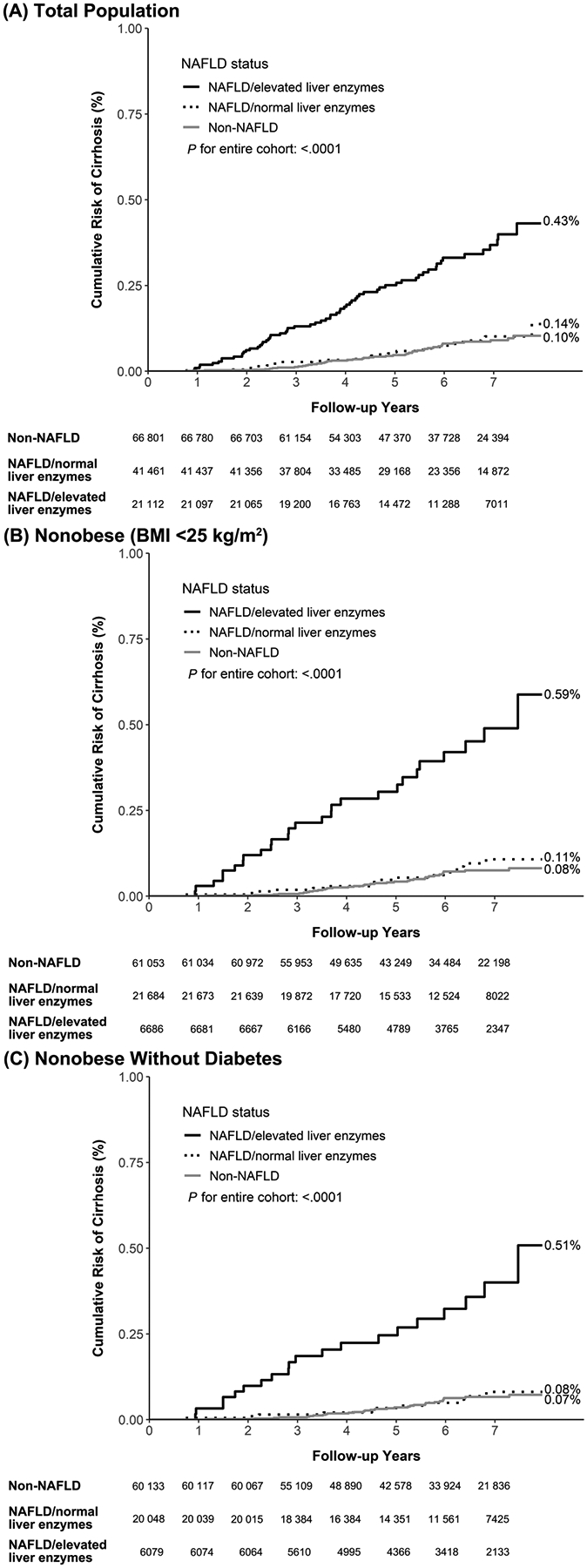
Cumulative Risk of Cirrhosis Among (A) Total Population, (B) Nonobese (BMI <25 kg/m2) Group, and (C) Nonobese Without Diabetes Group.
In total, 71 cases of HCC occurred after 753 753 person-years of follow-up in the entire cohort, yielding an HCC incidence rate of 9.4 per 100 000 person-years. The incidence of HCC was highest in the individuals with NAFLD and elevated liver enzyme levels: 20.7 per 100 000 person-years as compared to 7.2 per 100 000 person-years in the individuals without NAFLD and with normal liver enzyme levels and 7.5 per 100 000 person-years in those with NAFLD and normal liver enzyme levels (Table 2). At the end of follow-up, approximately 0.17% of the participants with NAFLD and elevated liver enzymes had received an HCC diagnosis compared with 0.06% of participants with NAFLD and normal liver enzyme levels (P = .0005, Figure 3A).
Figure 3.
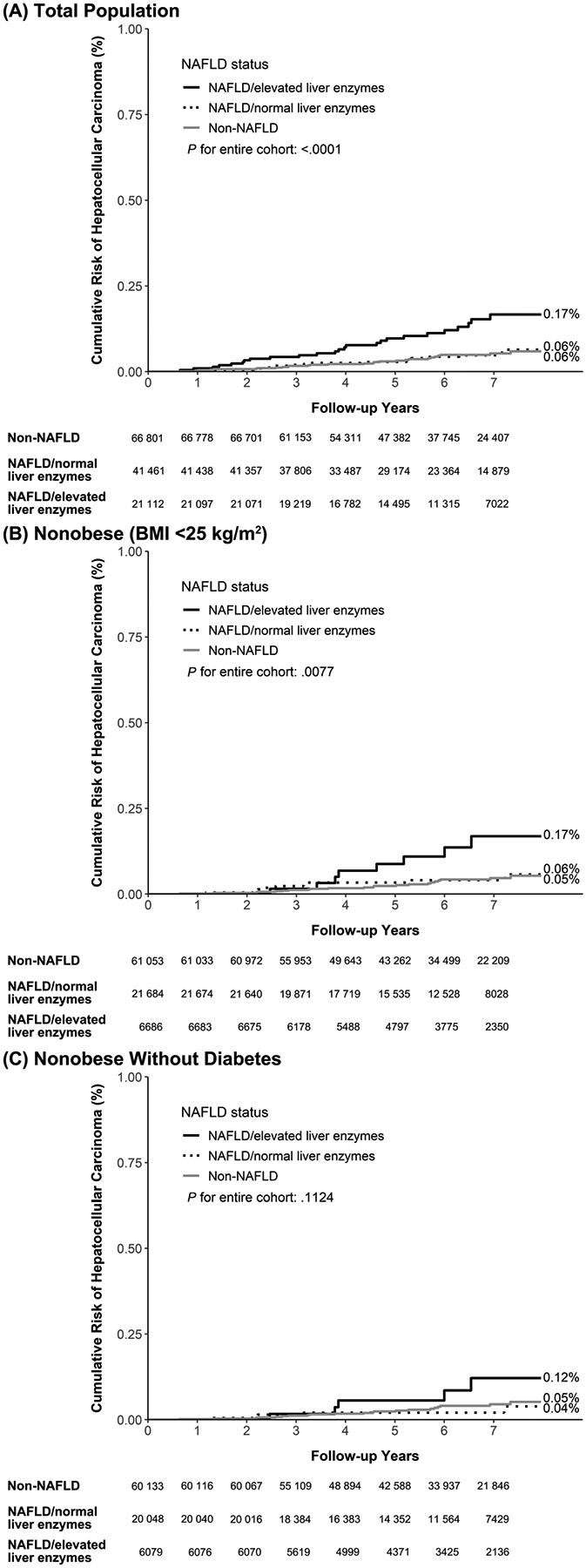
Cumulative Risk of Hepatocellular Carcinoma Among (A) Total Population, (B) Nonobese (BMI <25 kg/m2) Group, and (C) Nonobese Without Diabetes Group.
Relative Risks of Cirrhosis and HCC According to NAFLD and Liver Enzyme Levels
Table 3 displays the multivariate-adjusted HRs of cirrhosis, which were respectively 0.73 (0.46–1.16) and 3.51 (2.36–5.22) for the individuals with NAFLD and normal liver enzyme and those with NAFLD and elevated liver enzymes, compared with those without NAFLD and with normal liver enzyme levels as a reference group (P for trend < .001). For HCC, the multivariate-adjusted HRs were 0.57 (0.31–1.04) and 1.91 (1.08–3.38), respectively, again compared to those without NAFLD and with normal liver enzymes (P for trend < .001). Advanced age, male sex, low platelet count (<200 × 103/μL), high alpha-fetoprotein (≥5 ng/mL), and diabetes were significantly associated with increased risk of cirrhosis and HCC (P < .05).
Table 3.
Risk for Liver Cirrhosis and Hepatocellular Carcinoma According to NAFLD Status
| Crude | Adjusted | |||
|---|---|---|---|---|
| Outcome | HR (95% CI) | P value | HR (95% CI) | P value |
| Cirrhosis | ||||
| Total population | ||||
| Non-NAFLD | 1.00 (reference) | 1.00 (reference) | ||
| NAFLD/normal liver enzymes† | 1.09 (0.69–1.72) | .7064 | 0.73 (0.46–1.16) | .1832 |
| NAFLD/elevated liver enzymes | 4.47 (3.05–6.53) | <.0001 | 3.51 (2.36–5.22) | <.0001 |
| P for trend | <.0001 | P for trend | <.0001 | |
| Nonobese (BMI <25 kg/m2) | ||||
| Non-NAFLD | 1.00 (reference) | 1.00 (reference) | ||
| NAFLD/normal liver enzymes† | 1.28 (0.71–2.31) | .4176 | 0.77 (0.42–1.41) | .3980 |
| NAFLD/elevated liver enzymes | 7.05 (4.27–11.64) | <.0001 | 4.48 (2.66–7.55) | <.0001 |
| P for trend | <.0001 | P for trend | <.0001 | |
| Nonobese and without diabetes | ||||
| Non-NAFLD | 1.00 (reference) | 1.00 (reference) | ||
| NAFLD/normal liver enzymes† | 1.09 (0.55–2.18) | .8028 | 0.79 (0.39–1.60) | .5158 |
| NAFLD/elevated liver enzymes | 6.60 (3.75–11.62) | <.0001 | 5.22 (2.94–9.27) | <.0001 |
| P for trend | <.0001 | P for trend | <.0001 | |
| Hepatocellular carcinoma | ||||
| Total population | ||||
| Non-NAFLD | 1.00 (reference) | 1.00 (reference) | ||
| NAFLD/normal liver enzymes† | 1.04 (0.58–1.88) | .8970 | 0.57 (0.31–1.04) | .0646 |
| NAFLD/elevated liver enzymes | 2.90 (1.69–4.98) | .0001 | 1.91 (1.08–3.38) | .0256 |
| P for trend | .0004 | P for trend | .0005 | |
| Nonobese (BMI <25 kg/m2) | ||||
| Non-NAFLD | 1.00 (reference) | 1.00 (reference) | ||
| NAFLD/normal liver enzymes† | 1.14 (0.53–2.48) | .7345 | 0.67 (0.31–1.49) | .3292 |
| NAFLD/elevated liver enzymes | 3.32 (1.48–7.46) | .0036 | 2.27 (0.99–5.23) | .0542 |
| P for trend | .0147 | P for trend | .0405 | |
| Nonobese and without diabetes | ||||
| Non-NAFLD | 1.00 (reference) | 1.00 (reference) | ||
| NAFLD/normal liver enzymes† | 0.71 (0.27–1.88) | .4896 | 0.49 (0.18–1.31) | .1561 |
| NAFLD/elevated liver enzymes | 2.36 (0.89–6.26) | .0845 | 1.99 (0.74–5.30) | .1707 |
| P for trend | .3201 | P for trend | .0888 |
Abbreviation: NAFLD: nonalcoholic fatty liver disease; CI: confidence interval; HR: hazard ratio.
Adjusted for age, sex, platelet count, alpha-fetoprotein, and diabetes.
Normal liver enzymes defined by ALT < 40 and AST < 37 IU/L for male and ALT < 31 and AST < 31 IU/L for female participants.
Risks of Cirrhosis and HCC Among Individuals Classified as Nonobese and Those Classified as Nonobese Without Diabetes
In our study cohort, 61 053 (68%) individuals without NAFLD and with normal liver enzymes, 21 684 (24%) individuals with NAFLD and normal liver enzymes, and 6686 (7%) individuals with NAFLD and elevated liver enzymes were considered nonobese (Table 2). Similar to the findings in the entire cohort, nonobese participants with NAFLD and elevated liver enzymes exhibited a higher incidence of cirrhosis and HCC (Table 2). As illustrated in Figure 2B and Figure 3B, the participants with NAFLD and elevated liver enzyme levels consistently displayed high cumulative risks of advanced liver disease, with cumulative incidences of 0.59% for cirrhosis and 0.17% for HCC. After controlling for other risk factors, elevated liver enzyme levels were associated with increased risk of cirrhosis and HCC among nonobese individuals (P for trend < .05), while no significant differences were observed in the risk of cirrhosis or HCC among participants with normal liver enzyme levels, either with or without NAFLD (Table 3).
The findings remained similar even after we restricted the analysis to those nonobese participants without diabetes (n = 86 260). Individuals with NAFLD and elevated liver enzymes still had higher cumulative risk of cirrhosis compared with NAFLD with normal liver enzymes (P < .001, Figure 2C). The cumulative risk of HCC for NAFLD with elevated liver enzymes remained high compared with the NAFLD with normal liver enzymes (P = .04). After adjustment for other risk factors, NAFLD with elevated liver enzyme had higher risk for cirrhosis (P for trend < .001) and HCC (P for trend = .09) compared with non-NAFLD group (Table 3).
Discussions
This large-scale study revealed that the risk of advanced liver disease was low among individuals with NAFLD and normal liver enzymes. However, NAFLD with elevated liver enzymes was associated with increased risks of cirrhosis and HCC. These associations remained similar even when we restricted the analysis to the participants without obesity or those without obesity or diabetes. These findings suggest that patients with elevated liver enzymes should be monitored in clinical settings.
Although the incidence of NAFLD-related HCC is considerably lower than that of other HCC-related etiologies, such as chronic hepatitis B and C virus infection, the prevalence of NAFLD is higher than that of chronic viral hepatitis. Moreover, individuals with NAFLD typically have other comorbidities21. In a study that identified NAFLD using proton magnetic resonance spectroscopy plus intrahepatic triglyceride content of ≥5%, the prevalence of NAFLD in a Chinese population was 28.6%22. However, only approximately 4% of patients with NAFLD had advanced fibrosis, suggesting that the prevalence of severe liver disease was low despite the high NAFLD prevalence22. Risk stratification for the targeting of patients with NAFLD for HCC surveillance would allow clinicians to more effectively plan secondary prevention strategies.
In Asian countries, metabolic fatty liver disease is rapidly increasing because of sedentary behavior, low levels of physical activity, high calorific intake, and Western diets23. Among patients with nonalcoholic and noncirrhotic HCC, at least 35% had fat content of >5% in hepatocytes, suggesting the relevance of NAFLD in Asian populations24. The incidence of HCC over a median of 5.6 follow-up years in Japanese patients with ultrasonography-diagnosed NAFLD was 0.25%, with an estimated annual rate of 0.043%25. These low rates are similar to our findings and those of other studies conducted in Western countries26, 27. Another Korean study reported that the estimated incidence of HCC in NAFLD was 23 per 100 000 person-years28. In the present study, we found that the adults with NAFLD had incidences of cirrhosis and HCC of 25.9 and 11.8 (per 100 000 person-years), respectively. Compared with the findings of the Korean study, the incidence of reported HCC is lower in our study. However, the Korean study only monitored patients in the hospital and was unable to take death into account; thus, the HCC incidence rates may have been overestimated28.
ALT is an enzyme primarily present in the liver that is involved in the transfer of amino groups of alanine to ketoglutaric acid, whereas AST is mostly found within the mitochondria. Although normal ALT and AST levels may not exclude the possibility of NAFLD, NAFLD and NASH are reportedly the most common causes of elevated liver enzymes29. Abnormal levels of ALT and AST among patients with suspected NAFLD should prompt referral for in-depth assessment of disease severity and inform decision-making on whether to perform liver biopsy as per clinical guidelines30. Our study supported the notion that elevated ALT and AST could be used as a prognostic marker among individuals with NAFLD19. In addition, the findings were consistent for NAFLD in nonobese individuals or in those without diabetes, suggesting that these findings are not driving by obesity or diabetes and that elevated liver enzyme levels indicate increased risk of advanced liver disease across all patients with NAFLD. At least 13% of patients with NAFLD had normal ALT7, suggesting that ALT alone was not a satisfactory prognostic factor for patients with NAFLD. Our results suggest that the addition of AST can help identify those at high risk of developing cirrhosis and HCC19.
The epidemiological characteristics of NAFLD and its associated risk for HCC vary in Asian and non-Asian populations. The considerable differences between these populations emphasize the need for more studies to be conducted in Asia. The body compositions of Asian and non-Asian individuals differ notably. Generally, non-Asian individuals are considered lean if their BMI is below 25 kg/m2, whereas Asians are considered lean if their BMI is below 23 kg/m2. In our study population, few HCC cases among lean adults were observed; thus, we did not estimate their specific risks for cirrhosis or HCC. In some cases, Asian individuals accumulate abdominal and visceral fat despite low BMI31. Liver diseases appear to be more severe among Asian than non-Asian patients14. Multiple genetic variants are associated with hepatic steatosis across ancestries, and missense variants in PNPLA3 and GCKR genes are likely functional across multiple ancestries32.
Large-scale studies may provide more useful information, particularly regarding the influence of NAFLD in Asian patients, for which evidence remains limited. Previous population-based studies investigating the clinical course of NAFLD have primarily originated from the National Health and Nutrition Examination Survey (NHANES) program19, 33 or from the US Veterans Health Administration system8, 34, 35. However, among the Veterans study participants, nearly 90% were men, 70% were White, 65% had a BMI of ≥30 kg/m2, and 35% had diabetes. The mean age in the Veteran study was nearly 60 years8, which is older than that of our study population (44.3 years). Moreover, 55% of the veterans enrolled had abnormal ALT levels, resulting in higher cirrhosis and HCC incidence rates8. Moreover, the NHANES studies were limited by a lack of granular clinical data and incomplete diagnoses of cirrhosis and HCC19, 33. The findings of these Western studies may or may not apply to Asian populations, highlighting the importance of our study.
Ultrasonography-diagnosed NAFLD is not associated with increased mortality, but advanced fibrosis is a significant predictor of liver-specific mortality33. One study that enrolled patients with biopsy-proven NAFLD consistently found that HCC incidence rates increased monotonically across categories of steatosis, nonfibrotic steatohepatitis, noncirrhotic fibrosis, and cirrhosis36. We stratified NAFLD into 2 groups according to non-elevated or elevated liver enzyme level, which is regarded as a proxy for NASH19. Although our study was distinct in from the Western studies in several respects, our findings are consistent with those of previous studies, indicating that NAFLD with elevated liver enzyme was associated with liver disease progression and requires ongoing clinical monitoring8, 33, 36. Our study revealed that NAFLD with elevated liver enzymes was associated with increased risk of advanced liver disease, a key mechanism of which might be NAFLD-induced liver inflammation.
The strengths of our study include the large-scale and well-characterized population of men and women, a long-term follow-up, and endpoints determined on the basis of reliable nationwide registries. We were also able to adjust for multiple confounders; by excluding the individuals with chronic viral hepatitis and excessive alcohol consumption, we avoided the impacts of these factors. Previous studies have defined NAFLD according to elevated ALT levels34, 35 or fatty liver index37. Although ultrasound has limitations and liver biopsy is regarded as the gold standard, ultrasound remains the recommended noninvasive tool for accurate diagnosis of NAFLD and is used in clinical and population-based studies. Additionally, ultrasound is more feasible for diagnosis of NAFLD in the general population. However, if individuals with less severe steatosis were mistakenly given a diagnosis of not having NAFLD, we may have overestimated cirrhosis and HCC risk in the non-NAFLD control group. Another limitation that should be acknowledged is that the participants’ liver enzymes were assessed only once. Changes in the levels of ALT and AST may have occurred during follow-up. However, this limitation underscores the importance of carefully observing and following-up cases of NAFLD with abnormal liver enzyme levels. The nondifferential misclassification of normal or elevated liver enzymes among participants with NAFLD may attenuate the estimated risks.
In conclusion, this large-scale study revealed that NAFLD with elevated ALT or AST levels significantly increased the risks of cirrhosis and HCC. The findings were consistent among the nonobese and nonobese without diabetes groups. It is needed to differential NAFLD patients with or without elevated liver enzymes for more close surveillance. Individuals with NAFLD and elevated liver enzyme levels should be monitored and guided in terms of behavioral modifications.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND
Incorporating imaging in addition to elevated concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) might provide insights for nonalcoholic fatty liver disease (NAFLD) patients on the risk stratification for hepatocellular carcinoma (HCC) surveillance.
FINDINGS
The prospective study revealed that NAFLD patients with elevated liver enzymes had increased risk for cirrhosis and HCC. Individuals with normal liver enzyme levels had similar risks of cirrhosis and HCC regardless the presences of NAFLD. These associations remained consistent among the subgroup participants without obesity or those without obesity or diabetes.
IMPLICATIONS FOR PATIENT CARE
Individuals with NAFLD and elevated ALT or AST levels significantly increased the risks of cirrhosis and HCC, and should be monitored and guided in terms of behavioral modifications.
Financial support:
This study was supported by the Ministry of Science and Technology, Taipei, Taiwan (grant: 109-2926-1010-504; 109-2628-B-010-010 and 110-2628-B-A49A-507), the National Health Research Institute, Taiwan (grant: NHRI-EX111-11117PI) and by general funds from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (DCEG). None of the funding organization played a role in the study design and conduct; data collection, management, analysis, and interpretation; data preparation and review; or manuscript approval.
All or part of the data used in this research were authorized by, and received from MJ Health Research Foundation (Authorization Code: MJHRF2019005A). Any interpretation or conclusion described in this paper does not represent the views of MJ Health Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The authors disclose no conflicts of interest.
References
- 1.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–86. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–30. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–55. [DOI] [PubMed] [Google Scholar]
- 4.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1–10. [DOI] [PubMed] [Google Scholar]
- 5.Sheka AC, Adeyi O, Thompson J, et al. Nonalcoholic steatohepatitis: A review. JAMA. 2020;323(12):1175–83. [DOI] [PubMed] [Google Scholar]
- 6.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286–92. [DOI] [PubMed] [Google Scholar]
- 7.Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48(3):792–8. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan Y, Kramer JR, Yu X, et al. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology. 2020;72(4):1242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee TY, Wu JC, Yu SH, et al. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141(7):1307–14. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastrol Hepatol. 2017;15(4):474–85. [DOI] [PubMed] [Google Scholar]
- 11.Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52. [DOI] [PubMed] [Google Scholar]
- 12.Hagstrom H, Nasr P, Ekstedt M, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol Commun. 2018;2(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67(4):862–73. [DOI] [PubMed] [Google Scholar]
- 14.Mohanty SR, Troy TN, Huo D, et al. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50(4):797–804. [DOI] [PubMed] [Google Scholar]
- 15.Wen CP, Cheng TYD, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371(9631):2173–82. [DOI] [PubMed] [Google Scholar]
- 16.Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22(6):775–7. [DOI] [PubMed] [Google Scholar]
- 17.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. 2008;17(3):370–4. [PubMed] [Google Scholar]
- 18.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J. 1986;292(6512):13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez CS, Graubard BI, Thistle JE, et al. Attributable fractions of nonalcoholic fatty liver disease for mortality in the United States: results from the third National Health and Nutrition Examination Survey with 27 years of follow-up. Hepatology. 2020;72(2):430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang CJ, You SL, Chen CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45(3):291–6. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y, Cho J, Cho YK, et al. Alcoholic and Nonalcoholic Fatty Liver Disease and Incident Hospitalization for Liver and Cardiovascular Diseases. Clin Gastrol Hepatol. 2020;18(1):205–15 e7. [DOI] [PubMed] [Google Scholar]
- 22.Wong VW-S, Chu WC-W, Wong GL-H, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61(3):409–15. [DOI] [PubMed] [Google Scholar]
- 23.Zhou F, Zhou J, Wang W, et al. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology. 2019;70(4):1119–33. [DOI] [PubMed] [Google Scholar]
- 24.Huang SF, Chang IC, Hong CC, et al. Metabolic risk factors are associated with non-hepatitis B non-hepatitis C hepatocellular carcinoma in Taiwan, an endemic area of chronic hepatitis B. Hepatol Commun. 2018;2(6):747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura Y, Arase Y, Ikeda K, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107(2):253–61. [DOI] [PubMed] [Google Scholar]
- 26.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. [DOI] [PubMed] [Google Scholar]
- 27.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. [DOI] [PubMed] [Google Scholar]
- 28.Kim GA, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2018;68(1):140–6. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56(1):234–40. [DOI] [PubMed] [Google Scholar]
- 30.EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. [DOI] [PubMed] [Google Scholar]
- 31.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–40. [DOI] [PubMed] [Google Scholar]
- 32.Palmer ND, Musani SK, Yerges-Armstrong LM, et al. Characterization of European ancestry nonalcoholic fatty liver disease-associated variants in individuals of African and Hispanic descent. Hepatology. 2013;58(3):966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanwal F, Kramer JR, Li L, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71(3):808–19. [DOI] [PubMed] [Google Scholar]
- 36.Simon TG, Roelstraete B, Sharma R, et al. Cancer risk in patients with biopsyconfirmed nonalcoholic fatty liver disease: A population-based cohort study. Hepatology. 2021;74(5):2410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aberg F, Puukka P, Salomaa V, et al. Risks of Light and Moderate Alcohol Use in Fatty Liver Disease: Follow-Up of Population Cohorts. Hepatology. 2020;71(3):835–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


