Abstract
SpikoGen® is a subunit recombinant spike protein vaccine combined with Advax‐CpG55.2™ adjuvant. This COVID‐19 vaccine was shown to be safe, immunogenic and efficacious in previous clinical trials. This study aimed to assess the safety and immunogenicity of SpikoGen® vaccine as a homologous and heterologous booster vaccination. This double‐blind and randomized placebo‐controlled (5:1) trial was performed on 300 already vaccinated participants. SpikoGen® or saline placebo was administered as a booster dose to participants who had received a full two‐dose COVID‐19 vaccination course. Immunogenicity assessments were done 14 days after the booster dose with the primary immunogenicity outcome seroconversion rate of neutralizing antibodies. Safety outcomes included the incidence of solicited adverse events up to 7 days after the booster dose. SpikoGen® vaccine induced a robust humoral response both as a homologous and heterologous booster, when compared to the placebo. At Day 14, seroconversion of neutralizing antibodies was 76% (95% confidence interval [CI]: 69%–82%) in the SpikoGen® group versus 3% (95% CI: 0%–13%) in the placebo group. The most common local and systemic reported adverse events were injection site pain and fatigue. No serious adverse events were reported. The SpikoGen®‐booster induced cross‐neutralization of other SARS‐CoV‐2 variants. Irrespective of the primary vaccine course received, SpikoGen® vaccine showed promising effects as both a homologous and heterologous booster dose. This vaccine also had a good safety profile with no vaccine‐associated serious adverse events. On the basis of these results, SpikoGen® vaccine has been approved as a booster dose.
Keywords: booster, COVID‐19, cross‐neutralization, SARS‐CoV‐2, SpikoGen
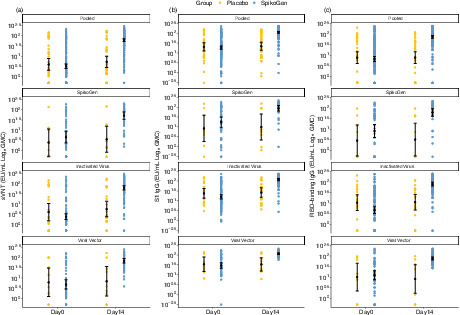
Shown are geometric mean concentration (GMC) in the per‐protocol set for sVNTw responses (panel a), S1w IgG responses (panel b) and RBDw IgG responses (panel c) at Days 0 and 14 (14 days after the booster dose). Antibody values below the LLOQ were replaced by 0.5 × LLOQ. The 95% CI was calculated based on the t‐distribution of the log‐transformed values for GMC and GM levels, then back‐transformed to the original scale for presentation. CI, confidence interval; RBDw, receptor‐binding domain (W subscript refers to the Wuhan‐Hu‐1 strain); S1w, S1w part of the spike protein (W subscript refers to the Wuhan‐Hu‐1 strain); sVNTw, surrogate virus‐neutralizing test (W subscript refers to the Wuhan‐Hu‐1 strain).
Abbreviations
- BAU
binding antibody unit
- COVID‐19
coronavirus disease 2019
- FDA
Food and Drug Administration
- GMC
geometric mean concentration
- GMFR
geometric mean fold rise
- NTD
N‐terminal domain
- RBDw
receptor‐binding domain (W subscript refers to the Wuhan‐Hu‐1 strain)
- S1w
S1w part of the spike protein (W subscript refers to the Wuhan‐Hu‐1 strain)
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- sVNTw
surrogate virus‐neutralizing test (W subscript refers to the Wuhan‐Hu‐1 strain)
- WHO
World Health Organization
INTRODUCTION
As of May 2022, SARS‐CoV‐2 remains an ongoing problem with waning natural and vaccine‐induced immunity and immune escape of new variants resulting in an increasing number of re‐infections and vaccine‐breakthrough infections worldwide. This has directed development efforts towards optimal vaccine boosters to restore waning immunity. Nevertheless, many important questions regarding COVID‐19 booster doses remain, including optimal timing, dose and whether mismatch between the primary vaccine course received and the booster vaccine affects the nature of the resulting immune response. A key consideration for COVID‐19 boosters is their ability to induce cross‐neutralizing antibodies against relevant current Omicron variants as opposed to the ancestral strains on which the current vaccines are based.
Studies have shown that homologous or heterologous vaccine boosters can enhance serum antibody levels while having an acceptable safety profile [1, 2]. Immunization by ChAdOx1 nCoV‐19A followed by BNT162b2 mRNA vaccine induced a strong antibody response [3]. Similarly, booster vaccination with a recombinant subunit vaccine following priming with an inactivated SARS‐CoV‐2 vaccine increased neutralizing titres [4]. A COVID‐19 booster vaccine should ideally increase the breadth of variant recognition rather than just amplify the antibody responses against the original vaccine strain. With Omicron strains currently dominant globally, a critical test for any current booster vaccine is its ability to induce Omicron cross‐neutralizing antibodies, with mounting evidence that Omicron is relatively vaccine‐resistant [5].
SpikoGen® is a stable S protein trimer and was optimized for insect cell expression as a soluble secreted product. This vaccine‐induced high titres of neutralizing antibodies in mice after two intramuscular doses and protected ferrets from nasal viral shedding [6]. A Phase 1 trial in Australia was performed with satisfactory safety and immunogenicity results. Phase 2 and 3 studies of SpikoGen® in Iran demonstrated positive safety, immunogenicity and efficacy resulting in emergency use authorization from Iran's food and drug organization [7]. Additional trials of SpikoGen® vaccine are currently in progress in children aged 5 years and above (NCT05231590).
This study aimed to assess the immunogenicity and safety of SpikoGen® booster shot following primary vaccination with inactivated, viral vector or SpikoGen® vaccine.
METHODS
Trial design and participants
This study is a parallel randomized and double‐blind placebo‐controlled trial performed on 300 COVID‐19 vaccinated participants with a 5:1 ratio and a follow‐up duration of 6 months after the booster dose. Adults 18 years and older with stable medical conditions were included in the study. Participants were allowed to enter the study if they had received their first vaccination course 4–9 months before the screening visit. The exclusion criteria included: pregnant or lactating women; clinical signs or symptoms of SARS‐CoV‐2; history of severe or progressive neurological or seizure disorders, immunosuppressive treatment; history of severe adverse reactions (e.g., anaphylaxis) to any components of SpikoGen® or other medications; receiving any other research product within 30 days before screening; history of COVID‐19 between the first vaccination series and booster dose; previous vaccination with any other authorized vaccine within 28 days before screening (or the intention to receive such a vaccine within 14 days after the booster dose); history of bleeding tendency disorders; administration of (or the intention to receive) any blood, plasma or immunoglobulin products within 90 days before screening; donation of more than or equal to 450 ml of blood within 28 days before screening; and any special circumstances that, in the researcher's opinion, may increase the risk of participating in the study or interfere with the evaluation of the initial objectives of the study.
All participants signed the informed consent form before enrolment. SpikoGen® or saline placebo was administered to the participants on Day 0. Fourteen days after the booster dose, the participants became unblinded and the trial continued as open‐label. The placebo group also received the SpikoGen® vaccine 14 days after the booster dose. Safety follow‐up will continue until 6 months after the booster dose.
The study was approved by the Iran National Committee for Ethics in Biomedical Research (ethics code number: IR.NREC.1400.015) and was registered at the Iranian Registry of Clinical Trials (IRCT20150303021315N26) and at ClinicaTrial.gov (NCT 05175625).
Randomization and masking
Eligible participants were assigned to groups using stratified permuted block randomization with a 5:1 allocation ratio (250 to the vaccine booster group, 50 to the placebo group) by R‐CRAN‐version 4.0.1. There were three strata for randomization based on the primary vaccine platform: received. Adenoviral vector (including ChAdOx1 or Sputnik V), inactivated whole virus (BBIBP‐CorV or COVIran Barekat) or recombinant protein (SpikoGen®). Most of the SpikoGen®‐prime participants were past participants of the SpikoGen® Phase 2 clinical trial. The appearance of the vaccine and the placebo were identical, and the participants, investigators, and laboratory staff were masked to the treatment allocation.
Outcomes
The primary outcome was seroconversion rate of neutralizing antibodies via surrogate virus‐neutralizing test (sVNTw). Secondary outcomes included the geometric mean concentrations (GMC) of S1w, receptor‐binding domainw (RBDw), and neutralizing antibodies in the two groups. Geometric mean fold rise (GMFR) in comparison to baseline along with seroconversion against S1w, RBDw and T‐cell responses were also assessed on Day 14.
Seroconversion was defined as a change in the status of antibody levels from negative to positive based on the prespecified commercial ELISA kits threshold and at least a fourfold rise in the antibody levels on Day 14 over the baseline values. T‐cell response was assessed on 40 participants in the SpikoGen® booster group only on Days 0 and 14. The test was performed based on QuantiFERON SARS‐CoV‐2 RUO (Qiagen) toolset. Tube 1 contained spike protein CD4+ peptide epitopes and tube 2 contained spike protein CD4+ and CD8+ peptide epitopes from the spike protein. The levels of interferon‐gamma in plasma samples were reported in international units per millilitre according to the manufacturer's instructions. The full data regarding the whole of these assays and thresholds are provided in the Supporting Information.
Safety outcomes included the incidence of local and systemic solicited adverse events for 7 days after the booster dose. Serious adverse events are being evaluated up to 6 months after the booster dose. Safety outcomes were reported based on the classifications provided in the Medical Dictionary for Regulatory Activities (MedDRA). Each patient's severity score was assessed based on the FDA toxicity grading scale [8].
Statistical analysis
This booster dose study included 300 participants. The sample size was not calculated based on statistical power. All the participants who received the booster dose were included in the safety population. Safety was presented as counts and percentages of participants with at least one solicited (local or systemic) adverse event for each group. The immunogenicity objectives were reported based on the per‐protocol set in which all randomly selected participants received a booster dose of the study treatment and did not have missing antibody results or major protocol deviation. Moreover, participants who were infected with SARS‐CoV‐2 were excluded from the per‐protocol population.
Missing data were not imputed. No multiplicity adjustments were made in this study. Continuous data were compared using t‐test and categorical data were assessed using a chi‐squared test or Fisher's exact test. Hypothesis testing was two‐sided, and p values less than 0.05 were considered significant. The 95% CIs for GMC and GMFR were calculated based on the t‐distribution of the log‐transformed values and then back‐transformed to the original scale at each time point for presentation. Wilcoxon tests were used to compare the paired samples to evaluate the difference in the concentrations of interferon‐gamma in the SpikoGen® group.
Sub‐group analyses were performed based on the type platform of vaccine used in the primary vaccination course. We used R (version 4.0.1), STATA 14 and Graphpad Prism for all statistical analyses. Results are presented as RU/ml per the assay kit results but were also converted into binding antibody units (BAUs) using the WHO standards (see Supporting Information).
RESULTS
The trial was initiated on 15 December 2021. In total, 250 participants were enrolled in the SpikoGen® booster group, and 50 received the saline placebo. Screening process of the participants is provided in the CONSORT diagram in Figure 1. Participants in the inactivated‐prime and viral vector‐prime had on average received their primary vaccination series only 4–5 months previously, whereas most in the SpikoGen® group had received their primary vaccination series approximately 6 months previously as part of the SpikoGen® Phase 2 trial. The baseline serology status of the participants is provided in Table 1.
FIGURE 1.
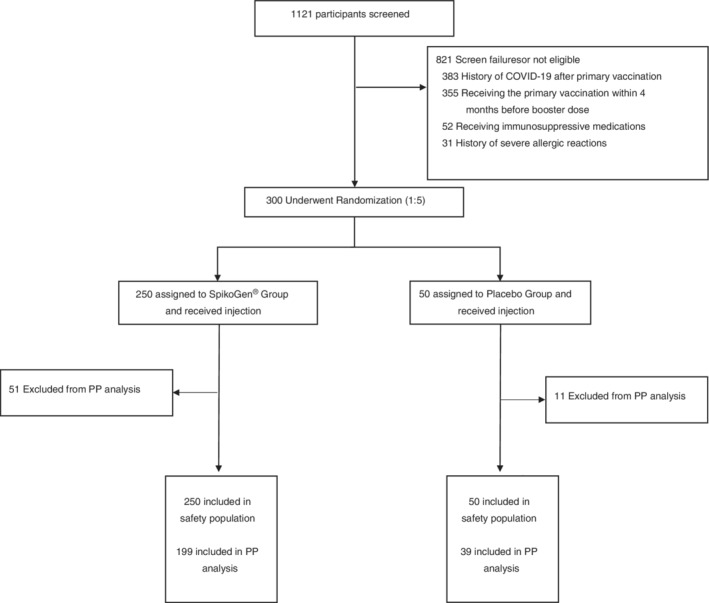
CONSORT flowchart showing schema of screening, randomization and analysis of participants
TABLE 1.
Baseline characteristics and past medical history of the participants
| Characteristics | SpikoGen® | Placebo |
|---|---|---|
| N = 250 | N = 50 | |
| Sex—n (%) | ||
| Male | 114 (45.60) | 18 (36) |
| Age (years)—mean ± SD | 44.04 ± 16.05 | 48.99 ± 18.10 |
| First vaccination platform—n (%) | ||
| Inactivated whole virus | 142 (56.8) | 28 (56.0) |
| Viral vector | 56 (22.4) | 12 (24.0) |
| SpikoGen® | 52 (20.8) | 10 (20.0) |
| Seropositive at baseline—n (%) | ||
| Inactivated whole virus | 54 (38.03) | 11 (39.29) |
| Viral vector | 29 (51.79) | 5 (41.67) |
| SpikoGen® | 28 (53.85) | 5 (50) |
| Medical history—frequency (%) a | ||
| Anxiety/depression | 4 (1.6) | 3 (6) |
| Arrhythmia | 5 (2) | 0 (0) |
| Asthma | 2 (0.8) | 0 (0) |
| Cerebrovascular accident | 18 (7.2) | 7 (14) |
| Deep vein thrombosis | 13 (5.2) | 4 (8) |
| Diabetes mellitus | 15 (6) | 2 (4) |
| Dyslipidemia | 18 (7.2) | 6 (12) |
| Hepatic steatosis | 18 (7.2) | 4 (8) |
| Hypothyroidism | 2 (0.8) | 0 (0) |
| Malignancy | 9 (3.6) | 2 (4) |
| Multiple sclerosis | 5 (2) | 2 (4) |
| Ischemic heart disease | 6 (2.4) | 1 (2) |
| Nephrolithiasis | 12 (4.8) | 2 (4) |
| Osteoarthritis | 5 (2) | 1 (2) |
| Rheumatoid arthritis | 2 (0.8) | 1 (2) |
| Other autoimmune disorders | 17 (6.8) | 7 (14) |
Malignancy includes Prostate cancer and breast cancer. Other autoimmune disorders include Ankylosing spondylitis, Autoimmune thyroiditis, Behcet's syndrome, Cutaneous vasculitis, Hyperthyroidism, Inflammatory bowel disease, Lichen planus, Psoriasis and Psoriatic arthropathy.
As Table 1 shows, at baseline, 33 (53.23%) of participants in the SpikoGen® prime group, 65 (38.24%) in the inactivated whole virus prime group and 34 (50%) participants in the viral vector prime group were still seropositive. Demographic data and medical histories of the participants are also presented in Table 1.
Immunogenicity outcomes
By Day 14 post‐booster, seroconversion of neutralizing antibodies in the pooled population was 76% (95% CI: 69%–82%) in the SpikoGen® booster group versus 3% (95% CI: 0% to 13%) in the placebo group. Seroconversion of binding IgG against the S1w protein in the pooled population was 56% (95% CI: 49% to 63%) for SpikoGen® group versus 0% (95% CI: 0%–9%) for the placebo group and seroconversion of binding IgG against the RBDw protein in the pooled group was 64% (95% CI: 57%–70%) for SpikoGen® group versus 0% (95% CI: 0%–9%) for the placebo group. The results of seroconversion based on the type of the primary vaccination course are provided in Table 2.
TABLE 2.
Seroconversion rate in the participants
| Pooled | SpikoGen® | Inactivated virus | Viral vector | |||||
|---|---|---|---|---|---|---|---|---|
| SpikoGen® | Placebo | SpikoGen® | Placebo | SpikoGen® | Placebo | SpikoGen® | Placebo | |
| sVNTw | ||||||||
| SCR | 151 (76) | 1 (3) | 28 (68) | 0 (0) | 87(80) | 1 (5) | 36 (73) | 0 (0) |
| 95% CI | (69–82) | (0–13) | (52–82) | (0–34) | (71–87) | (0–24) | (59–85) | (0–34) |
| S1w IgG | ||||||||
| SCR | 111 (56) | 0 (0) | 14 (34) | 0 (0) | 74 (68) | 0 (0) | 23 (47) | 0 (0) |
| 95% CI | (49–63) | (0–9) | (20–51) | (0–34) | (58–77) | (0–16) | (33–62) | (0–34) |
| RBDw | ||||||||
| SCR | 127 (64) | 0 (0) | 18 (44) | 0 (0) | 84 (77) | 0 (0) | 25 (51) | 0 (0) |
| 95% CI | (57–70) | (0–9) | (28–60) | (0–34) | (68–85) | (0–16) | (36–66) | (0–34) |
Note: Percentages for seroconversion rate (SCR) were calculated as a number of subjects who reported the event divided by the number of subjects in the Per‐Protocol Set at Day 14 with non‐missing data multiply 100. The 95% confidence interval (CI) for SCR was calculated using the exact Clopper–Pearson method.
The results of the concentrations of antibodies at baseline and 14 days after the booster dose are shown in Figure 2.
FIGURE 2.
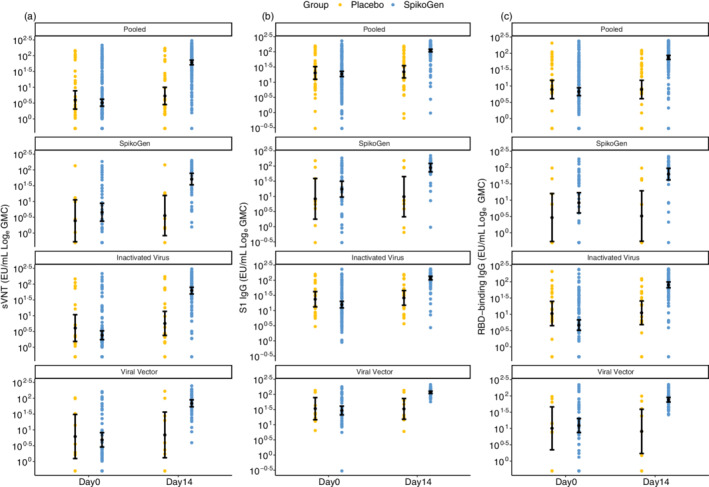
Shown are geometric mean concentration (GMC) in the per‐protocol set for sVNTw responses (panel a), S1w IgG responses (panel b) and RBDw IgG responses (panel c) at Days 0 and 14 (14 days after the booster dose). Antibody values below the LLOQ were replaced by 0.5 × LLOQ. The 95% CI was calculated based on the t‐distribution of the log‐transformed values for GMC and GM levels, then back‐transformed to the original scale for presentation. CI, confidence interval; RBDw, receptor‐binding domain (W subscript refers to the Wuhan‐Hu‐1 strain); S1w, S1w part of the spike protein (W subscript refers to the Wuhan‐Hu‐1 strain); sVNTw, surrogate virus‐neutralizing test (W subscript refers to the Wuhan‐Hu‐1 strain).
The use of the WHO standards allowed us to convert the results into BAUs. The results of these concentrations based on BAU are provided in Table S1.
The fold‐rise of the antibodies over baseline values is shown in Table 3. The geometric mean fold rise in neutralizing antibodies following prime vaccination with the inactivated whole virus platform, viral vector platform, and SpikoGen® were 25.77 (95% CI: 18.51–35.87), 14.32 (95% CI: 8.81–23.27) and 11.33 (95% CI: 6.45–19.92), respectively. As the table shows, these increases are more prominent in participants whose first vaccinations were based on the inactivated whole virus platform (which includes BBIBP‐CorV and COVIran Barekat).
TABLE 3.
GMFR of S1w IgG, RBDw IgG and sVNTw in the participants
| Pooled | SpikoGen® | Inactivated virus | Viral vector | |||||
|---|---|---|---|---|---|---|---|---|
| SpikoGen® | Placebo | SpikoGen® | Placebo | SpikoGen® | Placebo | SpikoGen® | Placebo | |
| sVNTw | ||||||||
| Day 14 (n) | 199 | 39 | 41 | 9 | 109 | 21 | 49 | 9 |
| GMFR | 18.83 | 1.34 | 11.33 | 1.46 | 25.77 | 1.40 | 14.32 | 1.13 |
| 95% CI | (14.71–24.10) | (1.15–1.56) | (6.45–19.92) | (1.04–2.04) | (18.51–35.87) | (1.09–1.79) | (8.81–23.27) | (0.97–1.31) |
| S1w‐IgG | ||||||||
| Day 14 (n) | 199 | 39 | 41 | 9 | 109 | 21 | 49 | 9 |
| GMFR | 5.92 | 1.09 | 5.11 | 1.18 | 7.49 | 1.11 | 3.97 | 0.98 |
| 95% CI | (4.94–7.10) | (0.99–1.21) | (3.10–8.44) | (0.89–1.56) | (5.93–9.45) | (0.98–1.25) | (2.91–5.42) | (0.74–1.30) |
| RBDw‐IgG | ||||||||
| Day 14 (n) | 199 | 39 | 41 | 9 | 109 | 21 | 49 | 9 |
| GMFR | 11.24 | 1 | 7.59 | 1.11 | 17.06 | 1.05 | 6.81 | 0.81 |
| 95% CI | (8.77–14.42) | (0.87–1.15) | (4.29–13.41) | (0.87–1.43) | (12.25–23.77) | (0.93–1.18) | (3.89–9.83) | (0.47–1.40) |
Note: The 95% confidence interval (CI) for geometric mean fold rise (GMFR) was calculated based on the t‐distribution of the log‐transformed values, then back‐transformed to the original scale for presentation.
Cross‐neutralization of other variants results
Next, we sought to assess the impact of the SpikoGen® booster on cross‐neutralizing the major variants of concern using a range of lentivirus pseudotype assays. A random sample of 42 baseline sera (14 from each of the 3 primary vaccine groups) was first assessed for neutralization activity against the prevailing Delta or Omicron BA.1 variants (Figure 3). Seropositivity was defined as a pVNT titre of greater than 16. Of the baseline samples tested, 37/42 (88.1%) were seropositive for neutralization activity against Delta, whereas only 12/42 (28.6%) of baseline sera were seropositive against Omicron (Figure 3a). Overall, Omicron titres at baseline were 7.2‐fold lower than for Delta. When broken down by primary vaccine group, baseline Delta neutralization activity was highest for the SpikoGen®‐prime (GMT 89, 95% CI: 44–179), followed by inactivated‐prime (GMT 73, 95% CI: 38–139) and viral vector‐prime (GMT 59, 95% CI: 29–120) groups, although these differences were not significant. Baseline Omicron BA.1 neutralization titres by subgroup went from viral vector‐prime (GMT 12, 95% CI: 6.2–24), to SpikoGen®‐prime (GMT 11, 95% CI: 5.6–23) to inactivated‐prime (GMT 7.5, 95% CI: 5.0–11) groups.
FIGURE 3.
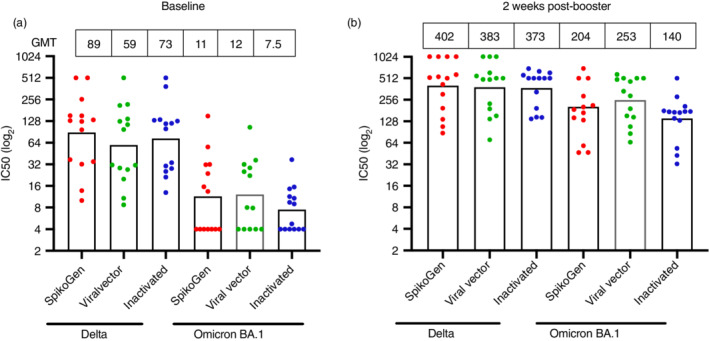
Lentivirus pseudotyping neutralization test for Delta and Omicron BA.1 in a random subset of 14 baseline sera from each primary vaccine group (a). Results from the same individuals, 2 weeks after the SpikoGen® booster dose (b). Column height shows GMT
Next, at Day 14, post‐booster sera from the 42 subjects were tested for their ability to neutralize Alpha, Beta, Gamma, Delta, Lambda and Omicron BA.1 pseudotype lentiviruses (Figure 4). All boosted sera showed high neutralization of Alpha (GMT 422, range 94–1024) and Delta (GMT 384.9, range 70.4–1024), variants. Neutralization titres against Beta, Gamma, Lambda and Omicron BA.1 variants were all significantly less than for Alpha, with the geometric mean fold ratio reduction from Alpha, being Beta (1.5), Lambda (1.7), Omicron (2.2) and Gamma (2.5) (Figure 4b). When the Day 14 post‐booster Delta or Omicron BA.1 responses were broken down by primary vaccine subgroup, no significant differences were seen between groups. Notably, the post booster fold‐rise in Omicron pVNT titres for each of the primary vaccine groups was higher than the fold‐rise in Delta pVNT titres, with the difference between Delta and Omicron neutralization reduced from more than 7‐fold lower before the boost, to ~2‐fold lower after the boost (Figure 3b).
FIGURE 4.
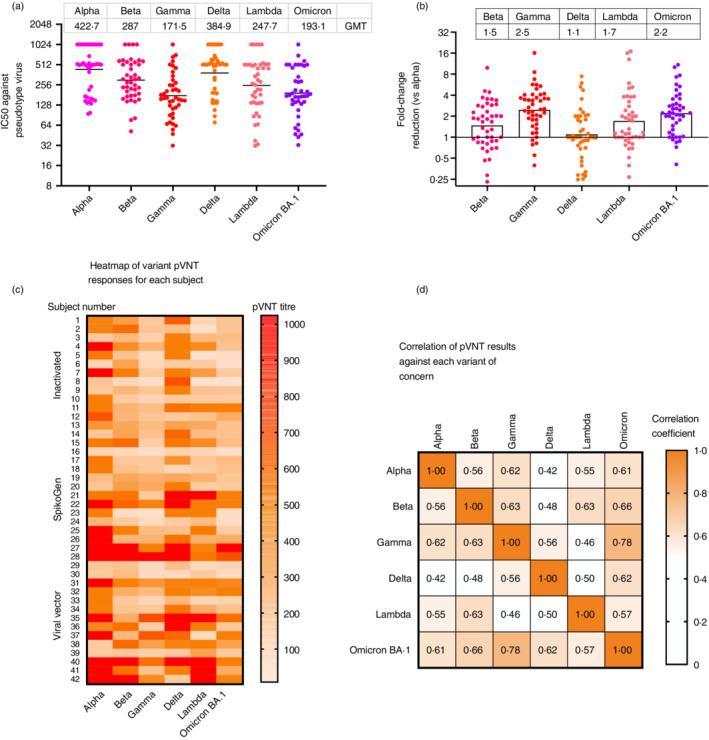
Lentivirus pseudotype viral neutralization test (pVNT) for each major variants of concern was performed on 42 trial subjects (14 per primary vaccine group). Baseline sera were tested for neutralization titres against Delta and Omicron. Individual titres and group GMT (top bar) for Day 14 post‐booster sera against each of the variants of concern are shown for the selected subjects (a). The fold‐change in pVNT for each variant was calculated by reference to the Alpha ancestral strain (b). The pVNT results against each variant for each individual subject are shown as a heatmap where the intensity of the shading reflects the level of the pVNT titre as shown by the colour scale on the right (c). Pairwise correlations were performed of pVNT results against each variant of concern for Day 14 post‐booster sera of 44 subjects and depicted as a heatmap with the correlations shown in each cell and colour scale depicting the extent of correlation shown on the right (all correlations shown were significant at p < 0.05 (d)
To more closely examine the pattern of neutralization of variants, we constructed a heatmap showing each subject's response against each of the major variants (Figure 4c). While a small number of subjects showed similar responses against all variants, most subjects showed a high level of variability, with high titres against some variants but low responses against others. To see which variants might be closest serologically, we assessed the correlation of the Day 14 post‐booster pVNT responses between variants. Alpha showed a high correlation to Gamma (r = 0.63) and Omicron responses (r = 0.61), whereas Omicron showed the highest correlation to Gamma (r = 0.77) and Beta (r = 0.65) responses (Figure 4d). Finally, we compared the fold increase in Omicron BA.1 pVNT titres from baseline to 2‐week post‐boost for each group. The SpikoGen®‐prime group had the greatest response with a 17.8‐fold increase in Omicron pVNT responses post booster, then the inactivated‐prime group with a 14.4‐fold increase and the viral vector‐prime group with a 13.2‐fold increase (data not shown).
T‐cell responses
In the SpikoGen® group, the median [IQR] interferon‐gamma concentrations changed from 0.10 [0.07–0.21] at baseline to 0.15 [0.08–0.30] on Day 14 (p = 0.006) after stimulation with CD4+ epitopes. Moreover, the median [IQR] interferon‐gamma concentration changed from 0.11 [0.08–0.21] at baseline to 0.17 [0.09–0.39] on Day 14 (p = 0.005) after stimulation with CD4+ and CD8+ epitopes. These results are also provided in Table S2.
Safety outcomes
A graphical representation of the incidence of solicited adverse events after the booster injection is shown in Figure 5. As can be seen, 174 (70%) participants developed at least one adverse event following the booster dose of SpikoGen® compared to 18 (36%) in the placebo group. Considering local complications, injection site pain was the most common adverse event among the two groups (66% in the SpikoGen® group vs. 16% in the placebo group). Among systemic complications, fatigue was the most reported adverse event, being detected in 70 (28%) participants in the SpikoGen® group versus 7 (14%) participants in the placebo group.
FIGURE 5.
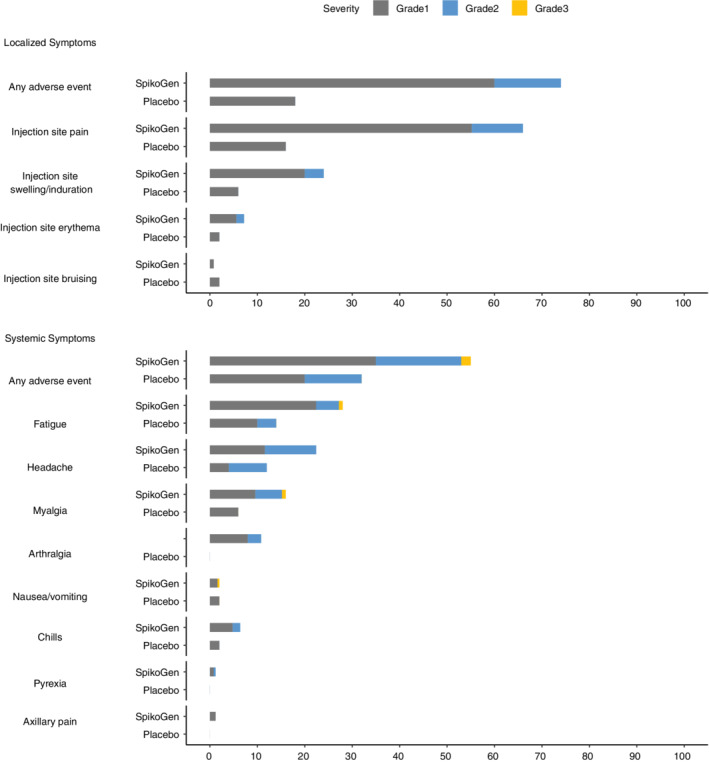
Solicited local and systemic adverse events. Shown is the percentage of participants in each group (SpikoGen®, placebo) with solicited local (upper figure) and systemic (lower figure) adverse events according to the toxicity grading scale during the 7 days after the booster. There were no grade 4 (life‐threatening) events
Exploratory results
In this study, 37 participants in the SpikoGen® primed stratum were the participants of Phase 2 trial of SpikoGen®. Hence, we assessed the increase in the S1w and RBDw levels after the booster dose in comparison with 2 weeks after the second dose of the primary vaccination in Phase 2. The results are shown in Figure 6. GMC of IgGs against S1w at 2 weeks after the second dose was 39.99 (95% CI: 22.83–70.06) versus 66.38 (95% CI: 44.16–99.79) 2 weeks after the booster dose. GMC of IgGs against RBDw at 2 weeks after the second dose was 22.47 (95% CI: 10.1–49.96) versus 41.4 (95% CI: 23.55–72.79) 2 weeks after the booster dose.
FIGURE 6.
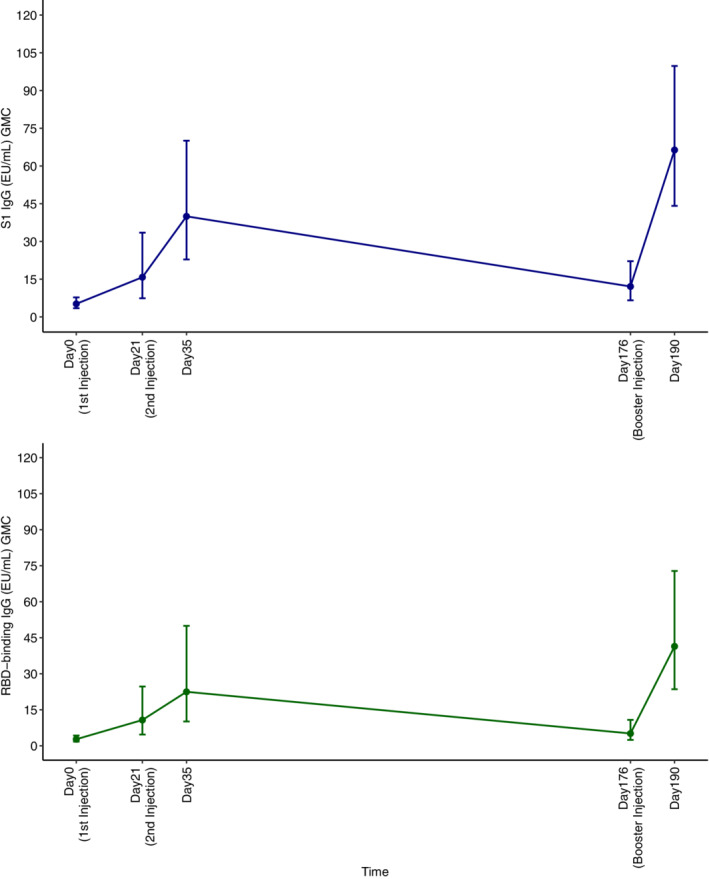
The trend of S1w and RBDw antibodies in primary vaccination and booster dose of SpikoGen®. RBDw, receptor‐binding domain (W subscript refers to the Wuhan‐Hu‐1 strain); S1w, S1w part of the spike protein (W subscript refers to the Wuhan‐Hu‐1 strain).
DISCUSSION
Based on the solid rise in antibody levels 2 weeks after administration of the SpikoGen® booster dose in all groups, SpikoGen® looks to be a promising option as either a homologous or a heterologous adjuvanted protein‐booster after priming with itself or with inactivated whole virus or viral vector vaccines.
Irrespective of vaccine platform used, it is now well recognized that serum neutralizing antibody levels fall rapidly from their peak, with antibodies after vaccination waning at similar rates to antibodies induced by natural infection, with an average of ∼90% loss in RBD IgG levels within 90 days [9]. It was estimated that after two doses of BNT162b2 mRNA COVID‐19 vaccine, the antibody half‐life in initially seronegative subjects was 55 days (95% CI: 37–107 days), although it was estimated to be longer (80 days, 95% CI: 46–303) in previously infected subjects who were seropositive prior to vaccination [10]. A rapid decline in antibody levels post‐vaccination was consistent with our baseline study data which showed that just 4–6 months after a primary course of inactivated, viral vector or recombinant protein vaccine, already ~50% of vaccinees no longer had detectable serum neutralizing antibodies by sVNTw. This was true irrespective of primary vaccine type subjects had received, albeit there was a trend to lower baseline sVNTw levels in the inactivated‐prime vaccinees. As serum neutralizing antibody levels are considered the best predictor of protection [11], our baseline sVNTw data might suggest receipt of a third booster dose may be beneficial at an interval of less than 4 months after the second dose. This may be particularly relevant given the rise of the new vaccine‐resistant variants such as Omicron to which vaccine immunity may wane even faster [12].
The size of the sVNTw response to the SpikoGen® booster dose was particularly robust in the participants who had received a primary course of inactivated whole virus vaccine. Inactivated virus vaccines typically present only low and variable amounts of spike protein to the immune system due to the low ratio of spike protein to total virus protein content in the inactivated vaccine and because some cleavage of S1 from S2 occurs during the beta‐propiolactone inactivation process [13]. Hence, in individuals primed with inactivated vaccine, exposure to the much larger 25 μg dose of spike protein in SpikoGen® may stimulate a particularly strong recall memory B cell response, helping explain the large rise in sVNTw in this group. The Advax‐CpG adjuvant in the SpikoGen® booster dose is also likely to have contributed to this strong response. A similar situation may apply if viral vector vaccines only induce low endogenous levels of spike protein that result in suboptimal B cell priming, with no way to quantitate the amount of spike protein generated in vivo by this vaccine. These results are consistent with the idea that boosting with a heterologous vaccine platform may give a stronger response than boosting with a homologous vaccine. Indeed, another COVID‐19 vaccine study showed following priming with two doses of inactivated virus vaccine, a heterologous protein subunit booster elicited a strong anti‐RBD and neutralizing antibody responses [4]. In another study, after priming with two doses of inactivated CoronaVac vaccine, boosting with a viral vector vaccine performed better than a third homologous inactivated booster [14].
These studies and our own suggest that a heterologous booster dose after inactivated whole virus may often give a better response than a homologous booster dose.
In the 37 participants of Phase 2 trial of spikoGen® who received the booster dose, GMFR of antibodies against S1w and RBDw after the booster dose was ~1.5 in comparison to the second dose. This is in line with a trial of a booster dose of homologous inactivated vaccine that showed a 1‐ to 3‐fold increase after the third dose compared to the second dose [1]. Fourteen days after a third homologous ChAdOx1 nCoV‐19 adenovirus boost, there was a 2.1‐fold increase in GMT levels over the second dose [15].
Most of the participants in the SpikoGen® primed subgroup in this study were previously enrolled in the original Phase 2 trial and had received their second dose of SpikoGen® almost 6 months before receiving the booster dose in this study. Considering this group had a second to booster dose interval was on average about a month longer than the other groups, the frequency of positive neutralizing antibody levels 6 months after the SpikoGen® primary vaccine course (53.23%) trended higher than for participants who had received the inactivated whole virus vaccines (38.24%). The frequency of positive neutralizing antibody after the Adenovirus viral vector® primary vaccine course was near to SpikoGen® primary vaccine course (50%).
How might the spike antibody levels achieved with the booster in our study, translate into SARS‐CoV‐2 protection? The use of the WHO standards allowing conversion of results into BAU/ml allows some comparisons to be made between studies. One study using data from a ChAdOx1 nCoV‐19 adenoviral vaccine study reported a vaccine efficacy of 80% against symptomatic infection with SARS‐CoV‐2 Alpha (B.1.1.7) equated to an anti‐S1w level of 264 BAU/ml, a level of 113 BAU/ml, to an efficacy of 70% and a level of 54 BAU/ml to an efficacy of 60% [16]. The overall levels in our booster study are favourably comparable to these levels and based on these predictions would be consistent with an efficacy of 80% against symptomatic infection with SARS‐CoV‐2 Alpha variant. Goldblatt et al. used samples from a number of studies using different vaccine technologies broad to define a serological cut‐off in the BAU whereby the percentage of subjects in a population above that cut‐off predicted the population level of protection [17]. The GMC to spike WT following immunization with mRNA‐1273 and BNT162b2 vaccines were 5530 BAU/ml and 2653 BAU/ml, respectively, compared to 196 BAU /ml and 61 BAU/ml following ChAdOx1 nCoV‐19 and J&J vaccines, respectively. Across all the vaccine studies analysed, the mean protective threshold was 154 BAU/ml for WT virus. Using this cut‐off, our results are well in the protective levels after the booster dose.
Attempts can similarly be made to correlate S1‐binding IgG levels with protection against other SARS‐CoV‐2 variants, after adjustments are made for any relative reduction in neutralization titres [16]. As neutralizing antibody levels against variants such as beta, gamma or omicron are typically lower than against the wildtype virus, the cut‐off level of S1w‐binding IgG predictive of protection against these variants would need to be adjusted accordingly. Hence, to predict protection against a variant with a 3‐fold reduction in neutralizing titre, would require using an adjusted S1w‐binding IgG cut‐off of 3 × 60 = 180 BAU/ml.
To identify what correction factor might be required, we first sought to determine the relative ability of the booster sera from randomly selected subjects to neutralize a broad range of variants of concern using a lentivirus pseudotyping assay. The sera showed high neutralization of the Alpha (GMT 427, range 94–1024) and Delta (GMT 384.9, range 70.4–1024) variants. Neutralization of Beta, Gamma, Lambda and Omicron were all significantly lower than Alpha, and compared to Alpha, neutralization of Delta was reduced 1.1‐fold, Beta 1.5‐fold, Lambda 1.7‐fold, Omicron 2.2‐fold and Gamma 2.4‐fold. These relatively small around 2‐fold reductions in neutralization compared to Alpha were surprising, as another study reported mRNA vaccine immune sera had 127‐ to 187‐fold lower ability to neutralize Omicron than wildtype virus and convalescent sera also had 32‐ to 60‐fold lower neutralization capacity against Omicron [5]. Although higher neutralization of Omicron was seen in that study after a third mRNA booster, or after two mRNA vaccines in previously infected subjects, neutralization of Omicron was still 10‐20‐fold lower than for wild‐type virus. Another study found sera of mRNA immunized subjects showed 7.6‐fold lower neutralization of Beta than wildtype virus, and AstraZeneca immunized subjects showing 9‐fold lower neutralization of Beta virus [18]. How SpikoGen® vaccine is able to induce such broadly cross‐neutralizing antibodies extending to Omicron is not known. SpikoGen® vaccine is based on the full extracellular domain of the spike protein consisting of both S1 and S2, and thereby includes both the RBD and the N‐terminal domain (NTD). Studies have shown neutralizing antibodies can be directed not just to the RBD but also to the NTD. Hence, the inclusion of both RBD and NTD in SpikoGen® may induce a broader repertoire of neutralizing antibodies than just against the RBD. Furthermore, the spike protein in SpikoGen® is manufactured in insect cells which glycosylate the spike protein using paucimannose‐type glycans, whereas for inactivated virus, mRNA and adenoviral vaccines, the spike protein is produced either in vitro or in vivo by mammalian cells which confer mostly complex‐ and high‐mannose‐type glycans [19]. This difference in glycosylation could conceivably mean more spike protein epitopes are accessible in the insect cell‐expressed protein, thereby rendering it more immunogenic [20]. In addition to potential antigen differences, the Advax‐CpG adjuvant in SpikoGen® has previously been shown to have the ability to induce extremely broadly cross‐neutralizing antibodies. For example, mice immunized with an inactivated Japanese encephalitis virus (iJEV) antigen with Advax‐CpG adjuvant cross‐neutralized and were protected against lethal infection with West Nile virus infection [21]. Sera from these JEV‐immunized mice cross‐neutralized an extremely broad repertoire of flaviviruses including West Nile virus, Murray Valley encephalitis virus, St Louis encephalitis virus and even dengue viruses [22]. In the current study, the SpikoGen®‐prime group had the largest increases in Omicron pVNT responses (17.8‐fold) post‐booster compared to the increases in the inactivated‐prime group (14.35‐fold) and adenovirus‐prime group (13.2‐fold). Hence, by virtue of its unique antigen and adjuvant, SpikoGen® vaccine may induce a broader variant cross‐neutralizing response than seen with other vaccines.
T‐cell responses were also increased after the booster dose of SpikoGen®. In a study that was performed on subjects who received a protein‐based booster after primary vaccination with inactivated whole virus, the interferon‐gamma levels rose significantly in the ELIspot assay after stimulation with S2, whereas stimulation with S1 did not induce interferon‐gamma [4]. In our study, stimulation with both CD4+ and CD8+ cells epitopes resulted in a significant rise in interferon‐gamma in comparison with baseline and T‐cell interferon‐gamma responses were at least modestly increased by the SpikoGen® booster. A limitation was the low number of participants in the T‐cell response assay and the lack of a control group for this T‐cell evaluation.
Considering vaccine safety, the results showed that the vaccine was well tolerated. The observed local and systemic adverse events were consistent with those normally seen after vaccination and were predominantly in the mild category and transient with full recovery. Hence, the favourable safety profile of the SpikoGen® vaccine that was observed in the previous clinical trials of SpikoGen® was further confirmed in the current clinical trial.
In conclusion, a single booster dose of SpikoGen® vaccine given 4–9 months after primary vaccination with a variety of different vaccine types including inactivated whole virus, viral vector or homologous recombinant protein, induced a strong rise in SARS‐CoV‐2 neutralizing antibodies 2 weeks post the booster. Notably, extremely broad cross‐neutralization was observed against all the major variants of concern, including Delta and Omicron BA.1. The ability to induce broad cross‐neutralization plus its strong safety profile makes SpikoGen® vaccine a promising option as a broad‐purpose COVID‐19 vaccine booster.
AUTHOR CONTRIBUTIONS
Payam Tabarsi conceptualized and coordinated the study. Nassim Anjidani recruited the participants and organized the framework. Ramin Shahpari performed statistical analysis. Khashayar Roshanzamir, Newsha Fallah and Greiciely Andre were involved in conducting, and technical support of the study. Nikolai Petrovsky was involved in conceptualizing the study. Saghar Barati was involved in the trial design, conceptualization and drafted the manuscript. All authors critically reviewed the manuscript and approved the final version. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
CONFLICTS OF INTEREST
NA, RSH, NF and SB are members of the Orchid Pharmed medical department which is in collaboration with CinnaGen company with respect to conducting clinical trials. KhR is in CinnaGen Medical Biotechnology Research Center. GA and NP are members of the Vaxine Pty Ltd. The remaining authors have no other relevant affiliations.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENTS
The authors are thankful to study participants and Data and Safety Monitoring Board committee. This study was funded by CinnaGen Co.
Tabarsi P, Anjidani N, Shahpari R, Roshanzamir K, Fallah N, Andre G, et al. Immunogenicity and safety of SpikoGen®, an adjuvanted recombinant SARS‐CoV‐2 spike protein vaccine as a homologous and heterologous booster vaccination: A randomized placebo‐controlled trial. Immunology. 2022. 10.1111/imm.13540
Funding information CinnaGen
Contributor Information
Nassim Anjidani, Email: Anjidani.N@orchidpharmed.com.
Saghar Barati, Email: barati.s@orchidpharmed.com.
DATA AVAILABILITY STATEMENT
Anonymous participant data will be available upon a reasonable request to the corresponding author.
REFERENCES
- 1. Guo W, Duan K, Zhang Y, Yuan Z, Zhang YB, Wang Z, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18 years or older: a randomized, double‐blind, placebo‐controlled, phase 1/2 trial. EClinicalMedicine. 2021;38:101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falsey AR, Frenck RW Jr, Walsh EE, Kitchin N, Absalon J, Gurtman A, et al. SARS‐CoV‐2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hillus D, Schwarz T, Tober‐Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime‐boost immunisation with ChAdOx1 nCoV‐19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9(11):1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ai J, Zhang H, Zhang Q, Zhang Y, Lin K, Fu Z, et al. Recombinant protein subunit vaccine booster following two‐dose inactivated vaccines dramatically enhanced anti‐RBD responses and neutralizing titers against SARS‐CoV‐2 and variants of concern. Cell Res. 2022;32(1):103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt F, Muecksch F, Weisblum Y, Da Silva J, Bednarski E, Cho A, et al. Plasma neutralization of the SARS‐CoV‐2 omicron variant. N Engl J Med. 2022;386(6):599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li L, Honda‐Okubo Y, Huang Y, Jang H, Carlock MA, Baldwin J, et al. Immunisation of ferrets and mice with recombinant SARS‐CoV‐2 spike protein formulated with Advax‐SM adjuvant protects against COVID‐19 infection. Vaccine. 2021;39(40):5940–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tabarsi P, Anjidani N, Shahpari R, Mardani M, Sabzvari A, Yazdani B, et al. Safety and immunogenicity of SpikoGen®, an Advax‐CpG55.2‐adjuvanted SARS‐CoV‐2 spike protein vaccine: a phase 2 randomized placebo‐controlled trial in both seropositive and seronegative populations. Clin Microbiol Infect. 2022, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Popmihajlov Z, Pang L, Brown E, Joshi A, Su SC, Kaplan SS, et al. A post hoc analysis utilizing the FDA toxicity grading scale to assess injection site adverse events following immunization with the live attenuated Zoster Vaccine (ZVL). Hum Vaccin Immunother. 2018;14(12):2916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibarrondo FJ, Hofmann C, Fulcher JA, Goodman‐Meza D, Mu W, Hausner MA, et al. Primary, recall, and decay kinetics of SARS‐CoV‐2 vaccine antibody responses. ACS Nano. 2021, Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 10. Favresse J, Bayart JL, Mullier F, Elsen M, Eucher C, Van Eeckhoudt S, et al. Antibody titres decline 3‐month post‐vaccination with BNT162b2. Emerg Microbes Infect. 2021;10(1):1495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205–11. [DOI] [PubMed] [Google Scholar]
- 12. Evans JP, Zeng C, Carlin C, Lozanski G, Saif LJ, Oltz EM, et al. Neutralizing antibody responses elicited by SARS‐CoV‐2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022;14(637):eabn8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu C, Mendonça L, Yang Y, Gao Y, Shen C, Liu J, et al. The architecture of inactivated SARS‐CoV‐2 with postfusion spikes revealed by cryo‐EM and cryo‐ET. Structure. 2020;28(11):1218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, et al. Heterologous prime‐boost immunization with CoronaVac and Convidecia. medRxiv. 2021;2021.09.03.21263062. [Google Scholar]
- 15. Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV‐19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(11):2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldblatt D, Fiore‐Gartland A, Johnson M, Hunt A, Bengt C, Zavadska D, et al. Towards a population‐based threshold of protection for COVID‐19 vaccines. Vaccine. 2022;40(2):306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, et al. Evidence of escape of SARS‐CoV‐2 variant B.1.351 from natural and vaccine‐induced sera. Cell. 2021;184(9):2348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi X, Jarvis DL. Protein N‐glycosylation in the baculovirus‐insect cell system. Curr Drug Targets. 2007;8(10):1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Urbanowicz RA, Wang R, Schiel JE, Keck ZY, Kerzic MC, Lau P, et al. Antigenicity and immunogenicity of differentially glycosylated hepatitis C virus E2 envelope proteins expressed in mammalian and insect cells. J Virol. 2019;93(7): 12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petrovsky N, Larena M, Siddharthan V, Prow NA, Hall RA, Lobigs M, et al. An inactivated cell culture Japanese encephalitis vaccine (JE‐ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross‐protective memory B cells and neutralizing antibody. J Virol. 2013;87(18):10324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Komiya T, Honda‐Okubo Y, Baldwin J, Petrovsky N. An Advax‐adjuvanted inactivated cell‐culture derived Japanese encephalitis vaccine induces broadly neutralising anti‐flavivirus antibodies, robust cellular immunity and provides single dose protection. Vaccines. 2021;9(11): 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
Anonymous participant data will be available upon a reasonable request to the corresponding author.


