Abstract
Aim
To evaluate the tolerability of casirivimab and imdevimab (CAS/IMB) therapy in pregnant women with COVID‐19 in Japan and its impact on the neonate and process of delivery.
Methods
Eight cases of pregnancy complicated by COVID‐19 and requiring hospitalization during the delta variant epidemic were included. Gestational age, initial symptoms, pregnancy complications and outcome, severity of illness, blood test findings at the time of treatment initiation and on days 3–5 after administration, body temperature at administration, and 8, 24, and 48 h post‐administration, delivery outcome, and neonatal findings were recorded. Ten pregnant women who required hospitalization at the same time and did not receive CAS/IMB were used as controls.
Results
Of the eight cases, seven were mild, and one case was of moderate severity. Body temperature in the CAS/IMB group was significantly higher at 8 h post‐administration than that at the time of administration. However, body temperature significantly reduced at 24 and 48 h post‐administration in the CAS/IMB group compared with that in the control group. There were no apparent adverse events after CAS/IMB administration.
Conclusions
Maternal administration of CAS/IMB was safe. Although it was difficult to evaluate the improvement in disease by blood test findings, the fever improved within 24 h, which suggests rapid improvement in patient condition.
Keywords: body temperature, COVID‐19, monoclonal antibody, neonates, pregnancy
Introduction
A highly transmissible coronavirus, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), emerged in China in late 2019 and spread rapidly worldwide, subsequently becoming a pandemic in March 2020. 1 SARS‐CoV‐2 infection can be asymptomatic or associated with an acute respiratory illness called coronavirus disease 2019 (COVID‐19), which can be mild to fatal. 2
The Delta (B.1.617.2) variant of SARS‐CoV‐2 has been the predominant variant from July to December 2021 in the United States and Japan. 3 This variant has been reported to increase COVID‐19‐related morbidity, 4 particularly in pregnant females with low vaccine acceptance. 5
COVID‐19 morbidity and mortality during pregnancy have significantly increased. 6 However, despite reports on the tolerability of vaccination during pregnancy, 7 vaccination coverage in the pregnant population is lower than that in the nonpregnant population. 8 Under these circumstances, in addition to preventive vaccination, neutralizing monoclonal antibodies (mAbs) targeting the SARS‐CoV‐2 spike protein have been developed and shown to reduce hospitalizations in high‐risk populations with worsening COVID‐19 symptoms. 9 One such combination of mAbs, casirivimab, and imdevimab (CAS/IMB), is a co‐packaged formulation of two neutralizing immunoglobulin gamma 1 (IgG1) human mAbs (casirivimab and imdevimab: CAS/IMB) against SARS‐CoV‐2.
In July 2021, CAS/IMB was first approved in Japan for the treatment of mild or moderate COVID‐19, and in August 2021, it was conditionally approved in the United Kingdom for the prevention and treatment of acute COVID‐19 infections. 10
The FDA added pregnancy as a high‐risk criterion to warrant the use of casirivimab and imdevimab in May 2021, 11 but data on their use in pregnant patients with COVID‐19 are limited. After the first case reported by Hirshberg et al., 12 Mayer et al. reported the efficacy and safety of CAS/IMB in two patients with moderate disease. 13 Chang et al., who reviewed 30 pregnancies which were affected between December 2020 and October 2021 and received mAbs, mentioned the possibility of potential effects due to the administration of various mAbs on the mother and fetus. 14 Richley et al. administered either of the two mAbs (Bamlanivimab/etesevimab or Casirivimab/imdevimab) to 15 pregnant cases with COVID‐19 between April 2021 and October 2021, and reported that although the outcome of the administered cases was generally favorable, adverse events due to systemic reactions during infusion were observed in the mothers and effects on the fetus (maternal respiratory abnormalities and associated fetal heart rate abnormalities) were also noted. 15 However, in these reports, the number of cases was small and the key limitations were that different mAbs were administered and the relevant variants of concern were not mentioned.
In this study, we report the administration of CAS/IMB to pregnant women affected by COVID‐19 during the period when the Delta (B.1.617.2) variant was predominant, with a focus on the tolerability of this antibody preparation.
Methods
Eight pregnant women infected with COVID‐19 between August 2021 and October 2021, when the delta variant was prevalent, and receiving CAS/IMB were included in the study. Among them, polymerase chain reaction (PCR) testing was performed on the swab samples of symptomatic participants or those who were close contacts of a patient with COVID‐19. A positive nasopharyngeal PCR test was adopted for the definitive diagnosis of COVID‐19.
Inclusion criteria
Eligibility for the use of CAS/IMB was derived from the FDA's Emergency Use Authorization, 11 and was based on the following criteria: age, 12 years or older; weight, 40 kg or more; positive SARS‐CoV‐2 PCR test; infection symptoms within 10 days; and the ability to provide informed consent. In addition, pregnant women affected by COVID‐19 with mild to moderate severity 16 and who were unvaccinated were included in the study. CAS/IMB was administered to the patient after obtaining informed consent. It was administered as a single combined dose of 600 mg of casirivimab and 600 mg of imdevimab, based on the manufacturer's prescribing information. 17
Evaluation method
Pregnancies complicated with COVID‐19 were managed in the hospital at the time of diagnosis. Treatment with CAS/IMB was then initiated at the time of diagnosis of mild to moderate disease. The treatment strategy for mild to moderate COVID‐19 at each facility was different. All patients in this study were hospitalized.
The management policy after hospitalization was as follows:
Oxygen to be administered to patients with SpO2 < 95.
Vital signs, including maternal body temperature, SpO2 level, and blood pressure, to be measured at least thrice a day.
Acetaminophen for antipyretic purposes not to be used in routine care.
In addition to the above strategies, anticoagulants, antivirals, and steroids were administered at some facilities.
Treatment methods were inconsistent among facilities, and most patients were hospitalized for bed rest, although oxygen, steroids, anticoagulants, and antivirals were also administered on a case‐by‐case basis. Acetaminophen was used to treat fever, not routinely. Per patient management policy, vital signs, including maternal body temperature, SpO2 levels, and blood pressure, were measured at least thrice a day during hospitalization.
The primary endpoint was the change in the maternal severity of clinical disease, which was assessed based on the US National Institutes of Health (NIH) classification. 16
The secondary endpoints included changes in symptoms during hospitalization and the duration of hospitalization. Maternal background information regarding age, gestational age, comorbidity, obesity, and pregnancy outcomes was collected. Because the clinical manifestations of anaphylaxis are not always characteristic of the onset of allergy or adverse events after administration, the following symptoms shown in previous reports are useful in diagnosing anaphylaxis: skin symptoms (e.g., acute urticaria, angioedema, flushing, mucosal swelling), sudden onset of respiratory symptoms (e.g., dyspnea, cough, and wheezing), sudden decrease in blood pressure, or clinical manifestations (e.g., collapse, tachycardia, and incontinence), or gastrointestinal tract disturbances (e.g., abdominal pain and vomiting). 18 , 19
Maternal adverse events were evaluated based on subjective symptoms and Common Terminology Criteria for Adverse Events (CTCAE) (National Cancer Institute, Division of Cancer Treatment and Diagnosis). 20 However, for symptoms present at the time of admission, the worsening of symptoms, if any, should be evaluated for at least 24 h. New symptoms were evaluated, regardless of severity. Changes in body temperature were statistically assessed for fever at the time of CAS/IMB administration, and at 8, 24, and 48 h after administration. The data were compared with the results obtained for 10 cases of pregnancy with COVID‐19 at the same time, who did not receive CAS/IMB. In the CAS/IMB group, blood tests were performed at the time of drug administration and 3–5 days after admission. In the non‐CAS/IMB group, blood tests were performed at admission and 3–5 days after admission. The following blood tests, known to be associated with poor prognosis, were selected for observation: white blood cell count (WBC), hemoglobin (Hb), platelet count, and C‐reactive protein (CRP) to assess inflammatory response, and D‐dimer to determine coagulation. 21 , 22 , 23 , 24 These parameters were compared between the CAS/IMB and non‐CAS/IMB groups; the results of blood samples taken at admission and during hospitalization within the same group were also compared.
Statistical evaluation
The Mann–Whitney U test was used to compare parity, age, maternal body mass index (BMI), gestational age of onset, SpO2 at hospitalization, days from onset to hospitalization, neonatal information (gestational age at delivery, weight [g, %ile], APGAR score [1 min, 5 min]), umbilical artery blood test (pH, base excess [BE]), placental weight (g), and blood test parameters between groups. The Wilcoxon signed‐rank test was used to compare the body temperature and blood test parameters at the time of admission and during hospitalization. SPSS (version 25; IBM Corporation, Armonk, NY, USA) was used for analysis.
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Mie University Hospital (No. H2021‐224). All participants gave their written informed consent prior to participation in this study.
Results
The patient characteristics are presented in Table 1, and delivery and neonatal information are presented in Table 2.
TABLE 1.
Maternal clinical characteristics
| Group | Case | Age | Parity | Onset GA (weeks) | Initial symptoms | Image findings | SpO2 | Comorbidity | The days from the onset to administration/hospitalization | Exacerbation after admission |
|---|---|---|---|---|---|---|---|---|---|---|
| CAS/IMB | 1 | 33 | 2 | 20 | Head ache, fever | 98 | 1 | No | ||
| 2 | 27 | 0 | 24 | Fever, cough, dysgeusia | Pneumonia | 37 | Asthma | 4 | No | |
| 3 | 35 | 2 | 28 | Fever, cough, | Pneumonia | 97 | 4 | No | ||
| 4 | 25 | 1 | 36 | Fever, cough, | 98 | Obesity | 5 | No | ||
| 5 | 20 | 0 | 34 | Fever, cough, abdominal pain | Pneumonia | 98 | 4 | No | ||
| 6 | 31 | 3 | 32 | Fever, cough, | Pneumonia | 96 | Obesity | 4 | No | |
| 7 | 34 | 0 | 33 | Fever, cough, | 98 | Panic disorder | 2 | No | ||
| 8 | 37 | 1 | 18 | Fever, cough, | Pneumonia | 97 | 5 | No | ||
| non‐CAS/IMB | 1 | 36 | 1 | 25 | Fever | 96 | 6 | No | ||
| 2 | 35 | 0 | 12 | Nausea fever | 97 | Obesity | 1 | Yes | ||
| 3 | 43 | 4 | 34 | Fever | 96 | 4 | Yes | |||
| 4 | 37 | 0 | 32 | Fever | Pneumonia | 96 | 13 | Yes | ||
| 5 | 29 | 0 | 13 | Fever | Pneumonia | 97 | 4 | Yes | ||
| 6 | 30 | 0 | 35 | Fever, sore throat | Pneumonia | 96 | 3 | No | ||
| 7 | 20 | 0 | 32 | Sore throat | Pneumonia | 98 | 4 | No | ||
| 8 | 41 | 1 | 24 | Fever, head ache | 98 | 1 | No | |||
| 9 | 22 | 0 | 30 | Fever | 99 | 1 | No | |||
| 10 | 27 | 0 | 11 | Fever, cough | 96 | 3 | No |
Abbreviations: CAS/IMB, casirivimab and imdevimab; GA, gestational age.
TABLE 2.
Delivery methods and neonatal background
| Group | Case | Delivery | Adaptation | Delivery weeks | Sex | Weight (g) | Weight (percentile) | APGAR (1 min) | APGAR (5 min) | pH | BE | Placental weight (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAS/IMB | 1 | VD | 38 | F | 3008 | 60.6 | 9 | 10 | — | — | 478 | |
| 2 | VD | 38 | M | 2776 | 33.8 | 10 | 10 | 7.33 | −5.5 | 470 | ||
| 3 | CS | Previous CS | 37 | F | 2296 | 9.2 | 10 | 10 | 7.29 | −4.2 | 479 | |
| 4 | CS | Placental abruption | 36 | F | 2474 | 38.8 | 2 | 3 | 7.26 | −6.7 | 501 | |
| 5 | VD | 40 | F | 2510 | 4.9 | 9 | 9 | 7.41 | −2.0 | 412 | ||
| 6 | CS | Previous CS | 38 | F | 2739 | 37.1 | 8 | 9 | 7.37 | 0.7 | 470 | |
| 7 | VD | 41 | F | 3300 | 72.1 | 9 | 9 | 7.33 | −1.2 | 610 | ||
| 8 | CS | Previous CS | 38 | M | 3244 | 81.0 | 9 | 10 | — | — | 630 | |
| non‐CAS/IMB | 1 | VD | 40 | M | 3450 | 73.6 | 9 | 10 | 7.24 | −8.0 | 623 | |
| 2 | VD | 40 | F | 4234 | 100 | 8 | 9 | 7.35 | −1.9 | 720 | ||
| 3 | CS | COVID‐19 | 35 | F | 2830 | 96.3 | 5 | 8 | 7.33 | −3.6 | 650 | |
| 4 | VD | 40 | F | 2905 | 27.6 | 8 | 9 | 7.32 | −3.2 | 560 | ||
| 5 | VD | 40 | F | 3148 | 39.7 | 9 | 10 | 7.30 | −6.8 | 630 | ||
| 6 | CS | COVID‐19 | 36 | M | 2672 | 76.4 | 8 | 9 | 7.27 | −7.0 | 610 | |
| 7 | VD | 40 | M | 3168 | 58.7 | 9 | 10 | 7.32 | −6.0 | 696 | ||
| 8 | VD | 40 | M | 3350 | 77.4 | 10 | 10 | 7.36 | −2.9 | 517 | ||
| 9 | VD | 38 | F | 3082 | 68 | 9 | 9 | 7.22 | −8.4 | 554 | ||
| 10 | VD | 39 | F | 2664 | 18.6 | 9 | 9 | 7.34 | −1.4 | 542 |
Abbreviations: —, Specimens not collected; APGAR, APGAR score; BE, base excess; CAS/IMB, casirivimab and imdevimab; CS, cesarean section; F, female; M, male; VD, vaginal delivery.
Maternal background
All eight patients administered CAS/IMB and all 10 patients who did not receive CAS/IMB were hospitalized and treated.
There were no significant differences between the two groups based on maternal parameters (parity: p = 0.460, age: p = 0.696, BMI: p = 0.274, gestational age at onset: p = 0.573, SpO2 at admission: p = 0.237, and days from onset to admission: p = 0.573).
All recruited patients were symptomatic and tested positive for COVID‐19 using PCR. In addition, all the patients did not take the recommended two doses of the vaccine. No cases of worsening of disease severity were reported after CAS/IMB administration. Seven patients had mild disease, and one patient had moderate disease. There were no cases of allergy‐like symptoms, other than fever, within 24 h after CAS/IMB administration, and no cases of symptoms worsening, or new symptoms reported at the time of administration.
Delivery process and neonatal background
No significant differences were observed in neonatal information of the two groups (gestational age at delivery: p = 0.408; weight [g]: p = 0.122; weight [%ile]: p = 0.146 [%ile]; APGAR score [1 min]: p = 0.515; APGAR score [5 min]: p = 0.829; umbilical artery blood test [pH]: p = 0.368; umbilical artery blood test [BE]: p = 0.220). However, there was a significant difference in placental weight between the two groups (p = 0.009).
Delivery and neonatal information were traced in all the cases. In the CAS/IMB group, four of the eight deliveries were by cesarean section, one by placental abruption, and three by elective cesarean section due to a previous cesarean section. Placental abruption was diagnosed before the administration of CAS/IMB, and an emergency cesarean section was performed after the administration. Regarding fetal growth, two cases of small for gestational age (SGA) were confirmed. In two cases, umbilical artery blood samples were not collected. The umbilical cord blood gas test showed no abnormalities in the cases where blood collection was possible.
Changes in body temperature after hospitalization
There was no significant difference in fever at the time of hospitalization, on CAS/IMB administration (p = 0.274), or 8 h after hospitalization/CAS/IMB administration, between the CAS/IMB and non‐CAS/IMB groups (p = 0.083). In contrast, a significant reduction in fever was observed in the CAS/IMB group at 24 and 48 h after hospitalization/administration of CAS/IMB (p < 0.001; Figure 1).
FIGURE 1.
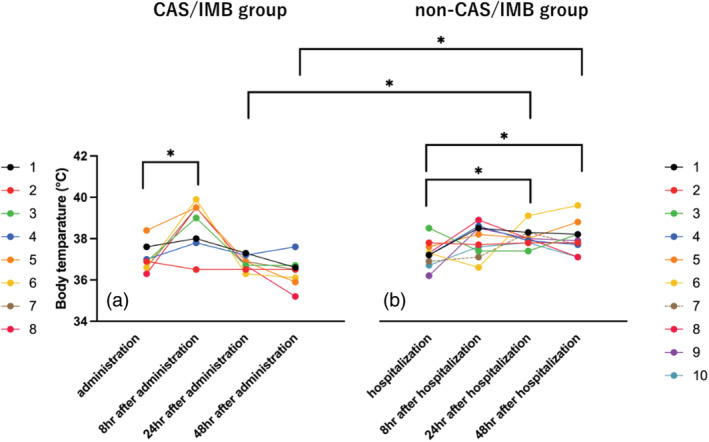
Changes in body temperature after hospitalization/administration of casirivimab and imdevimab (CAS/IMB). Panels (a) and (b) show time‐dependent changes in body temperature in the CAS/IMB and non‐CAS/IMB groups, respectively. In the CAS/IMB group, a transient increase in body temperature was observed 8 h after drug administration; thereafter, the body temperature decreased, and no significant difference was observed. In the non‐CAS/IMB group, there was a significant increase in body temperature at 24 and 48 h compared with that at the time of admission. In the intergroup comparison, a significantly lower body temperature was reported in the CAS/IMB group at 24 and 48 h after administration/hospitalization than that in the non‐CAS/IMB group.
In the CAS/IMB group, there was a significant increase in body temperature at 8 h post‐administration than that at the time of administration, but there was no significant difference in body temperature at 24 and 48 h after administration (p = 0.021, 0.069, and 0.069, respectively). In contrast, in the non‐CAS/IMB group, there was no significant difference in body temperature at 8 h of hospitalization than that at admission, but there was a significant increase in body temperature at 24 and 48 h after hospitalization, compared with that at hospitalization (p = 0.093, 0.037, and 0.021, respectively; Figure 1).
Blood test results
The blood test results in the CAS/IMB and non‐CAS/IMB groups are shown in Tables 3 and 4. There was no significant difference between the groups in any of the parameters at the time of admission or at the time of CAS/IMB administration (WBC: p = 0.274; Hb: p = 0.897; platelets: p = 0.237; CRP: p = 0.762; D‐dimer: p = 0.762).
TABLE 3.
Results of blood tests after CAS/IMB administration or hospitalization
| CAS/IMB group | Administration | 3–5 days after administration | Non‐CAS/IMB group | Hospitalization | 3–5 days after hospitalization |
|---|---|---|---|---|---|
| WBC (/μL) | 5540 (4207–6150) | 6080 (5325–8175) | WBC (/μL) | 6700 (5400–7430) | 4125 (3428–6063) |
| Hb (g/dL) | 11.1 (10.7–12.4) | 11.1 (10.0–12.0) | Hb (g/dL) | 11.7 (10.4–12.3) | 10.8 (10.0–11.9) |
| Plt (×10 000/μL) | 18.4 (17.3–22.2) | 20.8 (17.1–26.1) | Plt (×10 000/μL) | 20.7 (19.2–22.7) | 19.9 (16.3–21.2) |
| CRP (mg/L) | 2.0 (0.7–3.1) | 2.0 (1.1–3.7) | CRP (mg/L) | 2.3 (0.7–2.6) | 0.9 (0.6–5.0) |
| D‐dimer (μg/mL) | 2.1 (1.2–3.5) | 2.6 (1.5–3.3) | D‐dimer (μg/mL) | 2.0 (0.8–3.1) | 2.3 (1.3–4.3) |
Note: The parameters did not differ significantly between the two groups. Values are listed in median and inter‐quartile ranges.
Abbreviations: CAS/IMB, casirivimab and imdevimab; CRP, C‐reactive protein; Hb, hemoglobin; Plt, platelet count; WBC, white blood cell count.
TABLE 4.
Changes in blood test results between CAS/IMB administration or hospitalization and 3–5 days after administration/hospitalization
| CAS/IMB group | Non‐CAS/IMB group | |
|---|---|---|
| WBC (/μL) | 400 (−855 to 1860) | −1380 (−1880 to 50) |
| Hb (g/dL) | 0.05 (−0.55 to 0.33) | −0.10 (−0.60 to −0.10) |
| Plt (×10 000/μL) | 1.40 (−4.20 to 5.60) | −0.70 (−4.60 to 0.30) |
| CRP (mg/L) | −0.48 (−1.4 to 0.86) | 0.31 (−0.26 to 2.46) |
| D‐dimer (μg/mL) | −0.05 (−1.83 to 0.08) | −0.1 (−0.99 to 0.40) |
Note: None of the parameters differed significantly between the two groups. Values are listed in median and inter‐quartile ranges.
Abbreviations: CAS/IMB, casirivimab and imdevimab; CRP: C‐reactive protein; Hb, hemoglobin; Plt, platelet count; WBC, white blood cell count.
No significant differences were found in any of the parameters during hospitalization and post‐hospitalization within each group (CAS/IMB group: WBC, p = 0.575; Hb, p = 0.866; platelets, p = 0.726; CRP, p = 0.753; D‐dimer, p = 0.416; non‐CAS/IMB group: WBC, p = 0.690; Hb, p = 0.324; platelets, p = 0.208; CRP: p = 0.263; D‐dimer, p = 0.866; Table 3).
The changes in the blood test parameters are shown in Table 4. There were no significant differences between the groups for these parameters (WBC: p = 0.114; Hb: p = 0.673; platelets: p = 0.481; CRP: p = 0.864; D‐dimer: p = 0.755).
Discussion
In the present study, we report the outcome of CAS/IMB treatment in pregnant women with COVID‐19, affected by the Delta variant epidemic. No worsening of COVID‐19 symptoms was observed after CAS/IMB administration in all eight cases, suggesting the tolerability of the CAS/IMB treatment. There is little evidence on the use of mAbs during pregnancy and further enhancement of knowledge is desired. Therapies with mAbs, such as CAS/IMB, have not been specifically tested in pregnant women, and the National Institutes of Health has concluded that there is insufficient evidence regarding the use of mAbs in pregnant women with COVID‐19. 11
Patients should be informed that mAbs such as casirivimab and imdevimab may cross the placenta, and the effect on the fetus is unknown. 13 Compared to the placenta of the control women, those of women with COVID‐19 showed vascular changes, consistent with preeclampsia, which can cause abruption. 25 However, the phenomenon is still uncertain, since the state of systemic inflammation and hypercoagulability seen in nonpregnant patients with severe COVID‐19 is also characteristic of preeclampsia. 26 Among the eight cases in this study, one case of placental abruption and two cases of SGA were found, which could be followed up. This may reflect vascular endothelial damage, due to inflammation associated with COVID‐19. Although less frequent, some patients with COVID‐19 complain of abdominal pain, and it is important to differentiate COVID‐19 from other pregnancy‐related diseases. In these cases, there were no abnormalities in the postoperative course of the mother and child, and fortunately, COVID‐19 symptoms did not worsen. Because fetal heart rate had to be monitored in isolation in COVID‐19‐complicated pregnancies, caution needs to be exercised in future if pregnant women with COVID‐19 present with abdominal pain.
In the present study, the transient worsening of fever was observed within 24 h after the administration of CAS/IMB. However, fever did not persist for 24 h, and there was a significant improvement in fever compared with that observed in the non‐CAS/IMB group. Fever has earlier been associated with the use of mAbs in patients with COVID‐19. 27 However, similar to the present study, these symptoms have not been severe. In these reports, fever is believed to be a reaction to infusion. In addition, CAS/IMB does not suppress viral activity immediately after administration, and fever may be associated with the worsening of COVID‐19. Nonetheless, rapid improvement in fever following CAS/IMB administration was observed in this study. Complementing this finding, 3/10 (30%) of the control cases in this study were treated with steroids after hospitalization, but the CAS/IMB group showed an earlier improvement in fever than the control group.
So far, diverse types of adverse reactions to mAbs have been reported, and there are reports of adverse reactions in 0.2%–2.3% of nonpregnant patients in the treated group. 28 Most of these events were described as pruritus, flushing, rash, or facial swelling, which occurred during infusion and were mild in severity. In addition, fetal heart rate abnormalities associated with maternal respiratory abnormalities, reported previously, were not observed in these cases. 15 However, several cases (case 1, 2, and 8 in Table 1) in early gestational weeks were not indicated for fetal heart rate monitoring, and the impact of mAb administration on fetal heart rate monitoring could not be accurately evaluated.
Interestingly, no significant hematological findings were observed between the groups in this study. This indicates that there was no obvious difference in the hematological condition of the patients between the two groups at the time of admission or the administration of CAS/IMB. The reason for unaltered blood test findings despite the improvement in symptoms is that it is difficult to evaluate changes in disease severity in mild cases over time using the blood test parameters employed in this study. It is also known that serum inflammatory response markers peak slowly in response to acute inflammation. 29 The lack of significant differences in blood test findings between groups in this study may also be due to the short intervals between blood tests in this study.
The limitations of this study include the small sample size and the timing of blood tests performed—that is, more than 3 days after the initial collection. In future, it will be necessary to accumulate sufficient number of cases and evaluate the effect of CAS/IMB by grouping patients according to gestational week, maternal background, and the severity of illness. The vaccination history could not be confirmed for the study participants and it is presumed that some of the recruited participants may have taken only one vaccination dose.
Author Contributions
Shoichi Magawa: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. Masafumi Nii: Supervision, Writing – review & editing. Shintaro Maki: Data curation, Supervision. Naosuke Enomoto: Data curation, Supervision, Formal analysi. Sho Takakura: Data curation, Supervision, Resources. Yuka Maegawa: Data curation, Supervision, Visualization. Kazuhiro Osato: Data curation, Supervision, Methodology. Hiroaki Tanaka: Funding acquisition. Eiji Kondo: Supervision. Tomoaki Ikeda: Writing – review & editing, Project administration, Supervision.
Conflict of interest
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request due to privacy/ethical restrictions. The data that support the findings of this study are available on request. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2021;19:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324:782–93. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . COVID data tracker: variant proportions. 2021. Available from: https://covidcdcgov/covid‐datatracker/?CDC_AA_refValhttps%3A%2F%2Fwwwcdcgov%2Fcoronavirus%2F2019‐ncov%2Fcases‐updates%2Fvariant‐proportionshtml#variant‐proportions. Accessed September 2, 2021
- 4. Adhikari EH, SoRelle JA, McIntire DD, Spong CY. Increasing severity of COVID‐19 in pregnancy with Delta (B.1.617.2) variant surge. Am J Obstet Gynecol. 2022;226:149–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, et al. COVID‐19 vaccination coverage among pregnant women during pregnancy: eight integrated health care organizations, United States, December 14, 2020‐May 8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID‐19: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu W, Sivajohan B, McClymont E, Albert A, Elwood C, Ogilvie G, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID‐19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2022;156:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shamshirsaz AA, Hessami K, Morain S, Afshar Y, Nassr AA, Arian SE, et al. Intention to receive COVID‐19 vaccine during pregnancy: a systematic review and meta‐analysis. Am J Perinatol. 2022;39:492–500. [DOI] [PubMed] [Google Scholar]
- 9. Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. Bamlanivimab plus etesevimab for mild or moderate Covid‐19. N Engl J Med. 2021;385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deeks ED. Casirivimab/Imdevimab: First approval. Drugs. 2021;81:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. United States Food and Drug Administration . Fact sheet for health care providers. Emergency use authorization (EUA) of REGEN‐COVTM (casirivimab and imdevimab). Available from: https://www.fda.gov/media/145611/download. Accessed December 10, 2021.
- 12. Hirshberg JS, Cooke E, Oakes MC, Odibo AO, Raghuraman N, Kelly JC. Monoclonal antibody treatment of symptomatic COVID‐19 in pregnancy: initial report. Am J Obstet Gynecol. 2021;225:688–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayer C, VanHise K, Caskey R, Naqvi M, Burwick RM. Monoclonal antibodies Casirivimab and Imdevimab in pregnancy for coronavirus disease 2019 (COVID‐19). Obstet Gynecol. 2021;138:937–9. [DOI] [PubMed] [Google Scholar]
- 14. Chang MH, Cowman K, Guo Y, et al. A real‐world assessment of tolerability and treatment outcomes of COVID‐19 monoclonal antibodies administered in pregnancy. Am J Obstet Gynecol. 2022;226(5):743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richley M, Rao RR, Afshar Y, Mei J, Mok T, Vijayan T, et al. Neutralizing monoclonal antibodies for coronavirus disease 2019 (COVID‐19) in pregnancy: a case series. Obstet Gynecol. 2022;139:368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institutes of Health . COVID‐19 treatment guidelines. 2021. Available from: https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_43.pdf. Accessed August 8, 2021. [PubMed]
- 17. Chugai Pharmaceutical Co Ltd . Ronapreve. for intravenous infusion set: Japanese prescribing information. 2021. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/450045_62505A0A1023_1_01. Accessed September 13, 2021.
- 18. Sampson HA, Munoz‐Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J Allergy Clin Immunol. 2006;117:391–7. [DOI] [PubMed] [Google Scholar]
- 19. Klimek L, Bergmann KC, Brehler R, Pfützner W, Zuberbier T, Hartmann K, et al. Practical handling of allergic reactions to COVID‐19 vaccines: a position paper from German and Austrian allergy societies AeDA, DGAKI, GPA and OGAI. Allergo J Int. 2021;30:79–95. 10.1007/s40629-021-00165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Common Terminology Criteria for Adverse Events v5.0. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_60. Accessed January 5, 2021.
- 21. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021–8. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Zhao K, Wei H, Chen W, Wang W, Jia L, et al. Dynamic relationship between D‐dimer and COVID‐19 severity. Br J Haematol. 2020;190:e24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taneri PE, Gomez‐Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID‐19: a systematic review and meta‐analysis. Eur J Epidemiol. 2020;35:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wool GD, Miller JL. Impact of COVID‐19 disease on platelets and coagulation. Pathobiology. 2021;88:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID‐19. Am J Clin Pathol. 2020;154:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narang K, Enninga EAL, Gunaratne M, et al. SARS‐CoV‐2 infection and COVID‐19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95:1750–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ganesh R, Pawlowski CF, O'Horo JC, et al. Intravenous bamlanivimab use associates with reduced hospitalization in high‐risk patients with mild to moderate COVID‐19. J Clin Invest. 2021;131:e151697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with COVID‐19. N Engl J Med. 2021;384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pepys MB, Hirschfield GM. C‐reactive protein: a critical update. J Clin Investig. 2003;15:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions. The data that support the findings of this study are available on request. The data are not publicly available due to privacy or ethical restrictions.


