Abstract
Purpose
Rural communities are among the most underserved and resource‐scarce populations in the United States. However, there are limited data on COVID‐19 outcomes in rural America. This study aims to compare hospitalization rates and inpatient mortality among SARS‐CoV‐2‐infected persons stratified by residential rurality.
Methods
This retrospective cohort study from the National COVID Cohort Collaborative (N3C) assesses 1,033,229 patients from 44 US hospital systems diagnosed with SARS‐CoV‐2 infection between January 2020 and June 2021. Primary outcomes were hospitalization and all‐cause inpatient mortality. Secondary outcomes were utilization of supplemental oxygen, invasive mechanical ventilation, vasopressor support, extracorporeal membrane oxygenation, and incidence of major adverse cardiovascular events or hospital readmission. The analytic approach estimates 90‐day survival in hospitalized patients and associations between rurality, hospitalization, and inpatient adverse events while controlling for major risk factors using Kaplan‐Meier survival estimates and mixed‐effects logistic regression.
Findings
Of 1,033,229 diagnosed COVID‐19 patients included, 186,882 required hospitalization. After adjusting for demographic differences and comorbidities, urban‐adjacent and nonurban‐adjacent rural dwellers with COVID‐19 were more likely to be hospitalized (adjusted odds ratio [aOR] 1.18, 95% confidence interval [CI], 1.16‐1.21 and aOR 1.29, CI 1.24‐1.1.34) and to die or be transferred to hospice (aOR 1.36, CI 1.29‐1.43 and 1.37, CI 1.26‐1.50), respectively. All secondary outcomes were more likely among rural patients.
Conclusions
Hospitalization, inpatient mortality, and other adverse outcomes are higher among rural persons with COVID‐19, even after adjusting for demographic differences and comorbidities. Further research is needed to understand the factors that drive health disparities in rural populations.
Keywords: COVID‐19, hospitalization, mortality, SARS‐CoV‐2, urban‐rural health
INTRODUCTION
The novel coronavirus (SARS‐CoV‐2) was the third leading cause of death in the United States in 2020 1 and is responsible for more than 1 million US deaths to date. 2 During the initial 4 months of the US SARS‐CoV‐2 epidemic, cases were most concentrated in urban areas. However, by late 2020, rural communities experienced a surge in SARS‐CoV‐2 infections, with some of the highest case rates in the nation. 3 Nonmetropolitan areas constitute 97% of the US land area, with approximately 20% of the population residing in rural areas. 4 Rural compared with urban inhabitants are older, less likely to engage in behaviors to prevent SARS‐CoV‐2 infection, 5 and have a higher prevalence of comorbidities (eg, obesity) associated with more severe COVID‐19 (C19) and death. 6 They have also experienced a greater disparity in life expectancy over the last 50 years, 7 which is likely multifactorial, resulting from, but not limited to, decreased access to care, increased disability, and socioeconomic factors. 8 Population‐level analyses conducted early in the SARS‐CoV‐2 pandemic demonstrated higher mortality rates in metropolitan areas than rural or micropolitan counties in the United States. 9 Subsequent studies have shown differences in infection clustering and higher C19 mortality rates in rural areas of the United States, driven by social determinants, 10 social vulnerability, and the differences in mitigation policies 11 between rural and urban communities.
The relative paucity of rigorous research and surveillance data concerning rural dwellers relative to those dwelling in urban settings and communities is well documented in the social sciences literature. 12 This research and information gap extends to the biomedical sciences. Community hospital and public health data from rural communities are often sparse and preclude meaningful comparison across regions. Rural disparities are complex and diverse, with varying challenges across different regions and communities. A potent societal stressor, the C19 pandemic, both creates acute problems and spotlights chronic weaknesses of rural health and health care systems. The unique challenges in optimally responding to C19 among rural dwellers include fewer intensive care beds per capita; more limited access to relevant infectious disease and other specialty care providers; greater baseline comorbidities, including age, obesity, and diabetes; often relatively adverse social determinants of health, such as income and education; and physical travel distances resulting in a delay in treatment. 13
To better understand potential drivers of C19 outcomes in rural America, we assessed hospitalization and mortality using the National COVID Cohort Collaborative (N3C), a National Institutes of Health‐supported data enclave containing electronic health record (EHR) information on nearly 9 million persons tested for SARS‐CoV‐2 across 65 US sites and more than 2.9 million patients with a definitive diagnosis or lab result of SARS‐CoV‐2 infection.
While the relationship between rural and urban hospitalization and mortality has been studied for chronic conditions, limited research has evaluated SARS‐CoV‐2 infected rural‐urban discrepancies. To our knowledge, this is the largest cohort of C19 cases in North America using data at the patient level, which provides detail unavailable in population‐based studies. Previous large‐scale studies have been restricted to single states 14 or utilized public health reporting systems. 15
The purpose of this study was to (1) estimate differences in hospitalization and mortality among rural and urban individuals with SARS‐CoV‐2 infection using real‐world data adjusted for underlying demographic differences and comorbid burden and (2) quantify differences in rural outcomes based on region and degree of rurality. We hypothesized that rural dwellers would have outcomes similar to their urban counterparts after adjustment for demographic differences and comorbid conditions.
METHODS
This retrospective cohort study received Institutional Review Board approval from each investigator's institution and was reviewed and approved by the N3C Data Access Committee. Our study cohort includes patients diagnosed between January 1, 2020, and June 30, 2021. This study followed the Enhancing the Quality and Transparency of Health Research (EQUATOR) reporting guidelines, Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD). 16 Analyses were performed within the N3C Enclave using SQL, Python, and R v.3.5.1. in accordance with N3C privacy and download review policies.
N3C Data Enclave
N3C has broad inclusion criteria, harmonizing data from 65 sites across the United States. 17 N3C collects longitudinal EHR or health information exchange data (with a 2‐year “lookback” period to January 1, 2018) on all patients with a C19 diagnostic code without a confirmed positive diagnostic (polymerase chain reaction [PCR] or Ag) test (22% of all patients) or a positive SARS‐CoV‐2 PCR or antigen test (78% of all patients) as well as uninfected patients serving as controls. N3C collects and aggregates data on definitive SARS‐CoV‐2‐infected patients and a demographically matched comparison group of SARS‐CoV‐2‐uninfected persons (2:1 SARS‐CoV‐2 uninfected: infected); matching is performed as part of the N3C ingestion process based on site, age, gender, race, and ethnicity. 18 Source system C19 testing protocols are mapped to standard terminologies for labs (LOINC) and conditions (ICD‐10 CM and SNOMED CT) by the N3C Data Ingestion and Harmonization Workstream, which maintains a computable phenotype for defining the presence of C19. 19 To capture patients during the early stages of the pandemic (before 5/1/2020), patients with 2 weak diagnostic codes (such as ICD10 J80* Acute Respiratory Distress Syndrome and R43.0 Anosmia) probabilistic of C19 are also included (see Supplementary Methods for a summary of the ingestion and harmonization process, sampling approaches, concept definitions, and computable phenotypes). 20
Cohort identification
Rural and urban categories were identified by 5‐digit ZIP Codes that were then mapped to the 2010 Rural‐Urban Continuum Codes (RUCA), distinguished by population density, degree of urbanization, and adjacency to metropolitan areas. 21 For purposes of this study, 3 categories are defined: urban, urban‐adjacent rural (UAR), and nonurban‐adjacent rural (NAR). 22 , 23 This classification has been commonly used to attribute rurality based on census tract or ZIP Code. 24 , 25 To validate the representativeness of the cohort population with the overall US population, we compared the population percentages for each category in N3C with the US population using public datasets (Table S1). 2 , 26 , 27
N3C data partners are contributing institutions encompassing multiple providers and potentially numerous care sites. We developed and utilized a data robustness screening matrix to determine minimum fact reporting per patient across key domains for each data partner. This follows a similar approach used by the 4 source data models that all rely on data quality dashboards to enhance site reporting for inclusion in network studies: Observational Medical Outcomes Partnership (OMOP), 28 Accrual to Clinical Trials (ACT), 29 TriNetX, 30 and Patient‐Centered Clinical Research Network (PCORnet). 31
Where possible, we categorically excluded data partners rather than individual participants based on minimum data reporting requirements and robustness measures. We excluded data partners with limited data robustness (less than 1 standard deviation below mean reporting) in 2 key domains: death reporting and measurement reporting, which was used to calculate body mass index (BMI). We excluded patients with missing age, gender, and 5‐digit ZIP Codes across all data partners (Figure 1).
FIGURE 1.
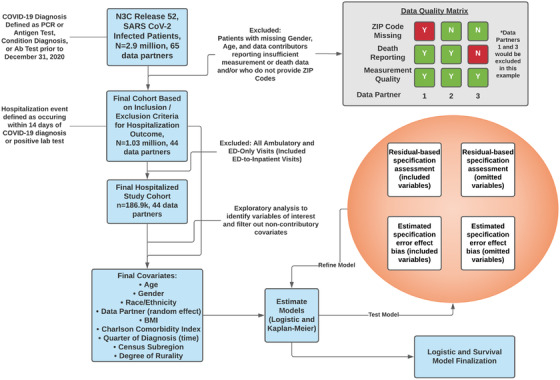
Data analysis plan. Figure 1 documents the data analysis plan, including steps for inclusion and exclusion of data partners based on the availability of 5‐digit ZIP Codes and robustness based on covariates of interest (measurement domain to calculate BMI and death domain for primary outcome). We also excluded patients with missing age or gender
Data extraction
Data were extracted on November 5, 2021, (N3C release 52) in the OMOP Common Data Model version 5.3.1. 17 This facilitates a 4‐month window for data reporting from our diagnostic cutoff (June 30, 2021) to support 90‐day outcomes analyses and comprehensive reporting from data partners. All clinical concept sets were created collaboratively within the N3C Enclave, with at least 1 informatician and 1 clinical subject‐matter expert reviewing each relevant concept set. Concept sets 32 contain standardized terminology corresponding to clinical domains (eg, LOINC, SNOMED CT, ICD‐10, and RxNorm). Logistic models were calculated with all C19 patients. All‐cause mortality was collected on hospitalized patients as published literature suggests that the most reliable and timely death data are available in hospitalized patients, representing 64% of all death certificates in the final quarter of 2020. 33
Covariates
N3C provides patient ZIP Codes for most patients (∼66% of all subjects). The majority of missing ZIP Code information is from specific data providers, who elect not to provide 5‐digit ZIP Codes in their data transmissions––these sites were excluded from this study. Based on data availability, we relied on a RUCA code crosswalk to match ZIP Codes and RUCA classifications. 34 Current RUCA codes derive from the 2010 Census and the 2006‐2010 American Community Survey. 21 We defined rural areas broadly according to the Office of Management and Budget and Federal Office of Rural Health Policy (FORHP) definitions (primary RUCA code between 1 and 3 corresponding to urban, and 4 and 10 corresponding to rural). Based on FORHP definitions, we further divided rural areas into 2 categories: UAR (RUCA codes 4‐5, 7‐8) and NAR (RUCA codes 6, 9‐10). 35
Outcomes
Primary outcomes are hospital admission and all‐cause mortality (any reported death or a discharge to hospice) among hospitalized patients as observed during their initial post‐C19 hospitalization. Survival analyses assessed mortality at 90 days posthospitalization. Secondary outcomes included implementation of supplemental oxygen, invasive mechanical ventilation, vasopressor support, extracorporeal membrane oxygenation (ECMO), or the occurrence of major adverse cardiovascular event (MACE), and hospital readmission following initial C19 hospitalization.
Statistical analyses
Summary statistics using Pearson chi‐squared tests for nominal data and Kruskal‐Wallis tests for numerical data were calculated on all C19 patients and hospitalized C19 patients stratified by rural‐urban categories. 36 Covariates examined include gender, age, race, ethnicity, BMI, Charlson Comorbidity Index (CCI) 37 composite score (a higher score indicating worse health), comorbidity categories, tobacco usage, hospitalization, and Census subregion. Mixed‐effects logistic regression models were calculated for hospitalization and adverse event in the hospitalized cohort. All models included fixed effects for gender, race, ethnicity, BMI, age at visit start, CCI score, Census subregion, and rural category and random effects for data partner.
We assessed model specification using a methodology that checked for specification change influence on the estimated rural versus urban effect. Among the possible specification changes assessed were (1) transformations of main effects included in the model, (2) all 2‐way interactions of main effects included in the model, (3) potential addition of available comorbidities not included in the model, and (4) inclusion of data‐providing organization as a fixed or random effect. In addition, a stepwise assessment approach was used to determine if combinations of any such potential changes resulted in a difference in the estimated rural versus urban effect. The only specification change identified was the need to include the data provider organization as a fixed or random effect. The choice of effect type (fixed vs random) was found to be irrelevant. As a result, the initial model was modified to include data‐providing organization as a random effect. This helps mitigate the possibility that our final model's estimated rural effects are artifacts of mortality differences across those organizations’ patient populations as well as differences in reporting practices.
The risk adjustment process employed in our modeling used information about pre‐COVID comorbidities. As patient data in N3C differ in the availability of pre‐COVID clinical data, ranging from none to 2 years of pre‐COVID clinical data, we examined the possibility that estimated rural effects stemmed from rural and urban patients differing in the extent of pre‐COVID comorbidity information. To evaluate baseline differences in outcomes, we ran the same analyses on the SARS‐CoV‐2‐uninfected patient population, evaluating differences in inpatient death or transfer to hospice.
The variables associated with more severe outcomes in the logistic models––rurality, CCI, age, and period of SARS‐CoV‐2 diagnosis––were secondarily evaluated using Kaplan‐Meier estimates of the overall time to death or transfer to hospice, starting from hospital admission, censored at 90 days or upon nonhospice discharge.
RESULTS
Demographics
Our final cohort included data from 44 data partners (Figure 1) with 1,033,229 C19 diagnosed and 186,882 hospitalized C19 patients. Patient demographics in the entire C19 cohort demonstrated rural inhabitants to have a similar distribution of gender but to be older and less racially and ethnically diverse in all groups examined (Tables 1 and 2). Urban dwellers were 57% white and 17% Hispanic or Latinx, while UAR were 76% and 9.9%, and NAR were 82% and 4.9%, respectively. Patient rurality was evenly distributed along Census subregions, apart from a higher percentage of rural patients in the West North Central subregion and more urban patients in the Middle Atlantic subregion. While patient distribution is aggregated around N3C data contributors (Figure 2), this study includes patients from all US states. Our sample proportionally represents the distribution of urban‐rural distribution of the greater US population while closely mirroring the reported caseload and case fatality documented in public surveillance reporting systems (Figure S1).
TABLE 1.
Baseline characteristics of all SARS‐CoV‐2 infected by rural category, January 2020‐June 2021
| Characteristic |
Urban, N = 907,953 a |
Urban‐adjacent rural, N = 100,219 a |
Nonurban‐adjacent rural, N = 25,057 a |
P value b |
|---|---|---|---|---|
| Gender | <.001 | |||
| Female | 499,659 (55%) | 53,887 (54%) | 13,183 (53%) | |
| Male | 408,294 (45%) | 46,332 (46%) | 11,874 (47%) | |
| Age group | <.001 | |||
| <18 | 98,387 (11%) | 11,684 (12%) | 3,098 (12%) | |
| 18‐29 | 171,687 (19%) | 16,067 (16%) | 3,684 (15%) | |
| 30‐49 | 271,467 (30%) | 27,430 (27%) | 6,026 (24%) | |
| 50‐64 | 204,684 (23%) | 23,977 (24%) | 6,406 (26%) | |
| > = 65 | 161,728 (18%) | 21,061 (21%) | 5,843 (23%) | |
| Age, median (IQR) | 43 (27, 59) | 46 (27, 62) | 49 (28, 63) | |
| Race | <.001 | |||
| White | 519,903 (57%) | 76,052 (76%) | 20,448 (82%) | |
| Black or AA | 141,959 (16%) | 9,649 (9.6%) | 1,981 (7.9%) | |
| Asian or NHPI | 30,981 (3.4%) | 1,070 (1.1%) | 67 (0.3%) | |
| Other | 8,470 (0.9%) | 511 (0.5%) | 102 (0.4%) | |
| Missing/unknown | 206,640 (23%) | 12,937 (13%) | 2,459 (9.8%) | |
| Ethnicity | <.001 | |||
| Not Hispanic or Latino | 649,290 (72%) | 81,012 (81%) | 20,472 (82%) | |
| Hispanic or Latino | 158,049 (17%) | 9,893 (9.9%) | 1,217 (4.9%) | |
| Missing/unknown | 100,614 (11%) | 9,314 (9.3%) | 3,368 (13%) | |
| BMI category | <.001 | |||
| <18.5 | 28,283 (3.1%) | 3,164 (3.2%) | 853 (3.4%) | |
| 18.5‐24.9 | 133,424 (15%) | 12,547 (13%) | 2,960 (12%) | |
| 25‐29.9 | 140,691 (15%) | 14,214 (14%) | 3,717 (15%) | |
| >30 | 210,526 (23%) | 27,452 (27%) | 7,065 (28%) | |
| Unknown/missing | 395,029 (44%) | 42,842 (43%) | 10,462 (42%) | |
| Body mass index, median (IQR) | 28 (24, 33) | 29 (25, 35) | 29 (25, 35) | |
| Charlson Comorbidity Index Composite | <.001 | |||
| <1.0 | 637,254 (70%) | 67,528 (67%) | 16,948 (68%) | |
| 1.0‐2.0 | 175,839 (19%) | 20,142 (20%) | 4,955 (20%) | |
| >2.0 | 94,860 (10%) | 12,549 (13%) | 3,154 (13%) | |
| Composite score, median (IQR) | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | |
| Comorbidity incidence | ||||
| Hypertension | 192,522 (21%) | 24,970 (25%) | 6,080 (24%) | <.001 |
| Diabetes mellitus | 104,727 (12%) | 13,607 (14%) | 3,260 (13%) | <.001 |
| Myocardial infarction | 18,295 (2.0%) | 2,536 (2.5%) | 653 (2.6%) | <.001 |
| Congestive heart failure | 36,134 (4.0%) | 5,114 (5.1%) | 1,349 (5.4%) | <.001 |
| Peripheral vascular disease | 38,452 (4.2%) | 4,917 (4.9%) | 1,176 (4.7%) | <.001 |
| Stroke | 33,795 (3.7%) | 4,332 (4.3%) | 1,026 (4.1%) | <.001 |
| Dementia | 11,582 (1.3%) | 1,543 (1.5%) | 361 (1.4%) | <.001 |
| Chronic pulmonary disease | 105,481 (12%) | 12,403 (12%) | 3,069 (12%) | <.001 |
| Rheumatologic disease | 25,248 (2.8%) | 3,043 (3.0%) | 782 (3.1%) | <.001 |
| Mild or severe liver disease | 35,510 (3.9%) | 3,862 (3.9%) | 957 (3.8%) | .5 |
| Hemiplegia or paraplegia | 5,498 (0.6%) | 760 (0.8%) | 194 (0.8%) | <.001 |
| Renal disease | 44,480 (4.9%) | 6,579 (6.6%) | 1,635 (6.5%) | <.001 |
| Any malignancy (except skin) | 46,110 (5.1%) | 5,687 (5.7%) | 1,580 (6.3%) | <.001 |
| Metastatic solid tumor | 8,351 (0.9%) | 1,066 (1.1%) | 304 (1.2%) | <.001 |
| HIV/AIDS | 4,532 (0.5%) | 239 (0.2%) | 41 (0.2%) | <.001 |
| Multiple comorbidities | 318,584 (35%) | 38,765 (39%) | 9,575 (38%) | <.001 |
| Current or former smoker | 241,198 (27%) | 18,649 (19%) | 5,650 (23%) | <.001 |
| Outcomes | ||||
| Hospitalized after COVID diagnosis | 165,483 (18%) | 16,974 (17%) | 4,425 (18%) | <.001 |
| All‐cause mortality or hospice | 26,613 (2.9%) | 3,902 (3.9%) | 1,042 (4.2%) | <.001 |
| Quarter of diagnosis | <.001 | |||
| Jan‐Mar 2020 | 20,600 (2.3%) | 462 (0.5%) | 139 (0.6%) | |
| Apr‐Jun 2020 | 136,509 (15%) | 8,701 (8.7%) | 1,788 (7.1%) | |
| Jul‐Sep 2020 | 139,110 (15%) | 13,319 (13%) | 3,029 (12%) | |
| Oct‐Dec 2020 | 338,088 (37%) | 42,454 (42%) | 10,406 (42%) | |
| Jan‐Mar 2021 | 195,246 (22%) | 25,276 (25%) | 6,682 (27%) | |
| Apr‐Jun 2021 | 78,400 (8.6%) | 10,007 (10.0%) | 3,013 (12%) | |
| Subregion | <.001 | |||
| New England | 69,665 (7.7%) | 6,014 (6.0%) | 4,541 (18%) | |
| Middle Atlantic | 148,091 (16%) | 1,311 (1.3%) | 345 (1.4%) | |
| South Atlantic | 179,927 (20%) | 24,758 (25%) | 6,042 (24%) | |
| East South Central | 57,156 (6.3%) | 13,682 (14%) | 2,127 (8.5%) | |
| East North Central | 202,162 (22%) | 19,834 (20%) | 4,465 (18%) | |
| West North Central | 66,281 (7.3%) | 25,690 (26%) | 6,281 (25%) | |
| West South Central | 4,441 (0.5%) | 196 (0.2%) | 40 (0.2%) | |
| Mountain | 142,068 (16%) | 8,141 (8.1%) | 1,148 (4.6%) | |
| Pacific | 38,162 (4.2%) | 593 (0.6%) | 68 (0.3%) | |
Statistics presented: n (%).
Statistical tests performed: chi‐square test of independence, Kruskal‐Wallis test.
TABLE 2.
Baseline characteristics of hospitalized SARS‐CoV‐2 infected by rural category, January 2020‐June 2021
| Characteristic |
Urban, N = 165,483 a |
Urban‐adjacent rural, N = 16,974 a |
Nonurban‐adjacent rural, N = 4,425 a |
P value b |
|---|---|---|---|---|
| Gender | <.001 | |||
| Female | 83,363 (50%) | 8,316 (49%) | 2,048 (46%) | |
| Male | 82,120 (50%) | 8,658 (51%) | 2,377 (54%) | |
| Age group | <.001 | |||
| <18 | 7,334 (4.4%) | 796 (4.7%) | 178 (4.0%) | |
| 18‐29 | 14,736 (8.9%) | 1,311 (7.7%) | 266 (6.0%) | |
| 30‐49 | 36,285 (22%) | 3,163 (19%) | 665 (15%) | |
| 50‐64 | 43,245 (26%) | 4,536 (27%) | 1,235 (28%) | |
| > = 65 | 63,883 (39%) | 7,168 (42%) | 2,081 (47%) | |
| Age, median (IQR) | 59 (41, 72) | 61 (44, 73) | 63 (49, 74) | |
| Race | <.001 | |||
| White | 80,804 (49%) | 12,514 (74%) | 3,459 (78%) | |
| Black or AA | 38,488 (23%) | 2,358 (14%) | 606 (14%) | |
| Asian or NHPI | 6,677 (4.0%) | 140 (0.8%) | <20 c | |
| Other | 1,448 (0.9%) | 114 (0.7%) | <50 c | |
| Missing/unknown | 38,066 (23%) | 1,848 (11%) | 321 (7.3%) | |
| Ethnicity | <.001 | |||
| Not Hispanic or Latino | 116,360 (70%) | 14,261 (84%) | 3,690 (83%) | |
| Hispanic or Latino | 37,584 (23%) | 1,705 (10%) | 249 (5.6%) | |
| Missing/unknown | 11,539 (7.0%) | 1,008 (5.9%) | 486 (11%) | |
| BMI category | <.001 | |||
| <18.5 | 4,483 (2.7%) | 358 (2.1%) | 82 (1.9%) | |
| 18.5‐24.9 | 25,511 (15%) | 2,244 (13%) | 594 (13%) | |
| 25‐29.9 | 30,836 (19%) | 2,959 (17%) | 764 (17%) | |
| >30 | 55,183 (33%) | 6,667 (39%) | 1,834 (41%) | |
| Unknown/missing | 49,470 (30%) | 4,746 (28%) | 1,151 (26%) | |
| Body mass index, median (IQR) | 29 (25, 35) | 30 (26, 36) | 30 (26, 36) | |
| Charlson Comorbidity Index Composite | <.001 | |||
| <1.0 | 92,410 (56%) | 8,527 (50%) | 2,191 (50%) | |
| 1.0‐2.0 | 34,998 (21%) | 3,607 (21%) | 921 (21%) | |
| >2.0 | 38,075 (23%) | 4,840 (29%) | 1,313 (30%) | |
| Composite score, median (IQR) | 0.00 (0.00, 2.00) | 0.00 (0.00, 3.00) | 1.00 (0.00, 3.00) | |
| Comorbidity incidence | ||||
| Hypertension | 55,857 (34%) | 6,506 (38%) | 1,723 (39%) | <.001 |
| Diabetes mellitus | 35,694 (22%) | 4,247 (25%) | 1,089 (25%) | <.001 |
| Myocardial infarction | 8,950 (5.4%) | 1,103 (6.5%) | 306 (6.9%) | <.001 |
| Congestive heart failure | 18,359 (11%) | 2,252 (13%) | 648 (15%) | <.001 |
| Peripheral vascular disease | 14,695 (8.9%) | 1,839 (11%) | 472 (11%) | <.001 |
| Stroke | 13,700 (8.3%) | 1,651 (9.7%) | 424 (9.6%) | <.001 |
| Dementia | 6,379 (3.9%) | 644 (3.8%) | 185 (4.2%) | .5 |
| Chronic pulmonary disease | 25,521 (15%) | 3,078 (18%) | 811 (18%) | <.001 |
| Rheumatologic disease | 6,039 (3.6%) | 714 (4.2%) | 161 (3.6%) | .001 |
| Mild or severe liver disease | 10,784 (6.5%) | 1,211 (7.1%) | 368 (8.3%) | <.001 |
| Hemiplegia or paraplegia | 2,631 (1.6%) | 358 (2.1%) | 80 (1.8%) | <.001 |
| Renal disease | 21,145 (13%) | 2,717 (16%) | 758 (17%) | <.001 |
| Any malignancy (except skin) | 13,658 (8.3%) | 1,797 (11%) | 534 (12%) | <.001 |
| Metastatic solid tumor | 3,149 (1.9%) | 418 (2.5%) | 109 (2.5%) | <.001 |
| HIV/AIDS | 1,115 (0.7%) | 53 (0.3%) | <20 c | <.001 |
| Multiple comorbidities | 80,485 (49%) | 9,159 (54%) | 2,420 (55%) | <.001 |
| Current or former smoker | 53,254 (32%) | 3,571 (21%) | 1,102 (25%) | <.001 |
| Outcomes | ||||
| Any oxygen support | 15,310 (9.3%) | 2,112 (12%) | 486 (11%) | <.001 |
| Any mechanical ventilation | 15,289 (9.2%) | 2,428 (14%) | 674 (15%) | <.001 |
| Hospital readmission | 7,897 (4.8%) | 1,015 (6.0%) | 258 (5.8%) | <.001 |
| MACE | 17,425 (11%) | 2,684 (16%) | 803 (18%) | <.001 |
| ECMO | 880 (0.5%) | 151 (0.9%) | 40 (0.9%) | <.001 |
| All‐cause inpatient mortality or hospice | 21,580 (13%) | 2,943 (17%) | 800 (18%) | <.001 |
| Time to death in days, median (IQR) | 15 (7, 35) | 15 (7, 36) | 15 (7, 33) | .5 |
| Quarter of diagnosis | <.001 | |||
| Jan‐Mar 2020 | 8,995 (5.4%) | 126 (0.7%) | 40 (0.9%) | |
| Apr‐Jun 2020 | 35,175 (21%) | 1,695 (10.0%) | 451 (10%) | |
| Jul‐Sep 2020 | 19,319 (12%) | 2,497 (15%) | 617 (14%) | |
| Oct‐Dec 2020 | 51,346 (31%) | 6,412 (38%) | 1,593 (36%) | |
| Jan‐Mar 2021 | 35,340 (21%) | 4,180 (25%) | 1,150 (26%) | |
| Apr‐Jun 2021 | 15,308 (9.3%) | 2,064 (12%) | 574 (13%) | |
| Subregion | <.001 | |||
| New England | 10,622 (6.4%) | 558 (3.3%) | 399 (9.0%) | |
| Middle Atlantic | 43,115 (26%) | 110 (0.6%) | 39 (0.9%) | |
| South Atlantic | 32,000 (19%) | 5,191 (31%) | 1,483 (34%) | |
| East South Central | 9,839 (5.9%) | 3,923 (23%) | 730 (16%) | |
| East North Central | 40,878 (25%) | 3,305 (19%) | 672 (15%) | |
| West North Central | 8,005 (4.8%) | 2,646 (16%) | 807 (18%) | |
| West South Central | 631 (0.4%) | 75 (0.4%) | <30 c | |
| Mountain | 13,209 (8.0%) | 1,079 (6.4%) | 264 (6.0%) | |
| Pacific | 7,184 (4.3%) | 87 (0.5%) | <20 c | |
Statistics presented: n (%).
Statistical tests performed: chi‐square test of independence, Kruskal‐Wallis test.
Censored to remove small cell count or potential reidentification of small cell count.
FIGURE 2.
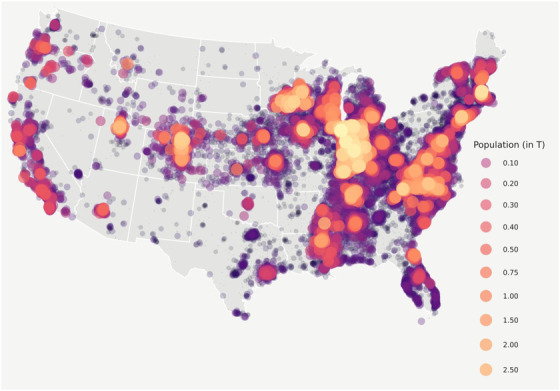
N3C patient distribution. Figure 2 shows the geospatial distribution of the N3C COVID‐19‐positive population. N3C contains data from 65 data contributors from across the United States, 52 of whom include sufficient location information to spatially map by ZIP Code centroid. Of those sites, we selected 44 whose data met our minimum robustness qualifications for inclusion in our study. This bubble map is to scale with larger bubbles representing more patients. Numbers represent population distribution, in thousands
Underlying health disparities and observable vulnerabilities
Among all patients, rural populations had higher rates of comorbidities across 14 of the 15 comorbidity categories and had notably higher rates of obesity (Table 1). Among our C19 cohorts, between 65% and 80% of patients in each category had prior visit history and between 63% and 74% had prior conditions reported in the pre‐COVID period, suggesting that our data robustness matrix (Figure 1) sufficiently captured patients with high‐fidelity data. The median number of pre‐C19 visits was 10 (IQR 3, 27) across all patients included in this study, with similar distribution across rural categories.
Assessing the date of SARS‐CoV‐2 infection and hospitalization, as a proxy for changes in clinical practice and treatment, we found that rural C19 patients were more likely to be diagnosed (Table 1) and subsequently hospitalized later in the pandemic (Table 2) when treatment practices were leading to better outcomes. Urban dwellers had higher caseloads in the first 3 quarters of 2020 (January‐September, 2020: 32% urban, 23% UAR, and 19% NAR; P<.001) and rural dwellers had higher caseloads in all subsequent periods (October, 2020‐June, 2021: 68% urban, 77% UAR, and 81% NAR; P<.001). Hospitalization rates were consistent with caseloads by rural categories across time periods with urban dwellers seeing greater hospitalization loads in the first 3 quarters of 2020 (January‐September, 2020: 38% urban, 25% UAR, and 25% NAR; P<.001), while rural dwellers saw higher hospitalization loads in all subsequent quarters (October, 2020‐June, 2021: 62% urban, 75% UAR, and 75% NAR; P<.001).
Hospitalization
As shown in Figure 3, crude hospitalization rates showed that persons in urban areas had lower odds of hospitalization than UAR (cOR 0.91, 95% CI, 0.90, 0.93) and NAR (cOR 0.96, 95% CI, 0.93‐0.99) dwellers over all time periods. After adjusting for differences in gender, race, ethnicity, BMI, age, CCI, quarter of diagnosis, and Census subregion, C19 patients in rural areas had increased odds of hospitalization: UAR (aOR 1.18, 95% CI, 1.16, 1.21) and NAR (aOR 1.29, 95% CI, 1.24, 1.34). Full model results for all adjusted models are provided in Table S2.
FIGURE 3.
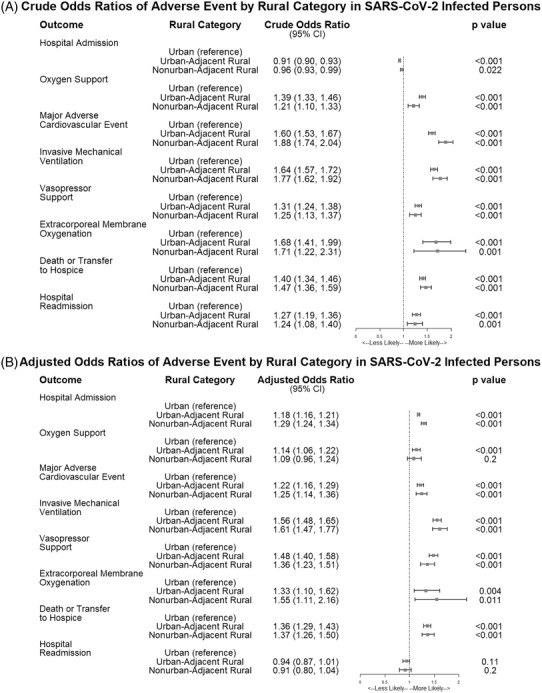
Forest plot showing the crude and adjusted odds ratios for adverse events by rural category in SARS‐CoV‐2‐infected persons in N3C, January 2020‐June 2021. Figure 3 shows the crude (A) and adjusted (B) odds ratios for being hospitalized, dying or being transferred to hospice after hospitalization, requiring any inpatient oxygen support, having a major adverse cardiovascular event, requiring invasive mechanical ventilation, requiring extracorporeal membrane oxygenation, or having a hospital readmission after initial hospitalization in the SARS‐CoV‐2‐infected population in N3C by rural category. Risk is similar between adjusted and unadjusted models, suggesting a real impact of rurality on adverse events. Adjusted models include adjustments for gender, race, ethnicity, BMI category, age, Charlson Comorbidity Index (CCI) composite score, rurality, quarter of diagnosis, and Census subregion. Data provider is included as a random effect in the adjusted models to account for differences across source data systems
Mortality
All‐cause 90‐day mortality or transfer to hospice during the study period was 3,054.2 per 100,000 persons, with higher rates among rural (3,946.5 per 100,000 persons) than urban dwellers (2,931.1 per 100,000 persons). The model estimates of all‐cause inpatient mortality or transfer to hospice after C19 diagnosis were significantly greater for rural compared to urban patients: UAR (OR 1.40, 95% CI, 1.34, 1.46) and NAR (OR 1.47, 95% CI, 1.36‐1.59) (Figure 3). After adjusting for differences in gender, race, ethnicity, BMI, age, CCI, quarter of diagnosis, and Census subregion, mortality remained approximately 36% greater for rural C19 hospitalized patients, UAR (aOR 1.36, 95% CI, 1.29‐1.43) and NAR (aOR 1.37, 95% CI, 1.26‐1.50) (Figure 3).
Kaplan‐Meier survival estimates demonstrate significantly higher mortality 90 days after hospitalization among rural C19 patients compared to their urban counterparts. As shown in Figure 4, hospitalized C19 patients with a higher CCI (indicating higher comorbid burden) and diagnosis earlier in the pandemic demonstrated significantly higher mortality (P<.0001).
FIGURE 4.
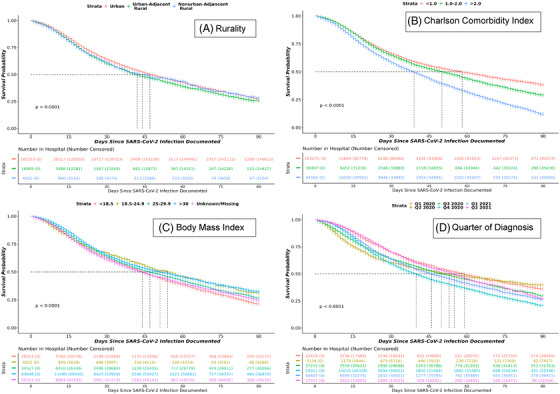
Kaplan‐Meier survival curves in SARS‐CoV‐2‐infected patients over 90 days from hospital admission. Figure 4 shows Kaplan‐Meier survival estimates in hospitalized SARS‐CoV‐2 persons in N3C by rurality (A), Charlson Comorbidity Index category (B), body mass index category (C), and quarter of diagnosis (D). Events were censored at day 90 or if patients left the hospital prior to 90 days
Secondary outcomes
We included several secondary outcomes reflective of C19 complications, including oxygen support, invasive mechanical ventilation, MACE, ECMO, and hospital readmission after initial hospitalization event, all of which were significantly elevated in rural compared to urban categories (Figure 3). Odds of hospital readmission was higher in rural dwellers, both UAR (cOR 1.27, 95% CI, 1.19, 1.36) and NAR (cOR 1.24, 95% CI, 1.08, 1.40). However, after adjustments, rural dwellers had lower odds of hospital readmission: UAR (aOR 0.94, 95% CI, 0.87, 1.01) and NAR (aOR 0.91, 95% CI, 0.80, 1.04) compared with their urban counterparts. The mean time to death was similar across all rural categories (Table 2).
SARS‐CoV‐2‐uninfected comparison group sensitivity analysis
To compare the baseline differences between SARS‐CoV‐2‐infected and ‐uninfected patients, we ran a sensitivity analysis using the same inclusion/exclusion criteria. We relied on earliest available negative SARS‐CoV‐2 lab test as the index date in the uninfected cohort. This cohort included 958,967 SARS‐CoV‐2‐uninfected patients (803,001 urban, 122,376 UAR, and 33,590 NAR).
Inpatient death or transfer to hospice was higher along rural lines in the uninfected cohort, albeit attenuated compared to the SARS‐CoV‐2‐infected population. After adjusting for differences in gender, race, ethnicity, BMI, age, CCI, quarter of earliest negative lab test, and Census subregion, odds of all‐cause mortality remained approximately 15% higher for rural C19 hospitalized patients, UAR (aOR 1.15, 95% CI, 1.12‐1.18) and NAR (aOR 1.16, 95% CI, 1.12‐1.21) (Table S3).
DISCUSSION
This retrospective cohort study from a large representative data enclave of C19 patients found significantly higher mortality rates among hospitalized rural C19 patients. Mortality was approximately 36% higher among rural C19 patients after adjustment for age, gender, race, ethnicity, BMI, CCI composite score, date of diagnosis, Census subregion, and differences derived from the data contributor.
The SARS‐CoV‐2 pandemic provides an important lens to examine all‐cause mortality by area of residence. SARS‐CoV‐2 is a new virus, rapidly transmitted in an immunologically naïve human population and initially without known effective treatment. The rapidity with which first urban then rural communities in the United States experienced surges of SARS‐CoV‐2 led us to hypothesize that an urban‐rural mortality differential would not be observed. That, however, is not the case. Although rural populations are older and have higher rates of comorbid conditions, 38 such as diabetes mellitus and obesity, 39 which have been associated with increased disease severity and death in SARS‐CoV‐2 infection, adjustment for these factors did not change the finding of higher rural mortality. 40 , 41
The gradient of risk for chronic diseases and mortality between urban and rural inhabitants is a relatively recent development in the United States. Prior to 1980, mortality rates in rural and urban areas of the United States were comparable. Since then, mortality rates in rural America have exceeded urban rates, and the gap has accelerated since 1999, even when adjusted for age. 39 , 40 , 42 This mortality disparity has been referred to as “the nonmetropolitan penalty,” 43 and some experts believe that structural urbanism is widening the gap. 44 Similar findings are noted among nonmetropolitan counties for common causes of death, including heart disease, cancer, chronic lower respiratory disease, unintentional injury, and stroke. 7
Given the research objectives, it would be illogical to adjust the rural versus urban comparison for clinical severity, such as clinical severity at the time of hospital admission. If a rural versus urban effect exists, it almost certainly would lead to rural versus urban differences in clinical severity at admission. Therefore, adjusting for clinical severity at admission would result in adjusting away the effect that the modeling seeks to evaluate. Our modeling showed that the rural versus urban effect was larger among patients hospitalized for C19 than among patients hospitalized for non‐COVID reasons. This suggests that rurality played a greater relative role in C19 outcomes than it plays in the outcomes resulting from most other (non‐COVID) hospitalizations.
The etiology of this “penalty” is likely multifactorial. Poverty, unemployment, and lower levels of educational attainment are more prevalent in rural areas, and these factors may partially explain the urban‐rural mortality disparity. Diminished access to care has also been described as contributing to rural‐urban mortality differences. One study concluded that having 1 or more specialist visits during the previous year was associated with 16.6% lower mortality for those with chronic conditions. 45 , 46 Among counties with defined shortages in primary care delivery, 56% are nonmetropolitan, while 19% are metropolitan, according to data reported by the Health Resources and Services Administration. 47
Although limited research has been done on socioeconomic risk factors and case‐fatality rates over the first year of the SARS‐Cov‐2 pandemic, a recent study from Japan observed higher C19‐related mortality in prefectures with the lowest household incomes. 48 A recent study conducted using spatial models in the United States found rurality to be one of several ecologic determinants of C19 mortality. 15
Our results again raise the question of why rural populations experience higher mortality rates after adjustment for multiple factors, even with a new pathogen, such as SARS‐CoV‐2. To what extent delays in care contribute to increased SARS‐CoV‐2‐related mortality among rural populations is unclear, as is the potential impact of environmental risk factors in rural areas. Further research is needed as to whether delays in care result in increased hospitalization and mortality across other acute and chronic medical conditions, perhaps more generally explaining systemic discrepancies in rural‐urban outcomes observed in with C19 in this large N3C cohort. In particular, whether these potential delays arise prior to or after contact with health care, or both. If proven, the former would likely require educational efforts, proactive care, and more rapidly available, low‐barrier access, such as telehealth. If demonstrated, posthealth care system contact delays might be addressed with system‐based changes, including greater integration and telehealth support from advanced centers.
In addition to possible delays as an explanatory factor for the “rural penalty,” identifying and understanding other potential causes of urban‐rural health disparities, including mortality across both chronic and acute conditions, may inform study of rational and cost‐effective mitigation strategies. Others 45 , 49 , 50 have demonstrated that attribution of rurality does not provide a one‐size‐fits‐all means to prescribe approaches to reduce health disparities. There are many other possible contributors to the observed disparity, highlighting the urgent need for a robust research agenda that will address the root causes, which may differ by geographic region.
The C19 pandemic appears to highlight and extend the apparent relative vulnerability of rural populations to acute health conditions. While confirming poorer baseline health status, the N3C data also suggest that rural dwellers have incremental vulnerability to C19 as an acute health condition. Poorer baseline health measures do not fully explain the disparately adverse C19 acute outcomes among rural dwellers. Recent scholarship points to a dynamic, ecological model for addressing rural adversity based on tailored approaches to individual community needs. 51 Additional observational and experimental research is needed to potentially identify practical evidence‐based steps to improve health outcomes among rural dwellers for both acute and chronic health conditions.
Limitations
N3C is an observational registry compiling data from multiple diverse participating sites. Therefore, some information may be entirely or partially and nonrandomly missing from the database in rural versus urban residents. In our C19‐positive cohort, we report the incidence of comorbidities in more than two‐thirds of our study population, which is similar to those reported in a COVID‐19 study across OCHIN, a network of 396 community health centers across 14 states. 52 Nonetheless, we examined the possibility that estimated rural effects stemmed from rural and urban patients differing in the extent of pre‐COVID comorbidity information and found this not to be the case. To some degree, such potential bias can be partly assessed by future analyses, which would include data based on all diagnosed C19 populations in geographic areas rather than data limited to C19 patients who received care at N3C collaborating provider systems.
We believe the sample to be a good representation of the United States in terms of raw distribution of both region and rurality, but further research using community health centers would provide additional insight into differences in outcomes across the severity spectrum and would provide less uncertainty about the severity of disease at admission. Additionally, health care organizations contributing data to N3C may have cared for more severely ill patients, providing a potential source of bias. In any case, understanding differences in those persons requiring treatment at tertiary care centers provides value; in addition, the relative distribution of deaths is similar to that for the overall population reported in public health systems, which suggests a nondifferential risk of misclassification.
Other limitations of the N3C data source include data aggregated from different health systems with different local practices, regulations, and data models, resulting in potential reporting differences, despite our application of data robustness checks. Additionally, the comparison group is limited only to those prematched at the site level from patients who have had a confirmed negative SARS‐CoV‐2 test. Although 54% of American Indian and Alaska Native populations in the United States live in rural areas and have been disproportionately affected by C19, 53 , 54 their racial demographic is unavailable for explicit study in accordance with tribal sovereignty policies. Finally, these analyses are limited to residents in the United States and may not be generalizable to other countries.
CONCLUSIONS
Hospitalization, death, and other adverse events were significantly higher among rural C19 patients than their urban counterparts after adjusting for multiple factors, including age, sex, race, Census subregion, and comorbidities. These data provide evidence‐based documentation of rural health disparities. Further research is needed to understand this disparity for both acute and chronic health conditions.
ADDITIONAL DATA PARTNERS WHO HAVE SIGNED DTA AND DATA RELEASE PENDING
The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children's National (CTSA‐CN) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Cincinnati Children's Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • Aurora Health Care — UL1TR002373: Wisconsin Network For Health Research • Brown University — U54GM115677: Advance Clinical Translational Research (Advance‐CTR) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • #N/A — UL1TR001445: Langone Health's Clinical and Translational Science Institute • Children's Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • HonorHealth — None (Voluntary) • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE‐CTR) Network • Arkansas Children's Hospital — UL1TR003107: UAMS Translational Research Institute.
Supporting information
Supporting information
TableS1‐S3
ACKNOWLEDGMENTS
The project described was supported by the National Institute of General Medical Sciences, U54GM104942‐05S2, U54GM115458, U54GM104940, U54GM104938, U54GM115516, U54GM115677, U54GM115428, and U54GM104941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors wish to thank Dr. Michele McGuirl, Chief of the Research Advancement Programs Branch, National Institute of General Medical Sciences, for her support of the Institutional Development Award (IDeA) Centers for Translational Research (CTRs), and the N3C Rural Health Domain Team (https://covid.cd2h.org/rural‐health).
The analyses described in publication were conducted with data or tools accessed through the NCATS N3C Data Enclave (https://covid.cdh2.org/)] and supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organizations (https://ncats.nih.gov/n3c/resources/data‐contribution/data‐transfer‐agreement‐signatories) and scientists who have contributed to the ongoing development of this community resource (https://doi.org/10.1093/jamia/ocaa196).
We gratefully acknowledge contributions from the following N3C core teams:
(Asterisks indicate leads) • Principal Investigators: Melissa A. Haendel*, Christopher G. Chute*, Kenneth R. Gersing, Anita Walden
• Workstream, subgroup, and administrative leaders: Melissa A. Haendel*, Tellen D. Bennett, Christopher G. Chute, David A. Eichmann, Justin Guinney, Warren A. Kibbe, Hongfang Liu, Philip R.O. Payne, Emily R. Pfaff, Peter N. Robinson, Joel H. Saltz, Heidi Spratt, Justin Starren, Christine Suver, Adam B. Wilcox, Andrew E. Williams, Chunlei Wu
• Key liaisons at data partner sites
• Regulatory staff at data partner sites
• Individuals at the sites who are responsible for creating the datasets and submitting data to N3C • Data Ingest and Harmonization Team: Christopher G. Chute*, Emily R. Pfaff*, Davera Gabriel, Stephanie S. Hong, Kristin Kostka, Harold P. Lehmann, Richard A. Moffitt, Michele Morris, Matvey B. Palchuk, Xiaohan Tanner Zhang, Richard L. Zhu
• Phenotype Team (Individuals who create the scripts that the sites use to submit their data, based on the COVID and Long COVID definitions): Emily R. Pfaff*, Benjamin Amor, Mark M. Bissell, Marshall Clark, Andrew T. Girvin, Stephanie S. Hong, Kristin Kostka, Adam M. Lee, Robert T. Miller, Michele Morris, Matvey B. Palchuk, Kellie M. Walters
• Project Management and Operations Team: Anita Walden*, Yooree Chae, Connor Cook, Alexandra Dest, Racquel R. Dietz, Thomas Dillon, Patricia A. Francis, Rafael Fuentes, Alexis Graves, Julie A. McMurry, Andrew J. Neumann, Shawn T. O'Neil, Usman Sheikh, Andréa M. Volz, Elizabeth Zampino
• Partners from NIH and other federal agencies: Christopher P. Austin*, Kenneth R. Gersing*, Samuel Bozzette, Mariam Deacy, Nicole Garbarini, Michael G. Kurilla, Sam G. Michael, Joni L. Rutter, Meredith Temple‐O'Connor
• Analytics Team (Individuals who build the Enclave infrastructure, help create codesets, variables, and help Domain Teams and project teams with their datasets): Benjamin Amor*, Mark M. Bissell, Katie Rebecca Bradwell, Andrew T. Girvin, Amin Manna, Nabeel Qureshi
• Publication Committee Management Team: Mary Morrison Saltz*, Christine Suver*, Christopher G. Chute, Melissa A. Haendel, Julie A. McMurry, Andréa M. Volz, Anita Walden
• Publication Committee Review Team: Carolyn Bramante, Jeremy Richard Harper, Wenndy Hernandez, Farrukh M Koraishy, Federico Mariona, Amit Saha, Satyanarayana Vedula
Data partners with released data:
Stony Brook University — U24TR002306 • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA‐Clinical & Translational Research • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE‐CTR) Network • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • George Washington Children's Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children's National (CTSA‐CN) • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • University of Washington — UL1TR002319: Institute of Translational Health Sciences • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • University of Wisconsin‐Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • Children's Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • Nemours — U54GM104941: Delaware CTR ACCEL Program • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Tulane University — UL1TR003096: Center for Clinical and Translational Science • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • OCHIN — INV‐018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks
Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS‐CoV‐2‐infected persons in rural America. J Rural Health. 2022;1‐16. 10.1111/jrh.12689
Authorship was determined using ICMJE recommendations.
N3C Consortium Collaborators/Authors:
1. Christopher G. Chute, DrPH, MD, MPH—Schools of Medicine, Public Health, and Nursing, Johns Hopkins University, Baltimore, MD
2. Melissa A. Haendel, PhD—Center for Health AI, University of Colorado Anschutz Medical Campus, Aurora, CO
3. Anita Walden, MS—Center for Health AI, University of Colorado Anschutz Medical Campus, Aurora, CO
4. Davera Gabriel, RN—Institute for Clinical and Translational Research, Johns Hopkins University School of Medicine, Baltimore, MD
REFERENCES
- 1. Ahmad FB, Cisewski JA, Miniño A, Anderson RN. Provisional mortality data—United States, 2020. Morb Mortal Wkly Rep. 2021;70(14):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time [published correction appears in Lancet Infect Dis. 2020 Sep; 20(9): e215]. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. COVID‐19 Stats: COVID‐19 Incidence, by Urban‐Rural Classification — United States, January 22–October 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Census Bureau . American Community Survey: 2011–2015. US Census Bureau; 2010. [Google Scholar]
- 5. Callaghan T, Lueck JA, Trujillo KL, Ferdinand AO. Rural and urban differences in COVID‐19 prevention behaviors. J Rural Health. 2021;37(2):287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.]. Whitfield GP, Carlson SA, Ussery EN, Fulton JE, Galuska DA, Petersen R. Trends in meeting physical activity guidelines among urban and rural dwelling adults – United States, 2008–2017. MMWR Morb Mortal Wkly Rep. 2019;68(23):513‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh GK, Siahpush M. Widening rural‐urban disparities in life expectancy, U.S., 1969–2009. Am J Prev Med. 2014;46(2):e19‐e29. [DOI] [PubMed] [Google Scholar]
- 8. Pathman DE, Konrad TR, Dann R, Koch G. Retention of primary care physicians in rural health professional shortage areas. Am J Public Health. 2004;94(10):1723‐1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karim SA, Chen HF. Deaths for COVID‐19 in rural, micropolitan, and metropolitan areas: a county‐level comparison. J Rural Health. 2021;37:124‐132. [DOI] [PubMed] [Google Scholar]
- 10. Paul R, Arif A, Pokhrel K, Ghosh S. The association of social determinants of health with COVID‐19 mortality in rural and urban counties. J Rural Health. 2021;37(2):278‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson SL, Derakhshan S, Blackwood L, et al. Spatial disparities of COVID‐19 cases and fatalities in United States counties. Int J Environ Res Public Health. 2021;18(16):8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mueller JT, McConnell K, Burow PB, Pofahl K, Merdjanoff AA, Farrell J. Impacts of the COVID‐19 pandemic on rural America. Proc Natl Acad Sci. 2021;118(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Infectious Diseases Society of America (IDSA) . COVID‐19 Policy Brief: Disparities Among Rural Communities in the United States Version: December 22, 2020. Arlington, VA: IDSA; 2021. [Google Scholar]
- 14. Dixon BE, Grannis SJ, Lembcke LR, Valvi N, Roberts AR, Embi PJ. The synchronicity of COVID‐19 disparities: statewide epidemiologic trends in SARS‐CoV‐2 morbidity, hospitalization, and mortality among racial minorities and in rural America. PLoS One. 2021;16(7):e0255063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kathe NJ, Wani RJ. Determinants of COVID‐19 case fatality rate in the United States: spatial analysis over one year of the pandemic. J Health Econ Outcomes Res. 2021;8(1):51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haendel MA, Chute CG, Bennett TD, et al; N3C Consortium . The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28(3):427‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfaff E, Kostka K, Miller R, et al. 2021. COVID‐19 Phenotype Documentation, Version 3.3. N3C Phenotype & Data Acquisition Workstream. https://github.com/National‐COVID‐Cohort‐Collaborative/Phenotype_Data_Acquisition/wiki/Latest‐Phenotype. Accessed November 3, 2021.
- 19. Pfaff E, Kostka K, Miller R, et al. 2021. N3C Phenotype & Data Acquisition Workstream. Github Repository. https://github.com/National‐COVID‐Cohort‐Collaborative/Phenotype_Data_Acquisition. Accessed November 3, 2021.
- 20. Bennett TD, Moffitt RA, Hajagos JG, et al. Clinical characterization and prediction of clinical severity of SARS‐CoV‐2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open. 2021;4(7):e2116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US Department of Agriculture, Economic Research Service . Rural‐Urban Commuting Area Codes. https://www.ers.usda.gov/data‐products/rural‐urban‐commuting‐area‐codes/. Accessed November 15, 2021.
- 22. University of Washington . Rural‐Urban Commuting Area Codes (RUCAs). https://depts.washington.edu/uwruca/. Accessed November 15, 2021.
- 23. Rahman M, White EM, Thomas KS, Jutkowitz E. Assessment of rural‐urban differences in health care use and survival among Medicare beneficiaries with Alzheimer disease and related dementia. JAMA Netw Open. 2020;3(10):e2022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Onega T, Weiss JE, Alford‐Teaster J, Goodrich M, Eliassen MS, Kim SJ. Concordance of rural‐urban self‐identity and ZIP Code‐derived Rural‐Urban Commuting Area (RUCA) Designation. J Rural Health. 2020;36(2):274‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chappel AR, Zuckerman RS, Finlayson SR. Small rural hospitals and high‐risk operations: how would regionalization affect surgical volume and hospital revenue? J Am Coll Surg. 2006;203(5):599‐604. [DOI] [PubMed] [Google Scholar]
- 26. Smith J. Comparison of COVID‐19 case and death counts in the United States reported by four online trackers: January 22–May 31, 2020. MedRxiv. 2020.
- 27. Centers for Disease Control and Prevention . COVID‐19 Vaccinations in the United States, County. https://data.cdc.gov/Vaccinations/COVID‐19‐Vaccinations‐in‐the‐United‐States‐County/8xkx‐amqh. Accessed November 21, 2021.
- 28. Dixon BE, Wen C, French T, Williams JL, Duke JD, Grannis SJ. Extending an open‐source tool to measure data quality: case report on Observational Health Data Science and Informatics (OHDSI). BMJ Health Care Inform. 2020;27(1):e100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Visweswaran S, Becich MJ, D'Itri VS, et al. Accrual to Clinical Trials (ACT): a Clinical and Translational Science Award Consortium Network. JAMIA Open. 2018;1(2):147‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Topaloglu U, Palchuk MB. Using a federated network of real‐world data to optimize clinical trials operations. JCO Clin Cancer Inform. 2018;2:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bian J, Lyu T, Loiacono A, et al. Assessing the practice of data quality evaluation in a national clinical data research network through a systematic scoping review in the era of real‐world data. J Am Med Inform Assoc. 2020;27(12):1999‐2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anzalone AJA; Rural Health Domain Team . Concept Sets. Github Repository. 2021.. https://github.com/National‐COVID‐Cohort‐Collaborative/CS‐Rural‐Health/tree/main/rural‐mortality‐and‐hospitalization/concept‐sets. Accessed November 30, 2021.
- 33. United States, January–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:523‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. US Department of Agriculture, Economic Research Service . Rural‐Urban Commuting Area Codes. ZIP Code File. 2010.. https://www.ers.usda.gov/webdocs/DataFiles/53241/RUCA2010zipcode.xlsx?v=4790.9. Accessed November 15, 2021.
- 35. US Department of Health and Human Services . HRSA Federal Office of Rural Health Policy. Defining Rural Population. 2021. https://www.hrsa.gov/ruralhealth/aboutus/definition.html. Accessed November 15, 2021.
- 36. Sjoberg DD, Whiting K, Curry M. Reproducible summary tables with the gtsummary Package. R J. 2021;13:570. [Google Scholar]
- 37. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676‐682. [DOI] [PubMed] [Google Scholar]
- 38. Weeks WB, Kazis LE, Shen Y, et al. Differences in health‐related quality of life in rural and urban veterans. Am J Public Health. 2004;94:1762‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hale NL, Bennett KJ, Probst JC. Diabetes care and outcomes; disparities across rural America. J Community Health. 2010;35:365‐374. [DOI] [PubMed] [Google Scholar]
- 40. Tan AX, Hinman JA, Abdel Magid HS, Nelson LM, Odden MC. Association between income inequality and county‐level COVID‐19 cases and deaths in the US. JAMA Netw Open. 2021;4(5):e218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matthews KA, Ullrich F, Gaglioti AH, Dugan S, Chen MS, Hall DM. Nonmetropolitan COVID‐19 incidence and mortality rates surpassed metropolitan rates within the first 24 weeks of the pandemic declaration: United States, March 1–October 18, 2020. J Rural Health. 2021;37(2):272‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cross SH, Califf RM, Warraich HJ. Rural‐urban disparity in mortality in the US from 1999 to 2019. JAMA. 2021;325(22):2312‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cosby AG, Neaves TT, Cossman RE, et al. Preliminary evidence for an emerging nonmetropolitan mortality penalty in the United States. Am J Public Health. 2008;98:1470‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Probst J, Eberth JM, Crouch E. Structural urbanism contributes to poorer health outcomes for rural America. Health Aff (Millwood). 2019;38(12):1976‐1984. [DOI] [PubMed] [Google Scholar]
- 45. Johnston KJ, Wen H, Joynt Maddox KE. Lack of access to specialists associated with mortality and preventable hospitalizations of rural Medicare beneficiaries. Health Aff (Millwood). 2019;38(12):1993‐2002. [DOI] [PubMed] [Google Scholar]
- 46. Singh GK, Siahpush M. Widening rural‐urban disparities in all‐cause mortality and mortality from major causes of death in the USA, 1969–2009. J Urban Health. 2014;91:272‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Health Resources and Services Administration . Data Downloads: Health Professional Shortage Areas (HPSAs), HPSA‐Primary Care. https://data.hrsa.gov/data/download. Accessed November 30, 2021.
- 48. Yoshikawa Y, Kawachi I. Association of socioeconomic characteristics with disparities in COVID‐19 outcomes in Japan. JAMA Netw Open. 2021;4(7):e2117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cohen SA, Cook SK, Kelley L, Foutz JD, Sando TA. A closer look at rural‐urban health disparities: associations between obesity and rurality vary by geospatial and sociodemographic factors. J Rural Health. 2017;33(2):167‐179. [DOI] [PubMed] [Google Scholar]
- 50. Alanazy ARM, Wark S, Fraser J, Nagle A. Factors impacting patient outcomes associated with use of emergency medical services operating in urban versus rural areas: a systematic review. Int J Environ Res Public Health. 2019;16(10):1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lawrence‐Bourne J, Dalton H, Perkins D, et al. What is rural adversity, how does it affect wellbeing and what are the implications for action? Int J Environ Res Public Health. 2020;17(19):7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huguet N, Schmidt T, Larson A, et al. Prevalence of pre‐existing conditions among community health center patients with COVID‐19: implications for the Patient Protection and Affordable Care Act. J Am Board Fam Med. 2021;34(Suppl):S247‐S249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dewees S, Marks B. Twice Invisible: Understanding Rural Native America. First Nations Development Institute; 2017. [Google Scholar]
- 54. Brodt E, Empey A. American Indians and Alaska Natives in the COVID‐19 pandemic: the grave burden we stand to bear. Health Equity. 2021;5(1):394‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
TableS1‐S3


