Abstract
The COVID‐19 pandemic caused by SARS‐CoV‐2 virus quickly spread globally, infecting over half a billion individuals, and killing over 6 million*. One of the more unusual symptoms was patients' complaints of sudden loss of smell and/or taste, a symptom that has become more apparent as the virus mutated into different variants. Anosmia and ageusia, the loss of smell and taste, respectively, seem to be transient for some individuals, but for others persists even after recovery from the infection. Causes for COVID‐19‐associated chemosensory loss have undergone several hypotheses. These include non‐functional or destroyed olfactory neurons and gustatory receptors or of their supporting cells, disruption of the signaling protein Neuropilin‐1, and disruption in the interaction with semaphorins, key molecules in the gustatory and olfactory axon guidance. The current paper will review these hypotheses and chart out potential therapeutic avenues.
Keywords: chemosensory disorders, COVID‐19, olfaction, SARS CoV‐2, smell loss, taste, taste loss
1. INTRODUCTION
In the first 2 months of the COVID‐19 pandemic, unusual symptoms of smell and taste dysfunction in 43.8% of patients associated with an emerging global pandemic of COVID‐19 patients were reported (Guan et al., 2020). Since mid‐March of 2020, when a German virologist first reported the prevalence of smell and taste dysfunction in COVID‐19 patients at 66.7%, many subsequent studies have confirmed this important symptom (Streeck, 2020). The range of chemosensory dysfunction in infected patients varied. While some experienced hypogeusia, a reduced sense of taste, others complained of dysgeusia, a distorted sense of taste, or ageusia, a complete loss of it. Those infected could also experience parosmia, a distortion of smell, hyposmia, reduced smell, or partial/complete anosmia, loss of smell (Dicpinigaitis, 2021). For many scientists, this was the first time they learned the medical terms that define chemosensory disorders a debilitating condition affecting nutrition and the quality of life. A recent systematic review of 22 studies shows pooled prevalence of all COVID‐19‐associated olfactory dysfunction of 55%, and 40%, each for anosmia and hyposmia, respectively (Esmaeili et al., 2021). In the same study, the frequency of any gustatory dysfunction, and separately, ageusia and dysgeusia were estimated to be 41%, 31%, and 34%, respectively.
Although the sense of taste and smell are both detecting chemicals, olfactory and gustatory dysfunctions in COVID‐19 patients appear to be independent of each other. To understand the background of both chemosensory disorders, this review will cover them separately.
2. CLINICAL FEATURES, PREVALENCE, AND SUSCEPTIBILITY
The clinical features of COVID‐19 disease are diverse and vary from individual to individual. Some patients are completely asymptomatic, while others experience cold‐like symptoms similar to other viral respiratory system infections (Ripa et al., 2021). The COVID‐19 literature is replete with chemosensory dysfunctions as a common reported symptom in infected patients. Anosmia and/or ageusia, therefore, are essential in distinguishing between COVID‐19 from other upper respiratory tract infections (Mastrangelo et al., 2021). The timing of onset of the chemosensory disorder symptoms were also relevant. Symptoms of anosmia developed on average after 4.4 days after the onset of SARS‐CoV‐2 infection and lasted on average 9 days. Of these patients, 98% recovered within 28 days (Klopfenstein et al., 2020). PCR testing of 77,167 potentially COVID‐19‐infected patients found that anosmia and dysgeusia were early symptoms of SARS‐CoV‐2 infection, and that COVID‐19‐positive patients were 27.1‐fold more likely to present anosmia or dysgeusia than COVID‐19‐negative patients with other upper respiratory illness (Wagner et al., 2020).
Age distribution of the COVID‐induced mortality in 22 studies across 45 countries was found lowest in the 5–9‐year‐old age group, increasing by age in a linear‐logarithmic fashion (O'Driscoll et al., 2020). These data correlate with cytomegalovirus (CMV) infection in epithelial cells in which the presence of CMV increased with age. CMV, like SARS‐CoV‐2 infection, is linked to the dysfunction and constant activation of T cells leading to a massive recruitment of immune cells. Another important finding was that Neuropilin 1 a cell‐surface receptor for a variety of physiological ligands was observed as critical host factor for CMV infection (Gudowska‐Sawczuk & Mroczko, 2021; Lane et al., 2020). The very same molecule appears to be implicated in the mechanisms of chemosensory loss (see further).
The prevalence of COVID‐19 seems to correlate significantly with the geography, ethnicity, genetics, age, gender, and severity of the disease (Bartheld et al., 2020; Butowt & von Bartheld, 2021). Studies outside of Asia have shown threefold higher prevalence of smell and/or taste dysfunction with COVID‐19 in Caucasians than in Asian patients (Bartheld et al., 2020; Butowt & von Bartheld, 2021).
A recent study determined genetic susceptibility for self‐reported anosmia or ageusia in COVID‐19 disease. Genome‐wide association study found such a link with uridine −5′ phosphate glucuronosyltransferase family 2A1/A2 (UGT2A1/UGT2A2) locus. UGT2A proteins are found in the nasal epithelium and are part of a family of enzymes that breakdown odorants that bind to olfactory receptors. These findings may help with predictability and early diagnosis for chemosensory disorders in COVID‐19 (Shelton et al., 2022).
3. PHYSIOLOGY OF SMELL AND TASTE
As air flows through the nasal air passage, airborne chemical compounds are detected by specialized olfactory receptors located in the olfactory neuroepithelium, covering the upper and middle turbinate. The olfactory neuroepithelium contains 25 million bipolar olfactory sensory neurons (OSN) that send their axons across the cribriform plate, before reaching the olfactory bulb, one of oldest segments of the brain.
In addition to the OSNs, the olfactory neuroepithelium also contains sustentacular cells (SuCs), microvillar cells, Bowman glands, and basal cells (Fodoulian et al., 2020). The exposed aspect of OSN displays olfactory cilia where odor molecules bind to approximately 400 specific olfactory receptors. There are at least 1000 olfactory receptor genes but at least half of them are pseudogenes (Menashe et al., 2003). Each olfactory neuron is thought to be attuned to a single odorant. The axons of the olfactory neurons synapse in the olfactory bulb, where depending on the odorant stimuli, a different neuronal activity pattern is generated and interpreted accordingly in the brain. The olfactory bulb contains several layers that include the glomerular layer, where the primary neuronal axons synapse and the mitral and tufted cell layer, the second order of olfactory neurons. Roughly 25,000 primary olfactory neurons synapse with each glomerulus. Each glomerulus receives input from the sensory neurons expressing the same olfactory receptor protein (OR). The glomerulus also receives the axons of about 100‐second order olfactory neurons, the mitral and tufted cells. This implies that every second‐order olfactory neuron receives input from several thousands of primary olfactory neurons. Second‐order olfactory neurons send projections to the inferior surface of the temporal lobe where the primary olfactory (piriform) cortex is located (Spielman et al., 2019).
ORs are structurally part of the G‐Protein Coupled Receptor family. Following binding, the receptor undergoes a conformational change and activate elevation of the second messenger, cAMP leading to calcium influx and opening of calcium‐dependent Cl− channels leading to further depolarization and action potential (Kaupp, 2010).
The ability to taste involves linking taste receptor cells from the oral cavity to the brainstem, where information is propagated to the insular lobe buried deep under the frontal, temporal and parietal lobes for processing. Taste receptor cells, unlike olfactory neuronal receptors, are epithelial in nature. They are embedded in the tongue epithelium, the palate and pharynx, arise from the basal layer of the taste bud and mature to become one of several specialized gustatory receptor cells (Figure 1) (Lee et al., 2017; Roper & Chaudhari, 2017). The half‐life of a taste receptor cell is approximately 10–12 days, but mature cells exposed to the oral cavity are limited to a few hours before they shed into the oral cavity. We use different taste cells for each major meal.
FIGURE 1.
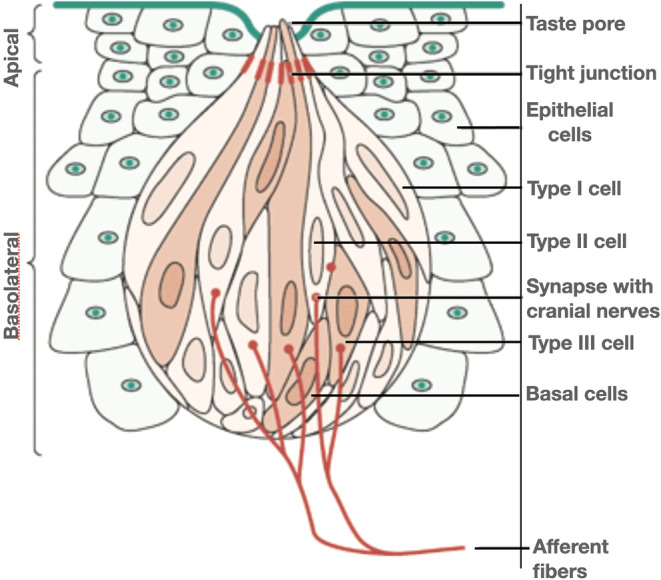
Structure of a taste bud (Spielman et al., 2019, reproduced with permission of John Wiley & Sons)
The taste cells are connected to the gustatory centers via several cranial nerves. Each taste receptor cell detects a single taste quality (Barretto et al., 2015). The fast‐reproducing epithelial taste receptor cells must reconnect with never‐reproducing cranial nerves to provide signal fidelity for each taste quality.
This is achieved through two molecules belonging to the semaphorin axon guidance family of molecules (SEMA3A and SEMA7A). Semaphorins help maintain the “labelled line principle” between peripheral bitter or sweet receptors and their respective central projection area in the gustatory center (Lee et al., 2017; Spielman & Brand, 2018). The role of semaphorins in both gustatory and olfactory systems and COVID‐associated chemosensory disorders will be explored in the next sections.
4. CHEMOSENSORY DYSFUNCTION
Anosmia is a common chemosensory disorder, although it has been brought to the forefront of public attention as an early symptom of COVID‐19. Post‐viral olfactory dysfunction has been well‐characterized before. It is assumed to be due to the virus‐induced damage to the olfactory epithelium. It is common to upper respiratory infections caused by rhinovirus, parainfluenza virus, Epstein–Barr virus, and other previously known coronaviruses (Dicpinigaitis, 2021). The virus could be detected in the nasal discharge of anosmic patients.
Typical symptoms of viral respiratory infections such as fever, congestion, mucus secretion, or cough are the result of the host's immune system to hinder viral replication and an attempt to trap and expel the virus. Surprisingly, chemosensory dysfunction symptoms in COVID‐19 appear to be without mucosal congestion (Shelton et al., 2022; Vaira et al., 2020). This suggests that compared to previously documented viral upper respiratory infections, SARS‐CoV‐2 may take an evasive pathway to diminish the hosts' immune response. Although most cases of anosmia are transient, viral or post‐viral anosmia can be truly debilitating for individuals infected with the SARS‐CoV‐2 virus irrespective of its duration.
Compared to olfaction, the gustatory system is relatively robust. Because of the epithelial (not neuronal) nature of taste receptor cells and wide distribution and innervation of the peripheral gustatory system, taste loss is a relatively rare condition, even with aging (Spielman, 2019). Most patients sense a decrease in the flavor of food, primarily because of olfactory loss, rather than true taste loss.
Most COVID‐19 patients in the first 2 days are asymptomatic, while many experience a few symptoms, of which olfactory and gustatory loss seem to be among the first. COVID‐19 patients with ageusia and anosmia do not seem to present symptoms of rhinitis, typically associated with virus‐induced upper respiratory illness (Shelton et al., 2022; Vaira et al., 2020). In a recent study, the prevalence of overall taste loss in COVID‐19 patients was 37% (Hannum et al., 2022) in line with a previous study (Esmaeili et al., 2021).
5. MECHANISMS BY WHICH SARS‐COV‐2 MAY CAUSE ANOSMIA
To affect olfaction, SARS‐CoV‐2 must interact directly or indirectly with components of the odorant processing pathway. These include the olfactory neuroepithelium, the olfactory bulb, or the olfactory cortex (Sharma et al., 2019). Here, we provide four hypotheses for olfactory dysfunction as they evolved over the course of the pandemic.
6. THE OLFACTORY SENSORY NEURON DESTRUCTION THEORY
At the beginning of the pandemic, the first mechanism of SARS‐CoV‐2‐induced anosmia was based on the analysis of data from post‐viral anosmia in rhinitis patients that also experienced damage to the nasal olfactory epithelium (Han et al., 2020). These data showed that even prior to COVID‐19, other viral conditions lead to post‐viral anosmia. The symptoms of olfactory loss seemed to persist for weeks to months until all damaged parts of the nasal olfactory epithelium presumably regenerated (Yamagishi et al., 1994). Based on prior viral pandemics, the COVID‐19 experience led to the initial hypothesis that a viral infection directly caused the destruction of OSNs and consequent anosmia (Doty, 2021; Han et al., 2020). While other upper respiratory infections caused nasal congestion and possibly loss of OSN, these symptoms proved inconsistent with the experience of anosmia. A multinational systematic study with a cohort of over 30,000 patients, nasal congestion, and loss of OSN were not the cause of anosmia. In 17 of these studies, 58.6% of patients did not experience nasal congestion, and in nine studies, the mean duration of subjective anosmia was only 9 days. Such inconsistency cannot support the neuronal destruction hypothesis. Patients were recovering from anosmia on average in 8–10 days, while the turnover rate for new olfactory neurons is much longer (Butowt & von Bartheld, 2021).
7. THE OLFACTORY SUSTENTACULAR CELL DAMAGE THEORY
The next hypothesis for anosmia focused on the role of the sustentacular cells and the role of proteins, ACE‐2, the angiotensin‐converting enzyme 2 and TMPRSS, the transmembrane serine protease, a cell‐surface protein, in the viral binding and cell entry (Hoffmann et al., 2020). The level of ACE2 expression in the nasal epithelium is still controversial. One study found ACE2 expression on ciliated OSN cells, while others demonstrated ACE2 expression in the basal layer of the nasal epithelium (Hamming et al., 2004; Sims et al., 2005). A single‐cell RNA‐sequence approach identified the specific cells of the olfactory epithelium that co‐express ACE2 and TMPRSS for SARS‐CoV‐2 binding and entry, and it was the non‐neuronal supporting cells, the sustentacular cells (Han et al., 2020). As a result, the hypothesis to understand the mechanism of anosmia shifted from damage of OSNs to sustentacular support cells. Several further studies confirmed this hypothesis. In the first study, a cohort of 12 golden Syrian hamsters were infected with two different SARS‐CoV‐2 isolates followed by examination of the nasal cavity. The olfactory epithelium (OE) was well‐preserved in the control animals but damaged by day two post‐infection. After the complete destruction of the olfactory epithelium at day four, the OE began to recover and reached pre‐infection thickness by 14 days (Bryche et al., 2020). The quick 4‐day recovery rate indicated that the target could not have been the OSN which requires a much longer replacement and ciliary maturation (Butowt & von Bartheld, 2021). Double staining of the olfactory mucosa with specific markers of mature OSNs and sustentacular cells clearly identified that only sustentacular cells contained viral antigens. To establish a secondary, immune‐cell mediated destruction of the OSN, the same study demonstrated the presence of Ionized Calcium‐binding Adapter Molecule (Iba1), a marker for monocyte/macrophage immune cells, in the olfactory mucosa of infected animals, but not controls (Bryche et al., 2020). These results confirmed that sustentacular cells were targeted for infection by SARS‐CoV‐2 and the damage caused to the olfactory epithelium was a result of death of SuCs and recruitment of immune cells and inflammation of the OE (Han et al., 2020). Figure 2 displays a theory of the SARS‐CoV‐2‐induced destruction of sustentacular cells and subsequent inflammation of the olfactory neuroepithelium.
FIGURE 2.
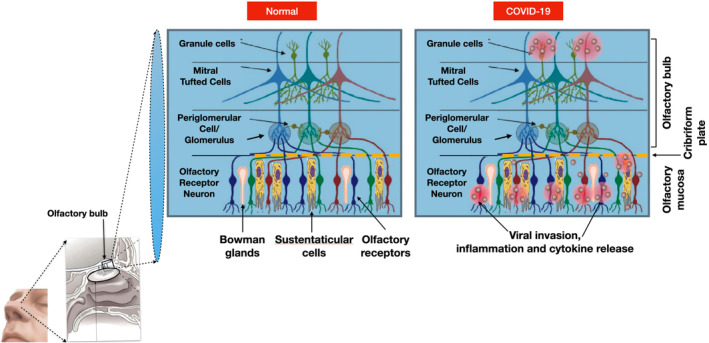
Olfactory neuroepithelium in health and in COVID‐19‐induced anosmia
In a follow‐up study (Fodoulian et al., 2020), collecting biopsies via nasal endoscopic surgery from four adult patients, expression of ACE2 and TMPRSS within the olfactory and respiratory epithelia was confirmed. Bulk RNA sequencing was then performed on the tissue to map out respiratory and olfactory epithelia datasets. A differential expression analysis between the two datasets found that olfactory‐specific genes, including olfactory receptor genes OMP and ERMN (a specific marker of SuCs), were abundant in the olfactory samples. After evaluating the co‐expression levels of ACE2 and TMPRSS in different cellular populations, they recorded that sustentacular cells in the olfactory epithelium and ciliated cells in the respiratory epithelium showed the highest levels of expression of both proteins (Fodoulian et al., 2020). With SARS‐CoV‐2 entering through the ACE2 receptor, damage to sustentacular cells seems to explain most of the transient anosmia experienced by COVID‐19 patients. This hypothesis, however, is still tenuous because the mechanism of how a destroyed SuC signals to its nearby OSN, or the mechanism by which SARS‐CoV‐2 enters olfactory receptor neurons from sustentacular cells is unclear. This led into alternative hypotheses that considers a different route of entry for SARS‐CoV‐2 (Cantuti‐Castelvetri et al., 2020).
8. THE NEUROPILIN‐1 THEORY
This theory involves the signaling protein Neuropilin‐1 (NRP‐1), a protein, that like ACE2 and TMPRSS, helps SARS‐CoV‐2 to enter a cell. The transmembrane form of NRP‐1 has a ligand‐binding site intended for growth factors, a site that SARS‐CoV‐2 uses to attach and evade the host immune system (Daly et al., 2020; Mayi et al., 2021). Unlike the ACE2 receptor, NRP‐1 is abundant in the olfactory and upper respiratory epithelium (Butowt & von Bartheld, 2021) and a binding site for Semaphorine‐3A necessary for olfactory axonal guidance (Schwarting et al., 2000). Similarly in taste, NRP‐1 acts as a co‐receptor for Semaphorin‐3A (SEMA3A), that guides signal transmission from bitter taste receptor cells to their respective bitter target neurons (Lee et al., 2017; Spielman & Brand, 2018). While NRP‐1 has a ligand‐binding site in its transmembrane form meant for growth factors like VEGF, viruses such as SARS‐CoV‐2 have seized this binding site to infect the host cell. In response, the host cell protease, furin, cleaves Spike protein of SARS‐CoV‐2 into S1 and S2 polypeptides, revealing a C‐terminal motif rich in lysine and arginine, also known as the C‐end rule (CendR) (Li & Buck, 2021). The CendR motif in S1 protein binds to NRP‐1, thus increasing infectivity of SARS‐CoV‐2. In another study, isolated cells from bronchoalveolar lavage of patients suffering from severe‐COVID‐19 disease were analyzed for RNA expression of Neuropilin‐1, NRP‐1 expression was found in SARS‐CoV‐2‐positive cells but not in control uninfected cells (Cantuti‐Castelvetri et al., 2020). Abundant in the olfactory and respiratory epithelia and as a receptor of SEMA3A, NRP‐1 appears to be a good candidate in the mechanism of anosmia associated with SARS‐CoV‐2 infection.
A congenital condition, Kallman syndrome, a disease that causes anosmia with delayed or absent puberty, received a renewed focus following the NRP‐1 hypothesis and potentially an impaired interaction between NRP‐1 and SEMA3A. Although Kallman syndrome is a congenital disease that occurs from improper fetal neuronal development, adult neuronal maintenance and regeneration still requires the signaling pathway of NRP‐1 and SEMA3A interaction. Therefore, it is reasonable to hypothesize whether SARS‐CoV‐2 infects olfactory receptor cells via NRP‐1, the resulting damage would prevent its normal interaction with SEMA3A, leading to anosmia. It should be noted that SARS‐CoV‐2 does not infect cells that express NRP‐1 alone without ACE2 co‐expression. It appears that SARS‐CoV‐2 binds to both NRP‐1 and ACE2 with ACE‐2 as a necessary component, although the exact timing and mechanism is still unclear (Gudowska‐Sawczuk & Mroczko, 2021; Li & Buck, 2021). This adds knew knowledge but also complexity to our understanding of the mechanism of SARS‐CoV‐2‐associated anosmia and the pathogenesis of the viral infection itself.
9. THE OLFACTORY RECEPTOR DOWNREGULATION THEORY
A fourth theory on the mechanism of SARS‐CoV‐2 induced anosmia could be caused by a non‐cell‐autonomous process, in which in addition to all of the above factors—damage to OSNs, the death of sustentacular cells, disruption of NRP‐1, SEMA3A signaling, and inflammation—come together, downregulation of olfactory receptors and OR signaling genes is taking place (Zazhytska et al., 2022).
Persistent anosmia in human olfactory epithelium is due to SARS‐CoV‐2 infection when damage of SuCs causes destruction of the OE and alteration of genome organization of olfactory receptor clusters in cis and trans in OSN. This results in a delayed restoration of OR transcription because OR architecture only forms during differentiation, which may take weeks to months to be replaced. Although the downregulation in olfactory receptors and OR signaling genes was not directly observed to be the cause of anosmia, reduced gene expression in each step of the pathway, from olfactory receptor proteins, to their signaling molecules, and to the ion channels that direct axon potentials were considered together.
This complex mechanism ties the cell‐mediated viral damage to sustentacular cells and OSN to non‐cell autonomous mechanism through impairment of OR transcriptome and SEM3A expression (?). With the role of semaphorins in axonal guidance directly affecting NRP‐1 and SEMA3A interactions, this non‐cell‐autonomous mechanism includes both ACE2 and NRP‐1 hypotheses in one complex theory (Zazhytska et al., 2022).
10. MECHANISMS BY WHICH SARS‐COV‐2 MAY CAUSE TASTE DYSFUNCTION
Similar to the mechanisms of anosmia, several potential mechanisms of SARS‐CoV‐2‐mediated taste disruption have been put forward. Many statistics of chemosensory disorders seem to indicate that ageusia is a more common occurrence than anosmia; however, due to the nature of many of these studies being self‐reported, it is likely that reported ageusia is confounded with olfactory loss (Deems et al., 1991). While more recent studies have attempted to help patients distinguish between olfactory and gustatory losses by specifying changes in taste qualities such as salty, sour, sweet, bitter, or umami, it is still difficult to parse the mechanism of virus‐induced ageusia, from other chemosensory disturbances of other origin. Most cases of gustatory loss, however, are either standalone symptoms or combined with olfactory loss. With these considerations, there are two possible mechanisms for SARS‐CoV‐2 to affect gustatory loss: directly or via indirect infection. Either the virus directly infects and injures the taste receptor cells, or taste cell injury occurs indirectly by inflammatory cytokines (Cooper et al., 2020). Most cases of ageusia are transient. This is consistent with the idea that an inflammatory immune response around taste cells may play a role in gustatory loss through physical injury. This could be because by the time the viral infection resolves, taste cells turn over and symptoms of ageusia subside (Brann et al., 2020).
11. THE GUSTATORY RECEPTOR CELL DESTRUCTION THEORY
The earliest hypothesis for ageusia focused on the destruction of the peripheral gustatory epithelial cells. Taste receptor cells are epithelial in nature, reproduce from the basal layer, mature into individual receptors or supporting cells in 10–12 days, and are exposed to gustatory stimuli only for hours. Any destruction of the taste cells by the SARS‐CoV‐2 virus would be instantly replaced and any virus‐induced cell destruction would be limited. Furthermore, if the destruction was due to viral invasion of the peripheral cells, one would have to demonstrate the presence of the virus in taste receptor cells, and their absence in control patients.
Looking for the expression of ACE2 and TEMPRSS in peripheral taste tissue was an obvious choice since it is the main receptor of SARS‐CoV‐2 cell entry. Not surprisingly immunohistochemistry revealed ACE2 and TMPRSS positive taste cells, gingival tissues, salivary glands, surface epithelial cells of the tongue, and the exfoliated epithelia in saliva (Doyle et al., 2021; Koyama et al., 2021). With ACE2 receptors present on the specialized epithelial taste cells, it was possible to infer a mechanism for SARS‐CoV‐2 to enter and disrupt normal gustatory functions. However, immunohistochemistry could not detect SARS‐CoV‐2 in taste tissue from COVID‐19 patients (Figure 3, Sunavala‐Dossabhoy and Spielman, unpublished). This would imply that impairment of taste is not due to direct transduction of virus into peripheral taste cells but rather a different mechanism either further up in the signaling pathway or via an indirect mechanism.
FIGURE 3.
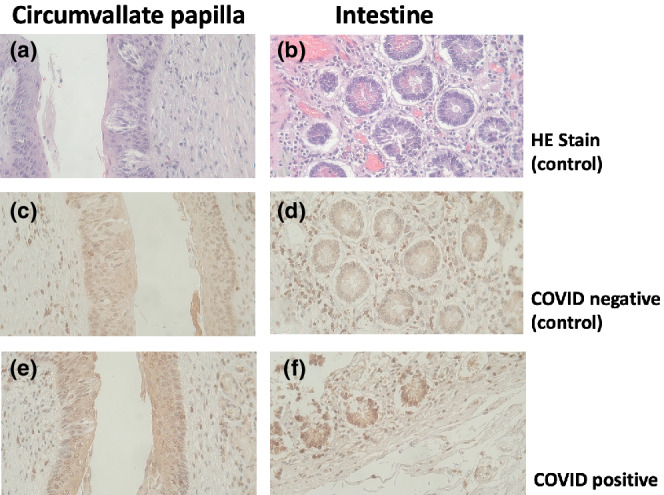
Thin sections from taste papillae and intestine of healthy (control) (a–d) and COVID+ (e, f) individuals were stained either with hematoxylin and eosin (a, b), or anti‐SARS‐CoV‐2 nucleocapsid antibodies (e, f; Invitrogen cat # PA5‐114532, 0.2 μg/ml, 4°C overnight). Image magnified at 40× obj. (Sunavala‐Dossabhoy and Spielman, unpublished)
12. THE NEUROPILIN‐1 THEORY
As shown previously, NRP‐1 resides in both olfactory and respiratory epithelia, and along with ACE‐2 and TMPRSS‐2, are co‐expressed in the gustatory nerve fibers VII, IX, and X (Vitale‐Cross et al., 2022). NRP‐1 is important in axonal guidance through its interaction with SEMA3A that receives information from bitter taste receptor cells. This increases the likelihood, but it is not yet proof that NRP‐1 plays a role in SARS‐CoV‐2‐associated dysgeusia or ageusia.
13. THE INFLAMMATORY CYTOKINE THEORY
A further theory of taste‐loss in COVID‐19 patients is an indirect bacterial‐induced inflammatory cytokine storm that can damage gustatory nerves (Iebba et al., 2021; Srinivasan, 2021). It is well known that taste cell turnover, renewal, and function are affected by the release of pro‐inflammatory cytokines expressed in taste bud cells (Wang et al., 2007). In healthy individuals, bacterial populations in the oral cavity are well balanced and rarely lead to inflammation and tissue damage. However, during a viral infection, the commensal bacterial population shifts with several pathogens disproportionately increasing (Wu et al., 2021). This imbalance is followed by an inflammatory response that can lead to tissue damage. Several viral infections like Herpes, HIV, influenza, all show bacterial shifts in the oral cavity consistent with the above hypothesis (Srinivasan, 2021). The increase in oral Prevotella salivae and Veionella infantium in oral samples of COVID‐19 patients were correlated with an increase in inflammatory cytokines (Iebba et al., 2021). The viral damage may be directly affecting taste cells or indirectly via activated macrophages, release of cytokines and injury to peripheral cranial nerves (Figure 4). However, association is not causation. While no direct proof of inflammatory cytokine‐induced receptor cell or neural damage has been shown, the pathogenesis hypothesized here is plausible but speculative.
FIGURE 4.
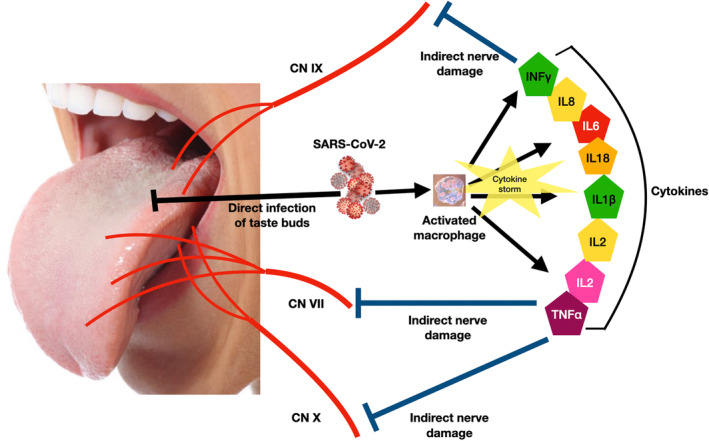
Inflammatory cytokine theory of ageusia
14. THERAPEUTIC STRATEGIES
It is difficult to develop therapeutics for COVID‐19‐induced anosmia or ageusia when the mechanisms of chemosensory loss are still not fully understood. As of now, there is no effective treatment for any chemosensory loss whether they are COVID‐19 associated or other viral/upper respiratory infection related. The enormous attention chemosensory loss has garnered since the start of the recent pandemic will only help find therapeutic solutions to this forgotten sensory infirmity. With the knowledge that we have so far treatment could involve olfactory training using essential oils to help improve olfactory function without drug intervention (Addison et al., 2021; Hummel et al., 2009). A taste training method involving both essential oils and particular diets is also in development for use in clinical settings (Koyama et al., 2021).
Based on the various mechanisms described in this paper, one can start targeting specific molecules with drugs. If there is evidence of a peripheral downregulation of the signal transduction in olfaction, several molecules could be targeted. A boost in cAMP by pentoxifylline, caffeine, and theophylline could be considered. These agents inhibit phosphodiesterase that otherwise degrades cAMP.
Insight into the pathogenesis specific to SARS‐CoV‐2 may help research into the NRP‐1 pathway for SARS‐CoV‐2 infection and targeting the region where NRP‐1 binds to the S1 of SARS‐CoV‐2 spike protein (Mayi et al., 2021). Alternatively, if there is evidence of damage to the olfactory or gustatory nerves, the use of neuroprotective agents such as statins, antibacterial like minocycline, intranasal vitamin A, omega 3, and melatonin is warranted. Corticosteroids, statins, and melatonin can block inflammatory effects triggered by viral infection and the indirect damage via cytokine storms. So far, these inhibitors have been studied in very small sample sizes with insufficient frequency and limited reliability. In the context of COVID‐19, corticosteroids are the most studied among potential treatment options, and despite its ability to block inflammation, its use for SARS‐CoV‐2‐induced anosmia and ageusia may pose other health risks (Khani et al., 2021).
For treatment of ageusia, studies in rodents have shown that topical application of a chemical compound, β‐caryophyllene, improves re‐epithelialization of cutaneous wounds and RNA sequencing of the treated skin (Koyama et al., 2019). Thus, essential oils and plants containing β‐caryophyllene, such as lavender, rosemary, peppermint, sage, may be good candidates for taste training (Koyama et al., 2021).
15. CONCLUSIONS
The field of SARS‐CoV‐2 research has exploded since the start of the COVID‐19 pandemic. Based on a PubMed search prior to 2020, roughly 14,000 papers were published on coronavirus. In the past 28 months, up till the end of April 2022, an additional 160,000 studies have been completed. The compilation of clinical features, epidemiology, prevalence, and mechanisms of the virus already lead to a better understanding of the chemosensory symptoms. While studies have proposed a variety of possible mechanisms for transient anosmia and ageusia caused by SARS‐CoV‐2 infection, less is known about what predicts the permanence of these chemosensory changes. Thus, future research should clarify the mechanism and prognosis of SARS‐CoV‐2‐induced anosmia and ageusia. Better understanding will inevitably lead to better treatments and improvement of the quality of life for such patients.
We have made considerable progress since the start of the COVID‐19 pandemic in understanding and preventing this disease. In record setting time, five vaccines have been developed and authorized in Europe, three in the United States, with a fourth awaiting emergency authorization at the FDA. As of April 24, 2022, over 5 billion people worldwide have been vaccinated, roughly 67% of the world population. Despite the half a billion infections, over −6 million deaths worldwide*, major economic downturn and societal disruptions, science has made enormous progress during the past 2 years in understanding COVID‐19. The methods used in preventing and countering this pandemic will provide improved tools for generation of vaccines for similar or new pandemics.
(*). Although the official number of COVID‐19 death is at 6 million, several studies suggest that this number is underreported. Studies on the excess death suggest the true number may be as high as 18 million (Adam, 2022; Wang et al., 2022).
AUTHOR CONTRIBUTIONS
Winnie Xu: Writing – review and editing. Gulshan Sunavala‐Dossabhoy: Resources; writing – review and editing. Andrew Spielman: Resources; supervision; writing – review and editing.
ACKNOWLEDGMENTS
This study was not supported by outside funding agencies. It was supported by institutional funds from NYU and LSU for time and effort.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.14300.
Xu, W. , Sunavala‐Dossabhoy, G. , & Spielman, A. I. (2022). Chemosensory loss in COVID‐19. Oral Diseases, 00, 1–10. 10.1111/odi.14300
REFERENCES
- Adam, D. (2022). COVID's true death toll: Much higher than official records. Modelling suggests that by the end of 2021, some 18 million people had died because of the pandemic. Nature, 603, 562. [DOI] [PubMed] [Google Scholar]
- Addison, A. B. , Wong, B. , Ahmed, T. , Macchi, A. , Konstantinidis, I. , Huart, C. , Frasnelli, J. , Fjaeldstad, A. W. , Ramakrishnan, V. R. , Rombaux, P. , Whitcroft, K. L. , Holbrook, E. H. , Poletti, S. C. , Hsieh, J. W. , Landis, B. N. , Boardman, J. , Welge‐Lüssen, A. , Maru, D. , Hummel, T. , & Philpott, C. M. (2021). Clinical olfactory working group consensus statement on the treatment of postinfectious olfactory dysfunction. The Journal of Allergy and Clinical Immunology, 147(5), 1704–1719. 10.1016/j.jaci.2020.12.641 [DOI] [PubMed] [Google Scholar]
- Barretto, R. , Gillis‐Smith, S. , Chandrashekar, J. , Yarmolinsky, D. A. , Schnitzer, M. J. , Ryba, N. J. P. , & Zuker, C. S. (2015). The neural representation of taste quality at the periphery. Nature, 517, 373–376. 10.1038/nature13873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartheld, V. C. S. , Hagen, M. M. , & Butowt, R. (2020). Prevalence of chemosensory dysfunction in COVID‐19 patients: A systematic review and meta‐analysis reveals significant ethnic differences. ACS Chemical Neuroscience, 11(19), 2944–2961. 10.1101/2020.06.15.20132134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann, D. H. , Tsukahara, T. , Weinreb, C. , Lipovsek, M. , Van Den Berge, K. , Gong, B. , Chance, R. , Maccaulay, I. C. , Chou, H. J. , Fletcher, R. B. , Das, D. , Street, K. , Debezieux, H. R. , Choi, Y. G. , Risso, D. , Dudoit, S. , Purdom, E. , Mill, J. , Hachem, R. A. , … Datta, S. R. (2020). Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19‐associated anosmia. Science Advances, 6(31), eabc5801. 10.1126/sciadv.abc5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryche, B. S. , St Albin, A. , Murri, S. , Lacôte, S. , Pulido, C. , Ar Gouilh, M. , Lesellier, S. , Servat, A. , Wasniewski, M. , Picard‐Meyer, E. , Monchatre‐Leroy, E. , Volmer, R. , Rampin, O. , le Goffic, R. , Marianneau, P. , & Meunier, N. (2020). Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS‐CoV‐2 in golden Syrian hamsters. Brain, Behavior, and Immunity, 89, 579–586. 10.1016/j.bbi.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt, R. , & von Bartheld, C. S. (2021). Anosmia in COVID‐10: Underlying mechanisms and assessment of an olfactory route to brain infection. The Neuroscientist, 27(6), 582–603. 10.1177/1073858420956905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti‐Castelvetri, L. , Ojha, R. , Pedro, L. D. , Djannatian, M. , Franz, J. , Kuivanen, S. , van der Meer, F. , Kallio, K. , Kaya, T. , Anastasina, M. , Smura, T. , Levanov, L. , Szirovicza, L. , Tobi, A. , Kallio‐Kokko, H. , Österlund, P. , Joensuu, M. , Meunier, F. A. , Butcher, S. J. , … Simons, M. (2020). Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science, 370(6518), 856–860. 10.1126/science.abd2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, K. W. , Brann, D. H. , Farruggia, M. C. , Bhutani, S. , Pellegrino, R. , Tsukahara, T. , Weinreb, C. , Joseph, P. V. , Larson, E. D. , Parma, V. , Albers, M. W. , Barlow, L. A. , Datta, S. R. , & di Pizio, A. (2020). COVID‐19 and the chemical senses: Supporting players take center stage. Neuron, 107(2), 219–233. 10.1016/j.neuron.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, J. L. , Simonetti, B. , Klein, K. , Chen, K. E. , Williamson, M. K. , Antón‐Plágaro, C. , Shoemark, D. K. , Simón‐Gracia, L. , Bauer, M. , Hollandi, R. , Greber, U. F. , Horvath, P. , Sessions, R. B. , Helenius, A. , Hiscox, J. A. , Teesalu, T. , Matthews, D. A. , Davidson, A. D. , Collins, B. M. , … Yamauchi, Y. (2020). Neuropilin‐1 is a host factor for SARS‐CoV‐2 infection. Science, 370(6518), 861–865. 10.1126/science.abd3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deems, D. A. , Doty, R. L. , Settle, R. G. , Moore‐Gillon, V. , Shaman, P. , Mester, A. F. , Kimmelman, C. P. , Brightman, V. J. , & Snow, J. B., Jr. (1991). Smell and taste disorders: A study of 750 patients from the University of Pennsylvania Smell and taste center. Archives of Otorhinolaryngology‐Head & Neck Surgery, 117, 519–528. 10.1001/archotol.1991.01870170065015 [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis, P. V. (2021). Post‐viral anosmia (loss of sensation of smell) did not begin with COVID‐19! Lung, 199(3), 237–238. 10.1007/s00408-021-00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty, R. L. (2021). The mechanisms of smell loss after SARS‐CoV‐2 infection. Lancet Neurology, 20(9), 693–695. 10.1016/S1474-4422(21)00202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, M. E. , Appleton, A. , Liu, Q. R. , Yao, Q. , Mazucanti, C. H. , & Egan, J. M. (2021). Human type II taste cells express angiotensin‐converting enzyme 2 and are infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). American Journal of Pathology, 191(9), 1511–1519. 10.1016/j.ajpath.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili, M. , Abdi, F. , Shafiee, G. , Asayesh, H. , Abdar, Z. E. , Baygi, F. , & Qorbani, M. (2021). Olfactory and gustatory dysfunction in 2019 novel coronavirus: An updated systematic review and meta‐analysis. International Journal of Preventive Medicine, 12(1), 170. 10.4103/ijpvm.IJPVM_484_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodoulian, L. , Tuberosa, J. , Rossier, D. , Landis, B. N. , Carleton, A. , & Rodriguez, I. (2020). SARS‐CoV‐2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. iScience, 23(12), 101839. 10.1101/2020.03.31.013268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , Liu, L. , Shan, H. , Lei, C. L. , Hui, D. S. C. , du, B. , Li, L. J. , Zeng, G. , Yuen, K. Y. , Chen, R. C. , Tang, C. L. , Wang, T. , Chen, P. Y. , Xiang, J. , … China Medical Treatment Expert Group for Covid‐19 . (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382, 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudowska‐Sawczuk, M. , & Mroczko, B. (2021). The role of Neuropilin‐1 (NRP‐1) in SARS‐CoV‐2 infection: Review. Journal of Clinical Medicine, 10(13), 2772. 10.3390/jcm10132772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , Bulthuis, M. L. , Lely, A. T. , Navis, G. , & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, A. Y. , Mukdad, L. , Long, J. L. , & Lopez, I. A. (2020). Anosmia in COVID‐19: Mechanisms and significance. Chemical Senses, 45(6), 423–428. 10.1093/chemse/bjaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum, M. E. , Koch, R. J. , Ramirez, V. A. , Marks, S. S. , Toskala, A. K. , Herriman, R. D. , Lin, C. , Joseph, P. V. , & Reed, D. R. (2022). Taste loss as a distinct symptom of COVID‐19: A systematic review and meta‐analysis. Chemical Senses, 47, bjac001. 10.1093/chemse/bjac001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N. H. , Nitsche, A. , Müller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, T. , Rissom, K. , Reden, J. , Hähner, A. , Weidenbecher, M. , & Hüttenbrink, K. (2009). Effects of olfactory training in patients with olfactory loss. Laryngoscope, 119(3), 496–499. 10.1002/lary.20101 [DOI] [PubMed] [Google Scholar]
- Iebba, V. , Zanotta, N. , Campisciano, G. , Zerbato, V. , Di Bella, S. , Cason, C. , Luzzati, R. , Confalonieri, M. , Palamara, A. T. , & Comar, M. (2021). Profiling of oral microbiota and cytokines in COVID‐19 patients. Frontiers in Microbiology, 12. 10.1101/2020.12.13.422589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp, U. B. (2010). Olfactory signaling in vertebrates and insects: Differences and commonalities. Nature Reviews Neuroscience, 11(3), 188–200. 10.1038/nrn2789 [DOI] [PubMed] [Google Scholar]
- Khani, E. , Khiali, S. , Beheshtirouy, S. , & Entezari‐Maleki, T. (2021). Potential pharmacologic treatments for COVID‐19 smell and taste loss: A comprehensive review. European Journal of Pharmacology, 912, 174582. 10.1016/j.ejphar.2021.174582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein, T. , Kadiane‐Oussou, N. J. , Toko, L. , Royer, P. Y. , Lepiller, Q. , & Gendrin, V. (2020). Features of anosmia in COVID‐19. Médicine et Maladies Infectieuses, 50(5), 436–439. 10.1016/j.medmal.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, S. , Kondo, K. , Ueha, R. , Kashiwadani, H. , & Heinbockel, T. (2021). Possible use of phytochemicals for recovery from COVID‐19‐induced anosmia and ageusia. International Journal of Molecular Sciences, 22, 8912. 10.3390/ijms22168912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, S. , Purk, A. , Kaur, M. , Soini, H. A. , Novotny, M. V. , Davis, K. , Kao, C. C. , Matsunami, H. , & Mescher, A. (2019). Beta‐caryophyllene enhances wound healing through multiple routes. PLoS One, 14, e0216104. 10.1371/journal.pone.0216104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. K. , Guo, H. , Fisher, A. D. , Diep, J. , Lai, Z. , Chen, Y. , Upton, J. W. , Carette, J. , Mocarski, E. S. , & Kaiser, W. J. (2020). Necroptosis‐based CRISPR knockout screen reveals Neuropilin‐1 as a critical host factor for early stages of murine cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America, 117, 20109–20116. 10.1073/pnas.1921315117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. , Macpherson, L. J. , Zuker, C. S. , & Ryba, N. J. P. (2017). Rewiring the taste system. Nature, 548(7667), 330–333. 10.1038/nature23299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , & Buck, M. (2021). Neuropilin‐1 assists SARS‐CoV‐2 infection by stimulating the separation of spike protein domains S1 and S2. Biophysical Journal, 120(14), 2828–2837. 10.1101/2021.01.06.425627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo, A. , Bonato, M. , & Cinque, P. (2021). Smell and taste disorders in COVID‐19: From pathogenesis to clinical features and outcomes. Neuroscience Letters, 748, 135694. 10.1016/j.neulet.2021.135694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayi, B. S. , Leibowitz, J. A. , Woods, A. T. , Ammon, K. A. , Liu, A. E. , & Raja, A. (2021). The role of Neuropilin‐1 in COVID‐19. PLoS Pathogens, 17(1), e1009153. 10.1371/journal.ppat.1009153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashe, I. , Man, O. , Lancet, D. , & Gilad, Y. (2003). Different noses for different people. Nature Genetics, 34(2), 143–144. 10.1038/ng1160 [DOI] [PubMed] [Google Scholar]
- O'Driscoll, M. , Ribeiro Dos Santos, G. , Wang, L. , Cummings, D. A. T. , Azman, A. S. , Paireau, J. , Fontanet, A. , Cauchemez, S. , & Salje, H. (2020). Age‐specific mortality and immunity patterns of SARS‐CoV‐2. Nature, 590, 140–145. 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- Ripa, M. , Galli, L. , Poli, A. , Oltolini, C. , Spagnuolo, V. , Mastrangelo, A. , Muccini, C. , Monti, G. , de Luca, G. , Landoni, G. , Dagna, L. , Clementi, M. , Rovere Querini, P. , Ciceri, F. , Tresoldi, M. , Lazzarin, A. , Zangrillo, A. , Scarpellini, P. , Castagna, A. , & COVID‐BioB Study Group . (2021). Secondary infections in patients hospitalized with COVID‐19: Incidence and predictive factors. Clinical Microbiology and Infections, 27(3), 451–457. 10.1016/j.cmi.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper, S. D. , & Chaudhari, N. (2017). Taste buds: Cells, signals, and synapses. Nature Reviews Neuroscience, 18(8), 485–497. 10.1038/nrn.2017.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting, G. A. , Kostek, C. , Ahmad, N. , Dibble, C. , Pays, L. , & Püschel, A. W. (2000). Semaphorin 3A is required for guidance of olfactory axons in mice. Journal of Neuroscience, 20(20), 7691–7697. 10.1523/JNEUROSCI.20-20-07691.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. , Kumar, R. , Aier, I. , Semwal, R. , Tyagi, P. , & Varadwaj, P. (2019). Sense of smell: Structural, functional, mechanistic advancements and challenges in human olfactory research. Current Neuropharmacology, 17, 891–911. 10.2174/1570159X17666181206095626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton, J. F. , Shastri, A. J. , Fletez‐Brant, K. , The 23andMe COVID‐19 Team , Aslibekyan, S. , & Auton, A. (2022). The UGT2A1/UGT2A2 locus is associated with COVID‐19‐related loss of smell or taste. Nature Genetics, 54, 121–124. 10.1038/s41588-021-00986-w [DOI] [PubMed] [Google Scholar]
- Sims, A. C. , Baric, R. S. , Yount, B. , Burkett, S. E. , Collins, P. L. , & Pickles, R. J. (2005). Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: Role of ciliated cells in viral spread in the conducting airways of the lungs. Journal of Virology, 79(24), 15511–15524. 10.1128/JVI.79.24.15511-15524.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman, A.I. (2019). Chemosensory disorders. In Clinician's guide ‐ salivary and chemosensory disorders Brennan, M. &. Fox, P.C. Eds. (2nd ed.). The American Academy of Oral Medicine, p.30. [Google Scholar]
- Spielman, A. I. , & Brand, J. G. (2018). Wiring taste receptor cells to the central gustatory system. Oral Diseases, 24(8), 1388–1389. 10.1111/odi.12833 [DOI] [PubMed] [Google Scholar]
- Spielman, A. I. , Ozdener, M. H. , & Brand, J. G. (2019). Chemosensory systems. In eLS. John Wiley & Sons, Ltd. 10.1002/9780470015902.a0000038.pub3 [DOI] [Google Scholar]
- Srinivasan, M. (2021). Taste dysfunction and long COVID‐19. Frontiers in Cellular and Infection Microbiology, 11. 10.3389/fcimb.2021.716563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck, H. (2020). Neue corona‐symptome entdeckt. Frankfurter Allgemeine Zeitung. https://www.faz.net/aktuell/gesellschaft/gesundheit/coronavirus/neue‐corona‐symptome‐entdeckt‐virologe‐hendrikstreeck‐zum‐virus‐16681450.html?GEPC=s3 [Google Scholar]
- Vaira, L. A. , Salzano, G. , Deiana, G. , & De Riu, G. (2020). Anosmia and ageusia: Common findings in COVID‐19 patients. Laryngoscope, 130(7), 1787. 10.1002/lary.28692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale‐Cross, L. , Szalayova, I. , Scoggins, A. , Palkovits, M. , & Mezey, E. (2022). SARS‐CoV‐2 entry sites are present in all structural elements of the human glossopharyngeal and vagal nerves: Clinical implications. eBioMedicine, 78, 103981. 10.1016/j.ebiom.2022.103981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, T. , Shweta, F. , Murugadoss, K. , Awasthi, S. , Venkatakrishnan, A. , Bade, S. , Puranik, A. , Kang, M. , Pickering, B. W. , O'Horo, J. C. , Bauer, P. R. , Razonable, R. R. , Vergidis, P. , Temesgen, Z. , Rizza, S. , Mahmood, M. , Wilson, W. R. , Challener, D. , Anand, P. , … Soundararajan, V. (2020). Augmented curation of clinical notes from a massive EHR system reveals symptoms of impending COVID‐19 diagnosis. eLife, 9, e58227. 10.7554/eLife.58227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Paulson, K. R. , Pease, S. A. , Watson, S. , Comfort, H. , Zheng, P. , Aravkin, A. Y. , Bisignano, C. , Barber, R. M. , Alam, T. , Fuller, J. E. , May, E. A. , Jones, D. P. , Frisch, M. E. , Abbafati, C. , Adolph, C. , Allorant, A. , Amlag, J. O. , Bang‐Jensen, B. , … Murray, C. J. L. (2022). Estimating excess mortality due to the COVID‐19 pandemic: A systematic analysis of COVID‐19‐related mortality, 2020‐21. The Lancet, 399(10334), 1513–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Zhou, M. , Brand, J. , & Huang, L. (2007). Inflammation activates the interferon signaling pathways in taste bud cells. The Journal of Neuroscience, 27, 10703–10713. 10.1523/JNEUROSCI.3102-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Cheng, X. , Jiang, G. , Tang, H. , Ming, S. , Tang, L. , Lu, J. , Guo, C. , Shan, H. , & Huang, X. (2021). Altered oral and gut microbiota and its association with SARS‐CoV‐2 viral load in COVID‐19 patients during hospitalization. npj Biofilms Microbiomes, 7, 61. 10.1038/s41522-021-00232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi, M. , Fujiwara, M. , & Nakamura, H. (1994). Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology, 32(3), 113–118. [PubMed] [Google Scholar]
- Zazhytska, M. , Kodra, A. , Hoagland, D. A. , Frere, J. , Fullard, J. F. , Shayya, H. , McArthur, N. , Moeller, R. , Uhl, S. , Omer, A. D. , Gottesman, M. E. , Firestein, S. , Gong, Q. , Canoll, P. D. , Goldman, J. E. , Roussos, P. , ten Oever, B. , Overdevest, J. B. , & Lomvardas, S. (2022). Non‐cell‐autonomous disruption of nuclear architecture as a potential cause of COVID‐19‐induced anosmia. Cell, 185(6), 1052–1064.e.12. 10.1016/j.cell.2022.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]


