Abstract
Objective
While there are literature reporting increased incidence of hair loss in COVID‐19 patients, insufficient evidence exists on the topic to date. This review aims to identify the existing evidence and clinical characteristics of hair loss with COVID‐19 infection.
Methods
Following the PRISMA Extension for Scoping Reviews, MEDLINE and EMBASE were searched for all peer‐reviewed articles with relevant keywords including “Alopecia,” “Telogen Effluvium (TE),” and “COVID‐19” from their inception to November 20, 2021.
Results
A total of 26 articles, with 9 observational studies and 17 case reports or series (a total of 58 cases), were included. Most studies dealt with TE. There were no clear trends between COVID‐19 severity and the extent of hair loss. Analysis of the 58 cases also found similar results with most of the cases being female (82.8%), the median onset of hair loss of 2.0 months, and the median time to recovery of hair loss of 5.0 months with a resolution rate of 95%.
Conclusion
While this systematic review revealed uncertainty and a lack of strong evidence regarding the association of COVID‐19 and hair loss, hair loss in COVID‐19 may mainly include TE and be reversible in nature. Future studies are warranted to determine the detailed pathophysiology and risk factors of hair loss in COVID‐19, including possible roles of estrogen, progesterone, and pro‐inflammatory cytokines.
Keywords: alopecia, COVID‐19, scoping review, systematic review, telogen effluvium
1. INTRODUCTION
While coronavirus disease 2019 (COVID‐19) is known to cause severe respiratory illness, patients with COVID‐19 also experience various symptoms, including dermatologic manifestations such as simultaneous herpetic lesions and hair loss. 1 Hair loss can occur commonly in reaction to an acute disease process or autoimmune etiology and was also reported as a symptom in previous coronavirus infections such as severe acute respiratory syndrome (SARS) or the Middle East respiratory syndrome (MERS). 2 , 3 , 4 , 5 Given its aesthetic impact, hair loss has garnered considerable attention from the general public. 6 , 7 Surprisingly, hair loss or hair changes in COVID‐19 have an estimated prevalence of up to around 20.4%. 8 , 9 , 10 Although individuals with post‐acute sequelae of SARS‐CoV‐2 infection (PASC) or “long COVID” have reported a high prevalence of cutaneous changes, the prevalence of hair loss in patients with PASC has yet to be elucidated. The spectrum of hair loss in COVID‐19 is broad and includes acute and chronic telogen effluvium, exacerbation of androgenic alopecia, and alopecia areata. 11
To date, several studies have been done to clarify the association between COVID‐19 and hair loss. In particular, reversibility of hair loss, duration, and association with COVID‐19 severity were areas of interest, with hypotheses that extent and type of hair loss might be associated with the severity of COVID‐19. Additionally, it was hypothesized that hair loss may be an initial symptom of COVID‐19 or a harbinger of future development of PASC. The results of existing studies are mixed without unified conclusions. Population‐based studies regarding hair changes and COVID‐19 noted that the extent of hair loss and loss of hair pigments were correlated with the severity of COVID‐19 infection. 12 Other studies report contradicting results, such as a lack of evidence suggesting an association between alopecia areata and COVID‐19. 13
Despite the uncertain level of evidence with regard to hair loss and COVID‐19, hair loss has certainly attracted the attention of the medical community with a number of case reports or small‐sized studies published. In this study, we aimed to summarize the current level of evidence regarding hair loss and COVID‐19 in order to identify its clinical characteristics and clinical utility by systematic review of literature.
2. MATERIALS AND METHODS
2.1. Study design
We performed a systematic scoping review in accordance with the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) extension for scoping reviews (PRISMA‐ScR). 14 , 15 Appendix 1 is PRISMA‐ScR Checklist of the present study.
2.2. Search strategy
MEDLINE and EMBASE searches were conducted for all peer‐reviewed articles from inception to November 20, 2021. We employed no filters for study design and language. Additional relevant articles were screened with the reference lists of all articles that satisfied the eligibility criteria. The search strategy harbored relevant keywords, including “Hair Loss,” “Alopecia,” “Telogen Effluvium,” and “COVID‐19.” Two authors (TC and YN) conducted the search independently. See Appendix 2 for detailed search terms.
2.3. Eligibility criteria
The criteria for the inclusion of articles are the following:
Peer‐reviewed articles describing the relationship between hair loss and COVID‐19, or cases of hair loss in patients with laboratory‐confirmed COVID‐19.
Randomized controlled trials, case–control studies, cohort studies (prospective or retrospective), cross‐sectional studies, and case series
The exclusion criteria included the following:
Qualitative studies, review articles, and commentaries.
Conference abstracts.
Diagnosis of COVID‐19 made without confirmatory polymerase chain reaction testing.
2.4. Study selection
TC and YN assessed selected articles for full‐text assessment independently using EndNote 20 reference management software. Articles considered eligible were subsequently evaluated in full length.
2.5. Data extraction and definition
We used a standardized data collection form that followed the PRISMA and Cochrane Collaboration guidelines for systematic reviews to obtain the following information from each study: title, name of authors, year of publication, country of origin, study characteristics, target outcome, aims, study and comparative groups, key findings, and limitations. We also summarized data from included cases to identify clinical characteristics of hair loss in COVID‐19.
3. RESULTS
3.1. Search results and study selection
Figure 1 illustrates a PRISMA flow diagram that depicts the process of identification, screening, eligibility, and inclusion or exclusion of the studies. The initial search of MEDLINE and EMBASE databases yielded 150 and 129 articles, respectively. Forty‐seven duplicate studies were removed. 232 articles were screened based on their relevance and type of article. 143 articles that were either review articles, editorials, or conference abstracts were excluded from the study. Eighty‐nine articles were then evaluated for full‐text review for study inclusion per our eligibility criteria. Seventy‐six reviews, opinion articles, articles with irrelevant topics were excluded. A total of 26 articles with nine observational studies and 17 case reports or series were included in the review.
FIGURE 1.
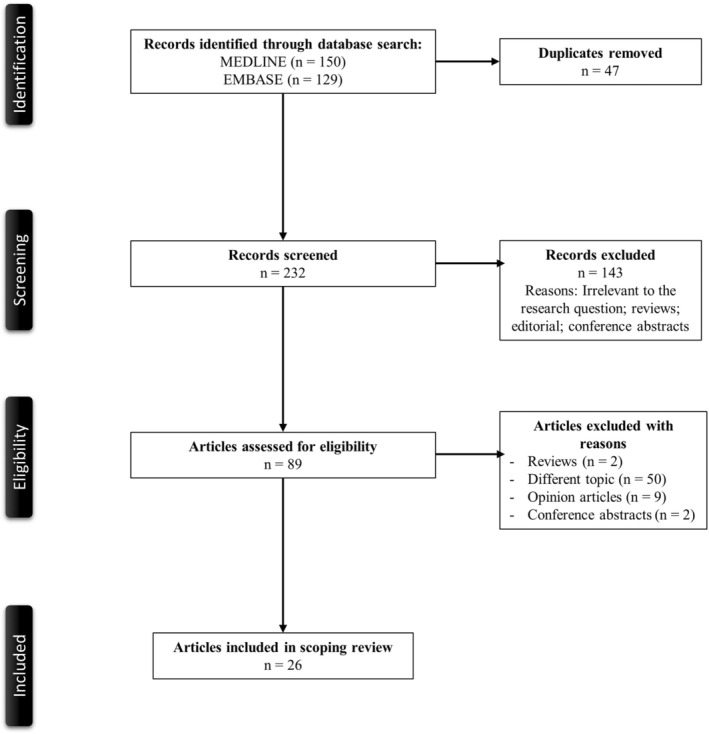
PRISMA flowchart of the search strategy
3.2. Description of included studies
Table 1 describes the main characteristics of nine observational studies from the scoping review. 13 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Overall, six studies were observational investigational studies without comparative groups to evaluate the characteristics of hair loss in COVID‐19. Except for a large retrospective cohort study from Korea which investigated the risk of alopecia areata (AA) among COVID‐19 patients, other studies focused on telogen effluvium (TE).
TABLE 1.
Main characteristics of the included observational studies in the scoping reviews
| Author Year Country | Study Type | Aim | Outcome | Population | Comparative groups | Detail of hair loss | Key findings | Limitations |
|---|---|---|---|---|---|---|---|---|
| Abrantes et al. 2021 USA | Observational | To evaluate the onset and duration of acute TE post‐COVID‐19 | Investigational | Patients with hair loss after COVID‐19 (n = 30) | N/A |
The onset of acute TE at a median of 45 days (IQR 13 days) The median duration of TE was 47.5 days (IQR 45 days) 20/30 (66.7%) had resolution of TE during the follow‐up (March to July 2020) |
Acute TE after COVID‐19 could occur sooner than TE of other causes (3 to 6 months to recover) |
Small sample size Potential other concomitant causes |
| Aksoy et al. 2021 Turley | Prospective case–control | To assess the incidence of TE developed following COVID‐19 and the correlation between the development of TE and the severity of infection | Characteristics of hair loss | Patients with TE due to COVID‐19 (n = 57) | Patients without TE (n = 147) | The onset of COVID‐19‐associated TE was an average 53.8 ± 23.8 days after PCR positivity |
TE more frequently occurred in those required hospitalization due to COVID‐19 (31.7% vs. 24.3%, p = 0.238) The proportion of women was significantly higher in patients with COVID‐19‐associated TE than men (42.3% vs. 6.2%, p < 0.001) No changes in the extent of stress among those with TE and without TE |
The authors did not use objective methods such as trichogram or modified wash test for TE diagnosis No recorded information about trichodynia No long‐term follow‐up to evaluate the recovery |
| Babaei 2021 Iran | Cross‐sectional | To evaluate the characteristics of TE in COVID‐19 | Investigational | 526 patients with documented TE that recovered from COVID‐19 | N/A |
The onset of hair loss was an average 53.6 ± 12.2 days after COVID‐19 27.9% had concurrent alopecia (78.2% androgenic, 19.0% alopecia areata, 2.7% cicatricial alopecia) |
8.4%, 7.2%, and 4.8% had concurrent vitamin D deficiency, hypothyroidism, and iron‐deficiency anemia, respectively |
Lack of description about recovery Failure to exclude other potential causes of hair loss Cross‐sectional design |
| Cline 2021 USA | Observational | To evaluate the prevalence of TE pre‐ and during COVID‐19 pandemic | Description of race, ethnicity, and comorbidities of patients with TE | Patients evaluated by the dermatology departments of eight safety‐net hospitals in New York City during the pandemic; from March 1 to October 1, 2020 (n = 14 827) | Patients evaluated by the dermatology departments of eight safety‐net hospitals in New York City pre‐pandemic; from August 1, 2019 to February 29, 2020 (n = 15 507) |
39 patients had TE pre‐pandemic and 108 had TE during the pandemic Among 108 patients, 10 had positive COVID‐19. Those with positive COVID‐19 were more likely to have extensive comorbidities and be Hispanic. All of them were female |
Those who had TE during the pandemic were more likely to be Hispanic (1.22% vs. 0.30%) |
Lack of follow‐up Lack of data about time course after COVID‐19 diagnosis and the onset of hair loss |
| Di Landro 2021 Italy | Observational | To describe characteristic of TE after COVID‐19 | Pathobiology of hair loss in TE after COVID‐19 | 39 patients with TE after COVID‐19 | N/A |
The onset of hair loss ranged from 8 weeks to 3 months after clinical manifestation of COVID‐19 7/39 (17.9%) patients had trichodynia The presence of empty follicular ostia and telogen club hairs visible on the scalp surface were noted in 3/39 patients Recovery occurred in 2–4 months |
Mean age 64.4 years (range: 48–73) 30/39 (76.9%) patients were female 16/39 (41.0%) required hospitalization due to COVID‐19 |
Small sample size No description about mean/median time to the onset or recovery of hair loss |
| Kim 2021 Korea | Retrospective cohort | To investigate the risk of developing alopecia areata among COVID‐19 patients | New diagnosis of alopecia areata based on ICD‐10‐Code L63 | 7958 patients with COVID‐19 during the study period (January 1 to June 4, 2020) | 218 779 patients without COVID‐19 during the study period | 18/7958 (0.2%) with COVID‐19 and 195/218584 (0.1%) had alopecia areata based on ICD‐10‐Code | The adjusted incidence rate ratio of developing alopecia areata using a log‐link Poisson regression model was 0.60 (95% CI: 0.35–1.03) in those with COVID‐19 compared with those without COVID‐19 |
Lack of detailed time course and description concerning hair loss Low prevalence of COVID‐19 in the study area during the period Diagnosis of alopecia areata was based on ICD‐10‐Code |
| Moreno‐Arrones 2021 Spain | Observational | To characterize acute TE after COVID‐19 | Investigational | 191 patients with acute TE and prior diagnosis of COVID‐19 | N/A |
The onset of COVID‐19‐associated TE was an average 57.1 ± 18.3 days after COVID‐19 diagnosis Severity of hair loss based on Sinclair score; 4.7% had a score of 1, 10.5% of 2, 12.6 of 3, 20.4% of 4, 22% of 5, and 29.8% of 6 27.2% recovered within 4 weeks |
78.5% female with mean age of 47.4 years (range: 15–88) 71.2% of the patients did not require hospitalization 59.7% received any form of minoxidil for acute TE treatment |
Small sample size with lack of long‐term follow‐up |
| Sharquie 2021 Iraq | Cross‐sectional | To characterize acute TE after COVID‐19 | Investigational | 39 patients with acute TE after COVID‐19 | N/A |
All patients experienced hair loss within 2–3 months after COVID‐19 The pull tests were strongly positive (> 10–50% with a mean of 35% of pulled hair away from scalp) in every patient 43.6% had the diffuse type, 30.8% bitemporal type, 12.8% occipital, 7.7% frontovertical, and 5.1% bitemporo‐frontal |
No patients required hospitalization for COVID‐19 Mean age 41.3 ± 11.6 years 92.3% females No patients had alopecic patches or scaling, erythema, or other dermatological abnormalities |
Small sample size at a single‐center design No description about recovery Lack of a comparison group |
| Starace 2021 Italy | Cross‐sectional | To evaluate the presence of trichodynia and TE in patients with COVID‐19 | Investigational | 128 patients with TE and/or trichodynia with a prior history of COVID‐19 | N/A |
The authors could collect detailed data of 101 patients In patients with trichodynia without TE, TR occurred in 2 weeks (IQR 1–3) after COVID‐19 Those with TE without trichodynia had TE 13 weeks (IQR 10.5–13.2) after COVID‐19 Those with both TE and TR had TE in 3 weeks after COVID‐19 (IQR 2–7.5) Complete resolution of TE or trichodynia occurred in 91/101 (90.1%) |
Patients who had anosmia and headache had higher odds of having trichodynia without TE (OR 6.7 and 6.3, respectively) |
Survey‐based design causing selection bias Only those who came to dermatology clinic were included Lack of a control group |
Abbreviations: COVID‐19, coronavirus disease 2019; ICD, International Classification of Diseases; IQR, interquartile range; OR, odds ratio; TE, telogen effluvium.
Abrantes et al. conducted a dermatologist‐led observational study by recruiting patients from the United States (US), Brazil, and Spain. While their sample size was small (n = 30), they found that the onset of acute TE was a median of 45 days after the onset of COVID‐19, and TE in COVID‐19 patients frequently self‐resolves. 16 A prospective case–control study by Aksoy et al., which included 204 patients, noted the onset of COVID‐19‐associated TE was 53.8 days on average after positive SARS‐CoV‐2, which was similar to the results of the study by Abrantes et al. 17 Cline et al. performed an observational study to compare the incidence of TE before and during the COVID‐19 pandemic in New York City. While the net prevalence of TE in their study was low (0.25% in the cohort of the pre‐COVID‐19 pandemic period and 0.73% in the cohort during the pandemic), they noted a significant increase in the prevalence of TE during the pandemic. 19 While eight included studies suggested a possible correlation between TE or other types of hair loss with COVID‐19, a large retrospective cohort study by Kim et al. from Korea reported no significant difference in the prevalence of alopecia areata based on ICD‐10 data analysis. 13 Except for studies by Babaei et al., Cline et al., and Kim et al., all other studies suffered from small sample sizes.
3.3. Review of included cases
Table 2 delineates the demographics and clinical characteristics of patients with hair loss and COVID‐19 (n = 58), with a predominance of female patients (17.2% male and 82.8% female). 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 Hair loss type was predominately TE (74.1%), with the next most common hair loss type being early‐onset effluvium (12.1%). Only a small number of individuals had other types of hair loss including AA (5.2%), fibrosing alopecia (3.4%), and one individual each with alopecia areata totalis (1.7%), alopecia areata universalis (1.7%), and anagen effluvium (1.7%). The median onset of hair loss was 2.0 months (interquartile range [IQR] 1.0–3.0) after diagnosis of COVID‐19. The outcome of hair loss was described only in 20 cases. Among the 20 cases, 19 (95.0%) had resolution of the symptom with the median time of recovery being 5.0 months (IQR 0.50–5.0). Among 38 cases with treatment described, most patients received either topical lotion such as clobetasol or minoxidil. Only one patient (2.6%) was prescribed low‐dose systemic corticosteroids. Two patients (5.3%) were treated with either hydroxychloroquine or oral minoxidil, but the long‐term outcome of hair loss was not described. 18.4% of the included cases only received reassurance.
TABLE 2.
Clinical characteristics of the 58 patients from case reports and case series
| Prevalence (%) a | Median (IQR) | |
| Age (years) | 58/58 (100) | 49.0 (38.0–57.3) |
| Sex | ||
| Male | 10/58 (17.2) | |
| Female | 48/58 (82.8) | |
| Type of hair loss | ||
| Telogen effluvium | 43/58 (74.1) | |
| Early‐onset effluvium | 7/58 (12.1) | |
| Alopecia areata | 3/58 (5.2) | |
| Fibrosing alopecia | 2/58 (3.4) | |
| Alopecia areata totalis | 1/58 (1.7) | |
| Alopecia areata universalis | 1/58 (1.7) | |
| Anagen effluvium | 1/58 (1.7) | |
| Onset of hair loss (months) | 57/58 (98.3) | 2.0 (1.0–3.0) |
| Resolution | 19/20 (95.0) | |
| Time to recovery (months) | 5.0 (0.50–5.0) | |
| Treatment | 38/58 (65.5) | |
| Clobetasol cream or lotion | 17/38 (44.7) | |
| Minoxidil lotion | 9/38 (23.7) | |
| Reassurance | 7/38 (18.4) | |
| Triamcinolone injection | 3/38 (7.9) | |
| Oral minoxidil | 2/38 (5.3) | |
| Hydroxychloroquine | 2/38 (5.3) | |
| Vitamin D | 2/38 (5.3) | |
| Low‐dose systemic cortico‐steroids | 1/38 (2.6) | |
| Antihistamine | 1/38 (2.6) | |
Abbreviations: IQR, interquartile range.
Prevalence here is defined as the number of cases reported the variable divided by the number of the total cases.
4. DISCUSSION
This study is the first systematic scoping review and analysis of existing case reports and series of hair loss in COVID‐19. Our results suggest a possible reversibility of hair loss in COVID‐19. However, patients could still experience prolonged time to recover from hair loss, and there were no clear trends between COVID‐19 severity and the extent of hair loss. Since most of the included studies dealt with TE without long‐term follow‐up, there is insufficient evidence regarding PASC and hair loss. Interestingly, we found a strong tendency for female patients to experience hair loss in COVID‐19.
To date, the underlying pathophysiology of hair loss in COVID‐19 has yet to be elucidated. One proposed theory is that interleukin‐6 (IL‐6), a pro‐inflammatory cytokine involved in severe and critical COVID‐19, may be playing a role with hair loss. IL‐6 is suspected to predispose and exacerbate hair loss by inhibiting hair shaft elongation and hair follicle proliferation. 41 , 42 Hair loss seems to have occurred mainly in mild COVID‐19 patients as only a few patients required hospitalization in the included studies. Given the considerable female dominance in patients having hair loss, female sex hormones such as estrogens and progesterone may also be critical in the underlying pathophysiology. Estrogens and progesterone are known to have immunomodulatory and anti‐inflammatory effects inhibiting pro‐inflammatory cytokines. 43 , 44 A study has been ongoing to repurpose estrogens and progesterone to COVID‐19 treatment. 45 Estrogen and progesterone additionally work protectively at the hair follicle. Estradiol is known to alter hair follicle growth and hair cycle through its receptors, and progesterone may decrease the conversion of testosterone to dihydrotestosterone, an active form of testosterone leading to hair loss. 46 Thus, female‐dominance hair loss in COVID‐19 may be due to acute insult with the viral infection causing a significant relative reduction in systemic estrogens and progesterone levels in female patients. Future studies are warranted to see a relationship between the extent of hair loss and female sex hormones.
Given an increasing number of patients suffering from PASC, long‐term follow‐up of recovered COVID‐19 patients has become crucial. Due to relatively short follow‐up periods in the included studies, it may be challenging to draw conclusions regarding the prevalence of hair loss as a PASC symptom. Detailed dermatologic examinations may need to be a part of routine examinations when seeing recovered COVID‐19 patients.
Limitations of this current study include that hair loss in COVID‐19 patients were commonly made as a clinical diagnosis without the use of histopathological assay or specific biomarkers. Thus, there might have been either under‐ or over‐diagnosis of hair loss in COVID‐19 patients. Also, given that hair loss is a relatively common dermatologic finding, hair loss and COVID‐19 in the included cases might have occurred solely by chance. Also, because most of the included cases were diagnosed as TE based on clinical course without pathologic findings, the prevalence of TE could have been overestimated. Given the clinical variability and benign nature of hair loss, it is important to consider that patients may not have had access to or sought out dermatologic care. Many may not have noticed mild hair loss symptoms. Thus, it is possible that severe cases may have been more likely to be reported, leading to selection bias. Another limitation is that there is a small number of observational studies with a relatively small participant numbers and multiple missing values, which decreases the quality of the data presented in their studies.
Despite these limitations, our results show detailed clinical presentations of COVID‐19 patients suffering from hair loss. While it may be reassuring for patients and clinicians that hair loss in COVID‐19 could potentially be reversible without specific treatment, a small number of patients experienced irreversible hair loss, without clear pathophysiology to date. Given the significant negative aesthetic effects of hair loss leading to psychological distress, clinicians need to be aware of the importance of dermatologic findings in COVID‐19 patients. With the growing number of patients experiencing long‐COVID symptoms, hair loss may rise in incidence and become a frequent patient concern during long‐term follow‐up. Future areas of research concerning hair loss and COVID‐19 may include the detailed histopathological analysis of hair follicles in COVID‐19 patients experiencing hair loss to identify the underlying mechanisms, as well as prospective analysis to see the correlation between estrogen, progesterone, pro‐inflammatory cytokines, and the extent of hair loss, as well as the significance of hair loss in those with PASC.
AUTHOR CONTRIBUTIONS
TC conceived the study, searched the literature, and drafted the manuscript. YN searched the literature, assessed the quality of the studies, revised the manuscript, and supervised the study.
CONFLICT OF INTEREST
None.
ETHICS STATEMENT
This article does not have any studies with human or animal subjects. The study was conducted in compliance with the ethical standards of the journal.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENT
None.
Czech T, Sugihara S, Nishimura Y. Characteristics of hair loss after COVID‐19: A systematic scoping review. J Cosmet Dermatol. 2022;00:1‐8. doi: 10.1111/jocd.15218
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aram K, Patil A, Goldust M, Rajabi F. COVID‐19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34(6):e15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Estiri H, Strasser ZH, Brat GA, Semenov YR, Patel CJ, Murphy SN. Evolving phenotypes of non‐hospitalized patients that indicate long COVID. BMC Med. 2021;19(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fagan N, Meah N, York K, et al. Shedding light on therapeutics in alopecia and their relevance to COVID‐19. Clin Dermatol. 2021;39(1):76‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID‐19 registries: updates and future directions. Dermatol Clin. 2021;39(4):575‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Esen‐Salman K, Akın‐Çakıcı Ö, Kardeş S, Salman A. Public interest in dermatologic symptoms, conditions, treatments, and procedures during the COVID‐19 pandemic: insights from Google trends. Dermatol Ther. 2021;34(2):e14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gunderson J, Mitchell D, Reid K, Jordan M. COVID‐19 information‐seeking and prevention behaviors in Florida, April 2020. Prev Chronic Dis. 2021;18:E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 9. Shinkai K, Bruckner AL. Dermatology and COVID‐19. Jama. 2020;324(12):1133‐1134. [DOI] [PubMed] [Google Scholar]
- 10. Yue H, Bai X, Wang J, et al. Clinical characteristics of coronavirus disease 2019 in Gansu province, China. Ann Palliat Med. 2020;9(4):1404‐1412. [DOI] [PubMed] [Google Scholar]
- 11. McMahon DE, Gallman AE, Hruza GJ, et al. Long COVID in the skin: a registry analysis of COVID‐19 dermatological duration. Lancet Infect Dis. 2021;21(3):313‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Müller Ramos P, Ianhez M, Amante MH. Alopecia and grey hair are associated with COVID‐19 severity. Exp Dermatol. 2020;29(12):1250‐1252. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Hong K, Gómez Gómez RE, Kim S, Chun BC. Lack of evidence of COVID‐19 being a risk factor of alopecia areata: results of a National Cohort Study in South Korea. Front Med (Lausanne). 2021;8:758069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGowan J, Straus S, Moher D, et al. Reporting scoping reviews‐PRISMA ScR extension. J Clin Epidemiol. 2020;123:177‐179. [DOI] [PubMed] [Google Scholar]
- 15. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. [DOI] [PubMed] [Google Scholar]
- 16. Abrantes TF, Artounian KA, Falsey R, et al. Time of onset and duration of post‐COVID‐19 acute telogen effluvium. J Am Acad Dermatol. 2021;85(4):975‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aksoy H, Yıldırım UM, Ergen P, Gürel MS. COVID‐19 induced telogen effluvium. Dermatol Ther. 2021;34(6):e15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Babaei K, Kavoussi H, Rezaei M, Kavoussi R. Characteristics of telogen effluvium in COVID‐19 in western Iran (2020). An Bras Dermatol. 2021;96(6):688‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cline A, Jacobs AK, Fonseca M, et al. Race, ethnicity, and comorbidities are critical factors in the diagnosis of telogen effluvium during the COVID‐19 pandemic. J Am Acad Dermatol. 2021;85(1):209‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Landro A, Naldi L, Glaser E, Paus R, Tosti A. Pathobiology questions raised by telogen effluvium and trichodynia in COVID‐19 patients. Exp Dermatol. 2021;30(7):999‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreno‐Arrones OM, Lobato‐Berezo A, Gomez‐Zubiaur A, et al. SARS‐CoV‐2‐induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2021;35(3):e181‐e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharquie KE, Jabbar RI. COVID‐19 infection is a major cause of acute telogen effluvium. Ir J Med Sci. 2021:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID‐19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Capalbo A, Giordano D, Gagliostro N, et al. Alopecia areata in a COVID‐19 patient: a case report. Dermatol Ther. 2021;34(2):e14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, beau lines, and acral peeling associated with COVID‐19 infection. JAAD Case Rep. 2021;13:138‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Domínguez‐Santás M, Haya‐Martínez L, Fernández‐Nieto D, Jiménez‐Cauhé J, Suárez‐Valle A, Díaz‐Guimaraens B. Acute telogen effluvium associated with SARS‐CoV‐2 infection. Aust J Gen Pract. 2020;49. [DOI] [PubMed] [Google Scholar]
- 27. Fivenson D. COVID‐19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. 2021;60(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayran Y, Yorulmaz A, Gür G, Aktaş A. Different hair loss patterns in two pediatric patients with COVID‐19‐associated multisystem inflammatory syndrome in children. Dermatol Ther. 2021;34(2):e14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lv S, Wang L, Zou X, et al. A case of acute telogen effluvium after SARS‐CoV‐2 infection. Clin Cosmet Investig Dermatol. 2021;14:385‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mieczkowska K, Deutsch A, Borok J, et al. Telogen effluvium: a sequela of COVID‐19. Int J Dermatol. 2021;60(1):122‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miola AC, Florêncio LC, Ribeiro MEB, Alcântara GP, Ramos PM, Miot HA. Early onset effluvium secondary to COVID‐19: a clinical and histological characterization. J Am Acad Dermatol. 2021;86:e207‐e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olds H, Liu J, Luk K, Lim HW, Ozog D, Rambhatla PV. Telogen effluvium associated with COVID‐19 infection. Dermatol Ther. 2021;34(2):e14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Özcan D, Vural AT, Özen Ö. Two cases of fibrosing alopecia in a patterned distribution after coronavirus disease 2019. Indian J Dermatol Venereol Leprol. 2021;87(6):848‐850. [DOI] [PubMed] [Google Scholar]
- 34. Rizzetto G, Diotallevi F, Campanati A, et al. Telogen effluvium related to post severe Sars‐Cov‐2 infection: clinical aspects and our management experience. Dermatol Ther. 2021;34(1):e14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossi A, Magri F, Michelini S, et al. New onset of alopecia areata in a patient with SARS‐CoV‐2 infection: possible pathogenetic correlations? J Cosmet Dermatol. 2021;20(7):2004‐2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rossi A, Magri F, Sernicola A, et al. Telogen effluvium after SARS‐CoV‐2 infection: a series of cases and possible pathogenetic mechanisms. Skin Appendage Disorders. 2021;7(5):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sgubbi P, Savoia F, Calderoni O, Longo R, Stinchi C, Tabanelli M. Alopecia areata in a patient with SARS‐Cov‐2 infection. Dermatol Ther. 2020;33(6):e14295. [DOI] [PubMed] [Google Scholar]
- 38. Shanshal M. COVID‐19 related anagen effluvium. J Dermatolog Treat. 2020;1–2:1114‐1115. [DOI] [PubMed] [Google Scholar]
- 39. Suzuki T, Kutsuna S, Saito S, et al. Clinical course of alopecia after COVID‐19. Int J Infect Dis. 2021;107:255‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vastarella M, Cantelli M, Nappa P, Fabbrocini G, Ocampo‐Garza SS. Black dots in trichoscopy after COVID‐19. can it be telogen effluvium? Dermatol Ther. 2021;34(5):e15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grifoni E, Valoriani A, Cei F, et al. Interleukin‐6 as prognosticator in patients with COVID‐19. J Infect. 2020;81(3):452‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone‐inducible IL‐6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012;132(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 43. Mauvais‐Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID‐19 outcomes. Endocrinology. 2020;161(9):bqaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al‐kuraishy HM, Al‐Gareeb AI, Faidah H, Al‐Maiahy TJ, Cruz‐Martins N, Batiha GES. The looming effects of estrogen in Covid‐19: a rocky rollout. Front Nut. 2021;8:649128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lovre D, Bateman K, Sherman M, Fonseca VA, Lefante J, Mauvais‐Jarvis F. Acute estradiol and progesterone therapy in hospitalised adults to reduce COVID‐19 severity: a randomised control trial. BMJ Open. 2021;11(11):e053684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grymowicz M, Rudnicka E, Podfigurna A, et al. Hormonal effects on hair follicles. Int J Mol Sci. 2020;21(15):5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


