Abstract
In early 2020, a global emergency was upon us in the form of the coronavirus disease 2019 (COVID‐19) pandemic. While horrific in its health, social and economic devastation, one silver lining to this crisis has been a rapid mobilization of cross‐institute, and even cross‐country teams that shared common goals of learning as much as we could as quickly as possible about the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and how the immune system would respond to both the virus and COVID‐19 vaccines. Many of these teams were formed by women who quickly realized that the classical model of “publish first at all costs” was maladaptive for the circumstances and needed to be supplanted by a more collaborative solution‐focused approach. This review is an example of a collaboration that unfolded in separate countries, first Canada and the United States, and then also Israel. Not only did the collaboration allow us to cross‐validate our results using different hands/techniques/samples, but it also took advantage of different vaccine types and schedules that were rolled out in our respective home countries. The result of this collaboration was a new understanding of how mucosal immunity to SARS‐CoV‐2 infection vs COVID‐19 vaccination can be measured using saliva as a biofluid, what types of vaccines are best able to induce (limited) mucosal immunity, and what are potential correlates of protection against breakthrough infection. In this review, we will share what we have learned about the mucosal immune response to SARS‐CoV‐2 and to COVID‐19 vaccines and provide a perspective on what may be required for next‐generation pan‐sarbecoronavirus vaccine approaches.
Keywords: antibodies, COVID‐19, gut, IgA, immune response, immunization, immunology, infection, mucosal immunity, saliva, SARS‐CoV‐2, spike, vaccination
1. INTRODUCTION
The predominant route of infection by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is inhalation, where the mucosa of the nasopharynx and oral cavity are directly exposed to virus from the environment. 1 , 2 Two receptors that facilitate the entry of SARS‐CoV‐2, angiotensin‐converting enzyme 2 (ACE2) and transmembrane protease, serine 2 (TMPRSS), are detected in the nasal, salivary, and intestinal epithelium. 1 , 2 Therefore, in order to understand and predict outcomes of infection, we must first understand the mucosal immune response occurring at the site of infection. If infection is established and virus penetrates the mucosa, virions can then disseminate systemically and progress into multiorgan infection and illness, known as coronavirus disease 2019 (COVID‐19).
Tools to identify individuals who have generated sufficient immune protection from SARS‐CoV‐2, either through prior infection or through vaccination, have attracted tremendous interest. While serum has been used to intensively study the kinetics and affinity of immunoglobin G (IgG) neutralizing antibody (nAb) production in response to either SARS‐CoV‐2 infection or COVID‐19 immunization, less is known about antibodies produced at mucosal surfaces. Moreover, while a clearer picture emerges regarding correlates of protection from disease, 3 correlates of protection from infection and transmission remain largely unidentified.
Knowledge of mucosal antibody immune responses to SARS‐CoV‐2 remain understudied but are essential to understand and predict outcomes to infection, as well as improve our vaccination strategies to further prevent person‐to‐person transmission, morbidity, and mortality. Highlighting research from our laboratories and others, we discuss comparative studies of antibody responses to SARS‐CoV‐2 in the blood and saliva of convalescent as well as vaccinated individuals.
2. LOCATION, LOCATION, LOCATION
2.1. Infection of SARS‐CoV‐2 along “the tube”
Although SARS‐CoV‐2 is a respiratory virus, it has the capacity to infect epithelial cells along the entire mucosal tract from mouth to gut, that is, “the tube”. Specifically, the human nasal epithelium has been shown to highly express SARS‐CoV‐2 entry factors in vitro 4 and ex vivo. 1 , 2 SARS‐CoV‐2 has also been shown to infect and replicate in the olfactory epithelium of the nasal turbinates in mice, 5 , 6 hamsters, 7 , 8 , 9 , 10 and rhesus macaques. 11 Notably, the viral temperature dynamics of SARS‐CoV‐2 changed from the original SARS‐CoV in the human respiratory epithelium, with SARS‐CoV‐2 having the ability to preferentially replicate in the upper airway. 12 This adaptation is a marked advantage for SARS‐CoV‐2 since it allows for increased transmissibility prior to more invasive infection of the lower airway and arguably could contribute to more asymptomatic spread. Interestingly, with the emergence of SARS‐CoV‐2 variants throughout the pandemic, fluctuations in viral replication levels across different mucosal surfaces of the airway have also been noted, 13 as well as evidence of lingering viral antigen in the intestinal mucosa. 14 , 15 A significant gap in our knowledge exists regarding the interaction between host immunity and SARS‐CoV‐2 along the mucosal tract, in addition to compartmental variations.
2.2. SARS‐CoV‐2 in saliva of patients with COVID‐19
Testing for the presence of virus is done from anatomically relevant locations of the nasopharynx, mid‐turbinate nasal canal, cheek swabs, throat swabs, saliva swabs, or saliva. 16 , 17 Early in the pandemic, SARS‐CoV‐2 was detected in saliva of 91.7% of hospitalized patients with COVID‐19 from which live virus was propagated in culture. 18 This finding is consistent with the early detection and high viral loads of the original SARS‐CoV in the saliva of hospitalized patients, even before the development of lung lesions. 19 Wyllie et al. 16 demonstrated that saliva has been an accurate metric to screen for SARS‐CoV‐2 infection, equivalent to nasopharyngeal swab testing. In the context of the Omicron variant specifically, researchers found that saliva swabs were more sensitive for viral detection as compared to nasal swabs. 20 As new variants continue to arise, more attention to the oral cavity is required to ensure that testing does not result in false negatives.
2.3. Beyond blood—antibodies to SARS‐CoV‐2
Most antibody testing has focused on circulating antibodies sampled from serum/plasma resulting in significant insights into the kinetics and nature of the humoral immune response. Indeed, SARS‐CoV‐2 infection can produce a robust and durable systemic antibody response corresponding with protection from disease. 3 , 21 , 22 , 23 , 24 In addition to blood, saliva is a unique specimen to evaluate as it offers a view of the mucosal immune response, as well as a window into the plasma due to vascular leakage from the gingival crevicular epithelium. 25 , 26 The ease of sampling saliva, particularly in pediatric patients, is an appealing alternative to phlebotomy. Paired bio‐sampling of saliva and blood in patients with COVID‐19 has shown strong positive correlations for SARS‐CoV‐2 Spike (S) and Receptor‐Binding Domain (RBD) specific immunoglobulin (IgM, IgG, and IgA) levels, demonstrating that systemic immune response can be monitored through saliva. 27 We will expand upon this concept in section IV.
2.4. SARS‐CoV‐2 Spike vaccines protect against severe COVID‐19 disease remarkably well, but…
Despite the general understanding that SARS‐CoV‐2 infects the airways and induces a humoral immune response in the upper respiratory tract, initial vaccine strategies inoculate via the parenteral or intramuscular route. The choice of route is sensible given previously established experience delivering vaccines via the intramuscular route, the notion that generation of systemic immune memory confers strong protection against disease, as well as accessible and well‐defined metrics to assess correlates of protection against disease. With approximately 11 billion vaccine doses administered worldwide at the time of writing of this review (Q1 2022), COVID‐19 vaccines given via the intramuscular route have been a staggering success in protecting against disease and to a certain degree against infection. 28 In addition, these vaccines have incredible capacity to generate long‐term memory that is projected to last for years to come. 29 , 30
However, as antibody levels decline post‐vaccination, breakthrough infections have become inevitable. This fact is particularly relevant for more antigenically divergent SARS‐CoV‐2 variants like Omicron, 31 or variants that accumulate exceptionally high viral levels such as Delta. 32 , 33 Sterilizing immunity was never the goal of intramuscular COVID‐19 vaccines. The future and hopefully “final frontier” of vaccine strategies for this pandemic will generate robust, durable, and flexible immunity at the upper respiratory tract that confers protection against SARS‐CoV‐2 infection. To tackle this final frontier, we must consider the “first frontier”—the mucosa.
3. MUCOSA AND THE LOCAL IMMUNE RESPONSE
3.1. The moat and the castle—mucosal versus systemic immune responses
The human body has multiple lines of defense to protect against pathogens. While our outermost physical barrier of the skin is a key protective layer, there are several orifices where there is no keratinized epithelial barrier, and the sheer surface area of the body's mucosa dwarfs the size of the external keratinized skin by orders of magnitude. At these mucosal surfaces, a thin layer of epithelium serves as a barrier, which in addition to being a more permeable physical barrier, also has significant immune properties. A dense matrix of connective tissue sits directly below the thin layer of epithelium and connects it to the smooth muscle layer. This structure serves as an additional layer of protection from pathogens and certain body fluids (gastric acid, urine, vaginal secretions). 34
The mucosa also forms an ideal niche for immune cells, which can remain in the mucosa as sentinels, protecting the body against infection. Figure 1 illustrates how the mucosal immune system is a first line of defense, and distinct from the systemic immune system. In addition to mucosal resident myeloid cells, T cells, and innate lymphoid cells, the mucosa is also a rich reservoir of plasma cells (PCs), which secrete antibodies that shield the body from resident communities of commensal microbiota. The mucosal immune system works separately from the rest of the body by forming a “firewall” that under normal circumstances stops at the first draining lymph node (i.e., the castle wall). In the case of the intestine, the mucosal immune response typically does not stray beyond the mesenteric lymph node (MLN). 35 However, the mucosal immune response also works in tandem with the systemic immune response as recirculation of mucosal immune cells has been shown to occur and may be a way to distribute mucosal immune effector cells across multiple‐barrier sites. 36 , 37 , 38 After all, viruses like SARS‐CoV‐2 can impact the entire length of “the tube.”
FIGURE 1.
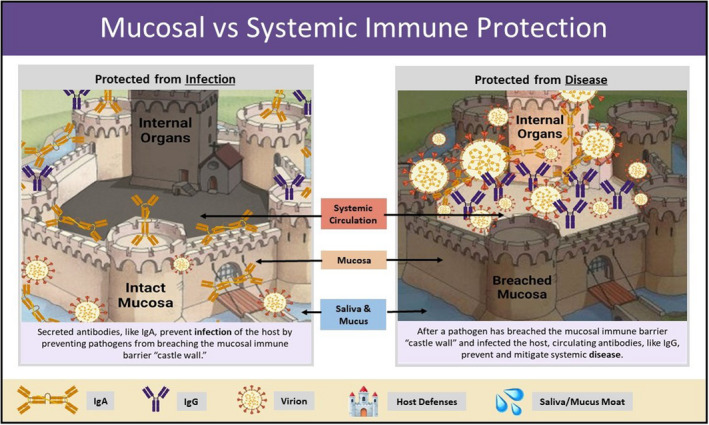
The castle and moat metaphor illustrates key differences between protective mucosal and systemic antibody responses
3.2. Induction of mucosal immune responses
There are three major Mucosa‐Associated Lymphoid Tissues (MALT): Gut‐Associated Lymphoid Tissue (GALT), Bronchus‐Associated Lymphoid Tissue (BALT), and Nasal‐Associated Lymphoid Tissue (NALT). 39 Priming against commensal or foreign antigen occurs most efficiently within organized lymphoid tissues. In the case of the GALT, MLN, and Peyer's Patches provide ideal environments for initiating immune responses within inducible germinal centers. In these germinal centers, cognate interactions between antigen‐specific B and T cells result in the secondary diversification of the B cell receptor, including antibody isotype class switch and affinity maturation. Select B cells become memory B cells or plasma cells (see below), and antigen‐specific T cells also acquire the capacity to become memory T cells. 40 The particular environment of the GALT programs these memory cells to home back to the gut lamina propria after they leave the MLN via the acquisition of homing receptors (integrin α₄ and β₇). 41 Memory cells that home to and lodge in the mucosa, and their progeny, serve to protect against repeat pathogen exposure, providing lasting immune memory at mucosal surfaces.
3.3. Mucosal antibodies
Germinal center B cells can differentiate into plasmablasts or plasma cells (PCs) and secrete antibodies of different isotypes including: IgD, IgM, IgA, IgE, and IgG. The class of antibody produced is largely dictated by the location of priming. For example, in the GALT and the NALT, cytokines such as transforming growth factor‐beta (TGFb), B cell‐activating factor (BAFF) and a proliferation‐inducing ligand (APRIL) provoke class switch to IgA, a topic that was recently reviewed in depth by Isho et al. 37 Focusing on the oral cavity, the major isotypes that predominate in the saliva are IgA (primarily) and IgG. While most IgG in the saliva are actually derived from the serum through passive diffusion and vascular leakage via the gingival crevicular epithelium, IgA can be actively transported across secretory epithelia of the oral mucosa and into the saliva. 42 IgD, IgM, and IgE can also be actively secreted into saliva under particular conditions or in specific disease states, but they are much less abundant in the oral cavity. 43 , 44
The process of IgA secretion into the oral cavity is similar to what occurs in the gut. Specifically, IgA secreting plasmablasts and PCs can secrete monomeric IgA or dimeric IgA, which consists of two IgA monomers bound together by the joining (J) chain. Dimeric IgA crosses through the cytoplasm of mucosal epithelial cells through transcytosis via the polymeric immunoglobin receptor (pIgR). Through this mechanism, dimeric IgA is then bound to the secretory component (SC), resulting in local production of a secretory form of IgA dimers (sIgA), which coats mucosal surfaces. The secretory component of SIgA provides protection against mucosal enzymatic destruction and degradation by resident commensal microbes. 45 SIga prevents infections through a phenomenon known as neutralization, where these antibodies bind to the proteins on the exterior of the pathogen, which then prevent it from binding to and infecting cells. In addition to local sIgA‐producing PC in the oral mucosa, a small amount of IgG antibodies are also produced locally by gingival, glandular, and tonsillar PCs. 25 , 26 , 42
4. HUMORAL IMMUNE RESPONSE TO SARS‐COV‐2 INFECTION
4.1. Systemic antibody response to SARS‐CoV‐2 infection
The ability of serum antibodies to bind to SARS‐CoV‐2 and neutralize virions after infection is significantly correlated with protection from morbidity and mortality due to COVID‐19. 3 , 46 , 47 , 48 , 49 In the absence of T cells, antibodies are sufficient for protection in mice 50 and macaques. 51 As shown in Figure 2, SARS‐CoV‐2 antibodies can be formed against different viral antigens such as the spike protein (anti‐S), receptor‐binding domain on the spike (anti‐RBD), and nucleocapsid (anti‐N). Multiple studies, including our own, have shown that the vast majority of patients with COVID‐19 develop detectable systemic antibody titers during infection. One serology study characterized antibody responses in 285 patients with COVID‐19 and found that 100% of these patients had virus‐specific IgG in their serum at 3 weeks post‐symptom onset, with median day for seroconversion at 13 days post‐symptom onset. 52 These findings were consistent with studies from Wajnberg et al. 53 which reported that 99% of patients with COVID‐19 who were monitored, seroconverted and developed anti‐S IgG antibodies. These antibodies are essential for host protection. Moreover, the kinetics and quality of the antibody response can dictate disease severity. 54
FIGURE 2.
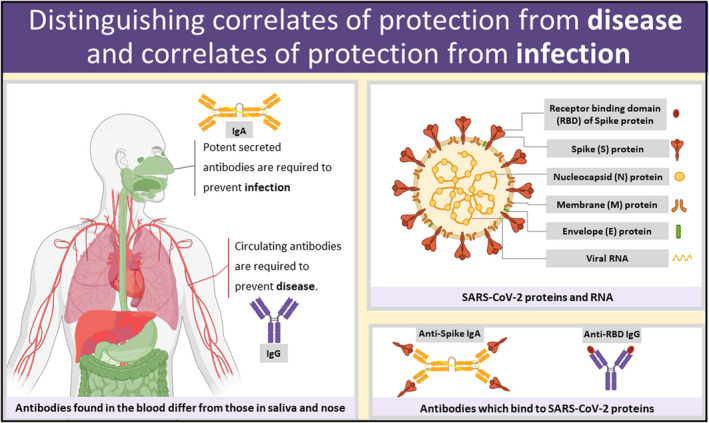
Not having local mucosal antibodies at the site of infection (upper airway) may put people at risk for infection, thus amplifying onward transmission. Correlates of protection allow us to predict protective responses without waiting to see the outcome of exposure to infection. Upper right, virion with protein and RNA components. Bottom right, antibodies bound to viral proteins. Left, an illustration of correlates of protection from disease (neutralizing IgG antibodies), and correlates of protection from infection (neutralizing IgA antibodies)
The durability of systemic antibody responses post‐SARS‐CoV‐2 infection is also of great interest. Multiple studies have reported persistence of anti‐S and anti‐RBD IgG titers in serum for at least 4 months following symptom onset. 27 , 53 Additionally, passive transfer of anti‐S and anti‐RBD nAb to rodents and macaques has been shown to be protective against severe symptoms and pulmonary inflammation. 55 , 56 Furthermore, one study showed that nAb titers correlated with anti‐RBD IgG in serum and that these titers were stable until at least 120 days post‐symptom onset. 57 Additionally, there are also reports to suggest more rapid decline of nAb titers in the serum of individuals with mild disease. 52 , 58 Taken together, these findings suggest a humoral antiviral response consists of a robust early plasmablast expansion phase (which accounts for high acute antibody levels in the serum/plasma), followed by a more protracted memory phase with lower levels of anti‐SARS‐CoV‐2 antibodies in the serum/plasma 59 that is sustained by plasma cells in the bone marrow. 60
4.2. Salivary antibody response to SARS‐CoV‐2 infection
As mentioned, the oral and nasopharyngeal cavities are the most likely routes of transmission of SARS‐CoV‐2, making neutralizing antibodies in these compartments one of the first lines of defense against infection. 11 B cells activated in mucosal induction sites (i.e., the MLN) are skewed towards IgA class switch due to the presence of IgA class switch factors such as TGFβ and APRIL. 61 Thus, humoral immunity in the oral and nasal mucosa is dominated by dimeric (mucosal) sIgA. In addition to sIgA, IgG antibodies are also present in the saliva, which mainly enter the saliva from the serum via transudation through the gingival crevicular fluid. 25 , 26
We and others have shown that IgA is readily detected against spike and RBD in the saliva of COVID‐19 acute and convalescent patients, although this response is quite variable and declines more rapidly than the IgG response. 16 , 27 In a longitudinal study analyzing serum and salivary antibody decay in convalescent patients, both serum and salivary IgG titers remained relatively stable up to 9 months post‐symptom onset. These investigators also showed that anti‐S IgA could be detected in the saliva of 55% of patients in early convalescence, with a gradual decline in salivary antibody titers until 8 months post‐symptom onset. Conversely, anti‐S IgG was detected in the saliva of 100% of patients, and demonstrated a much slower decline compared with IgA. 62
The level of anti‐SARS‐CoV‐2 IgG and IgA antibody responses in saliva and serum have been shown to correlate reasonably well between the two biofluids, 27 , 63 although the correlation between serum and saliva IgA is slightly weaker indicating potential compartmentalization of the mucosal immune response. 27 , 64 Nevertheless, because of the positive correlation between serum and saliva anti‐SARS‐CoV‐2 antibodies, researchers have proposed that saliva can be used as a less invasive substitute to serology for the detection of previous infection. Randad et al. 63 demonstrated that salivary and serum SARS‐CoV‐2‐specific IgG showed similar binding profiles in detection assays, and that salivary IgG alone could be used to detect previous infection with high sensitivity and specificity. These results were consistent with a study by Varadhachary et al., in which they developed an assay for detecting previous SARS‐CoV‐2 infection using salivary IgA titers. Their results showed a 92% positive predictive rate when salivary IgA was used to assess mucosal immune responses. 65
Salivary antibody responses following SARS‐CoV‐2 infection may also play a role in disease severity and outcome. In a study assessing saliva and serum from pediatric patients, asymptomatic pediatric patients had higher levels of salivary IgA than symptomatic cases, suggesting a strong neutralizing mucosal response may prevent systemic, symptomatic infection. 66 However, persistence of salivary IgA in adults has been associated with severe COVID‐19. 67 Although there have not been many studies analyzing the sIgA response and its relationship to COVID‐19 disease severity, reports indicating increased disease severity and mortality in IgA‐deficient patients 68 further highlight the importance of sIgA in protection at mucosal sites of infection. Lastly, Butler et al. 69 reported that IgA titers in nasal wash samples positively correlated with neutralizing activity.
Given that a confirmed correlate of protection against SARS‐CoV‐2 has not yet been identified, salivary antibodies provide an important proxy for both assessing immune responses to infection at the site of viral encounter, as well as a relatively simple method for monitoring population immunity. Most studies looking at correlations between anti‐S and anti‐RBD antibody titers and reinfection focus on serology. However, the exact contribution of salivary antibodies generated post‐infection in preventing reinfection has not yet been elucidated.
5. HUMORAL IMMUNE RESPONSE TO INTRAMUSCULAR VACCINATION
5.1. Systemic antibody response to COVID‐19 vaccination
The rapid speed with which COVID‐19 vaccines have been studied, tested, developed, approved, and distributed has been a testament to science and public health alike. These vaccines have been imperative to preventing severe COVID‐19 disease and death. In the quest to better understand the efficacy of these vaccines, quantitative and functional analysis of vaccine‐elicited antibodies are crucial. Here, we discuss systemic antibody responses to COVID‐19 vaccination, and their role in host protection.
Multiple reports have consistently shown that the majority of individuals who received a COVID‐19 vaccination develop anti‐S and anti‐RBD IgG in serum by 1 week post‐dose 2, irrespective of previous infection status. 70 , 71 , 72 , 73 , 74 Naaber et al. 71 reported elevated anti‐RBD IgG in sera of vaccinated participants 3 weeks post‐dose 1, with this response becoming significantly boosted post‐dose 2. Another study, by Chivu‐Economescu et al., analyzed antibody kinetics in sera following COVID‐19 vaccination in individuals previously seropositive and seronegative. They found that vaccination elicited anti‐S IgG and IgA antibodies in the serum of all participants, with peak anti‐S IgG titers reached at 6 weeks post‐dose 2. They also found that anti‐S and anti‐RBD antibody titers correlated well with nAb titers. 72 In a cohort of 137 previously seronegative and vaccinated healthcare professionals, Gianfanga et al. 73 found that 100% of these individuals had measurable IgG titers in serum 2 weeks post‐dose 2, demonstrating that COVID‐19 vaccines can elicit a robust and detectable systemic antibody response.
Previous infection status contributes to some variability in antibody responses. The study by Chivu‐Esconmescu et al. 72 also observed that vaccination elicited the highest titers of anti‐S IgA in participants who were seropositive prior to vaccination. This finding is consistent with reports from Zurac et al. 74 showing that serum IgA was over 5 times higher in vaccinated individuals who were previously seropositive. Interestingly, post‐dose 2 antibody titers in previously seronegative participants were boosted, which was not the case in the seropositive group. A study by Goel et al. 70 also found that nAb titers were boosted post‐dose 2 in only seronegative individuals.
One of the major indicators of vaccine efficacy is their ability to induce neutralizing antibodies (nAb), which is essential in host protection, as we have seen in patients with COVID‐19. Jackson et al. 75 observed that vaccine‐induced nAb titers could be detected in the sera of less than half of vaccinated participants after just 1 dose of mRNA‐1273 (Moderna), with a boost in nAb titers following the second dose. Given the emergence of multiple viral variants of SARS‐CoV‐2, whether antibody responses elicited by currently approved vaccines can provide protection against these variants is of great concern. Goel et al. 70 reported that a single dose of BNT162b2 (Pfizer/BioNTech) was insufficient at producing nAb titers against the Beta variant, but a second vaccine dose led to sufficient neutralization of virions. Sahin et al. demonstrated the breadth of nAb capacity of sera from vaccinated individuals against 16 SARS‐CoV‐2 variants using a surrogate neutralization assay. They showed sera collected 1 week post‐dose 2 was capable of neutralizing all tested variants. 76 These results are consistent with reports from Wang et al., in which they tested 17 of the most “potent” monoclonal antibodies that they were able to isolate from sera of vaccinated individuals and confirmed that a combination of antibodies was still effective at neutralizing multiple SARS‐CoV‐2 variants. 77 Lastly, Naaber et al. 71 demonstrated that messenger ribonucleic acid (mRNA) vaccine‐induced nAb were effective at neutralizing Alpha, Beta, Delta, Gamma and Kappa variants. Overall, evidence from multiple reports suggests that vaccine‐elicited nAb responses are sufficient for protection against the original Wuhan SARS‐CoV‐2 strain and multiple variants. The picture, however, is different for Omicron. This variant has approximately 30 mutations in the Spike gene resulting in significant antigenic drift. Remarkably, although the nAb response is significantly blunted when examining serum from individuals who received 2 doses of mRNA, 3 doses of mRNA appears to be a “sweet spot” for conferring sufficient memory B cell breadth to produce nAb that can neutralize Omicron. 78
In addition to the kinetics of vaccine‐elicited antibodies, persistence of this response (or lack thereof) is also of great interest and concern. Levin et al. 79 collected and analyzed serum samples from 2‐dose vaccinated healthcare professionals over 6 months and observed that peak antibody titers were detected between days 4–30 post‐dose 2 and decreased nearly 20‐fold over a 6 month period. They also observed a similar reduction in nAb titers, although the decrease in nAb titers was much slower than binding antibodies. Furthermore, Naaber et al. 71 reported that by 6 months post‐dose 2, antibody titers in serum had declined to levels comparable to post‐dose 1. Although circulating antibody titers in serum may decline over time, multiple reports have shown that COVID‐19 vaccination elicits stable memory B and T cell responses. 70 , 80 These studies observe a strong correlation between serum antibody titers and SARS‐CoV‐2‐specific memory B cell populations post‐dose 1, indicating strong recall responses and a potential role for memory B cells as a source of new antibody‐producing cells in the event of breakthrough infections.
Despite knowledge on the importance of anti‐SARS‐CoV‐2 antibody responses during infection, the precise titers of nAb required for protection against severe disease are unclear. One study aimed to estimate nAb titers based on data collected from patients who were classified as having either severe or mild illness as compared to vaccinated participants. Without taking into consideration potential cellular correlates of protection such as memory B and T cells, they observed that the mean nAb titers required for 50% protection against severe illness was 3% of the convalescent antibody titers. 3 For protection from any symptomatic illness, the neutralization level required was 20% of the mean convalescent titers. 3 Currently, there is no established correlate of protection against reinfection or breakthrough infections.
5.2. Salivary antibody response to COVID‐19 vaccination
Given our understanding of mucosal immunity and its significance to the COVID‐19 pandemic, one of the most urgent questions is whether an intramuscular vaccination can induce an immune response that prevents infection of the upper respiratory tract and onward transmission. We set out to ask: How much antibody is present at the site of SARS‐CoV‐2 exposure in individuals who have received different COVID‐19 vaccines, how do these levels compare to what is seen in the blood, and does this shine an additional light on vaccine breakthrough infections? Furthermore, due to infection surges and vaccine shortages, countries like Canada employed alternative vaccination strategies such as mixed dosing (Adenovirus‐based vaccine AZD1222 [AstraZeneca/Oxford] followed by mRNA vaccines) and dose spacing (4 months apart rather than 4 weeks apart), whereas in addition to mRNA vaccine regimes, millions of Americans received a single dose of Adenovirus vector Ad26.COV2.S (Johnson & Johnson/Janssen).
Measuring sIgA in saliva can be challenging, and Luminex‐style bead‐based assays seem particularly prone to high background binding of non‐specific salivary IgA. 81 , 82 Other types of assays report low sensitivity detecting sIgA, where vaccine‐induced IgG in saliva is detectable while IgA is not. 83 This issue becomes especially important in assessing salivary IgA produced in response to vaccination, which elicits lower levels of specific IgA than elicited in response to infection. 84 , 85 One approach to measuring sIgA efficiently is to pre‐adsorb the saliva in streptavidin coated plates, and then to use separate streptavidin coated plates with biotinylated SPIKE or RBD protein to bind vaccine‐induced salivary antibodies. 84
In Nahass et al. 81 we found striking differences in salivary antibodies between Ad26.COV2.S and mRNA vaccines. Namely, despite expected levels of circulating antibodies against receptor‐binding domain (RBD), individuals who had received Ad26.COV2.S had very low levels of antibodies in their saliva, and their saliva lacked the ability to neutralize the virus. 81 We found that individuals who had received mRNA vaccination with 1 or 2 doses of BNT162b2 or mRNA‐1273 also developed robust RBD‐specific antibodies with neutralizing ability in the plasma and unexpectedly also in the saliva. Out of these groups, we observed the highest percentage of vaccinated participants whose saliva sample could neutralize virus at levels comparable to that of convalescent individuals with prior SARS‐CoV‐2 infection had received 2 doses of mRNA vaccination 2–4 weeks prior, and made high levels of anti‐RBD IgA in their saliva. 81 However, we saw that the detectable neutralizing activity in saliva 2–4 weeks post‐vaccination was transient and showed significant decline at 3 months post‐vaccination. 86 Furthermore, we also saw that the amount of neutralizing activity, which could be measured in saliva, was quite low in comparison with the amount of neutralizing activity that can be measured from the blood. One of the most surprising observations was that Ad26.COV2.S vaccine did not induce a strong mucosal immune response or neutralizing salivary antibodies, even after a subsequent vaccination with an mRNA vaccine. This was in stark contrast to the salivary response induced by a different adenoviral vector vaccine, ChAdOx1‐S/nCOV‐19, which after a subsequent vaccination with an mRNA vaccine demonstrated similar levels of neutralizing activity in saliva to two doses of mRNA vaccination. 86 This illustrates that the formulation, dose, order and route of vaccine administration are just a few of many variables influencing the salivary antibody response.
In Sheikh‐Mohamed et al. 84 we found that anti‐S/RBD secretory chain associated IgA (sIgA) is readily provoked by one dose of mRNA in the saliva of almost all participants. This sIgA response rapidly declined at 2 weeks post‐dose 2, remaining positive in only 30% of participants, and at levels that were significantly lower than that observed in convalescents. This finding is very different from the IgG response, which is boosted by dose 2. IgG then significantly declines in the months following dose 2, as was seen also in Tu et al. 83 where they reported beyond the general decline, that 20% of the people followed had an extreme decline of 90% or more loss of vaccine‐induced salivary IgG within 3–4 months. In comparison with the significant decline in the IgG response by 6 months post‐dose 2, the anti‐S sIgA response is relatively resistant to decay in those participants (30%) who had positive anti‐S sIgA titers at 2 weeks post‐dose 2. 84 Since serum and saliva anti‐S/RBD IgA and IgG levels correlate at 2 weeks post‐dose 2, we examined serum antibodies in vaccinated individuals at this timepoint in two independent prospective cohorts from Toronto and Tel Aviv. 87 , 88 Comparing vaccination‐induced antibody levels in participants who did or did not acquire a subsequent breakthrough infection, we found that breakthrough infections are associated with significantly lower levels of anti‐S/RBD IgA that were elicited at 2 weeks post‐dose 2. No significant differences in anti‐S/RBD IgG were observed between cases and controls at that time point. This pattern of diminished IgA in breakthrough infections was observed in both cohorts even though the Toronto cohort was aged residents of a single long‐term care facility, immunized with mRNA‐1273, and experienced a Gamma outbreak, whereas the Tel Aviv cohort were healthcare professionals, vaccinated with BNT162b2 and experienced Alpha and Delta breakthrough infections. Together, our studies provide extremely pertinent information on the impact of different vaccine platforms, dosing, and schedules on mucosal immunity to SARS‐CoV‐2 of vaccination strategies that have been implemented in North America. 81 , 84
Several other studies also examined mucosal immune responses to COVID‐19 vaccination, including Mades et al. 89 that found IgG in nasal swabs and oral fluid in participants after mRNA‐1273 vaccination. Alternatively, Planas et al. 90 showed no observable neutralizing activity in nasal swabs, except in those with a history of both SARS‐CoV‐2 infection and vaccination. Ketas et al. 85 found that although less robust than participants with history of natural infection, IgA and IgG antibodies to the S‐protein and RBD were present in all serum samples and most saliva samples from individuals vaccinated with BNT162b2 or mRNA‐1273. However, in this same study, not all participants had IgA antibodies in the saliva and no nAbs were detected in the saliva by the assay utilized. 85 Most studies that observe antibodies in the saliva of vaccinated patients do not look at secretory component. For example, Azzi et al. 91 observed anti‐S IgG and IgA in the saliva of participants vaccinated with BNT162b2, but they could not confirm whether these antibodies were mucosa‐derived or systemically‐derived. However, like Sheikh‐Mohamed et al., Sano et al. were able to demonstrate that sIgA in the saliva by measuring secretory component, and this finding was particularly evident in participants who had experienced a combination of vaccination and infection. 92
Understanding the magnitude and kinetics of salivary antibody responses is paramount as we begin to shift from vaccination strategies that prevent severe illness and death, to strategies that also prevent infection and onward transmission.
6. FUTURE DIRECTIONS
6.1. How do intramuscular vaccines induce a (limited) sIgA response?
One particularly intriguing question that arises is how intramuscular mRNA vaccination induces weak but detectable sIgA responses in some individuals, and if there is a way to maximize this effect to induce not only protective systemic responses, but also protective mucosal responses. Indeed, in addition to the saliva, others have shown vaccine‐induced IgA in breast milk. 93 , 94
From our studies, mRNA vaccination is clearly better at inducing salivary antibodies (either IgG or sIgA) compared with Adenovirus vector vaccination. This finding may be due to the distribution of the Spike antigen. Highly sensitive SIMOA measurements have shown that Spike can be detected in the blood of people who received mRNA vaccination. 95 Therefore, in the context of mRNA vaccination, Spike antigen could be potentially distributed to mucosal lymph nodes that are further afield than the draining axillary lymph nodes, resulting in the generation of plasma cells expressing α₄ and β₇ mucosal homing integrins due to the mucosal lymph node priming environment. 96 Vaccines that can deliver antigen to lung dendritic cells or alveolar macrophages have shown to be capable of triggering production of mucosal IgA. 97 The observed lack of “boost‐ability” of the IgA response after dose 2 could be due to rapid opsonization of Spike antigen by pre‐existing IgG, thus precluding antigen distribution to distal sites. This finding could also have important implications for subsequent doses of vaccine as well, beyond dose 2, or in those with pre‐existing cross‐reactive immunity prior to vaccination.
Another possibility is that there could be transient upregulation of α₄ and β₇ integrins on primed immune cells in the draining lymph nodes that allows for some migration to mucosal sites, as observed with yellow fever vaccination. 98 Understanding mechanisms that provoke a mucosal immune response to COVID‐19 mRNA vaccination will require animal models. Even if this response is transient and limited, these studies are needed for us to learn how to potentially promote such responses.
6.2. Are we within spitting distance of vaccines that diminish infection and transmission?
While both unexpected and cheering that mRNA vaccines can induce a sIgA response in the oral cavity, a site of initial SARS‐CoV‐2 encounter, only 30% of vaccinated participants maintain this response and do so at levels significantly below what is observed in convalescents. Since we now know based on our work that low levels of antigen‐specific IgA are associated with vaccine breakthrough infections, our work provides a rationale for accelerating efforts to test the efficacy of intranasal SARS‐CoV‐2 boosts to prevent breakthrough infections, and consequently person‐to‐person transmission. Based on recent pre‐clinical data, this strategy also has the added benefit of protecting against SARS‐CoV‐2 variants as divergent as Beta (B.1.351), perhaps due to the high avidity of IgA dimers. 99 , 100 Oral or intranasal delivery of adenoviral vector vaccines can not only protect against disease, but also reduce transmission. 101 , 102 , 103 , 104 , 105 As we have shown, intramuscular delivery of adenoviral vector vaccines is not optimal for induction of mucosal IgA, 81 which suggests that existing adenoviral vector vaccines could be more protective with mucosal delivery. 101 , 104 Iwasaki and colleagues have shown that such a nasal boost can be conferred with even unadjuvanted stabilized Spike trimer, conferring protection against SARS‐CoV‐2 variants and even the original SARS‐CoV circa 2003. 106 Consequently, nasal boosts not only have potential for reducing infection, but are a possible route to a pan‐sarbecovirus vaccine.
Importantly, our priority should always be establishing strong systemic immunity with an initial intramuscular vaccine series to protect against disease. In fact, nasal boosts may require the foundation of systemic immunity to work effectively. Moreover, recent observations show that while Omicron infections provide strong boosting in previously vaccinated people (as measured by increased breadth of the antibody response), unvaccinated people who are infected with Omicron do not enjoy the same benefits. 107 Thus, cooperation between the moat AND the castle defenses may be what is needed to provide flexible immunity against an evolving enemy.
6.3. Accelerating shots into arms and boosts into noses
Over the course of this pandemic many scientists with relevant expertise stepped up to address big unfolding questions, with unprecedented real‐time data and preprint sharing prior to peer‐review. Social media platforms such as Twitter became places for scientists to learn about and discuss the most recent figure or study on immune responses to SARS‐CoV‐2. Indeed, the authors of this review learned of each other's work and formed new research collaborations through Twitter. In the case of the Gommerman lab study, previously unaffiliated research groups located in different countries (including the Tal, Weissman, Regev‐Yochay, McGeer, Straus, and Gingras groups) were brought together to make key observations and to replicate their findings in independent cohorts. Bringing these (largely female‐led) groups together to share samples and search for answers collaboratively, can serve as a model for global collaborations that impact public health amid an ongoing crisis.
In summary, if saliva can teach us as much about risk for transmission of SARS‐CoV‐2 as blood can teach us about protection from COVID‐19 disease, then this provides an avenue for pandemic management. We suggest that future surveillance of salivary antibodies could provide critical insight into who is at highest risk for breakthrough infection post‐vaccination and help to identify vaccination strategies that could provide local protection at the site of infection.
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest to declare.
Sheikh‐Mohamed S, Sanders EC, Gommerman JL, Tal MC. Guardians of the oral and nasopharyngeal galaxy: IgA and protection against SARS‐CoV‐2 infection. Immunol Rev. 2022;309:75‐85. doi: 10.1111/imr.13118
Salma Sheikh‐Mohamed and Erin C. Sanders contributed equally to this work.
Jennifer L. Gommerman and Michal Caspi Tal contributed equally to this work.
*
This article is part of a series of reviews covering SARS‐CoV‐2 Immunity appearing in Volume 309 of Immunological Reviews.
Funding information
We would like to acknowledge funding for this review through the Emily and Malcolm Fairbairn Family Gift Funding and CIHR team grant to CoVARR‐Net
Contributor Information
Jennifer L. Gommerman, Email: jen.gommerman@utoronto.ca.
Michal Caspi Tal, Email: mtal@mit.edu.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1. Huang N, Pérez P, Kato T, et al. SARS‐CoV‐2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892‐903. doi: 10.1038/s41591-021-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Wei Q, Alvarez X, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85(8):4025‐4030. doi: 10.1128/JVI.02292-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27:1205‐1211. doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 4. Sungnak W, Huang N, Bécavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681‐687. doi: 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golden JW, Cline CR, Zeng X, et al. Human angiotensin‐converting enzyme 2 transgenic mice infected with SARS‐CoV‐2 develop severe and fatal respiratory disease. JCI Insight. 2020;5(19):e142032. doi: 10.1172/jci.insight.142032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oladunni FS, Park J‐G, Pino PA, et al. Lethality of SARS‐CoV‐2 infection in K18 human angiotensin‐converting enzyme 2 transgenic mice. Nat Commun. 2020;11(1):6122. doi: 10.1038/s41467-020-19891-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan JF‐W, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID‐19) in a Golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020;71:2428‐2446. doi: 10.1093/cid/ciaa325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sia SF, Yan L‐M, Chin AWH, et al. Pathogenesis and transmission of SARS‐CoV‐2 in golden hamsters. Nature. 2020;583(7818):834‐838. doi: 10.1038/s41586-020-2342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhakal S, Ruiz‐Bedoya CA, Zhou R, et al. Sex differences in lung imaging and SARS‐CoV‐2 antibody responses in a COVID‐19 Golden Syrian hamster model. MBio. 2021;12:e0097421. doi: 10.1128/mBio.00974-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Melo GD, Lazarini F, Levallois S, et al. COVID‐19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596):eabf8396. doi: 10.1126/scitranslmed.abf8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou L, Ayeh SK, Chidambaram V, Karakousis PC. Modes of transmission of SARS‐CoV‐2 and evidence for preventive behavioral interventions. BMC Infect Dis. 2021;21(1):496. doi: 10.1186/s12879-021-06222-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. V'kovski P, Gultom M, Kelly JN, et al. Disparate temperature‐dependent virus–host dynamics for SARS‐CoV‐2 and SARS‐CoV in the human respiratory epithelium. PLoS Biol. 2021;19(3):e3001158. doi: 10.1371/journal.pbio.3001158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hui KPY, Ho JCW, Cheung M, et al. SARS‐CoV‐2 omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715‐720. doi: 10.1038/s41586-022-04479-6 [DOI] [PubMed] [Google Scholar]
- 14. Arostegui D, Castro K, Schwarz S, Vaidy K, Rabinowitz S, Wallach T. Persistent SARS‐CoV‐2 Nucleocapsid protein presence in the intestinal epithelium of a pediatric patient 3 months after acute infection. JPGN Rep. 2022;3(1):e152. doi: 10.1097/PG9.0000000000000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS‐CoV‐2. Nature. 2021;591(7851):639‐644. doi: 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wyllie AL, Fournier J, Casanovas‐Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS‐CoV‐2. N Engl J Med. 2020;383(13):1283‐1286. doi: 10.1056/NEJMc2016359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savela ES, Viloria Winnett A, Romano AE, et al. Quantitative SARS‐CoV‐2 viral‐load curves in paired saliva samples and nasal swabs inform appropriate respiratory sampling site and analytical test sensitivity required for earliest viral detection. J Clin Microbiol. 2022;60(2):e0178521. doi: 10.1128/jcm.01785-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. To KK‐W, Tsang OT‐Y, Yip CC‐Y, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841‐843. doi: 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W‐K, Chen S‐Y, Liu I‐J, et al. Detection of SARS‐associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis. 2004;10(7):1213‐1219. doi: 10.3201/eid1007.031113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marais G, Doolabh D, Enoch A, et al. Saliva swabs are the preferred sample for Omicron detection. medRxiv. Published online 2022. doi: 10.1101/2021.12.22.21268246 [DOI]
- 21. Brouwer PJM, Caniels TG, van der Straten K, et al. Potent neutralizing antibodies from COVID‐19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643‐650. doi: 10.1126/science.abc5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Premkumar L, Segovia‐Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS‐CoV‐2 patients. Sci Immunol. 2020;5(48):3. doi: 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184(4):861‐880. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med. 2020;26(7):1033‐1036. doi: 10.1038/s41591-020-0913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brandtzaeg P. Secretory immunity with special reference to the oral cavity. J Oral Microbiol. 2013;5(1):4. doi: 10.3402/jom.v5i0.20401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hettegger P, Huber J, Paßecker K, et al. High similarity of IgG antibody profiles in blood and saliva opens opportunities for saliva based serology. PLoS One. 2019;14(6):e0218456. doi: 10.1371/journal.pone.0218456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS‐CoV‐2 spike antigens in COVID‐19 patients. Sci Immunol. 2020;5(52):eabe5511. doi: 10.1126/sciimmunol.abe5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salo J, Hägg M, Kortelainen M, et al. The indirect effect of mRNA‐based COVID‐19 vaccination on healthcare workers' unvaccinated household members. Nat Commun. 2022;13(1):1162. doi: 10.1038/s41467-022-28825-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tarke A, Coelho CH, Zhang Z, et al. SARS‐CoV‐2 vaccination induces immunological T cell memory able to cross‐recognize variants from alpha to omicron. Cell. 2022;185(5):847‐859. doi: 10.1016/j.cell.2022.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS‐CoV‐2 and variants of concern. Science. 2021;374(6572):abm0829. doi: 10.1126/science.abm0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuhlmann C, Mayer CK, Claassen M, et al. Breakthrough infections with SARS‐CoV‐2 omicron despite mRNA vaccine booster dose. Lancet. 2022;399(10325):625‐626. doi: 10.1016/S0140-6736(22)00090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lipsitch M, Krammer F, Regev‐Yochay G, Lustig Y, Balicer RD. SARS‐CoV‐2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22(1):57‐65. doi: 10.1038/s41577-021-00662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levine‐Tiefenbrun M, Yelin I, Alapi H, et al. Viral loads of Delta‐variant SARS‐CoV‐2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med. 2021;27(12):2108‐2110. doi: 10.1038/s41591-021-01575-4 [DOI] [PubMed] [Google Scholar]
- 34. Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol. 2016;34(1):575‐608. doi: 10.1146/annurev-immunol-032414-112315 [DOI] [PubMed] [Google Scholar]
- 35. Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203(3):497‐500. doi: 10.1084/jem.20060227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rojas OL, Pröbstel A‐K, Porfilio EA, et al. Recirculating intestinal IgA‐producing cells regulate Neuroinflammation via IL‐10. Cell. 2019;176(3):610‐624. doi: 10.1016/j.cell.2018.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Isho B, Florescu A, Wang AA, Gommerman JL. Fantastic IgA plasma cells and where to find them. Immunol Rev. 2021;303(1):119‐137. doi: 10.1111/imr.12980 [DOI] [PubMed] [Google Scholar]
- 38. Fitzpatrick Z, Frazer G, Ferro A, et al. Gut‐educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587(7834):472‐476. doi: 10.1038/s41586-020-2886-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silva‐Sanchez A, Randall TD. Anatomical uniqueness of the mucosal immune system (GALT, NALT, iBALT) for the induction and regulation of mucosal immunity and tolerance. In: Kiyono H, Pascual DW, eds. Mucosal Vaccines. Elsevier; 2020:21‐54. [Google Scholar]
- 40. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30(1):429‐457. doi: 10.1146/annurev-immunol-020711-075032 [DOI] [PubMed] [Google Scholar]
- 41. Mora JR, von Andrian UH. Differentiation and homing of IgA‐secreting cells. Mucosal Immunol. 2008;1(2):96‐109. doi: 10.1038/mi.2007.14 [DOI] [PubMed] [Google Scholar]
- 42. Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann N Y Acad Sci. 2007;1098(1):288‐311. doi: 10.1196/annals.1384.012 [DOI] [PubMed] [Google Scholar]
- 43. Chorny A, Cerutti A. Regulation and function of mucosal IgA and IgD. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN, eds. Mucosal Immunology. Elsevier; 2015:683‐700. [Google Scholar]
- 44. Jafarzadeh A, Sadeghi M, Karam GA, Vazirinejad R. Salivary IgA and IgE levels in healthy subjects: relation to age and gender. Braz Oral Res. 2010;24(1):21‐27. doi: 10.1590/S1806-83242010000100004 [DOI] [PubMed] [Google Scholar]
- 45. Moon C, Baldridge MT, Wallace MA, et al. Vertically transmitted faecal IgA levels determine extra‐chromosomal phenotypic variation. Nature. 2015;521(7550):90‐93. doi: 10.1038/nature14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS‐CoV‐2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58(11):7. doi: 10.1128/JCM.02107-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS‐CoV‐2 in rhesus macaques. Science. 2020;369(6505):806‐811. doi: 10.1126/science.abc6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alter G, Seder R. The power of antibody‐based surveillance. N Engl J Med. 2020;383(18):1782‐1784. doi: 10.1056/NEJMe2028079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gilbert PB, Isbrucker R, Andrews N, et al. Immune correlates analysis of the mRNA‐1273 COVID‐19 vaccine efficacy trial. medRxiv. Published online August 15, 2021. doi: 10.1101/2021.08.09.21261290 [DOI]
- 50. Israelow B, Mao T, Klein J, et al. Adaptive immune determinants of viral clearance and protection in mouse models of SARS‐CoV‐2. PREPRINT Published Online May 19, 2021. [DOI] [PMC free article] [PubMed]
- 51. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature. 2021;590(7847):630‐634. doi: 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Long Q‐X, Liu B‐Z, Deng H‐J, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845‐848. doi: 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 53. Wajnberg A, Mansour M, Leven E, et al. Humoral response and PCR positivity in patients with COVID‐19 in the New York City region, USA: an observational study. Lancet Microbe. 2020;1(7):e283‐e289. doi: 10.1016/S2666-5247(20)30120-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang AT, Garcia‐Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704. doi: 10.1038/s41467-020-18450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rogers TF, Zhao F, Huang D, et al. Isolation of potent SARS‐CoV‐2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369(6506):956‐963. doi: 10.1126/science.abc7520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS‐CoV‐2. Nature. 2020;584(7821):443‐449. doi: 10.1038/s41586-020-2548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS‐CoV‐2 spike protein in COVID‐19 patients. Sci Immunol. 2020;5(52):eabe0367. doi: 10.1126/sciimmunol.abe0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti‐SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383(11):1085‐1087. doi: 10.1056/NEJMc2025179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xiang T, Liang B, Fang Y, et al. Declining levels of neutralizing antibodies against SARS‐CoV‐2 in convalescent COVID‐19 patients one year post symptom onset. Front Immunol. 2021;12:708523. doi: 10.3389/fimmu.2021.708523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Turner JS, Kim W, Kalaidina E, et al. SARS‐CoV‐2 infection induces long‐lived bone marrow plasma cells in humans. Nature. 2021;595(7867):421‐425. doi: 10.1038/s41586-021-03647-4 [DOI] [PubMed] [Google Scholar]
- 61. Spencer J, Sollid LM. The human intestinal B‐cell response. Mucosal Immunol. 2016;9(5):1113‐1124. doi: 10.1038/mi.2016.59 [DOI] [PubMed] [Google Scholar]
- 62. Alkharaan H, Bayati S, Hellström C, et al. Persisting salivary IgG against SARS‐CoV‐2 at 9 months after mild COVID‐19: a complementary approach to population surveys. J Infect Dis. 2021;224(3):407‐414. doi: 10.1093/infdis/jiab256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Randad PR, Pisanic N, Kruczynski K, et al. COVID‐19 serology at population scale: SARS‐CoV‐2‐specific antibody responses in saliva. medRxiv Prepr Serv Heal Sci. Published online May 26, 2020. doi: 10.1101/May24,2020.20112300 [DOI] [PMC free article] [PubMed]
- 64. Faustini SE, Jossi SE, Perez‐Toledo M, et al. Detection of antibodies to the SARS‐CoV‐2 spike glycoprotein in both serum and saliva enhances detection of infection. medRxiv Prepr Serv Heal Sci. Published online June 18, 2020. doi: 10.1101/June16,2020.20133025 [DOI]
- 65. Varadhachary A, Chatterjee D, Garza J, et al. Salivary anti‐SARS‐CoV‐2 IgA as an accessible biomarker of mucosal immunity against COVID‐19. medRxiv Prepr Serv Heal Sci. Published online August 11, 2020. doi: 10.1101/August7,2020.20170258 [DOI]
- 66. Dobaño C, Alonso S, Fernández de Sevilla M, et al. Antibody conversion rates to SARS‐CoV‐2 in saliva from children attending summer schools in Barcelona, Spain. BMC Med. 2021;19(1):309. doi: 10.1186/s12916-021-02184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Costantini VP, Nguyen K, Lyski Z, et al. Development and validation of an enzyme immunoassay for detection and quantification of SARS‐CoV‐2 salivary IgA and IgG. medRxiv Prepr Serv Heal Sci. Published online September 7, 2021. doi: 10.1101/2021.21263078 [DOI] [PMC free article] [PubMed]
- 68. Meyts I, Bucciol G, Quinti I, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147(2):520‐531. doi: 10.1016/j.jaci.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Butler SE, Crowley AR, Natarajan H, et al. Distinct features and functions of systemic and mucosal humoral immunity among SARS‐CoV‐2 convalescent individuals. Front Immunol. 2021;11:9. doi: 10.3389/fimmu.2020.618685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goel RR, Apostolidis SA, Painter MM, et al. Longitudinal analysis reveals distinct antibody and memory B cell responses in SARS‐CoV2 Naïve and recovered individuals following mRNA vaccination. medRxiv Prepr Serv Heal Sci. Published online March 6, 2021. doi: 10.1101/2021.21252872 [DOI] [PMC free article] [PubMed]
- 71. Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Heal Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chivu‐Economescu M, Bleotu C, Grancea C, et al. Kinetics and persistence of cellular and humoral immune responses to SARS‐CoV‐2 vaccine in healthcare workers with or without prior COVID‐19. J Cell Mol Med. 2022;26(4):1293‐1305. doi: 10.1111/jcmm.17186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gianfagna F, Veronesi G, Baj A, et al. Anti‐SARS‐CoV‐2 antibody levels and kinetics of vaccine response: potential role for unresolved inflammation following recovery from SARS‐CoV‐2 infection. Sci Rep. 2022;12(1):385. doi: 10.1038/s41598-021-04344-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zurac S, Nichita L, Mateescu B, et al. COVID‐19 vaccination and IgG and IgA antibody dynamics in healthcare workers. Mol Med Rep. 2021;24(2):9‐10. doi: 10.3892/mmr.2021.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med. 2020;383(20):1920‐1931. doi: 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sahin U, Muik A, Derhovanessian E, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594‐599. doi: 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 77. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature. 2021;592(7855):616‐622. doi: 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang K, Jia Z, Bao L, et al. Memory B cell repertoire from triple vaccinees against diverse SARS‐CoV‐2 variants. Nature. 2022;603:919‐925. doi: 10.1038/s41586-022-04466-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ciabattini A, Pastore G, Fiorino F, et al. Evidence of SARS‐CoV‐2‐specific memory B cells six months after vaccination with the BNT162b2 mRNA vaccine. Front Immunol. 2021;12:740708. doi: 10.3389/fimmu.2021.740708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nahass GR, Salomon‐Shulman RE, Blacker G, et al. Intramuscular SARS‐CoV‐2 vaccines elicit varying degrees of plasma and salivary antibody responses as compared to natural infection. medRxiv Prepr Serv Heal Sci. Published online August 30, 2021. doi: 10.1101/2021.08.22.21262168 [DOI]
- 82. Klingler J, Lambert GS, Itri V, et al. Detection of antibody responses against SARS‐CoV‐2 in plasma and saliva from vaccinated and infected individuals. Front Immunol. 2021;12:11. doi: 10.3389/fimmu.2021.759688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tu MK, Chiang SH, Bender RA, Wong DTW, Strom CM. The kinetics of COVID‐19 vaccine response in a community‐vaccinated population. J Immunol. 2022;208(4):819‐826. doi: 10.4049/jimmunol.2100919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sheikh‐Mohamed S, GYC Chao, Isho B, et al. A mucosal antibody response is induced by intra‐muscular SARS‐CoV‐2 mRNA vaccination. medRxiv Prepr Serv Heal Sci. Published online January 2, 2022. doi: 10.1101/2021.08.01.21261297 [DOI]
- 85. Ketas TJ, Chaturbhuj D, Cruz Portillo VM, et al. Antibody responses to SARS‐CoV‐2 mRNA vaccines are detectable in saliva. Pathog Immun. 2021;6(1):116‐134. doi: 10.20411/pai.v6i1.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tal MC. Indeed, if we Look across Different Vaccination Strategies and Compare Levels of Protective Antibodies in the Saliva, we See that there Are Low and Transient Levels of Salivary Neutralizing Antibodies, and that the Adenoviral Vaccinations . Twitter. Published 2022. https://twitter.com/ImmunoFever/status/1494332008679370767?s=20&t=2s7gop7Zzv‐O9sqVWzJw2A
- 87. Bergwerk M, Gonen T, Lustig Y, et al. Covid‐19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474‐1484. doi: 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Williams C, Al‐Bargash D, Macalintal C, et al. COVID‐19 outbreak associated with a SARS‐CoV‐2 P.1 lineage in a long‐term care home after implementation of a vaccination program – Ontario, April‐May 2021. Clin Infect Dis. 2022;74:1085‐1088. doi: 10.1093/cid/ciab617 [DOI] [PubMed] [Google Scholar]
- 89. Mades A, Chellamathu P, Kojima N, et al. Detection of persistent SARS‐CoV‐2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID‐19 mRNA vaccination. Sci Rep. 2021;11(1):24448. doi: 10.1038/s41598-021-03931-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS‐CoV‐2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27(5):917‐924. doi: 10.1038/s41591-021-01318-5 [DOI] [PubMed] [Google Scholar]
- 91. Azzi L, Dalla Gasperina D, Veronesi G, et al. Mucosal immune response in BNT162b2 COVID‐19 vaccine recipients. EBioMedicine. 2022;75:103788. doi: 10.1016/j.ebiom.2021.103788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sano K, Bhavsar D, Singh G, et al. Efficient mucosal antibody response to SARS‐CoV‐2 vaccination is induced in previously infected individuals. medRxiv Prepr Serv Heal Sci. Published online December 11, 2021. doi: 10.1101/2021.12.06.21267352 [DOI]
- 93. Gonçalves J, Juliano AM, Charepe N, et al. Secretory IgA and T cells targeting SARS‐CoV‐2 spike protein are transferred to the breastmilk upon mRNA vaccination. Cell Reports Med. 2021;2(12):100468. doi: 10.1016/j.xcrm.2021.100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Juncker HG, Mulleners SJ, Coenen ERM, van Goudoever JB, van Gils MJ, van Keulen BJ. Comparing human Milk antibody response after 4 different vaccines for COVID‐19. JAMA Pediatr. 2022;176:611‐612. doi: 10.1001/jamapediatrics.2022.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ogata AF, Cheng C‐A, Desjardins M, et al. Circulating SARS‐CoV‐2 vaccine antigen detected in the plasma of mRNA‐1273 vaccine recipients. Clin Infect Dis. 2021;74:715‐718. doi: 10.1093/cid/ciab465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mora JR, Iwata M, Eksteen B, et al. Generation of gut‐homing IgA‐secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157‐1160. doi: 10.1126/science.1132742 [DOI] [PubMed] [Google Scholar]
- 97. Bessa J, Jegerlehner A, Hinton HJ, et al. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J Immunol. 2009;183(6):3788‐3799. doi: 10.4049/jimmunol.0804004 [DOI] [PubMed] [Google Scholar]
- 98. Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710‐722. doi: 10.1016/j.immuni.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 99. Lapuente D, Fuchs J, Willar J, et al. Protective mucosal immunity against SARS‐CoV‐2 after heterologous systemic prime‐mucosal boost immunization. Nat Commun. 2021;12(1):6871. doi: 10.1038/s41467-021-27063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang Z, Lorenzi JCC, Muecksch F, et al. Enhanced SARS‐CoV‐2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577):14. doi: 10.1126/scitranslmed.abf1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hassan AO, Shrihari S, Gorman MJ, et al. An intranasal vaccine durably protects against SARS‐CoV‐2 variants in mice. Cell Rep. 2021;36:109452. doi: 10.1016/j.celrep.2021.109452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Langel SN, Johnson S, Martinez CI, et al. Oral and intranasal Ad5 SARS‐CoV‐2 vaccines decrease disease and viral transmission in a golden hamster model. medRxiv Prepr Serv Heal Sci. Published online October 5, 2021.
- 103. Yuan Y, Gao X, Ni F, et al. A novel intranasal administration adenoviral vector‐based platform for rapid COVID‐19 vaccine development. medRxiv Prepr Serv Heal Sci. Published online February 22, 2022.
- 104. Bricker TL, Darling TL, Hassan AO, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus‐vectored SARS‐CoV‐2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36(3):109400. doi: 10.1016/j.celrep.2021.109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim E, Weisel FJ, Balmert SC, et al. A single subcutaneous or intranasal immunization with adenovirus‐based SARS‐CoV‐2 vaccine induces robust humoral and cellular immune responses in mice. Eur J Immunol. 2021;51(7):1774‐1784. doi: 10.1002/eji.202149167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mao T, Israelow B, Suberi A, et al. Unadjuvanted intranasal spike vaccine booster elicits robust protective mucosal immunity against sarbecoviruses. medRxiv Prepr Serv Heal Sci. Published online January 26, 2022. [DOI] [PMC free article] [PubMed]
- 107. Suryawanshi RK, Chen IP, Ma T, et al. Limited cross‐variant immunity after infection with the SARS‐CoV‐2 omicron variant without vaccination. medRxiv Prepr Serv Heal Sci. Published online February 9, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


