CASE REPORT
We present a case of allergic contact dermatitis which coincided with the timing of the COVID‐19 vaccination, which was misdiagnosed as vaccine‐related hypersensitivity.
A 68‐year‐old woman with a history of acute recurrent vesicular hand eczema was referred for a suspected delayed‐type hypersensitivity reaction after Fosun Pharma/BioNTech Comirnaty BNT162b2 (BNT) vaccination.
She had received her first dose of BNT uneventfully and received her second dose at the recommended interval of 21 days. Concomitantly, around the time of her second dose, she experienced worsening of her hand eczema. She was self‐treated with chlorhexidine, budesonide cream and adhesive bandages (Elastoplast; Beiersdorf) daily with initial improvement.
A few days following her second dose of BNT, she developed well‐demarcated erythema over her fingers, which progressed into vesicles and eventually blistering lesions. The lesions were localized to her fingers only without mucosal involvement. In fear of vaccine‐associated reaction, she consulted her family physician who eventually diagnosed her with ‘COVID fingers’, or pernio triggered by COVID‐19 vaccination. In view of this vaccine‐associated ‘hypersensitivity’, she was advised to avoid receiving COVID‐19 vaccines in the future and referred for an Allergist opinion.
Upon review of her history and serial photos of her hands, sharply demarcated lesions and clinical course seemed more suggestive of allergic contact dermatitis instead (Figure 1). Routine bloods with autoimmune markers were unremarkable. The patient declined a skin biopsy. Patch testing was performed using IQ Ultra chambers with the International Standard Series (Chemotechnique Diagnostics), chlorhexidine digluconate (0.5% aq.) and the BNT vaccine (as is). Patch tests were applied for 48 h, and read on Day 2 (D2) and Day 4 (D4) in accordance with the International Contact Dermatitis Research Group criteria. There were strong positive reactions (erythema, papules, vesicles, infiltration) recorded at D4 (crescendo effect since D2) to colophonium (++), budesonide (++) and tixocortol‐pivalate (+). PT readings to other agents of the International Standard Series, chlorhexidine digluconate and BNT were negative.
FIGURE 1.
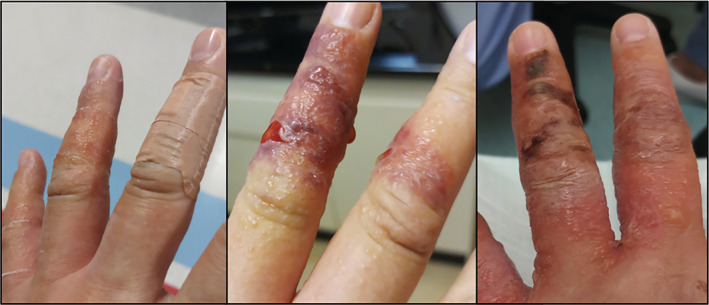
Clinical photos of the patients' hands following application of chlorhexidine, budesonide cream and adhesive bandages around the time of COVID‐19 vaccination
She was diagnosed with allergic contact dermatitis to budesonide (i.e., group B corticosteroid), with possible cross‐reactivity with group A corticosteroids, and colophonium. The patient was counselled to avoid these agents in the future and to proceed with her booster BNT vaccine. She tolerated her third dose of BNT without any subsequent reactions, excluding the diagnosis of delayed‐type hypersensitivity to BNT.
DISCUSSION
‘COVID toes and fingers’ have been described as a phenomenon seen in patients with COVID‐19 infection but have also been reported after COVID‐19 mRNA vaccinations. 1 , 2 However, genuine delayed‐type hypersensitivity reactions to COVID‐19 vaccines remain extremely rare. As illustrated, a case of allergic contact dermatitis was misdiagnosed as vaccine‐related hypersensitivity.
Red herrings for overdiagnosis in this case include coincidental timing of symptom presentation, suggestive appearance of cutaneous lesions and patients' strong opinions of their conditions, often self‐attributed to vaccination. Especially during the earlier phases of COVID‐19 vaccination rollout, suspected vaccine‐associated reactions have often been over‐diagnosed and ‐reported among academia and social media. This has generated significant anxiety and vaccine hesitancy among both patients and healthcare professionals. Misdiagnoses can lead to unnecessary vaccine avoidance and delays in achieving herd immunity. This case illustrates the importance for cautious review and to exclude more common dermatological conditions before labelling patients with COVID‐19 vaccine allergies.
INFORMED CONSENT
Informed consent was obtained from the patient to publish the case report.
AUTHOR CONTRIBUTIONS
Valerie Chiang: Writing – review and editing; writing – original draft; visualization; formal analysis; data curation; conceptualization. Jane C. Y. Wong: Conceptualization; methodology; investigation. Tik Suet Chan: Investigation; data curation. Chak Sing Lau: Supervision; project administration; writing – review and editing. Philip H. Li: Supervision; investigation; conceptualization; formal analysis; writing – review and editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Chiang V, Wong JCY, Chan TS, Lau CS, Li PH. Under‐awareness and over‐diagnosis of COVID‐19 vaccine ‘allergy’. Contact Dermatitis. 2022;1‐2. doi: 10.1111/cod.14193
REFERENCES
- 1. Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID‐19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36(2):172‐180. doi: 10.1111/jdv.17744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID‐19 pandemic. JAMA Dermatol. 2020;156(9):998‐1003. doi: 10.1001/jamadermatol.2020.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]


