Abstract
The genome of Chlamydia trachomatis, one of the most prominent human pathogens, contains two structural genes coding for proteins, herein called Npt1Ct and Npt2Ct (nucleoside phosphate transporters 1 and 2 of C. trachomatis), exhibiting 68 and 61% similarity, respectively, to the ATP/ADP transporter from the intracellular bacterium Rickettsia prowazekii at the deduced amino acid level. Hydropathy analysis and sequence alignments suggested that both proteins have 12 transmembrane domains. The putative transporters were expressed as histidine-tagged proteins in Escherichia coli to study their biochemical properties. His10-Npt1Ct catalyzed ATP and ADP transport in an exchange mode. The apparent Km values were 48 (ATP) and 39 (ADP) μM. ATP and ADP transport was specific since AMP, GTP, CTP, UTP, dATP, dCTP, dGTP, and dTTP did not inhibit uptake. In contrast, His10-Npt2Ct transported all four ribonucleoside triphosphates with apparent Km values of 31 μM (GTP), 302 μM (UTP), 528 μM (CTP), and 1,158 μM (ATP). Ribonucleoside di- and monophosphates and deoxyribonucleotides were not substrates. The protonophore m-chlorocarbonylcyanide phenylhydrazone abolished uptake of all nucleoside triphosphates by Npt2Ct. This observation indicated that His10-Npt2Ct acts as a nucleosidetriphosphate/H+ symporter energized by the proton motive force across the Escherichia coli cytoplasmic membrane. We conclude that Npt1Ct provides chlamydiae with energy whereas Npt2Ct catalyzes the net uptake of ribonucleoside triphosphates required for anabolic reactions.
The bacterial genus Chlamydia comprises four species which live as intracellular parasites in specialized vacuoles within eukaryotic cells (reviewed in reference 22). Chlamydia trachomatis is a human pathogen that infect mucous membranes in the urogenital epithelium and is the leading cause of sexually transmitted diseases. The chlamydial genome (1,043 kbp) is among the smallest of all known prokaryotic genomes (21). Such reduction of the genome size of obligate intracellular bacteria is possible because many intermediates required for their metabolism need not be synthesized by the complex pathways characteristic of free-living bacteria but are transported from the intermediate-rich host cell cytoplasm by unusual transport systems that often have no counterparts in free-living bacteria (16, 32).
Both chlamydiae (8) and Rickettsia prowazekii (30), another obligate intracellular bacterial parasite, are able to exchange their intracellular ADP for the host cell’s ATP. This is a system in which the net result is the transport of energy for the benefit of the parasite. The ATP/ADP transporter in R. prowazekii (R. prowazekii translocase [RpTLC]) comprises 497 amino acids and belongs to the family of solute transporters exhibiting 12 transmembrane domains (3, 29). Recently, two functional homologues to the rickettsial ATP/ADP transporter have been identified at the molecular level in higher-plant plastids from Arabidopsis thaliana (10, 15). The amino acid sequences of the plastidic ATP/ADP transporters exhibit 62 to 66% similarity to the evolutionarily widely distant rickettsial ATP/ADP transporter. The plastidic transporters, like the rickettsial homologue, catalyze ATP import in exchange to endogenous ADP (18).
It has been demonstrated that C. psittaci exhibits an ATP/ADP exchange similar to that in R. prowazekii (8). As both bacterial species are obligate intracellular parasitic bacteria but live in very different environmental niches (rickettsiae in the eukaryotic cytosol, chlamydiae in a modified phagosome), it is of interest to identify the transporter protein mediating ATP and ADP movement across the chlamydial cell membrane. From information available from the C. trachomatis genome program, it became evident that two putative membrane proteins with substantial homology to the rickettsial ATP/ADP transporter are encoded in the genome (24).
The demonstration that both rickettsial and plastidic ATP/ADP transporters can functionally be expressed in Escherichia coli (3, 12, 15, 27) suggested that this heterologous expression system could also be used to examine the putative chlamydial transporters. In this approach, we amplified the two structural chlamydial genes via PCR, cloned and expressed the products in E. coli, and analyzed the nucleotide transport properties of the chlamydial proteins across the E. coli cytoplasmic membrane. In this study we characterized the biochemical properties of both transporters, with special attention to whether both structural genes, C. trachomatis npt1 (nucleoside phosphate transporter 1) (npt1Ct) and C. trachomatis npt2 (npt2Ct), encode ATP/ADP transport proteins. Answers to these questions are crucial for understanding the physiology of one of the major human pathogens.
MATERIALS AND METHODS
Cloning of Npt1Ct and Npt2Ct.
Genomic C. trachomatis DNA (from serovar L2) was kindly provided by Grant McClarty (University of Manitoba, Winnipeg, Manitoba, Canada). DNA manipulations were performed essentially as described previously (20). The structural genes encoding Npt1Ct and Npt2Ct were amplified from the genomic DNA of C. trachomatis by PCR using Pfu DNA polymerase (Stratagene, Heidelberg, Germany), which has proofreading activity. The sense primers used, JT100 (5′-catatgactcaaaccgcggaaaaacc-3′) and JT200 (5′-tagattaggaaggagcatatgtcttccgagg-3′), were constructed on the basis of data from the C. trachomatis genome program with NdeI restriction sites at the start codons of npt1Ct and npt2Ct, respectively. The antisense primers, JT101 (5′-ttaagaaacaccttctatagcaggagcgg-3′) and JT201 (5′-ctataaagttgttacaggttcttctcgagac-3′), contained the stop codons of npt1Ct and npt2Ct, respectively. The PCR products obtained for npt1Ct and npt2Ct were gel purified and cloned into the EcoRV site of plasmid pBSK (Stratagene); the resulting plasmids were named pJT144 and pJT157, respectively. Both DNAs were sequenced on both strands by chain termination reaction (MWG-Biotech, Ebersberg, Germany). To construct E. coli plasmids expressing an N-terminal histidine tag (pJT167 [encoding His10-Npt1Ct] and pJT168 [encoding His10-Npt2Ct]), the NdeI/BamHI DNA inserts of plasmids pJT144 and pJT157 were introduced in frame into the NdeI/BamHI sites of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible T7 RNA polymerase bacterial expression vector pET16b (Novagen, Heidelberg, Germany). Transformation of E. coli was carried out according to standard protocols.
Heterologous expression of ntp1Ct and ntp2Ct in E. coli.
E. coli C43 cells transformed with plasmid pJT167 or pJT168 were grown in YT-ampicillin medium to an optical density (OD; A600) of 0.6. Induction of T7 RNA polymerase was initiated by addition of IPTG (final concentration, 1 mM). Cells were grown to an OD of 1.3 and collected by centrifugation for 8 min at 5,000 × g (4°C). The sediments were resuspended to an OD of 1.0 in potassium phosphate (50 mM, pH 7.5) (12) and stored on ice until use within 1 h.
Uptake of radioactively labeled nucleotides.
The washed E. coli cells described above (100 μl) were added to 100 μl of potassium phosphate (50 mM, pH 7.5) containing α-32P-labeled nucleotides (80 to 200 μCi/mmol). α-32P-labeled nucleoside triphosphates (NTPs) were purchased from NEN (Bad Homburg, Germany); synthesis of [α-32P]ADP from [α-32P]ATP and analysis of purity were carried out as described previously (27).
Uptake of nucleotides was carried out at 30°C and stopped at the indicated time by transfer of the cells onto a 0.45-μm-pore-size filter (25-mm diameter; Schleicher and Schüll, Hannover, Germany) previously exposed to potassium phosphate (50 mM, pH 7.5) and set under vacuum (31). The bacterial cells were washed twice with 1 ml of ice-cold potassium phosphate (50 mM, pH 7.5) to remove extracellular radioactivity. The filters were transferred into a 20-ml scintillation vial containing 5 ml of water, and radioactivity was quantified as Cerenkov radiation in a Canberra-Packard Tricarb-2500 counter (Canberra-Packard, Frankfurt, Germany). All data represent means of at least three independent experiments; the standard deviation was always less than 8% of the mean.
For back-exchange (efflux) experiments, the washed E. coli cells were preincubated at 30°C in potassium phosphate (50 mM, pH 7.5) containing 25 μM [α-32P]NTP (specific activity, 1 to 2 mCi/mmol). Uptake was stopped by centrifugation, and the sediment was resuspended in 1 ml of potassium phosphate (50 mM, pH 7.5) containing the indicated additions. The radioactivity in the cells was determined as described above.
Thin-layer chromatography of radioactively labeled guanosine nucleotides.
Polyethyleneamine-cellulose thin-layer chromatography was used to identify the chemical nature of the radioactivity accumulated by E. coli when incubated with GTP (13). Separation was carried out for 0.5 min with 0.5 M sodium formate (pH 3.4) and 2 min with 2 M sodium formate (pH 3.4), and the front was allowed to run for 15 cm with 4 M sodium formate (pH 3.4). Rf values of radioactively labeled nucleotides were determined after autoradiography and corresponded to Rf values of authentic standards visualized under UV light (13).
Nucleotide sequence accession numbers.
The amino acid sequences of Npt1Ct, Npt2Ct, and RpTLC are available under EMBL database accession no. AJ010586, AJ010587, and M28816, respectively.
RESULTS
Sequence and hydropathy analysis of Npt1Ct and Npt2Ct.
The genome of C. trachomatis contains two genes, named npt1Ct and npt2Ct, that show significant similarity at the deduced amino acid level to RpTLC. After amplification of npt1Ct and npt2Ct, we cloned the entire PCR products into the pBSK vector and sequenced the inserts. The deduced amino acid sequence of npt1Ct differed from the amino acid sequence determined in the C. trachomatis genome project at position 420, where we found a Leu instead of a Met (Fig. 1). Npt1Ct comprised 528 amino acids and exhibited 68% similarity (Genetics Computer Group PileUp program [2]) to RpTLC (Fig. 1).
FIG. 1.
Alignment of predicted amino acid sequences of Npt1Ct, Npt2Ct, and RpTLC. Numbers indicate amino acid positions; dots mark artificial sequence gaps introduced to improve similarity between the proteins.
The deduced amino acid sequence of Npt2Ct comprised 540 residues, showed 61.4% similarity to the RpTLC (Fig. 1), and differed from the genome project sequence at 10 amino acid positions. These changes were as follows: 161 Val (this study) for Ile, 208 Ser for Asn, 223 Met for Val, 256 Glu for Lys, 345 Ala for Thr, 361 Val for Ile, 370 Ile for Met, 506 Thr for Ala, 508 Val for Phe, and 512 Ala for Val (Fig. 1). Three independent PCR products of npt1Ct and npt2Ct have been sequenced and found to exhibit 100% identity, indicating that the differences in the amino acid sequences found were not due to misfunction of the Pfu polymerase.
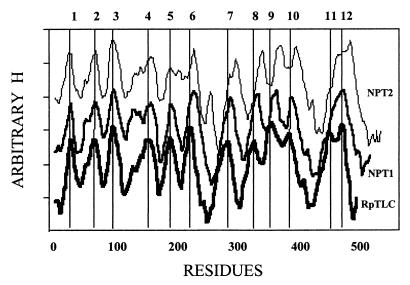
To gain insight into the molecular structures of Npt1Ct and Npt2Ct, we performed a hydropathy analysis and compared the putative structures of the chlamydial proteins with that of RpTLC. This analysis was carried out by using the algorithm developed by von Heijne (28), which attributes specifically weight to the amino acids deep in the membrane compared to those at the phospholipid-water interface. Npt1Ct and Npt2Ct are both highly hydrophobic proteins, and their hydrophobicity profile is consistent with their having 12 transmembrane domains, a topology that has been predicted for both the rickettsial and plastidic ATP/ADP transporters. The MEMSAT program (9) predicted RpTLC and Npt2Ct to be 12-transmembrane-domain proteins predicted that Npt2Ct had only 11 transmembrane domains because it failed to separate domains 11 and 12. The hydropathy profiles of all three proteins exhibited striking similarity with respect to the alteration and length of hydrophilic and hydrophobic domains. However, there were notable differences in that there were extra amino acid residues in the hydrophilic loop between TM6 and TM7 in Npt2Ct and a significantly longer C-terminal region in both chlamydial transporters (Fig. 1 and 2).
FIG. 2.
Hydropathy analysis of the predicted amino acid sequences of Npt1Ct, Npt2Ct, and RpTLC. Hydropathy analysis was carried out as described by von Heijne (28). The values of H, which are essentially identical, have been offset to separate the plots for clarity. The predicted transmembrane domains in RpTLC are shown at the top.
Analysis of His10-Npt1Ct.
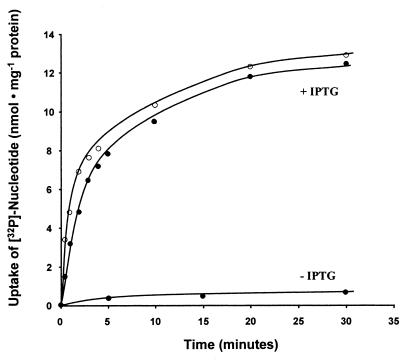
We examined the transport properties of Npt1Ct as an N-terminal His-tagged transporter fusion protein because an N-terminal histidine extension led to higher activities of the plastidic ATP/ADP transporters when expressed heterologously in E. coli (15, 27) and a histidine tag should allow purification of the fusion proteins in the future. The transport of ATP and ADP catalyzed by His10-Npt1Ct was linear for the first 2 min; after about 10 min of incubation, no substantial increase of radioactivity in the bacterial cells occurred (Fig. 3). In control experiments, uninduced E. coli cells did not import substantial radioactivity (Fig. 3; see also reference 27), and the activity of the unrelated glucose 6-phosphate and glucose transport systems did not increase after expression of His10-Npt1Ct (data not shown; see also reference 27). Increasing concentrations of exogenous [α-32P]ATP or [α-32P]ADP stimulated uptake to an apparent saturation at an external concentration of about 300 μM. A Lineweaver-Burk analysis of the data allowed us to estimate apparent Km values of 48 μM for ATP and 39 μM for ADP and Vmax values of 370 nmol of ATP · mg of protein−1 · h−1 and 625 nmol of ADP · mg of protein−1 · h−1.
FIG. 3.
Time dependency of [α-32P]ATP (●) and [α-32P]ADP (○) uptake into intact E. coli cells expressing npt1Ct. IPTG-induced E. coli cells harboring plasmid pJT167 were incubated with 50 μM [α-32P]ATP or [α-32P]ADP for the indicated time periods. Control cells were not induced (−IPTG). Data are means of three independent experiments; standard deviations were less than 8% of the mean values.
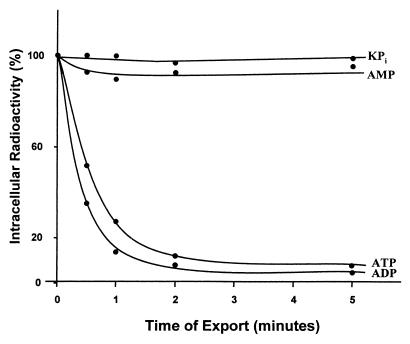
The rickettsial ATP/ADP transporter operates in an exchange mode that permits the influx or efflux of ADP or ATP only when there is a concomitant flux in the opposite direction of ADP or ATP (30). To determine if such mode of transport holds true for Npt1Ct transporter, we preloaded E. coli cells expressing npt1Ct with [α-32P]ATP, removed all extracellular nucleotide, and measured the dependence of the efflux of [α-32P]ATP on the presence of added unlabeled substrate in the medium. The addition of either unlabeled ATP or unlabeled ADP to the medium to enable exchange induced a rapid efflux of 95% of the intracellular radioactivity within 5 min (Fig. 4). In contrast, medium either with no additions or complemented with AMP did not support efflux, and the preloaded pool of radioactive nucleotide was retained (Fig. 4). The exchange nature of this transport system is also suggested by the time course of uptake (Fig. 3). Although the influx of ADP is almost twice as fast as the ATP influx, as predicted by the kinetic values and indicated by these data, the transport of both ATP and ADP reached the same steady-state level because it is determined by the identical intracellular pool of ADP plus ATP available for exchange.
FIG. 4.
Exchange-mediated efflux of intracellular radioactivity. IPTG-induced E. coli cells expressing npt1Ct were preloaded by incubation with [α-32P]ATP at 25 μM. After removal of external radioactivity, cells were resuspended in phosphate buffer with or without 1 mM AMP, ADP, or ATP.
To determine whether the heterologously expressed His10-npt1Ct, like RpTLC (12, 30), is specific for ATP and ADP transport, we measured the effects of related compounds on ATP uptake. In contrast to the marked inhibition by ADP, ATP transport is only slightly (about 20%) inhibited by GTP, and UTP, CTP, AMP, dATP, dGTP, dCTP, and dTTP were without effect (Tables 1 and 2).
TABLE 1.
Effects of various NTPs or CCCP on transport activity of Npt1Ct and Npt2Cta
| Effector | Rate of a NTP transport (nmol · mg of protein−1 · h−1)b
|
||||
|---|---|---|---|---|---|
| Npt1Ct, ATP | Npt2Ct
|
||||
| ATP | GTP | UTP | CTP | ||
| None | 130.0 | 53.4 | 75.7 | 86.3 | 91.1 |
| ATP | 77.9 | 49.4 | 61.7 | ||
| GTP | 105.0 | 3.5 | 3.4 | 7.3 | |
| UTP | 121.6 | 10.9 | 68.9 | 34.9 | |
| CTP | 130.4 | 16.5 | 70.6 | 39.9 | |
| CCCP | 91.2 | 7.1 | 10.6 | 19.8 | 20.9 |
Substrate concentrations for labeled nucleotides: for Npt1Ct, 50 μM (ATP); for Npt2Ct, 1,000, 50, 400, and 400 μM (ATP, GTP, UTP, and CTP, respectively). The unlabeled NTPs were used at a 2.5-fold-higher concentration; CCCP was present at 1 mM.
Mean of three independent experiments.
TABLE 2.
Effects of various nucleotides on transport activities of His10-Npt1Ct and His10-Npt2Ct
| Effector | Rate of nucleotide transport (%)a
|
|
|---|---|---|
| ATP import His10-Npt1Ct | GTP import His10-Npt2Ct | |
| None | 100.0 | 100.0 |
| dATP | 96.5 | 100.7 |
| dGTP | 100.6 | 92.6 |
| dCTP | 106.4 | 97.0 |
| dTTP | 108.7 | 94.7 |
| AMP | 100.5 | NM |
| GMP | NM | 90.5 |
| ADP | 12.0 | NM |
| GDP | NM | 89.1 |
| Guanosine tetraphosphate | NM | 97.1 |
ATP uptake was measured at a substrate concentration of 100 μM; GTP uptake was measured at a substrate concentration of 50 μM. The effectors were always present in a 2.5-fold-higher concentration than the substrates. ATP uptake by His10-Npt1Ct in the absence of effectors was 251.9 nmol · mg of protein−1 · h−1; GTP uptake by His10-Npt2Ct in the absence of effectors was 75.7 nmol · mg of protein−1 · h−1. NM, not measured. Data are means of three independent experiments.
Analysis of nucleotide transport catalyzed by His10-Npt2Ct.
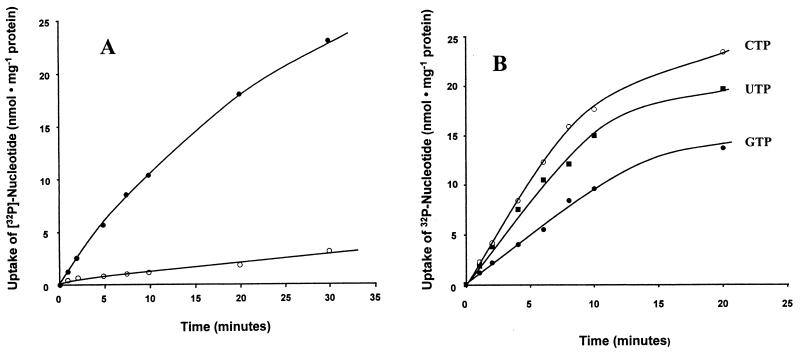
Although E. coli cells expressing npt2Ct were able to transport both [α-32P]ATP and [α-32P]ADP (Fig. 5A)—in contrast to nucleotide transport mediated by His10-Npt1Ct (Fig. 3)—transport failed to reach a rapid steady state and ADP uptake was substantially slower than ATP uptake. In addition, the substrate specificity of His10-Npt2Ct was much broader than that of His10-Npt1Ct. When GTP, UTP, and CTP were added as putative inhibitors at a concentration 2.5-fold higher than that of the substrate, [α-32P]ATP influx catalyzed by His10-Npt2Ct was reduced by 93, 80, and 69%, respectively (Table 1).
FIG. 5.
Time dependency of [α-32P]ATP, [α-32P]ADP, [α-32P]GTP, [α-32P]UTP, and [α-32P]CTP uptake into intact E. coli cells expressing npt2Ct. (A) Time dependency of [α-32P]ATP (0.8 mM; ●) or [α-32P]ADP (1 mM; ○) uptake. (B) Time dependency of [α-32P]GTP (0.05 mM), [α-32P]UTP (0.5 mM), and [α-32P]CTP (0.5 mM) uptake.
Since the marked inhibition of ATP uptake by nucleotides other than ADP suggested that His10-Npt2Ct transported other nucleotides, we measured the transport of α-32P-labeled GTP, UTP, and CTP. The nucleotides were transported by Npt2Ct with a time course similar to that seen with ATP (Fig. 5B).
The kinetic analysis revealed that GTP had the highest affinity for His10-Npt2Ct, (apparent Km of 31 μM), ADP had the lowest affinity (apparent Km of about 1.8 mM), and values for UTP and CTP were intermediate (Table 3). ATP, which had a Km of 48 μM for Npt1Ct, had a Km of only 1,158 μM for this transport system. The Vmax values ranged from 164 nmol · mg of protein−1 · h−1 for CTP to 109 nmol · mg of protein−1 · h−1 for GTP (Table 3).
TABLE 3.
Km and Vmax values of Npt2Ct for various nucleotides
| Nucleotide | Kinetic constant
|
|
|---|---|---|
| Km (μM) | Vmax (nmol · mg of protein−1 · h−1) | |
| GTP | 30.9 | 108.7 |
| UTP | 301.9 | 133.3 |
| CTP | 527.6 | 163.9 |
| ATP | 1,158.4 | 128.2 |
| ADP | 1,758.5 | 18.3 |
The inhibition by rNTPs at concentrations 2.5-fold higher than the substrate concentration affected the uptake of [α-32P]GTP, [α-32P]UTP, and [α-32P]CTP, as predicted by the kinetics of competitive inhibition if we assume that the Ki of a nucleotide was equal to the Km of the same nucleotide. ATP, UTP, and CTP at a 2.5-fold excess over [α-32P]GTP did not affect [α-32P]GTP uptake substantially. [α-32P]UTP uptake was inhibited strongly by GTP (4% residual activity) and to lesser extents by CTP (46% residual activity) and ATP (58% residual activity). [α-32P]CTP import was inhibited strongly by GTP (8% residual activity) and to lesser extents by UTP (38% residual activity) and ATP (68% residual activity) (Table 1). As demonstrated for His10-Npt1Ct, His10-Npt2Ct was not substantially affected by dNTPs and GTP uptake catalyzed by His10-Npt2Ct was specific for the triphosphate, since GMP, GDP, and guanosine tetraphosphate did not inhibit transport (Table 2).
As described above, His10-Npt1Ct catalyzed ATP/ADP exchange (Fig. 4). In contrast, His10-Npt2Ct is not able to exchange internal nucleotides with exogenously supplied nucleotides. We demonstrated that npt2Ct-expressing E. coli cells preloaded with either [α-32P]ATP or [α-32P]GTP do not release radioactivity into the medium after addition of unlabeled ATP, GTP, UTP, or CTP (data not shown).
Effect of CCCP on NTP uptake mediated by His10-Npt2Ct.
Several transport systems in bacteria are energized by the symport of H+ driven by the proton motive force. The uptake of all four α-32P-labeled rNTPs by E. coli cells expressing npt2Ct was markedly inhibited by the protonophore m-chlorocarbonyl cyanide phenyl/hydrazone (CCCP) (Table 1). In contrast, the exchange transport catalyzed by His10-Npt1Ct was only slightly inhibited. The uptake of [α-32P]GTP, the substrate for which His10-Npt2Ct exhibited the highest affinity, was 86% inhibited by CCCP (Table 1). Based on cell water determinations in E. coli (33), the intracellular concentration of GTP achieved at steady state in the experiment shown in Fig. 5B is approximately 1.8 mM. This represents a 36-fold (1.8/0.05) concentration gradient, of which 86% can be inhibited by CCCP. The lack of complete inhibition (in which the intracellular concentration equals the extracellular concentration) is most likely due to bound GTP and distribution of radioactivity into compounds other than NTPs. This interpretation was supported by analysis of the intracellular pool after uptake of [α-32P]GTP by thin-layer chromatography and autoradiography, which was demonstrated that about 80% of the extractable radioactivity was transportable [α-32P]GTP and 20% was nontransportable [α-32P]GDP (data not shown).
DISCUSSION
During the course of the C. trachomatis genome program, two putative ATP/ADP transporter proteins (herein called Npt1Ct and Npt2Ct) have been sequenced. In this study, we demonstrated that Npt1Ct and Npt2Ct catalyze nucleotide transport, but of very different sorts. There are obvious similarities in the amino acid sequences and the hydropathy profiles of Npt1Ct, Npt2Ct, and RpTLC. Npt1Ct and Npt2Ct exhibit 68 and 61% similarity to RpTLC (Fig. 1), comparable to the similarities between the two plastidic ATP/ADP transporters from A. thaliana and the rickettsial homologue (10, 15). npt1Ct and npt2Ct generated by PCR from genomic DNA exhibited few, but highly reproducible, differences in the deduced amino acid sequences compared to the sequences published by the C. trachomatis genome program. The differences are most likely due to the difference in serovars of C. trachomatis used for the genome analysis (serovar D) and for the experiments described here (serovar L2).
Hydropathy analysis revealed that both chlamydial proteins belong to the family of membrane-bound carriers exhibiting 12 transmembrane domains (Fig. 2), although they have no similarity in amino acid sequence to other members of this family (14). This configuration seem to represent a general feature of all nonmitochondrial nucleotide transporters analysed so far, although in a few cases 11 transmembrane domain models cannot be eliminated. The topological arrangement of the nonmitochondrial nucleotide transporters resembles those of most secondary carriers, which are constructed of 12 transmembrane domains irrespective of the substrates transported or the mode of transport (uniport, symport, or antiport) catalyzed (11). The main topological differences between the chlamydial and rickettsial transporter are significant C-terminal extensions in the chlamydial transporters, the extra amino acids in Npt2Ct, and the elimination in Npt1Ct of transmembrane domain 12 by the MEMSAT program (Fig. 1 and 2). Although small truncations at the C-terminal end of RpTLC resulted in drastically reduced transports rates (34) and a 10-his extension on the rickettsial C terminus did not interfere with activity (1), it remains to be established whether the C-terminal extensions of the chlamydial transporters influence their transport properties.
His10-Npt1Ct catalyzed ATP and ADP transport when expressed heterologously in E. coli (Fig. 3). The apparent affinities for the two compounds, Kms of 48 and 39 μM, respectively, are similar to the adenylate affinities of RpTLC (Km for ATP, 75 μM [29] or 100 μM [3]; Km for ADP, 50 μM [18a]). Hatch et al. (8) estimated in C. psittaci cells Km values for ATP and ADP of approximately 5 μM. The reason for this large difference is not known.
The observations that [α-32P]ATP uptake was strongly inhibited by ADP and that external ADP or ATP promoted efflux of preloaded [α-32P]ATP (Fig. 4) support the conclusions that the two compounds are transported by the same transport protein (namely, Npt1Ct) and that both metabolites move across the cytoplasm membrane in an exchange mode of transport. These conclusions are consistent with the biochemical properties of the rickettsial ATP/ADP transporter and the plastidic homologues in A. thaliana (12, 15, 18, 27, 30), as well as with findings for C. psittaci cells (8).
The physiological functions of the plastidic and rickettsial ATP/ADP exchange systems are obvious. For example, heterotrophic plastids are unable to synthesize ATP at sufficient rates and must import ATP for anabolic reactions to proceed (4). Both C. trachomatis and R. prowazekii are obligate intracellular bacterial parasites that exploit the host cells for both nutrients and energy. While rickettsiae exhibit both oxidative phosphorylation and the ability to transport ATP, the apparent absence of both oxidative activity and an electron chain in chlamydiae supported the assumption that these bacteria are energy parasites (16) and thus require an ATP/ADP exchange to satisfy their energy needs. Information obtained in the genome project suggests that, in fact, chlamydiae may have the ability to regenerate their own ATP. Thus, most likely the exchange of host cell ATP for bacterial ADP is an alternative mechanism to acquire energy in both organisms. In C. trachomatis, this transport is obviously mediated by Npt1Ct. After infection of eukaryotic cells with chlamydiae, the rate of glucose consumption is strongly increased and a concomitant increase of the ATP concentration occurs (19). Assuming that the inclusion membrane that surrounds the parasites is permeable to ATP, perhaps this facilitates the provision of the bacterial parasite with ATP. It is worth mentioning that little is known about transport across the inclusion membrane (6, 17).
Although Npt2Ct exhibits remarkable structural and topological similarities to both Npt1Ct and RpTLC (Fig. 1 and 2), Npt2Ct possesses totally different biochemical properties. Npt2Ct exhibited no substantial affinity for ADP, moderate affinity for ATP, UTP, and CTP, and very high affinity for GTP (Table 3). Npt2Ct does not mediate an exchange of NTPs (as catalyzed by Npt1Ct); rather, nucleotides accumulate in an energy-coupled manner inside the cell. For GTP, an approximately 30-fold gradient was mediated by this transporter. The inhibitory effect of CCCP on Npt2Ct-mediated uptake of NTPs (Table 1) indicated that NTPs enter the bacterial cells in an H+-cotransport mode. Such a mode of transport depends ultimately on the presence of a proton motive force across the parasitic cytoplasmic membrane. Indeed, it has been shown that such a force exists in obligate intracellular bacteria (35) and supports the accumulation of lysine (8, 23). The stoichiometry between proton and NTP cotransport is unknown but must be large, since GTP is assumed to be tetravalent in the cytoplasm. If the stoichiometry is 4, then the transported complex is neutral and the driving force is ΔpH and is independent of Δψ. Interestingly, with the high stoichiometry of 4, a ΔpH of 0.37 is adequate to support a 30-fold gradient of tetravalent GTP. If the stoichiometry is 3, then the transported complex is negative and a ΔpH of 1.2 would be necessary to obtain this gradient of GTP even assuming a modest Δψ of −120 mV (the situation would become worse as Δψ increased). If the stoichiometry is 5, then the complex is positively charged and no ΔpH would be needed, and a Δψ of 89 mV would suffice as a driving force.
But what is the physiological reason for a second type of nucleotide transport system mediating net influx of molecules? GTP, ATP, UTP, and CTP are required for RNA synthesis, the formation of lipids and carbohydrate-activated intermediates, protein synthesis, and signalling. However, C. trachomatis is auxotrophic for all NTPs except CTP, which can be derived from UTP (26). Therefore, RNA synthesis, and other processes, in C. trachomatis depends strictly on the exogenous provision of the bacterium with NTPs, in agreement with data for C. psittaci cells (7, 25).
It is remarkable that transport proteins with a high degree of structural similarity exhibit a totally different mode of transport. Npt1Ct catalyzes an exchange mode of transport with high substrate specificity for ATP and ADP, whereas Npt2Ct exhibits high substrate specificity toward GTP and other NTPs and is energized by the proton motive force. Keeping these differences in mind, how great do the molecular differences between two proteins have to be to allow significant changes in substrate specificity or mode of catalysis? There are examples indicating that comparable small changes in the molecular architecture suffice to alter such fundamental biochemical properties. For example, the exchange of only two amino acids in the strict NAD-specific leucine dehydrogenase from Thermoactinomyces intermedius leads to an enzyme which strongly prefers NADP as a coenzyme (5). Moreover, the chemical modification of four cysteine residues in the mitochondrial ADP/ATP carrier by application of thiol reagents alters the mode of transport from obligate exchange to uniport without loss of the maximal catalytic activity (2a). It will be interesting to study the structure-function relationships in more detail by generating site-directed mutants and chimeric proteins of Npt1Ct and Npt2Ct.
ACKNOWLEDGMENTS
H.E.N. thanks Renate Scheibe (University of Osnabrück) for support of this work.
This work was financially supported by the Deutsche Forschungsgemeinschaft (SFB 171-C16). C.S. is the recipient of a graduate student fellowship from the Federal State Niedersachsen. Work in the lab of H.H.W. was financially supported by Public Health Service grant AI-15035 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Alexeyen, M., and H. H. Winkler. Unpublished data.
- 2.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Dierks T, Salentin A, Heberger C, Krämer R. The mitochondrial asparate/glutamate and ADP-ATP carrier switch from obligate counterexchange to unidirectional transport after modification by SH-reagents. Biochim Biophys Acta. 1990;1028:268–280. doi: 10.1016/0005-2736(90)90176-o. [DOI] [PubMed] [Google Scholar]
- 3.Dunbar S A, Winkler H H. Increased and controlled expression of the Rickettsia prowazekii ATP/ADP translocase and analysis of cysteine-less mutant translocase. Microbiology. 1997;143:3661–3669. doi: 10.1099/00221287-143-11-3661. [DOI] [PubMed] [Google Scholar]
- 4.Emes M J, Neuhaus H E. Metabolism and transport in non-photo-synthetic plastids. J Exp Bot. 1998;48:1995–2005. [Google Scholar]
- 5.Galkin A, Kulakova L, Ohshima T, Esaki N, Soda K. Construction of a new leucine dehydrogenase with preferred specificity for NADP+ by site-directed mutagenesis of the strictly NAD+ specific enzyme. Protein Eng. 1997;10:687–690. doi: 10.1093/protein/10.6.687. [DOI] [PubMed] [Google Scholar]
- 6.Hackstadt T, Fischer E R, Scidmore M A, Rockey D D, Heinzen R A. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 7.Hatch T P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975;122:393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatch T P, Al-Hossainy E, Silverman J A. Adenine nucleotide and lysine transport in Chlamydia psittaci. J Bacteriol. 1982;150:662–670. doi: 10.1128/jb.150.2.662-670.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones D T, Taylor W R, Thornton J M. A model recognition approach to the prediction of all-helical membrane proteins structure and topology. Biochemistry. 1994;33:3038–3049. doi: 10.1021/bi00176a037. [DOI] [PubMed] [Google Scholar]
- 10.Kampfenkel K, Möhlmann T, Batz O, van Montagu M, Inzé D, Neuhaus H E. Molecular characterization of an Arabidopsis thaliana cDNA encoding a novel putative adenylate translocator of higher plants. FEBS Lett. 1995;374:351–355. doi: 10.1016/0014-5793(95)01143-3. [DOI] [PubMed] [Google Scholar]
- 11.Krämer R. Functional principles of solute transport systems: concepts and perspectives. Biochim Biophys Acta. 1994;1185:1–34. doi: 10.1016/0005-2728(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 12.Krause D C, Winkler H H, Wood D O. Cloning and expression of the Rickettsia prowazekii ADP/ATP translocator in Escherichia coli. Proc Natl Acad Sci USA. 1985;82:3015–3019. doi: 10.1073/pnas.82.9.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangold H K. Nucleinsäuren und Nucleotide. In: Stahl E, editor. Dünnschicht-Chromatographie. Ein Laboratoriumshandbuch. Heidelberg, Germany: Springer-Verlag; 1967. pp. 749–769. [Google Scholar]
- 14.Marger M D, Saier M H. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biol Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 15.Möhlmann T, Tjaden J, Schwöppe C, Winkler H H, Kampfenkel K H, Neuhaus H E. Occurrence of two plastidic ATP/ADP transporters in Arabidopsis thaliana: molecular characterisation and comparative structural analysis of homologous ATP/ADP translocators from plastids and Rickettsia prowazekii. Eur J Biochem. 1998;252:353–359. doi: 10.1046/j.1432-1327.1998.2520353.x. [DOI] [PubMed] [Google Scholar]
- 16.Moulder J M. The biochemistry of intracellular parasitism. Chicago, Ill: The University of Chicago Press; 1962. [Google Scholar]
- 17.Moulder J M. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuhaus H E, Thom E, Möhlmann T, Steup M, Kampfenkel K. Characterization of a novel ATP/ADP transporter from Arabidopsis thaliana L. Plant J. 1997;11:73–82. doi: 10.1046/j.1365-313x.1997.11010073.x. [DOI] [PubMed] [Google Scholar]
- 18a.Neuhaus, H. E., and H. H. Winkler. Unpublished data.
- 19.Ojcius D M, Degani H, Mispelter J, Dautryvarsat L. Enhancement of ATP levels and glucose metabolism during an infection by Chlamydia—NMR studies of living cells. J Biol Chem. 1998;273:7052–7058. doi: 10.1074/jbc.273.12.7052. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sarow I, Becker Y. Trachoma agent DNA. J Mol Biol. 1969;42:581–589. doi: 10.1016/0022-2836(69)90245-9. [DOI] [PubMed] [Google Scholar]
- 22.Sinai A P, Joiner K A. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 23.Smith D K, Winkler H H. Characterization of a lysine-specific active transport system in Rickettsia prowazekii. J Bacteriol. 1977;129:1349–1355. doi: 10.1128/jb.129.3.1349-1355.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens R S, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusol R L, Zhao Q, Koonin E V, Davis R W. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 25.Tamura A. Studies on RNA synthetic enzymes associated with meningo-pneumonitis virus. Ann Rep Inst Virus Res Kyoto Univ. 1967;10:26–36. [Google Scholar]
- 26.Tipples G, McClarty G. The obligate intracellular bacterium Chlamydia trachomatis is auxotrophic for three of the four ribonucleoside triphosphates. Mol Microbiol. 1993;8:1105–1114. doi: 10.1111/j.1365-2958.1993.tb01655.x. [DOI] [PubMed] [Google Scholar]
- 27.Tjaden J, Schwöppe C, Möhlmann T, Neuhaus H E. Expression of the plastidic ATP/ADP transporter gene in Escherichia coli lead to the presence of a functional adenine nucleotide transport system in the bacterial cytoplasmic membrane. J Biol Chem. 1998;273:9630–9636. doi: 10.1074/jbc.273.16.9630. [DOI] [PubMed] [Google Scholar]
- 28.von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;22:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 29.Williamson L R, Plano G V, Winkler H H, Krause D C, Wood D O. Nucleotide sequence of the Rickettsia prowazekii ATP/ADP translocase-encoding gene. Gene. 1989;80:269–278. doi: 10.1016/0378-1119(89)90291-6. [DOI] [PubMed] [Google Scholar]
- 30.Winkler H H. Rickettsial permeability: an ADP-ATP transport system. J Biol Chem. 1976;251:389–396. [PubMed] [Google Scholar]
- 31.Winkler H H. Membrane transport in Rickettsia. Methods Enzymol. 1986;125:253–259. doi: 10.1016/s0076-6879(86)25021-1. [DOI] [PubMed] [Google Scholar]
- 32.Winkler H H. Rickettsia species (as organisms) Annu Rev Microbiol. 1990;44:131–153. doi: 10.1146/annurev.mi.44.100190.001023. [DOI] [PubMed] [Google Scholar]
- 33.Winkler H H, Wilson T H. The role of energy coupling in the transport of β-galactosides by E. coli. J Biol Chem. 1966;241:2200–2211. [PubMed] [Google Scholar]
- 34.Winkler, H. H., and Y. Zhang. Unpublished data.
- 35.Zahorchak R J, Winkler H H. Transmembrane electrical potential in Rickettsia prowazekii and its relationship to lysine transport. J Bacteriol. 1983;153:665–671. doi: 10.1128/jb.153.2.665-671.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]