Abstract
Background
Patients with hematologic malignancies have impaired humoral immunity secondary to their malignancy and its treatment, placing them at risk of severe coronavirus disease‐19 (COVID‐19) infection and reduced response to vaccination.
Methods
The authors retrospectively analyzed serologic responses to initial and booster COVID‐19 vaccination in 378 patients with hematologic malignancy and subsequently tracked COVID‐19–related outcomes.
Results
Seroconversion occurred in 181 patients (48%) after initial vaccination; patients who had active malignancy or those who were recently treated with a B‐cell–depleting monoclonal antibody had the lowest rates of seroconversion. For initial nonresponders to vaccination, seroconversion after a booster dose occurred in 48 of 85 patients (56%). The seroconversion rate after the booster was similar for patients on (53%) and off (58%) active therapy (p = .82). Thirty‐three patients (8.8%) developed a COVID‐19 infection, and there were three COVID‐19–related deaths (0.8%). Although no significant association was observed between postvaccination seroconversion and the incidence of COVID‐19 infection, no patient with seroconversion died from COVID‐19, and no patient who received tixagevimab/cilgavimab (N = 25) was diagnosed with a COVID‐19 infection.
Conclusions
Booster vaccinations can promote seroconversion in a significant proportion of patients who are seronegative after the initial vaccination course regardless of the specific vaccine or on/off treatment status at the time of revaccination. Although postvaccination seroconversion may not be associated with a decrease in any (including asymptomatic) COVID‐19 infection, the authors' experience suggested that effective vaccination (including a booster), supplemented by passive immunization using tixagevimab/cilgavimab in case of lack of seroconversion, effectively eliminated the risk of COVID‐19 death in the otherwise high‐risk population.
Lay summary
Patients with hematologic malignancy, especially lymphoma, have an impaired response to coronavirus disease 2019 (COVID‐19) vaccination.
In this single‐institution review, less than one half of the patients studied made detectable antibodies.
For those who did not make detectable antibodies after initial vaccination, over one half (65%) were able to produce antibodies after booster vaccination.
By the end of February 2022, 33 of the original 378 patients had a documented COVID‐19 infection.
The only deaths from COVID‐19 were in those who had undetectable antibodies, and no patient who received prophylactic antibody therapy developed a COVID‐19 infection.
Keywords: booster, cancer, coronavirus, humoral immunity, lymphoma
Short abstract
Hematologic malignancies and their treatments impaired humoral immunity from coronavirus disease 2019 vaccination, and booster vaccine overcame a lack of initial response in 58% of patients, including 63% those on active therapy. The findings indicated that booster vaccinations should be strongly encouraged; however, even with boosters, many remain unprotected, which should inform infection prevention and treatment strategies.
INTRODUCTION
Coronavirus disease‐19 (COVID‐19) has caused death of millions of individuals worldwide and nearly a million deaths in the United States. People with cancer have a significantly increased risk of severe disease and death compared with the general public. 1 They also have an increased risk of breakthrough infection after vaccination. 2 Patients with hematologic malignancies have an impaired response to vaccination, including vaccination against COVID‐19, secondary to impaired humoral immunity from both treatment and disease. 3 , 4 , 5 , 6 From early in the COVID‐19 pandemic, receipt of anti‐CD20 therapy has been noted to increase the risk of severe disease, and patients with hematologic malignancies eagerly awaited vaccination that could lower their risk. 7 However, the phase 3 registration studies of COVID‐19 vaccines excluded patients who had immunosuppression or were receiving immunosuppressive therapies. 8 , 9 , 10 Despite this, professional organizations suggested vaccination, or even its prioritization, for patients with cancer. 11 With the realization that many groups remained at risk of COVID‐19 infection despite receiving a primary vaccine series consisting of a two‐dose series of messenger RNA (mRNA) coronavirus 2019 (COVID‐19) vaccine (Moderna COVID‐19 Vaccine [mRNA‐1273] or Pfizer‐BioNTech COVID‐19 Vaccine [BNT162b2]) or a single dose of Janssen COVID‐19 Vaccine (Ad26.COV2.S), the Centers for Disease Control and Prevention (CDC) began recommending booster vaccines for the immunocompromised in August of 2021 and in October approved a mix‐and‐match option to allow for changes in the booster from the original vaccine because both homogenous (same original vaccine and booster) and heterogenous (different original vaccine and booster) vaccine and booster appear to be safe and effective. 12 , 13 Of note, the initial CDC list of immunocompromising conditions qualifying an individual for a booster dose did not include patients with hematologic malignancies who were being followed using a watch‐and‐wait approach or who had completed therapy. 14 In contrast, recent studies have demonstrated that even those who were followed using a watch‐and‐wait approach or within 1 year of anti–B‐cell therapy demonstrated lower rates of seroconversion to vaccination. 3 , 15 A better understanding of who needs a booster and for whom it is likely to confer additional protection, juxtaposed to those unlikely to respond to repeat vaccination, remains paramount because COVID‐19 cases and deaths continue at high levels. The effectiveness of booster vaccination in patients with hematologic malignancies and which factors are associated with seroconversion in this population remain unknown. New therapies, including passive immunity and antiviral agents, provide hope for protection in those whose response to vaccination is impaired. The objectives of this review of vaccinated patients with hematologic malignancies were to provide information on the factors associated with seroconversion, to examine the risk of severe COVID‐19 after vaccination, and to assess the efficacy of available therapies.
MATERIALS AND METHODS
We conducted a retrospective study of adults with hematologic malignancies who received initial and booster vaccination with one of three US Food and Drug Administration‐authorized or approved COVID‐19 vaccines between February 2021 and February 2022. Inclusion criteria included age older than 18 years; any type of lymphoid, myeloid, or plasma cell malignancy; known treatment history and disease status; and clear documentation of vaccination type and time of vaccination. Disease status was classified as watchful waiting for those were never treated by either patient or clinician choice; active disease, indicating disease either during therapy or in need of imminent therapy; and in remission, for those who were without active disease after treatment. Patients without known malignancy, with incomplete vaccine history, or with a known history of COVID‐19 infection were excluded. In addition, data about seroconversion were collected from 31 individuals who were seen contemporaneously in our institution for nonmalignant diagnoses. Outcomes regarding COVID‐19 infections, as noted in the electronic medical record, were reviewed up to the data cutoff of February 28, 2022. The Institutional Review Board at Rhode Island Hospital approved the review with a waiver of patient informed consent because testing was part of routine medical care. SARS‐CoV‐2 antibodies were assessed using the qualitative SARS‐CoV‐2 Total Antibody Test (immunoglobulin G [IgG] or IgM against receptor‐binding domain [RBD]; Wondfo USA), the SARS‐CoV‐2 IgG test (IgG against nucleocapsid protein; Abbott), and the semiquantitative Abbott AdviseDx SARS‐CoV‐2 IgG II test (IgG against RBD). 16 In August 2021, the qualitative SARS‐CoV‐2 Total Antibody Test was retired and replaced by the semiquantitative Abbott AdviseDx SARS‐CoV‐2 IgG II test. To compare a binary positive or negative rate of seroconversion, anything above the minimum detection on the semiquantitative assay of 50 arbitrary units per milliliter (AU/ml) was considered positive. At minimum, antibody levels were assessed 7 days from the last vaccine because early phase vaccine studies demonstrated detectable antibodies in >90% of participants by this time point. 17 , 18 For univariate associations, we used Fisher exact tests for categorical variables and Wilcoxon rank‐sum tests for continuous variables. We further explored fractional polynomial fits to examine nonlinearity. Multivariate analysis included all factors that were considered clinically relevant, without consideration of statistical significance in univariate analysis. The multivariable logistic regression model reported the adjusted odds ratio with 95% confidence intervals. The cumulative incidence of COVID‐19 infection (documented in the medical record) or COVID‐19–related death was calculated starting from the date of the antibody test after vaccination (initial or booster) to avoid guarantee‐time bias. Time‐to‐event outcomes were compared using the log‐rank test stratified by age (65 years and older or younger than 65 years) and histology, where sample size allowed. All p values < .05 from statistical tests were considered significant. Biostatistical analyses were conducted using Stata/MP version 17.0 (StataCorp).
RESULTS
We identified 378 patients with hematologic malignancies who underwent initial vaccination (Table 1). Seroconversion after initial vaccination was noted in 181 patients (48%). Semiquantitative titers were available for 135 patients with hematologic malignancies, of whom 92 had positive seroconversion. The median anti‐RBD IgG level was 2233.6 AU/mL for those with seroconversion and < 50 AU/mL (on semiquantitative reporting) for those without seroconversion (p = .0001). Among patients with hematologic malignancies, in univariate analysis, seroconversion after initial vaccination was significantly associated with younger age (Figure 1A), type of hematologic malignancy, disease status, receipt of anti–B‐cell monoclonal antibody therapy, type of vaccine used, higher total white blood cell or lymphocyte count, longer time from last treatment to antibody testing, and later calendar time (possibly because most patients on active therapy were tested earlier in the year). There was not a significant association between seroconversion after initial vaccination and a history of stem cell transplantation.
TABLE 1.
Patient characteristics associated with seroconversion
| Variable | All patients | No seroconversion | Positive seroconversion | ||||
|---|---|---|---|---|---|---|---|
| No. | Row % | No. | Row % | No. | Row % | p | |
| No. of patients | 378 | 100.0 | 197 | 52.1 | 181 | 47.9 | |
| Age: Median (IQR), years | 69.7 (62.2–77.6) | 72.1 (64.7–78.9) | 67.4 (60.4–75.4) | .0037 | |||
| Sex | .065 | ||||||
| Women | 188 | 49.7 | 107 | 56.9 | 81 | 43.1 | |
| Men | 190 | 50.3 | 90 | 47.4 | 100 | 52.6 | |
| Histology | .0018 | ||||||
| Indolent B‐cell | 100 | 26.5 | 57 | 57.0 | 43 | 43.0 | |
| Aggressive B‐cell | 105 | 27.8 | 69 | 65.7 | 36 | 34.3 | |
| Other lymphoma | 39 | 10.3 | 16 | 41.0 | 23 | 59.0 | |
| Myeloid | 36 | 9.5 | 12 | 33.3 | 24 | 66.7 | |
| CLL | 48 | 12.7 | 23 | 47.9 | 25 | 52.1 | |
| Myeloma/MGUS | 50 | 13.2 | 20 | 40.0 | 30 | 60.0 | |
| Disease status | .00014 | ||||||
| Active | 158 | 41.8 | 100 | 63.3 | 58 | 36.7 | |
| Remission | 167 | 44.2 | 80 | 47.9 | 87 | 52.1 | |
| WW | 53 | 14.0 | 17 | 32.1 | 36 | 67.9 | |
| Disease status at time of vaccination | .099 | ||||||
| Active | 10 | 9.0 | 7 | 70.0 | 3 | 30.0 | |
| Remission | 97 | 91.0 | 62 | 63.9 | 35 | 36.1 | |
| Anti–B‐cell antibody | < .00001 | ||||||
| No | 164 | 43.4 | 58 | 35.4 | 106 | 64.6 | |
| Yes | 214 | 56.6 | 139 | 65.0 | 75 | 35.0 | |
| Stem cell transplantation | .24 | ||||||
| No | 351 | 92.9 | 186 | 53.0 | 165 | 47.0 | |
| Yes | 27 | 7.1 | 11 | 40.7 | 16 | 59.3 | |
| WBC: Median (IQR), ×109/L | 5.9 (4.4–8.0) | 5.5 (4.2–7.5) | 6.3 (4.8–8.6) | .0063 | |||
| ALC: Median (IQR) × 109/L | 1.2 | (0.8–1.8) | 1 | (0.6–1.5) | 1.5 | (0.9–2.1) | < .00001 |
| COVID vaccine | .0030 | ||||||
| BNT162b2/Pfizer | 214 | 56.6 | 125 | 58.4 | 89 | 41.6 | |
| mRNA‐1273/Moderna | 128 | 33.9 | 51 | 39.8 | 77 | 60.2 | |
| Ad26.COV2.S/J&J | 36 | 9.5 | 21 | 58.3 | 15 | 41.7 | |
| Time from vaccination to test: Median (IQR), days | 15.6 (8.4–25.1) | 15 (7.9–23.3) | 16 (8.9–26.9) | .029 | |||
| Time from last chemotherapy to vaccination: Median (IQR), months | 0 (0.0–23.4) | 0 (0.0–5.0) | 7.6 (0.0–31.3) | .00029 | |||
| Time from treatment, months | < .00001 | ||||||
| <12 | 204 | 69.2 | 134 | 65.7 | 70 | 34.3 | |
| ≥12 | 91 | 30.8 | 31 | 34.1 | 60 | 65.9 | |
| Anti‐RBD IgG: Median (IQR), AU/ml | 92 (1.00–2194.9) | 1 (1.00–4.85) | 2233.6 (489.1–8343.7) | < .00001 | |||
Abbreviations: Ad26.COV2.S, adenovirus serotype 26 against the severe acute respiratory‐coronavirus 2 spike protein; ALC, absolute lymphocyte count; anti‐RBD IgG, immunoglobulin G against receptor‐binding domain; AU, arbitrary units; CLL, chronic lymphocytic leukemia; COVID, coronavirus disease; IQR, interquartile range; J&J, Johnson & Johnson; MGUS, monoclonal gammopathy of undetermined significance; mRNA, messenger RNA; WW, watchful waiting.
Figure 1.
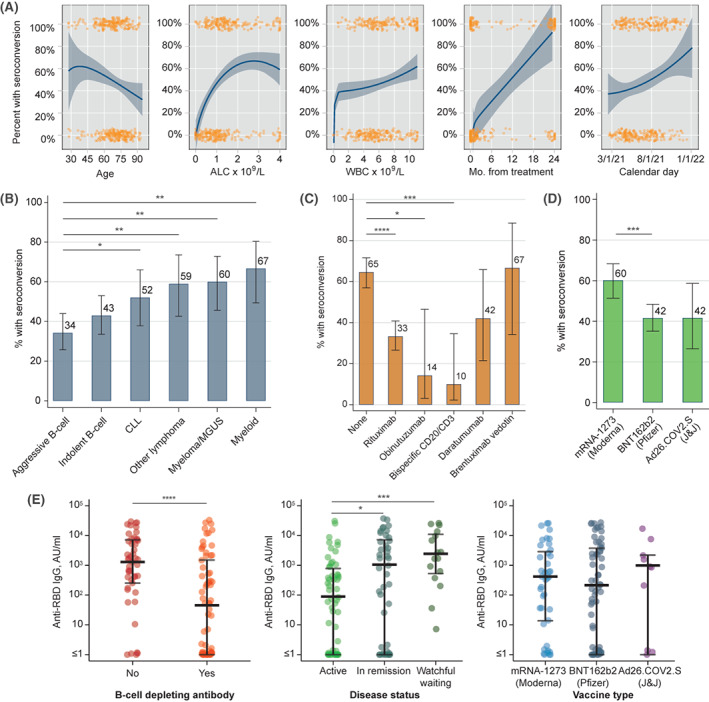
Association between seroconversion after initial COVID‐19 vaccination and (A) continuous variables, (B) type of hematologic malignancy, (C) prior exposure to specific monoclonal antibodies (only agents with n > 10 shown), and (D) specific COVID‐19 vaccine. (E) Levels of anti‐RBD IgG antibody after the initial vaccination according to prior exposure to B‐cell–depleting antibody, disease status, and type of initial vaccine. Variables in A include: age, absolute lymphocyte count (ALC) (capped at the upper limit of normal 4 × 10 9 /L), white blood cell count (WBC) (capped at upper limit of normal 11 × 10 9 /L), months (Mo.) from last disease‐directed therapy, and calendar month in 2021; orange markers indicate each individual data point, lines and shaded areas indicate fractional polynomial fit with 95% confidence interval. Asterisks in B–E indicate statistical significance on univariate testing: *p < .05, **p < .01, ***p < .001, ****p < .0001. Ad26.COV2.S indicates adenovirus serotype 26 against the severe acute respiratory‐coronavirus 2 spike protein; anti‐RBD IgG, immunoglobulin G against receptor‐binding domain; CLL, chronic lymphocytic leukemia; COVID‐19, coronavirus disease 2019; J&J, Johnson & Johnson; MGUS, monoclonal gammopathy of undetermined significance; mRNA, messenger RNA.
Among specific hematologic malignancy subtypes (Figure 1B), seroconversion after initial vaccination was least frequent and was <50% in those who had aggressive B‐cell lymphomas (36 of 105 patients; 34%) and indolent B‐cell lymphomas (43 of 100 patients; 43%), with higher observed rates in those who had chronic lymphocytic leukemia (CLL; 25 of 48 patients; 52%); other lymphomas, including Hodgkin and T‐cell lymphomas (23 of 39 patients; 59%); plasma cell disorders (30 of 50 patients; 60%); and myeloid malignancies (24 of 36 patients; 67%). Of the 214 patients who received B‐cell–depleting monoclonal antibody therapy, most received rituximab (N = 171), whereas others received daratumumab (N = 19), bispecific CD20/CD3 antibodies (N = 20), obinutuzumab (N = 14), brentuximab vedotin (N = 12), polatuzumab vedotin (N = 8), or tafasitamab (N = 1). Of the patients who had exposure to a B‐cell–depleting monoclonal antibody, 75 (35%) demonstrated seroconversion after the initial vaccination compared with 106 of 164 (65%) without this exposure (p < .00001). The rate of seroconversion was lowest among patients who were exposed to bispecific CD20/CD3 antibodies (10%), all of whom had diffuse large B‐cell lymphoma, and was higher among patients who were treated with daratumumab (42%) or brentuximab vedotin (67%; Figure 1C).
Vaccination using the mRNA‐1273/Moderna vaccine was associated with increased rates of seroconversion compared with using the BNT162b2/Pfizer vaccine (42%; p = .001) and the Ad26.COV2.S/J&J vaccine (42%; p = .058; Figure 1D). We also observed significantly lower amounts of the antibody in patients who were exposed to B‐cell–depleting monoclonal antibodies or who were receiving active therapy for their hematologic malignancies using the semiquantitative anti‐RBD IgG assessment, although the median levels of anti‐RBD IgG did not differ significantly between the vaccines (Figure 1D).
In a multivariable model, the rates of postvaccination seroconversion were statistically significantly different between the vaccines (Figure 2A), together with hematologic malignancy histology, disease status, absolute lymphocyte count, exposure to B‐cell–depleting monoclonal antibodies, and calendar time. The associations were consistent in the subpopulation of patients (N = 295) who had received prior therapy, adjusting further for the time from last chemotherapy (Figure 2B).
Figure 2.
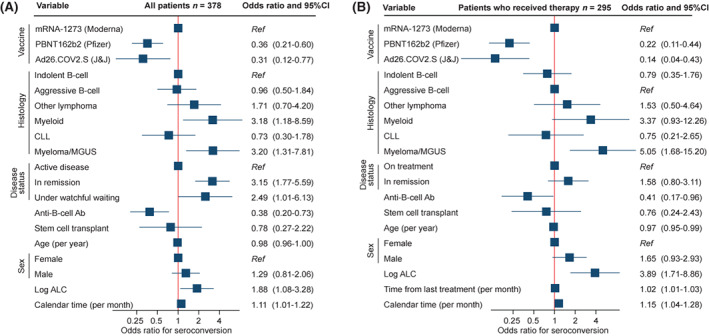
Multivariable models for the odds ratio of seroconversion after initial coronavirus disease 2019 vaccination among (A) all patients and (B) patients who received chemotherapy (also adjusting for the time from last treatment). Squares represent coefficients from the multivariable logistic model together with 95% confidence intervals (CIs) (blue lines). Ab indicates antibody; Ad26.COV2.S, adenovirus serotype 26 against the severe acute respiratory‐coronavirus 2 spike protein; ALC, absolute lymphocyte count; CLL, chronic lymphocytic leukemia; J&J, Johnson & Johnson; MGUS, monoclonal gammopathy of undetermined significance; mRNA, messenger RNA; Ref, reference category.
We examined subsequent seroconversion among 85 patients who had a negative anti–RBD IgG antibody test after the initial vaccination course and who then received a booster vaccination (Table 2). Forty‐eight of these patients (56%) demonstrated seroconversion after the booster. The median time from the initial vaccine to the booster was 6.4 months (interquartile range, 5.9–7.2 months). Seroconversion was noted in 32 of 52 patients (62%) who received a booster with the BNT162b2/Pfizer vaccine, in 13 of 28 (46%) who received a booster with the mRNA‐1273/Moderna vaccine, and in three of five (60%) who received a booster with the Ad26.COV2.S/J&J vaccine (Figure 3A). There was no statistically significant difference in these proportions when we compared specific booster vaccine products. Similarly, we observed no significant difference between seroconversion after a booster that was concordant (41 of 69 patients; 59%) or discordant (7 of 16 patients; 44%; p = .28; Figure 3B) with the initial vaccine product or when comparing patients who received a booster while on active anticancer therapy (16 of 30 patients; 53%) or not (32 of 55 patients; 58%; p = .82; Figure 3C).
TABLE 2.
Factors associated with seroconversion to booster in those without seroconversion to initial vaccine series
| Variable | All patients with no seroconversion before booster | No postbooster seroconversion | Positive postbooster seroconversion | ||||
|---|---|---|---|---|---|---|---|
| No. | Column % | No. | Row % | No. | Row % | p | |
| No. | 85 | 37 | 48 | ||||
| Age: Median (IQR), years | 73.4 (65.9–78.9) | 75.3 (65.5–79.0) | 72.9 (67.9–78.3) | .91 | |||
| Sex | .270 | ||||||
| Women | 50 | 58.8 | 19 | 38.0 | 31 | 62.0 | |
| Men | 35 | 41.2 | 18 | 51.4 | 17 | 48.6 | |
| Histology | < .001 | ||||||
| Indolent B‐cell | 19 | 22.4 | 3 | 15.8 | 16 | 84.2 | |
| Aggressive B‐cell | 35 | 41.2 | 25 | 71.4 | 10 | 28.6 | |
| Other lymphoma | 3 | 3.5 | 0 | 0.0 | 3 | 100.0 | |
| Myeloid | 5 | 5.9 | 2 | 40.0 | 3 | 60.0 | |
| CLL | 12 | 14.1 | 6 | 50.0 | 6 | 50.0 | |
| Myeloma/MGUS | 11 | 12.9 | 1 | 9.1 | 10 | 90.9 | |
| Disease status at the time of booster | .82 | ||||||
| Off treatment | 55 | 64.7 | 23 | 41.8 | 32 | 58.2 | |
| On treatment | 30 | 35.3 | 14 | 46.7 | 16 | 53.3 | |
| Anti–B‐cell antibody | .03 | ||||||
| No | 25 | 29.4 | 6 | 24.0 | 19 | 76.0 | |
| Yes | 60 | 70.6 | 31 | 51.7 | 29 | 48.3 | |
| Stem cell transplantation | .13 | ||||||
| No | 77 | 90.6 | 36 | 46.8 | 41 | 53.2 | |
| Yes | 8 | 9.4 | 1 | 12.5 | 7 | 87.5 | |
| WBC: Median (IQR), ×109/L | 5.3 | (3.85–6.35) | 5.1 | (3.8–6.4) | 5.4 | (3.9–6.3) | .77 |
| ALC: Median (IQR), ×109/L | 1.0 | (0.7–1.4) | 0.9 | (0.4–1.2) | 1.1 | (0.8–1.5) | .044 |
| COVID booster vaccine | .39 | ||||||
| BNT162b2/Pfizer | 52 | 61.2 | 20 | 38.5 | 32 | 61.5 | |
| mRNA‐1273/Moderna | 28 | 32.9 | 15 | 53.6 | 13 | 46.4 | |
| Ad26.COV2.S/J&J | 5 | 5.9 | 2 | 40.0 | 3 | 60.0 | |
Abbreviations: Ad26.COV2.S, adenovirus serotype 26 against the severe acute respiratory‐coronavirus 2 spike protein; CLL, chronic lymphocytic leukemia; COVID, coronavirus disease; IQR, interquartile range; MGUS, monoclonal gammopathy of undetermined significance; mRNA, messenger RNA; WW, watchful waiting.
Figure 3.
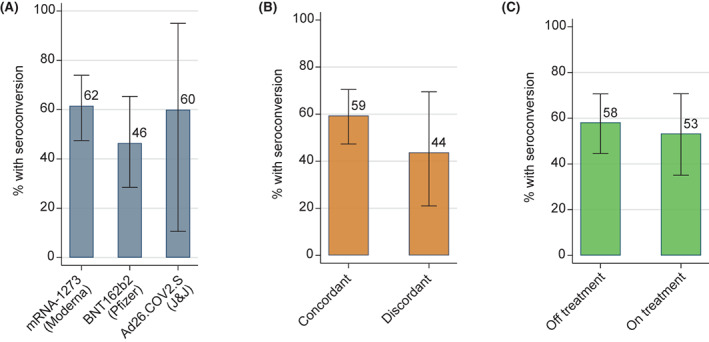
Percentage of patients attaining seroconversion after coronavirus disease 2019 booster vaccination by (A) type of vaccine, (B) vaccine concordance between original series and booster, and (C) treatment status at the time of booster administration. The percentage is listed at each bar; whisker bars indicate 95% exact binomial confidence intervals for the proportion estimate; none of the differences were statistically significant, so p values are not listed. Ad26.COV2.S indicates adenovirus serotype 26 against the severe acute respiratory‐coronavirus 2 spike protein; J&J, Johnson & Johnson; mRNA, messenger RNA.
Of the 378 patients we reviewed, 33 (8.8%) had a documented COVID‐19 infection (Figure 4A). Of these 33 infections, three resulted in death, all in seronegative patients (Figure 4B). One of the three deaths occurred after a booster. There was no evident association between seroconversion (after either primary vaccination or booster vaccination) and the cumulative incidence of any COVID‐19 infection (including asymptomatic infections; Figure 4A,C). In contrast, we observed as statistically significant (p = .046) reduction in COVID‐19–related deaths among patients who had seroconversion after a booster vaccination. The subgroup of patients who did not have evidence of seroconversion after a booster vaccination (N = 37) was eligible for pre‐exposure prophylaxis with tixagevimab/cilgavimab according to our institutional guideline. Among the 25 patients who received tixagevimab/cilgavimab, none were subsequently diagnosed with a COVID‐19 infection (compared with seronegative patients who did not receive pre‐exposure prophylaxis; p = .007; Figure 4E), whereas we observed three infections and one COVID‐19–related death among those who did not receive the pre‐exposure prophylaxis.
Figure 4.
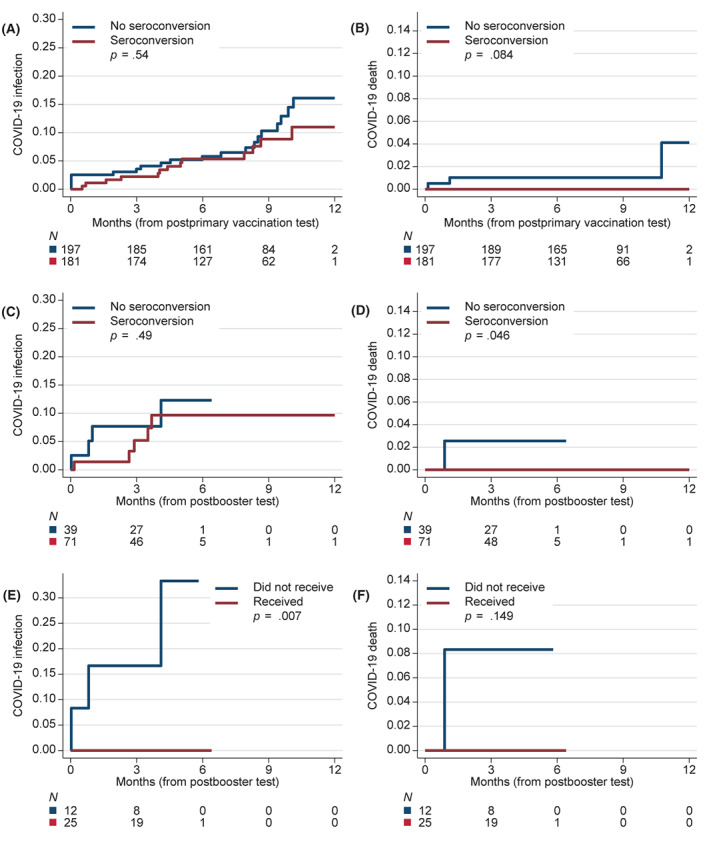
Cumulative incidence of documented COVID‐19 infection or COVID‐19–related death: (A) COVID‐19 infection according to seroconversion status after the initial vaccination course; (B) COVID‐19–related death according to seroconversion status after the initial vaccination course; (C) COVID‐19 infection and (D) COVID‐19–related death in the subgroup (N = 110) with known seroconversion status after booster vaccination (regardless of seroconversion status after initial vaccination course); and (E) COVID‐19 infection and (F) COVID‐19–related death in the subgroup (N = 37) with negative seroconversion status after the booster vaccination according to the receipt of prophylactic tixagevimab/cilgavimab. The p values in A–D are from log‐rank tests stratified by age (younger or older than 65 years) and histology of the hematologic cancer; p values in E and F are from unstratified log‐rank tests. COVID‐19 indicates coronavirus disease 2019.
DISCUSSION
In this retrospective study, we extended previous observations of decreased seroconversion after COVID‐19 vaccination in patients with hematologic malignancies to include an evaluation of seroconversion after booster vaccination and direct COVID‐19–related outcomes, including the preliminary efficacy of passive immunization with tixagevimab/cilgavimab. The current study has generated several novel findings of a significant, practical impact among patients and the clinicians caring for them. First, we observed that booster vaccinations can promote seroconversion in a significant proportion of patients who are seronegative after the initial vaccination course. Second, we found that, whereas seroconversion rates after the initial vaccination significantly differed between the vaccine products and according to disease status, booster vaccination produced additional seroconversion regardless of the specific vaccine or on‐treatment/off‐treatment status at the time of revaccination. Finally, although postvaccination seroconversion may not be associated with a decrease in any COVID‐19 infection (including asymptomatic infection), our experience suggests that effective vaccination (including a booster), supplemented by passive immunization using tixagevimab/cilgavimab in case of lack of seroconversion, effectively eliminated the risk of COVID‐19 death in the otherwise high‐risk population.
Several prior studies have explored disease‐specific, treatment‐specific, and time‐specific factors associated with response to vaccination in patients with hematologic malignancies. 3 , 4 Our findings in the prebooster cohort are consistent with published results. In a previous preliminary observation, we reviewed seroconversion after initial vaccination in less than one half of the current cohort (N = 160) to generate an alert for the scientific community regarding the low rate of seroconversion. 3 Our current study has uncovered additional specific prognostic factors and has extended the analysis of responses to include booster vaccination. Of the hematologic malignancies, B‐cell lymphoma consistently had the lowest rates of seroconversion, with reported rates from 42% to 75%. 3 , 4 , 19 In contrast, the rates of seroconversion among patients with plasma cell neoplasms (reported as 65%–95%) may be near rates in the general population. 4 , 19 Patients with active disease consistently have shown lower rates of seroconversion than those under watchful waiting or in remission. 3 , 4 Reported rates of seroconversion were as low as 3% after recent CD20‐directed therapy and remained low for the first year after completion of therapy, and then improved to 69%–80%. 3 , 15 Patients in receipt of Bruton tyrosine kinase inhibitors, primarily in CLL, similarly showed low rates of seroconversion at 40%. 4 An Israeli CLL study group recently published low rates of seroconversion to a third vaccine dose in patients who had CLL at 23.8% overall and at only 12% for those on active therapy. 20 Although these rates were lower than our findings, our cohort only included 12 patients with CLL, and our confidence intervals overlap with those findings (Table 2). Novel to our study is the first report of very low seroconversion rates (only 10%) for patients treated with bispecific (CD20‐directed and CD3‐directed) antibodies, which are experimental agents associated with a prolonged immune‐mediated depletion of B cells. 21 We postulate that these agents may decrease humoral immunity beyond traditional CD20‐directed therapies. Of note, similar low seroconversion rates have been reported among recipients of CD19‐directed chimeric antigen receptor T‐cell therapy, although, in current practice, that treatment is given to patients with relapsed/refractory lymphomas after multiple lines of immunosuppressive chemotherapy. 22 All of our patients received the bispecific CD20/CD3 antibodies on clinical trials, but many were received as first‐line therapy for diffuse large B‐cell lymphoma. We further demonstrate that the rate of seroconversion increases linearly over time from the last treatment without any specific cutoff. Rather than a specific time from treatment, persistent lymphopenia with an absolute lymphocyte count <1.0 × 10 9 /L was associated with a lower rate of response to COVID‐19 vaccines. Finally, specific vaccines have been associated with the rate of seroconversion in initial vaccine series. The mRNA‐1273/Moderna vaccine has previously been shown to have higher rates of seroconversion over those of the BNT162b2/Pfizer vaccine, although the degree of difference in efficacy varies between studies. 3 , 23 Although data on efficacy of the Ad26.COV2.S/J&J vaccine in patients with hematologic malignancy is less well studied, our data suggest that it is inferior at least to the mRNA‐1273/Moderna vaccine.
Given the low rates of seroconversion, there has been concern regarding the response to booster vaccines in hematologic malignancies and which factors would be associated with a booster response. Some clinicians have foregone the already debatable use of maintenance rituximab to hopefully improve vaccine response. Others have delayed boosters to allow time for immune recovery after prior therapy. Importantly, we found better than expected rates of seroconversion in previously seronegative patients at 56%, illustrating that boosters offer significant additional protection, even for patients who remain on active anticancer therapy. Our findings suggest that boosters should be given to patients with hematologic malignancies whenever they are eligible rather than delaying boosters until the completion of therapy. This is in contrast to a smaller study in which only six of 32 (19%) previously seronegative patients in receipt of anti‐CD20 therapy attained seroconversion after revaccination. 24 We note that our on‐treatment designation included any type of therapy, so lower rates might be observed among patients who continue anti‐CD20 therapy. Additional antigenic exposure, however, may improve low initial rates of seroconversion, raising the question of whether further vaccine doses might confer some protection to a larger proportion of immunocompromised patients or extend protection to emerging SARS‐CoV‐2 variants. In support of this concept, tandem influenza vaccine led to high rates of seroprotection in patients with plasma cell neoplasms. 25 The revaccination approach is already reflected in the authorized four‐dose series for immunocompromised patients, with an additional shot 1 month after the initial two‐shot series, followed by a booster (i.e., a fourth vaccine dose) 6 months later. 12 However, our patients did not receive this vaccination strategy.
Rates of seroconversion after booster vaccination were similar among the individual vaccines used. Therefore, using either of the mRNA vaccines to boost this population is reasonable given early data suggesting that these products may retain more efficacy against the omicron variant compared with the Ad26.COV2.S/J&J vaccine. 26 Although the Ad26.COV2.S/J&J vaccine as a booster led to high rates of seroconversion in our study (three of five patients; 60%), the small sample size makes conclusions difficult. The relatively higher rates of seroconversion after booster vaccination noted with the BNT162b2/Pfizer vaccine (62%), versus the mRNA‐1273/Moderna vaccine (46%), may reflect our small sample size but could also result from the reduced dose of the mRNA‐1273/Moderna booster. Discordance between initial vaccinations neither increased nor decreased seroconversion in our study, consistent with the CDC guidance for the general population. 12 , 13
This study used a retrospective design with a risk of selection bias for patient who were less likely to experience seroconversion. Patients without seroconversion after the original vaccination (who were the only patients retested for seroconversion after the booster) may be inherently less likely to respond to the booster vaccine compared with other individuals who have hematologic malignancies. In addition, our measurements of humoral immunity do not assess other facets of immunity, such as T‐cell function, so a lack of seroconversion is not equivalent with failure to respond to the vaccine. Whereas the US Food and Drug Administration states that, in general, antibody testing should not be used to assess for immunity from COVID‐19, such testing still may be informative for individuals with hematologic malignancies, who are less likely to have an adequate immune response to vaccination yet may benefit from passive immunization using tixagevimab/cilgavimab in the absence of high levels of endogenous antibodies. 27 In our practice, clinicians and patients found value in antibody testing, and, at the institutional level, we initially prioritized patients without seroconversion for pre‐exposure prevention using tixagevimab/cilgavimab, considering its limited supply. This approach is validated in our outcomes, in which seroconversion—thanks either to the vaccine or to the administration of tixagevimab/cilgavimab—was associated with no observed deaths from COVID‐19. Conversely, the mere presence of antibodies, even in relatively high titers, does not guarantee protection against any COVID‐19 infection, with breakthrough infections observed even in the general population. In the phase 3 trial of the mRNA‐1273/Moderna vaccine, antibodies were assessed as a correlate for the risk of COVID infection, with vaccine efficacy decreasing from 96% to 78% as the antibody levels dropped. 28 However, even with low humoral immunity, 77% of patients with hematologic malignancies who were infected with COVID‐19 had detectable SARS‐CoV‐2–specific T cells, and their numbers correlated with survival. 29 We also note that, although research has largely focused on the negative effect of CD20‐directed therapy on humoral immunity, many patients with hematologic malignancies receive chemotherapeutic agents like bendamustine, which have a substantial impact on T‐cell function and numbers. 30 Our measure of postvaccination COVID‐19 incidence is also limited by a small number of documented cases. With the availability of home tests or testing outside of the institution, some COVID‐19 cases may have been missed, although, in our practice, patients rapidly consulted with their oncologists in case of any positive test or significant exposure.
Although all patients with hematologic malignancies should remain vigilant against COVID‐19 infection, our study demonstrated an encouraging high rate of seroconversion after a booster vaccination among patients who did not have detectable anti–COVID‐19 antibodies after their initial vaccination course. Risk assessment using antibody testing can identify persistently seronegative patients at the highest risk of COVID‐19–related death and thus can help guide management, including prioritizing pre‐exposure prophylaxis. Clinicians and patients should be aware of additional methods for preventing infection and severe illness, including vaccination of all close contacts of patients, including friends, family, and medical staff. Hematologists should use the available pre‐exposure and postexposure therapeutics (monoclonal antibodies) while recognizing their potential limited efficacy against emerging SARS‐CoV‐2 variants like omicron. We conclude that a strategy that includes primary vaccination, booster vaccination, focused pre‐exposure prophylaxis using tixagevimab/cilgavimab, and risk assessment using the available serologic tests may be efficacious in preventing COVID‐19–related deaths among patients with hematologic malignancies.
AUTHOR CONTRIBUTIONS
Thomas A. Ollila: Participated in the design of the original concept, collected data, drafted the article, and reviewed and edited the article. Rebecca H. Masel: Collected data, drafted the article, and reviewed and edited the article. John L. Reagan: Participated in the design of the original concept and reviewed and edited the article. Shaolei Lu: Reviewed and edited the article. Ralph D. Rogers: Reviewed and edited the article. Kimberly J. Paiva: Reviewed and edited the article. Rashida Taher: Reviewed and edited the article. Ella Burguera‐Couce: Reviewed and edited the article. Adam S. Zayac: Reviewed and edited the article. Inna Yakirevich: Participated in the design of the original concept and reviewed and edited the article. Rabin Niroula: Reviewed and edited the article. Peter Barth: Reviewed and edited the article. Adam J. Olszewski: Collected data, performed the statistical analysis, drafted the article, and reviewed and edited the article.
CONFLICT OF INTEREST
Thomas A. Ollila reports a grant from the Rhode Island Foundation outside the submitted work. Peter Barth reports personal fees from Celgene and advisory board service at AbbVie, Janssen, and Sanofi‐Aventis outside the submitted work. Adam J. Olszewski reports research funding from Genentech, TG Therapeutics, Celldex Pharmaceuticals, and Precision Bio; and grants from Acrotech Pharma, Adaptive Biotechnologies outside the submitted work. The remaining authors made no disclosures.
Thomas A. Ollila and Rebecca H. Masel contributed equally to this manuscript.
REFERENCES
- 1. Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. 10.1016.j.ejca.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt AL, Labaki C, Hsu CY, et al. COVID‐19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022;33(3):340–346. doi: 10.1016/j.annonc.2021.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ollila TA, Lu S, Masel R, et al. Antibody response to COVID‐19 vaccination in adults with hematologic malignant disease. JAMA Oncol. 2021;7(11):1714–1716. doi: 10.1001/jamaoncol.2021.4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ribas A, Dhodapkar MV, Campbell KM, et al. How to provide the needed protection from COVID‐19 to patients with hematologic malignancies. Blood Cancer Discov. 2021;2(6):562–567. doi: 10.1158/2643-3230.Bcd-21-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bedognetti D, Zoppoli G, Massucco C, et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non‐Hodgkin's lymphoma patients treated with rituximab‐containing regimens. J Immunol. 2011;186(10):6044–6055. doi: 10.4049/jimmunol.1004095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teh JSK, Coussement J, Neoh ZCF, et al. Immunogenicity of COVID‐19 vaccines in patients with hematological malignancy: a systematic review and meta‐analysis. Blood Adv. 2022;6(7):2014–2034. doi: 10.1182/bloodadvances.2021006333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hueso T, Pouderoux C, Pere H, et al. Convalescent plasma therapy for B‐cell–depleted patients with protracted COVID‐19. Blood. 2020;136(20):2290–2295. doi: 10.1182/blood.2020008423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ribas A, Sengupta R, Locke T, et al. Priority COVID‐19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11(2):233–236. doi: 10.1158/2159-8290.Cd-20-1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). Media Statement from CDC Director Rochelle P. Walensky, MD, MPH, on Signing the Advisory Committee on Immunization Practices' Recommendation for an Additional Dose of an mRNA COVID‐19 Vaccine in Moderately to Severely Immunocompromised People. Accessed December 9, 2021. https://www.cdc.gov/media/releases/2021/s0813‐additional‐mRNA‐mrna‐dose.html
- 13. Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous covid‐19 booster vaccinations. N Engl J Med. 2022;386(11):1046–1057. doi: 10.1056/NEJMoa2116414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention (CDC). COVID‐19 Vaccines for Moderately or Severely Immunocompromised People. Accessed December 10, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/recommendations/immuno.html
- 15. Gurion R, Rozovski U, Itchaki G, et al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti‐CD2O antibodies. Haematologica. 2022;107(3):715–720. doi: 10.3324/haematol.2021.279216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paiva KJ, Grisson RD, Chan PA, et al. Validation and performance comparison of three SARS‐CoV‐2 antibody assays. J Med Virol. 2021;93(2):916–923. doi: 10.1002/jmv.26341 [DOI] [PubMed] [Google Scholar]
- 17. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid‐19 vaccine. N Engl J Med. 2021;384(19):1824–1835. doi: 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung DJ, Shah GL, Devlin SM, et al. Disease‐ and therapy‐specific impact on humoral immune responses to COVID‐19 vaccination in hematologic malignancies. Blood Cancer Discov. 2021;2(6):568–576. doi: 10.1158/2643-3230.Bcd-21-0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herishanu Y, Rahav G, Levi S, et al. Efficacy of a third BNT162b2 mRNA COVID‐19 vaccine dose in patients with CLL who failed standard 2‐dose vaccination. Blood. 2022;139(5):678–685. doi: 10.1182/blood.2021014085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abramson JS, Ghosh N, Smith SM. ADCs, BiTEs, CARs, and small molecules: a new era of targeted therapy in non‐Hodgkin lymphoma. ASCO Educ Book. 2020;40:302–313. doi: 10.1200/edbk_279043 [DOI] [PubMed] [Google Scholar]
- 22. Dahiya S, Luetkens T, Lutfi F, et al. Impaired immune response to COVID‐19 vaccination in patients with B‐cell malignancies after CD19 CAR T‐cell therapy. Blood Adv. 2022;6(2):686–689. doi: 10.1182/bloodadvances.2021006112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS‐CoV‐2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. doi: 10.1016/j.ccell.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidler D, Born A, Schietzel S, et al. Trajectories of humoraland cellular immunity and responses to a third dose of mRNA vaccines against SARS‐CoV‐2 in patients with a history of anti‐CD20 therapy. RMD Open. 2022;8(1):e002166. doi: 10.1136/rmdopen-2021-002166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Branagan AR, Duffy E, Gan G, et al. Tandem high‐dose influenza vaccination is associated with more durable serologic immunity in patients with plasma cell dyscrasias. Blood Adv. 2021;5(5):1535–1539. doi: 10.1182/bloodadvances.2020003880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization (WHO) . WHO Global Consultation—What evidence do we have that omicron is evading immunity and what are the implications? Accessed December 20, 2021. https://www.who.int/news‐room/events/detail/2021/12/15/default‐calendar/who‐global‐consultation—what‐evidence‐do‐we‐have‐that‐omicron‐is‐evading‐immunity‐and‐what‐are‐the‐implications
- 27. Tixagevimab and cilgavimab (Evusheld) for pre‐exposure prophylaxis of COVID‐19. JAMA. 2022;327(4):384–385. doi: 10.1001/jama.2021.24931 [DOI] [PubMed] [Google Scholar]
- 28. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA‐1273 COVID‐19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi: 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID‐19 and hematologic cancer. Nat Med. 2021;27(7):1280–1289. doi: 10.1038/s41591-021-01386-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ollila T, Butera J, Egan P, et al. Vincristine sulfate liposome injection with bendamustine and rituximab as first‐line therapy for B‐cell lymphomas: a phase I study. Oncologist. Published online March 7, 2022. doi: 10.1093/oncolo/oyab079 [DOI] [PMC free article] [PubMed]


