Abstract
Patients with coronavirus disease 2019 (COVID‐19) with cardiovascular diseases who are at higher risk of progressing to critical illness should be treated with nirmatrelvir/ritonavir (Paxlovid). Ritonavir, the booster in nirmatrelvir/ritonavir, modulates multiple drug metabolizing enzymes and transporters, complicating its use in real‐world clinics. We aimed to apply physiologically‐based pharmacokinetic (PBPK) modeling to simulate the complex drug–drug interactions (DDIs) of ritonavir with two anticoagulants, rivaroxaban and racemic warfarin, to address this important clinical conundrum. Simulations were implemented within Simcyp Simulator. Compound and population models were adopted from Simcyp and our previous studies. Upon verification and validation of the PBPK model of ritonavir, prospective DDI simulations with the anticoagulants were performed in both the general population (20–65 years) and geriatric subjects (65–85 years) with or without moderate renal impairment. Elevated rivaroxaban concentrations were simulated with nirmatrelvir/ritonavir treatment, where the impact was more profound among geriatric subjects with renal impairment. The overexposure of rivaroxaban was restored to normal range on day 4 post‐discontinuation of nirmatrelvir/ritonavir, corroborating with the recovery of enzyme activity. A lower 10 mg daily dose of rivaroxaban could effectively maintain acceptable systemic exposure of rivaroxaban during nirmatrelvir/ritonavir treatment. Treatment of ritonavir marginally declined simulated S‐warfarin concentrations, but substantially elevated that of R‐warfarin, resulting in a decrease in the international normalized ratio (INR). As INR only recovered 2 weeks post‐nirmatrelvir/ritonavir treatment, a longer surveillance INR for warfarin becomes important. Our PBPK‐guided simulations evaluated clinically important yet untested DDIs and supports clinical studies to ensure proper anticoagulation management of patients with COVID‐19 with chronic coagulative abnormalities when initiating nirmatrelvir/ritonavir therapy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Patients with coronavirus disease 2019 (COVID‐19) with cardiovascular diseases who are at higher risk of progressing to critical illness should be treated with nirmatrelvir/ritonavir (Paxlovid). Ritonavir, the booster in nirmatrelvir/ritonavir, modulates multiple drug metabolizing enzymes and transporters, complicating its concomitant use with two widely prescribed oral anticoagulants, rivaroxaban and warfarin.
WHAT QUESTION DID THIS STUDY ADDRESS?
For patients with COVID‐19 who undergo chronic anticoagulant therapy, what are the recommendations for appropriate oral anticoagulation management when initiating nirmatrelvir/ritonavir therapy?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
With the application of physiologically‐based pharmacokinetic modeling and simulations of complex disease‐drug–drug interactions, we provide scientific evidence of dose adjustment of rivaroxaban to 10 mg daily to mitigate the potential overexposure and up to 2‐weeks surveillance of international normalized ratio for warfarin treatment after nirmatrelvir/ritonavir discontinuation to avoid warfarin‐related thrombosis.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our novel findings are pertinent in supporting further clinical studies and/or systematic analyses of real‐world data to ensure efficacious and safe anticoagulant therapy while preventing progression to severe illness from COVID‐19 based on nirmatrelvir/ritonavir therapy.
Nirmatrelvir/ritonavir (Paxlovid) has received emergency use authorizations (EUAs) from the US Food and Drug Administration (FDA) for the treatment of patients with mild to moderate coronavirus disease (COVID‐19) at risk of progression to severe infection. 1 COVID‐19 Treatment Guidelines also strongly recommended the use of nirmatrelvir/ritonavir for treating nonhospitalized patients. 2 Treatment with nirmatrelvir/ritonavir within 5 days of the appearance of symptoms could effectively reduce the risk of hospitalization or death for high‐risk patients by 88%. 3 Nirmatrelvir/ritonavir comprises nirmatrelvir and ritonavir, where ritonavir, a potent synthetic HIV protease inhibitor, was given at a sub‐antiviral‐dose of 100 mg twice daily to boost the systemic exposure of the novel severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) Mpro inhibitor, nirmatrelvir. 1 , 3
Ritonavir yields potent mechanism‐based inactivation (MBI) of cytochrome P450 3A4 enzyme (CYP3A4) 4 and circumvents the rapid and extensive elimination of nirmatrelvir by CYP3A4. 5 Additionally, in vitro and in vivo studies have shown that ritonavir could remarkedly inhibit or induce several other CYP450 enzymes (e.g., CYP2C9 and CYP2J2) as well as multiple transporters (e.g., P‐glycoprotein (P‐gp) and organic anion transporters (OATs)). 4 , 6 , 7 Due to the unique co‐formulation of nirmatrelvir and ritonavir, the concomitant use of nirmatrelvir/ritonavir and certain other drugs may result in potentially significant drug–drug interactions (DDIs) and subsequent serious and/or life‐threatening reactions associated with elevated drug exposures. Such DDIs complicate the prescription of nirmatrelvir/ritonavir in real‐world clinics. 8 , 9
Patients with COVID‐19 have shown high incidence of thromboembolism and cardiac arrhythmias. 2 , 10 , 11 Several large cohort clinical studies evaluating anticoagulation therapy in hospitalized patients with COVID‐19 recommended the use of heparin analogues as effective interventions, whereas oral anticoagulants should be used with caution. 2 , 10 , 11 Hence, a clinical conundrum arises where patients with cardiovascular diseases who undergo chronic anticoagulant therapy are the most vulnerable cohort when diagnosed with COVID‐19, 12 necessitating nirmatrelvir/ritonavir treatment to prevent the progression of COVID‐19 to critical illness, whereas it is recommended that these patients should continue taking the oral anticoagulants after they receive a diagnosis of COVID‐19 infection. 2
Rivaroxaban and warfarin are two widely prescribed oral anticoagulants. S‐warfarin is predominantly metabolized by CYP2C9, 13 whereas CYP3A4 was responsible for the metabolism of R‐warfarin 13 and rivaroxaban. 14 Additionally, CYP2J2 and OAT3 are pivotally involved in hepatic metabolism and renal secretion of rivaroxaban, respectively. 14 EUA recommended close monitoring of warfarin if its co‐administration with nirmatrelvir/ritonavir is necessary, whereas concomitant use of nirmatrelvir/ritonavir with rivaroxaban should be avoided. 1 Clinical studies have reported that higher therapeutic doses of ritonavir (600 mg twice daily) increased systemic exposure of rivaroxaban by 2.53‐fold in healthy subjects, 15 and 100 mg once or twice daily dose of ritonavir could modestly decrease S‐warfarin concentration. 16 Furthermore, individual cases reported significant decline of international normalized ratio (INR) values by ritonavir during warfarin treatment. 17 Nevertheless, there remains a lack of direct clinical evidence in guiding appropriate oral anticoagulation management for patients with COVID‐19 during and post‐nirmatrelvir/ritonavir therapy. 8
To address this important clinical conundrum in the pharmacotherapy of patients with COVID‐19 with nirmatrelvir/ritonavir who are already prescribed with rivaroxaban or warfarin, we applied physiologically‐based pharmacokinetic (PBPK) and PBPK/pharmacodynamic (PD) modeling and simulations to investigate complex disease‐drug–drug interactions (DDDIs) and recommend dosage adjustment and surveillance in clinical scenarios where the DDDIs are potentially unfavorable. Our findings are pertinent in supporting clinicians in treating patients with COVID‐19 efficaciously and safely using both nirmatrelvir/ritonavir and oral anticoagulants.
METHODS
The models and simulations were implemented within the population‐based Simcyp Simulator (version 19, Sheffield, UK). Compound profiles for midazolam and digoxin were directly adopted from Simcyp. The ritonavir model provided by Simcyp was optimized based on previous studies. 6 , 7 , 18 Rivaroxaban PBPK model 14 and racemic warfarin PBPK/PD model 13 were judiciously constructed and directly utilized for our simulations. Details of model development are summarized in Supplementary Methods and Table S1 . Nirmatrelvir was not incorporated into the simulations due to its low DDI potential. 5 Healthy population (Healthy Volunteers), general White population (NEurCaucasian) and geriatric population (Geriatric NEC) provided in Simcyp were adopted as population models. The moderate renal impairment (creatinine clearance (CrCL) 30 to 49 mL/min) population model modified previously 19 , 20 were utilized for simulations. Each simulation was designed as a total size of 100 subjects (10 trials with 10 subjects for each).
The pharmacokinetic (PK) profiles of ritonavir following multiple oral dose administrations were first simulated to verify the performance of the modified ritonavir model. The model was further validated with clinically available DDI studies to assess the model liability for DDI simulations against different enzymes and transporters. The dosage regimens of drugs were matched to the design of the corresponding clinical studies. Details of model verification and validation are provided in Supplementary Methods .
Age is a critical factor that contributed to the COVID‐19‐related severe illness and deaths, 12 and could substantially affect the PK of compounds as revealed by our previous studies. 19 , 20 Prospective DDDI simulations were performed using both general population (20–65 years) and geriatric subjects (65–85 years). Because nirmatrelvir/ritonavir is prescribed for the treatment of mild to moderate COVID‐19, we postulated that the impact of COVID‐19 related cytokine storm on enzymes and transporters (e.g., CYP3A4) is marginal. The effect was not incorporated into our modeling. Ritonavir dose was set as 100 mg twice daily for 5 days. 1 In patients with moderate renal impairment, the dosage of nirmatrelvir/ritonavir is adjusted as 150 mg nirmatrelvir and 100 mg ritonavir twice daily, 1 and a dose reduction of rivaroxaban from 20 to 15 mg daily is also required for patients with moderate renal impairment during the treatment of non‐valvular atrial fibrillation. 21 Rivaroxaban was simulated with these two doses with or without moderate renal dysfunction, respectively. A lower 10 mg daily dose of rivaroxaban, which was approved for prophylaxis of deep vein thrombosis and venous thromboembolism, 21 was prospectively simulated as a dose adjustment strategy, starting from day 1 of nirmatrelvir/ritonavir treatment to day 3 post‐nirmatrelvir/ritonavir treatment. The daily dose of racemic warfarin was fixed as 5 mg.
The DDI potential with rivaroxaban was assessed based on simulated plasma concentrations of rivaroxaban and the fold‐change of area under curve (AUC) 20 on the fifth day of nirmatrelvir/ritonavir treatment (AUCD5,0–24 hours), which was further calculated for risk of major bleeding 22 ( Supplementary Methods ). INR was utilized for the safety evaluation of warfarin therapy.
RESULTS
Ritonavir PBPK model verification
As illustrated in Table S2 and Figure S1 , the ritonavir PBPK model successfully reproduced the nonlinear PKs of ritonavir with daily doses ranging from 100 to 1200 mg, where 23 out of 27 simulated PK parameters fell within fold‐error criterion of 0.67–1.50. DDI predictive performance of ritonavir was further validated using available PK data sets with multiple substrates, where digoxin, midazolam, S‐warfarin, and rivaroxaban validated the effects of ritonavir on P‐gp, CYP3A4, CYP2C9, and OAT3, respectively (Table S3 ).
Prospective PBPK DDDI simulation for rivaroxaban
DDDIs between rivaroxaban and ritonavir were simulated among virtual subjects with normal or impaired renal functions with clinically recommended dosage regimens. Elevated rivaroxaban concentrations were simulated for the four virtual populations along with nirmatrelvir/ritonavir treatment (Figure 1 ), resulting in AUCD5,0‐24h fold‐change exceeding the 1.43 threshold (Table S4 ). The impact was more profound among geriatric subjects with renal impairment, where simulated risk of major bleeding was as high as 24.03% (Table S4 ). As illustrated in Figure 1 , the overexposure of rivaroxaban normalized on day 4 post‐discontinuation of nirmatrelvir/ritonavir treatment, which corroborated with the recovery of simulated enzyme activities to 62.44, 82.12, and 77.58% for CYP3A4 (liver), CYP3A4 (gut), and CYP2J2, respectively (Figure S1 ). Simulation results of the lower 10 mg dose of rivaroxaban showed that the dose adjustment could effectively mitigate the bleeding tendency by half for all four cohorts while maintaining acceptable systemic exposure of rivaroxaban (Figure 1 , Table S4 ).
Figure 1.
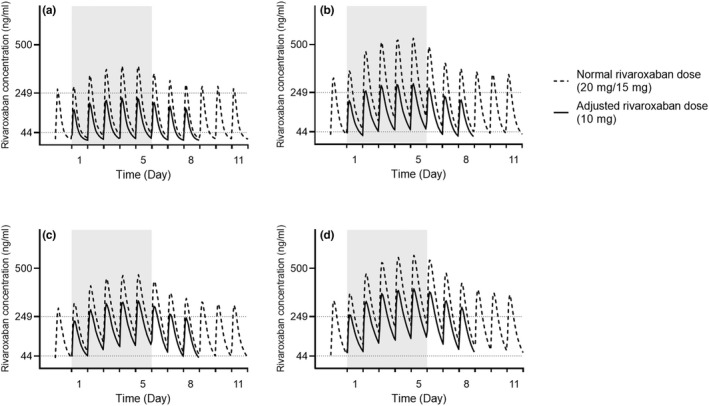
Plasma concentration profile over time of rivaroxaban in the general population (20–65 years) (a, c) and geriatric subjects (65–85 years) (b, d) with normal renal function a and b or moderate renal impairment (creatinine clearance (CrCL) 30 to 49 mL/min) c and d. Normal rivaroxaban daily dose of 20 mg and 15 mg was simulated for subjects with normal renal function or moderate renal impairment, respectively. Gray background represents 5‐days treatment of ritonavir of 100 mg twice daily. Data are presented as mean value. Plasma concentrations of 249 and 44 ng/mL were obtained from previous rivaroxaban Phase II studies 21 as maximum and minimum plasma concentration at steady‐state, respectively.
Prospective PBPK/PD simulation for warfarin
Expectedly, 5‐days’ treatment of ritonavir marginally declined S‐warfarin concentrations while substantially elevated that of R‐warfarin (Figure 2 a,b ). The polarized PKs of warfarin enantiomers resulted in a decrease in INR, which was more notable among older adults (Figure 2 c,d ). The simulated steady‐state INRs before nirmatrelvir/ritonavir treatment were 1.9 and 2.4, whereas the lowest INRs during treatment were 1.3 and 1.6 for the general population and older adults, respectively. In view of the indirect response of INR toward the changes of warfarin concentrations, INR values was simulated to return to pretreatment levels by week 2 post‐nirmatrelvir/ritonavir treatment (Figure 2 c,d ).
Figure 2.
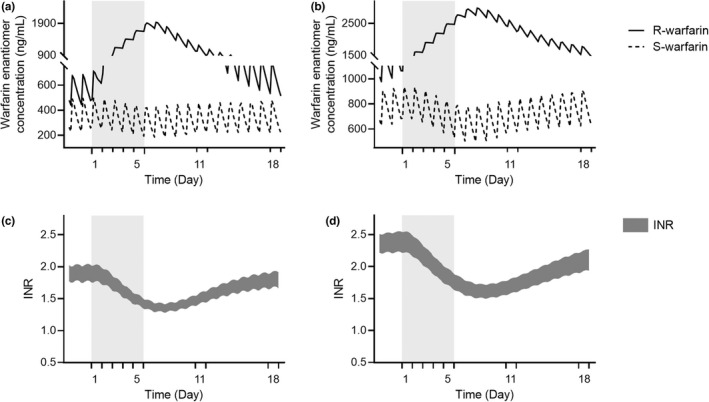
Plasma concentration profile of warfarin enantiomers (a, b) and INR profile (c, d) over time in the general population (20–65 years) a and c and geriatric subjects (65–85 years) b and d. Racemic warfarin daily dose of 5 mg was simulated. Gray background represents 5‐days treatment of ritonavir of 100 mg twice daily. Data are presented as mean value. INR, international normalized ratio.
DISCUSSION
COVID‐19 has been associated with inflammation and a prothrombotic state, resulting in high prevalence of cardiac arrhythmias and venous thromboembolism among patients with COVID‐19. 2 , 10 , 11 Meanwhile, patients with heart conditions and stroke disease are of high risk with COVID‐19 infection, as well as for progression to severe COVID‐19 condition. 12 Nirmatrelvir/ritonavir treatment should be initiated with these nonhospitalized patients as soon as possible while appropriately maintaining their anticoagulant therapy at the same time. 1 , 2 However, direct clinical data between nirmatrelvir/ritonavir and oral anticoagulants are still limited. 8 , 9 Notably, application of PBPK models in diseased and specific populations, in addition to DDIs, has demonstrated promising performance in the evaluation of clinically relevant yet untested scenarios. 23 PBPK‐guided dose recommendations, as an alternative to real‐world studies, have been approved in several drug labels in recent years, 23 , 24 and has been applied for nirmatrelvir/ritonavir‐related study. 25 In view of this urgent conundrum where clinical studies cannot be conducted in a timely manner, our study aimed to utilize a ritonavir PBPK model, which was validated against multiple DDI data and elimination pathways, to investigate the DDI potential of ritonavir with rivaroxaban and warfarin, and offer dosing and risk management strategy, during and after 5‐days of nirmatrelvir/ritonavir treatment.
Ritonavir inhibits OAT3‐ and P‐gp‐mediated transport and causes MBI of CYP3A4 and CYP2J2, 4 , 6 , 7 where these enzymes and transporters are implicated in the metabolism and excretion of rivaroxaban. 14 Considering the complex DDDIs, it remains critical to establish the currently arcane risk between rivaroxaban and nirmatrelvir/ritonavir comprising lower subtherapeutic dose of ritonavir, despite clinical DDI that had already been reported for rivaroxaban with 1,200 mg daily dose of ritonavir. 15 Our quantitative and mechanistical simulations verified the potent inhibition by both dosage regimens of ritonavir against enzymes and transporters associated with elimination of rivaroxaban, especially the comparable inhibition potency against CYP3A4 (Figure S2 A,B ). Corroborating our previous studies, 19 , 20 prospective simulations revealed that geriatric patients with moderate renal dysfunction were more susceptible to the DDDIs between rivaroxaban and ritonavir, who could potentially benefit from a further dose adjustment to 10 mg daily rivaroxaban while receiving nirmatrelvir/ritonavir treatment. Considering the enzyme half‐lives of 20–30 hours for CYP3A4 and CYP2J2 and simulated recoveries of CYP3A4 (liver), CYP3A4 (gut), and CYP2J2 activities post‐MBI by ritonavir were 62.4%, 82.1%, and 77.6%, respectively, the reduced dose of rivaroxaban could be maintained for 3 days post‐nirmatrelvir/ritonavir treatment, which was in accordance with recommendations by Marzolini et al. 9 and Hong et al. 25
As a racemate, S‐warfarin is metabolized by CYP2C9 whereas R‐warfarin is primarily metabolized by CYP3A4. 13 Unlike rivaroxaban, warfarin achieves its PD effect by indirectly interfering with vitamin K dependent clotting factors. 13 Several case reports found significantly decreased INR for patients taking warfarin after the initiation of antiretroviral therapy that included ritonavir, 17 whereas 15 days of oral treatment of ritonavir mildly decreased 24% of S‐warfarin systemic exposure in a clinical study, 16 attributing to the induction of CYP2C9 by ritonavir. Our simulations utilizing racemic warfarin additionally demonstrated up to 3.4‐fold increase of R‐warfarin concentrations during nirmatrelvir/ritonavir treatment, resulting from the inhibition of CYP3A4 by ritonavir. Because R‐warfarin was incorporated as a competitive antagonist of S‐warfarin against vitamin K epoxide reductase in our mechanistic PD model, 13 the elevated R‐warfarin concentration possibly contributed to the remarkable INR decline. As the increase in R‐warfarin concentrations was more profound than the decrease in S‐warfarin concentrations, it reasonably explained the clinically observed discrepancy between PKs and PDs of warfarin when interacting with ritonavir. 16 , 17 Because the appropriate dose of warfarin is determined on an individual basis and adjusted via INR monitoring, only a fixed 5 mg daily dose was simulated in current study without proposing further dose adjustment. Our prospective simulations highlighted the increased tendency of thrombosis during nirmatrelvir/ritonavir treatment, particularly for older adults. As 2 weeks were anticipated for the recovery of INR, our findings underscored the importance for a longer surveillance of INR of warfarin post‐nirmatrelvir/ritonavir therapy.
Taken together, for patients with chronic coagulative abnormalities and diagnosis of COVID‐19, our prospective simulations provided scientific evidence regarding rational and prudent oral anticoagulation management when initiating nirmatrelvir/ritonavir treatment. Clinical studies and/or systematic analyses of real‐world data are warranted to further confirm our recommendations and ensure the safety of anticoagulant therapy while preventing severe illness from COVID‐19 based on nirmatrelvir/ritonavir therapy.
FUNDING
Z.W. received postdoctoral fellowship from the National University of Singapore. E.C.Y.C. received research grant funding from the Joseph Lim Boon Tiong Urology Cancer Research Initiative (Grant: A‐0002678‐01‐00).
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
Z.W. and E.C.Y.C. designed the research and wrote the manuscript. Z.W. performed the research and analyzed the data.
Supporting information
Appendix S1
Figure S1
Figure S2
Table S1
Table S2
Table S3
Table S4
References
- 1. U.S. Food and Drug Administration . Paxlovid EUA Letter of Authorization <https://www.fda.gov/media/155049/download> (2021).
- 2. National Institutes of Health . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines <https://www.covid19treatmentguidelines.nih.gov/> (2021). [PubMed]
- 3. Burki, T.K. The role of antiviral treatment in the COVID‐19 pandemic. Lancet Respir. Med. 10, e18 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsu, A. , Granneman, G.R. & Bertz, R.J. Ritonavir: clinical pharmacokinetics and interactions with other anti‐HIV agents. Clin. Pharmacokinet. 35, 275–291 (1998). [DOI] [PubMed] [Google Scholar]
- 5. Eng, H. et al. Disposition of PF‐07321332 (Nirmatrelvir), an orally bioavailable inhibitor of SARS‐CoV‐2 3CL protease, across animals and humans. Drug Metab. Dispos. 10.1124/dmd.121.000801. [DOI] [PubMed] [Google Scholar]
- 6. Kaspera, R. et al. Investigating the contribution of CYP2J2 to ritonavir metabolism in vitro and in vivo. Biochem. Pharmacol. 91, 109–118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshida, K. , Maeda, K. & Sugiyama, Y. Transporter‐mediated drug–drug interactions involving OATP substrates: predictions based on in vitro inhibition studies. Clin. Pharmacol. Ther. 91, 1053–1064 (2012). [DOI] [PubMed] [Google Scholar]
- 8. Ratain, M.J. & Greenblatt, D.J. Drug interactions with a short course of Nirmatrelvir and ritonavir: prescribers and patients beware. J. Clin. Pharma. 10.1002/jcph.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marzolini, C. et al. Recommendations for the management of drug–drug interactions between the COVID–19 antiviral nirmatrelvir/ritonavir (Paxlovid®) and comedications. Clin. Pharma. Ther. 10.1002/cpt.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atallah, B. , Mallah, S.I. & AlMahmeed, W. Anticoagulation in COVID‐19. Eur. Heart J. Cardiovasc. Pharmacother. 6, 260–261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rattanawong, P.e.a. Guidance on short‐term Management of Atrial Fibrillation in coronavirus disease 2019. J. Am. Heart Assoc. 9, e017529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention COVID‐19 and Your Health . Centers for Disease Control and Prevention <https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html> (2020).
- 13. Wang, Z. et al. A physiologically based pharmacokinetic/pharmacodynamic modeling approach for drug–drug interaction evaluation of warfarin enantiomers with sorafenib. Drug Metab. Pharmacokinet. 39, 100362 (2021). [DOI] [PubMed] [Google Scholar]
- 14. Cheong, E.J.Y. , Teo, D.W.X. , Chua, D.X.Y. & Chan, E.C.Y. Systematic development and verification of a physiologically based pharmacokinetic model of rivaroxaban. Drug Metab. Dispos. 47, 1291–1306 (2019). [DOI] [PubMed] [Google Scholar]
- 15. Mueck, W. , Kubitza, D. & Becka, M. Co‐administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects: drug interactions with rivaroxaban. Br. J. Clin. Pharmacol. 76, 455–466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morcos, P.N. et al. A randomised study of the effect of danoprevir/ritonavir or ritonavir on substrates of cytochrome P450 (CYP) 3A and 2C9 in chronic hepatitis C patients using a drug cocktail. Eur. J. Clin. Pharmacol. 69, 1939–1949 (2013). [DOI] [PubMed] [Google Scholar]
- 17. Liedtke, M.D. & Rathbun, R.C. Drug interactions with antiretrovirals and warfarin. Expert Opin. Drug Saf. 9, 215–223 (2010). [DOI] [PubMed] [Google Scholar]
- 18. Marsousi, N. et al. Coadministration of ticagrelor and ritonavir: toward prospective dose adjustment to maintain an optimal platelet inhibition using the PBPK approach. Clin. Pharmacol. Ther. 100, 295–304 (2016). [DOI] [PubMed] [Google Scholar]
- 19. Wang, Z. & Chan, E. C. Y. Physiologically based pharmacokinetic modelling to investigate Baricitinib and tofacitinib dosing recommendations for COVID–19 in geriatrics. Clin. Pharmacol. Ther. 112, 291–296 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang, Z. , Cheong, E.J.Y. , Kojodjojo, P. & Chan, E.C.Y. Model‐based risk prediction of rivaroxaban with amiodarone for moderate renal impaired elderly population. Cardiovasc. Drugs Ther. 10.1007/s10557-021-07266-z. [DOI] [PubMed] [Google Scholar]
- 21. Mueck, W. , Stampfuss, J. , Kubitza, D. & Becka, M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 53, 1–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ismail, M. , Lee, V.H. , Chow, C.R. & Rubino, C.M. Minimal physiologically based pharmacokinetic and drug–drug‐disease interaction model of rivaroxaban and verapamil in healthy and renally impaired subjects. J. Clin. Pharmacol. 58, 541–548 (2018). [DOI] [PubMed] [Google Scholar]
- 23. Chu, X. et al. Clinical implications of altered drug transporter abundance/function and pbpk modeling in specific populations: an itc perspective. Clin. Pharmacol. Ther. 112, 501–526 (2022) [DOI] [PubMed] [Google Scholar]
- 24. Zhang, X. et al. Application of PBPK modeling and simulation for regulatory decision making and its impact on US prescribing information: an update on the 2018–2019 submissions to the US FDA's Office of Clinical Pharmacology. J. Clin. Pharmacol. 60(Suppl 1), S160–S178 (2020). [DOI] [PubMed] [Google Scholar]
- 25. Hong, E. , Almond, L.M. , Chung, P.S. , Rao, A.P. & Beringer, P.M. Physiologically–based pharmacokinetic–led guidance for patients with cystic fibrosis taking Elexacaftor–Tezacaftor–ivacaftor with Nirmatrelvir–ritonavir for the treatment of COVID–19. Clin. Pharmacol. Ther. 111, 1324–1333 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1
Figure S2
Table S1
Table S2
Table S3
Table S4


