Abstract
Neutralizing antibody is an important indicator of vaccine efficacy, of which IgG is the main component. IgG can be divided into four subclasses. Up to now, studies analysing the humoral response to SARS‐CoV‐2 vaccination have mostly focused on measuring total IgG, and the contribution of specific IgG subclasses remains elusive. The aim of this study is to investigate the kinetics of neutralizing antibodies and IgG subclasses, and to explore their relationships in people vaccinated with inactivated COVID‐19 vaccine. We conducted a prospective cohort study in 174 healthy adults aged 18–59 years old who were administrated 2 doses of CoronaVac 14 days apart and a booster dose 1 year after the primary immunization, and followed up for 15 months. Blood samples were collected at various time points after primary and booster immunization. We used live SARS‐CoV‐2 virus neutralizing assay to determine neutralizing ability against the wild‐type strain and 4 variants (Beta, Gamma, Delta and Omicron) and ELISA to quantify SARS‐CoV‐2 RBD‐specific IgG subclasses. The results showed that the 2‐dose primary immunization only achieved low neutralizing ability, while a booster shot can significantly enhance neutralizing ability against the wild‐type strain, Beta, Gamma, Delta and Omicron variants. IgG1 and IgG3 were the most abundant serum antibodies, and IgG2 and IgG4 were hardly detected at any time. The ratio of IgG1/IgG3 was positively associated with the neutralization ability. The underlying mechanism requires further exploration.
Keywords: IgG subclasses, neutralization ability, SARS‐CoV‐2 inactivated vaccine, variants
This study described the kinetics of neutralizing antibody and IgG subclasses against SARS‐CoV‐2 after the 2‐dose primary immunization and a third‐dose booster with inactivated COVID‐19 vaccine. IgG1 and IgG3 were the most abundant IgG subclasses. Subclass switching was quantified with the ratio of IgG1/IgG3, and was associated with neutralization ability.
INTRODUCTION
Since 2019, the COVID‐19 pandemic has been responsible for more than 6.18 million deaths [1]. As of April 5th, 2022, 35 COVID‐19 vaccines have been approved for use worldwide with more than 11.25 billion administered doses [2, 3]. Among these vaccines, inactivated vaccines have been approved for use in more than 60 countries, and are the main vaccine type used in China. Compared with other vaccines, COVID‐19 inactivated vaccines produced with traditional technology contain inactivated whole‐virion SARS‐CoV‐2, therefore they maintain the structure of epitopes on surface antigens, and are easier to store and transport [4]. Evidence from real‐world studies of inactivated vaccines in Chile [5], Brazil [6] and China [7] shows that a 2‐dose vaccination schedule can effectively prevent the infection of COVID‐19, and can offer greater protection against severe clinical outcomes.
Although vaccinations have markedly flattened COVID‐19 epidemic curves, active cases continue to surge as multiple variants of the SARS‐CoV‐2 continue to emerge and spread worldwide. Currently 5 variants of concern (VOCs) have been defined by the WHO, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron (B.1.1.529) [8]. All these VOCs have specific mutations within the spike regions [9], which may lead to escape from immunity induced by the prior infections or vaccinations, thereby potentially causing a large number of breakthrough infections [10, 11]. Although how new mutations can potentially affect the epidemic curves of the pandemic in the future is currently uncertain, the WHO still places high hopes on vaccination drive throughout world [3].
Since previous vaccination programs have faced the threat of circulating VOCs, booster immunization has become a standard practice to protect against variants, and has been started in more than 100 countries [3]. A preliminary evaluation has demonstrated that the additional dose could significantly reduce the number of new infections and symptomatic cases, and after the booster shot with CoronaVac the neutralization ability against the wild‐type strain increased by more than 30 times [12, 13, 14]. However, the kinetics of neutralizing antibodies after primary and booster immunization are still unclear.
Neutralizing antibodies are mainly consisted of IgG, IgA and IgM, among which IgG is the most abundant and multifunctional component of neutralizing antibodies [15, 16, 17]. IgG consists of 4 distinct subclasses, defined by the structure of their constant regions, including IgG1, IgG2, IgG3 and IgG4. The IgG subclasses induced by vaccination can serve as important determinants of vaccine efficacy [18, 19]. However, up to now, studies analysing the humoral response to SARS‐CoV‐2 vaccination have mostly focused on measuring total IgG in the serum. Therefore, the contribution of specific IgG subclasses remains elusive.
The aim of the present study was to comprehensively describe the kinetics of neutralizing antibodies against the wild‐type SARS‐CoV‐2 and 4 variants (Beta, Gamma, Delta and Omicron), to investigate the kinetics of IgG subclasses specific to the wild‐type SARS‐CoV‐2 RBD, and to explore the correlation between IgG subclasses and neutralizing ability in a large‐scale, long‐term prospective cohort study of 174 healthy adults vaccinated with inactivated COVID‐19 vaccines following primary and boost immunization.
MATERIALS AND METHODS
Study design, participants and serum collection method
Healthy, non‐pregnant adults aged 18 to 59 years old were recruited. The main exclusion criteria included a history of SARS‐CoV, SARS‐CoV‐2 or Middle East respiratory syndrome infection, a recent history (within 14 days before enrolment) of travel or exposure to infected individuals, axillary temperature > 37.0°C and reported allergy to any vaccine components. A complete list of exclusion criteria was included in the study protocol and approved by the Ethics Committee of Beijing Centre for Disease Prevention and Control (2020‐28). Written informed consents were obtained from all participants both before enrolment and before the administration of the booster shot. All participants were tested for SARS‐CoV‐2 nucleic acid biweekly according to the study protocol.
The participants were administered two doses of CoronaVac (Sinovac Life Sciences, Beijing, China) which is an inactivated vaccine against COVID‐19, at day 0 and day 14. A booster dose was given 12 months after the completion of the primary immunization. Blood samples were collected from the participants before vaccination and at 1, 3, 6 and 12 months after the second dose. The participants who received booster shots were randomly assigned to five groups, and their serum samples were collected on the 3rd, 7th, 10th, 14th and 21st days, respectively. The study design and sample collection schedule were summarized in Figure 1.
FIGURE 1.
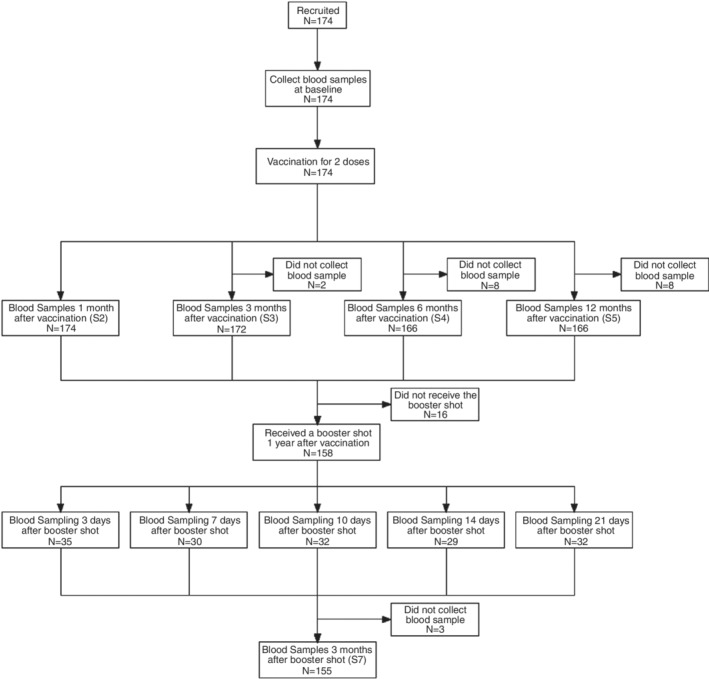
Study design and the sample collection
SARS‐CoV‐2 neutralization assay
Titres of neutralizing antibodies against live SARS‐CoV‐2 virus (wild‐type strain: SARS‐CoV‐2/human/CHN/CN1/2020, Beta: EPI_ISL_2536954, Gamma: EPI_ISL_1060876, Delta: EPI_ISL_1911197, Omicron provided by Sinovac Life Sciences, Beijing, China) were quantified using the micro cytopathogenic effect assay. Briefly, the serum samples were incubated at 56°C for 30 min and serially diluted with the cell culture medium. The diluted samples were incubated in duplicate with 50 μl of SARS‐CoV‐2 virus suspension at 37.0°C for 2 h. Vero cells (1.0–2.0 × 105 cells/mL) were thereafter added to the suspension and incubated at 36.5°C for 5 days. The cytopathic effects were recorded and the neutralizing antibody titres were then calculated by the dilution number of 50% protective condition. Seroconversion threshold of the neutralizing antibodies was defined as a titre of 8 [20].
Anti‐SARS‐CoV‐2 receptor binding domain (RBD) IgG subclasses assay
The anti‐SARS‐CoV‐2 RBD‐specific IgG subclasses serologic assay kit (ACROBiosystems) was used to measure the levels of IgG1, IgG2, IgG3 and IgG4 by an indirect enzyme linked immunosorbent assay (ELISA). The microplate was pre‐coated with the RBD of the spike protein. The sensitivity (lower detection limit) of SARS‐CoV‐2 RBD specific IgG1, IgG2, IgG3 and IgG4 monoclonal antibody was 10 ng/mL, 20 ng/mL, 0.2 ng/mL and 0.8 ng/mL, respectively. We used SARS‐CoV‐2 negative serum to validate its specificity. The specificity of IgG1, IgG2, IgG3 and IgG4 was 94.8%, 97.7%, 93.1% and 100%, respectively. The initial dilution of the sample was set to 1:20, since the dilution ratio of 1:20 can eliminate the background signal to the greatest extent while maintaining relatively high sensitivity. The cut‐off value was 0.1, as calculated by 2.1 times the standard deviation of the OD values of a large number of SARS‐CoV‐2 negative serum. If the OD value/cut‐off value (S/CO) was ≥1, then the test result was considered positive, whereas S/CO < 1 was negative. The corresponding level of IgG subclasses was calculated as S/CO × dilution folds. If saturated OD signals were observed, the samples were serially diluted until a negative test result was reached. The maximum dilution multiple of the positive test results was selected, and the corresponding OD value of the maximum dilution/cut‐off × dilution multiple was the antibody level corresponding to the sample.
Statistical analysis
IgG subclass levels were presented as S/CO × dilution folds with 95% confidence intervals (CIs). The neutralizing antibody titres were presented as dilution folds. The geometric mean titres (GMTs) and 95% CIs were calculated with log values of the titres followed by subsequent antilog‐transformation. Bonferroni's multiple comparison test was used to compare the titres of neutralizing antibodies against different strains. Mann–Whitney U test was used to compare the neutralizing antibody titres at different time points. Two‐sided p‐values < 0.05 were considered statistically significant. Statistical analyses were conducted with GraphPad Prism 8.0.1.
RESULTS
Demographic characteristics
A total of 174 participants were enrolled in this study. None of the participants was tested positive for SARS‐CoV‐2 nucleic acid until the end of the follow‐up. The oldest participant was 59 years old, and the youngest was 22 years old. The mean age of the cohort was 39.7 years old. 43.7% of the participants were male and 56.3% were female.
Immunogenicity and antibody persistence after 2‐dose primary immunization
Regarding the wild‐type strain, the GMT (geometric mean titre) of the neutralizing antibodies reached the peak of 7.1 at 1 month after the primary immunization, with a seroconversion rate of 50.6% (Figure 2a and Table S1). At 3 months, the GMT decreased to 4.5, and the seropositivity rate decreased to 21.5%. Interestingly, at 6 months, the GMT increased to 5.5, and the seropositivity rate increased to 37.3%. At 12 months, the GMT decreased again to 4.6, and the seropositivity rate decreased to 25.9%.
FIGURE 2.
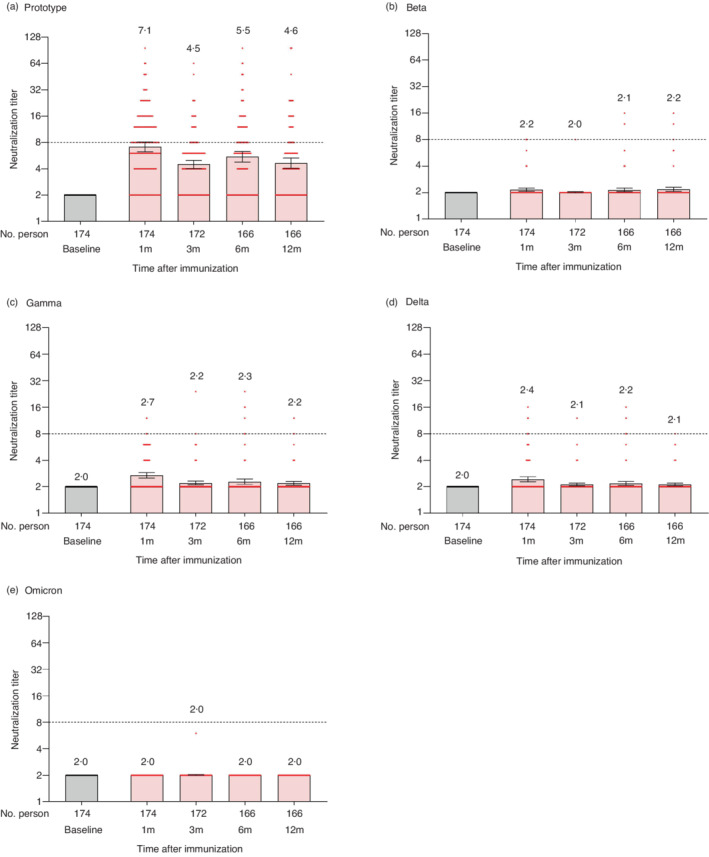
Neutralizing antibodies against the wild‐type strain and variants of SARS‐CoV‐2 after 2‐dose primary immunization. The results of neutralization assays against SARS‐CoV‐2 wild‐type strain (a), Beta variant (b), Gamma variant (c), Delta variant (d), and Omicron variant (e). Each dot represents the neutralizing antibody titre of an individual. The numbers indicated above the bars are the geometric mean titres (GMT), and the error bars indicate the 95% confidence intervals (CI) of GMT. The dotted horizontal line represents the seropositivity threshold of 1:8. The titres lower than the limit of detection (1:4) are presented as half the limit of detection (1:2)
The neutralization capacity against the variants was lower than that of the wild‐type. At 1 month, the GMT was 2.2, 2.7, 2.4 and 2.0 for Beta, Gamma, Delta and Omicron variants, respectively. The seroconversion rate was 2.9%, 2.9%, 4.6% and 0.0% for Beta, Gamma, Delta and Omicron variants, respectively (Figure 2b–e and Table S1). The GMT and seropositivity rate against all the analysed variants did not change significantly until 12 months. After the 2‐dose primary immunization, the GMT against the wild‐type and all the variants strain did not reach the positive threshold of 1:8 at any time.
Immunogenicity and antibody persistence after the booster shot
A booster shot was administered to 158 healthy participants who completed the 2‐dose primary immunization. Regarding the wild‐type strain, on the 3rd day after the booster shot, the GMT (3.7) and seroconversion rate (22.9%) of the neutralizing antibodies were comparable to that of 12 months after the primary immunization. The GMT increased significantly thereafter. On the 7th day, the GMT increased to 37.3, and the seroconversion rate was 90% (27/30). The GMT continued to increase on the 10th day (158.2), 14th day (228.2), and reached a peak of 290.6 on the 21st day, thereafter decreased to 143.6 at 3 months (Figure 3a and Table S1). The seroconversion rate reached 100% at the 10th day and remained 100% thereafter.
FIGURE 3.
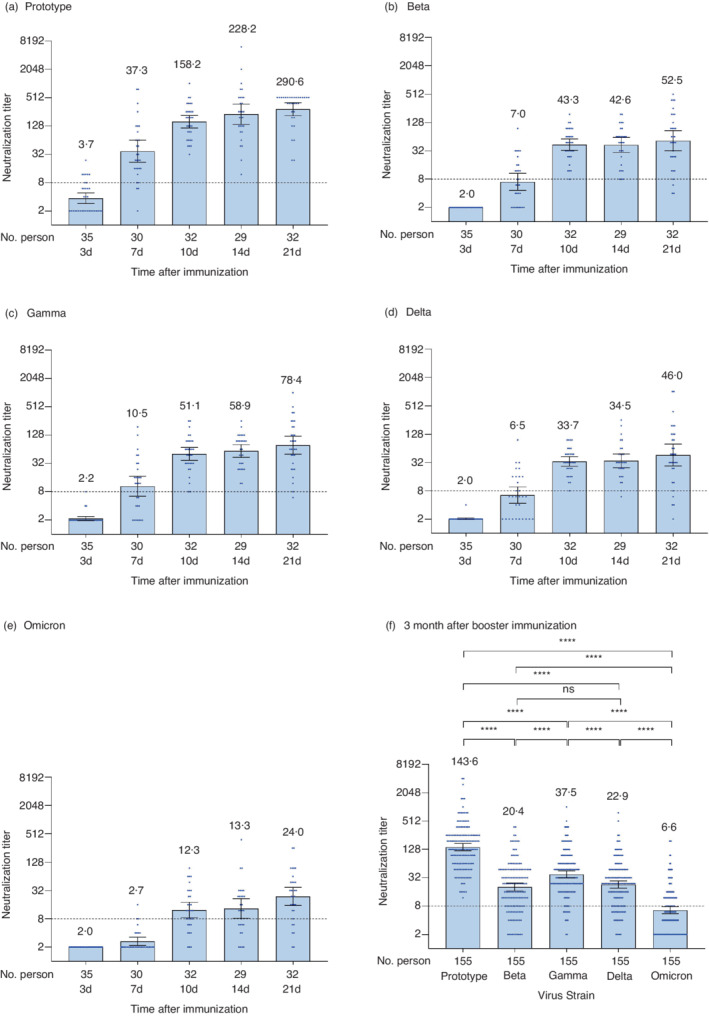
Neutralizing antibodies against the wild‐type strain and variants of SARS‐CoV‐2 after the booster shot. (a–e) show the results of the different neutralization assays against SARS‐CoV‐2 wild‐type strains (a), Beta variant (b), Gamma variant (c), Delta variant (d), and Omicron variant (e), while (f) shows the results at 3 months for the 5 strains. Each dot represents the neutralizing antibody titre of an individual. The numbers above the bars are GMTs, and the error bars indicate the 95% CIs of GMT. The dotted horizontal line represents the seropositivity threshold of 1:8. The titres lower than the limit of detection (1:4) are presented as half the limit of detection (1:2). Bonferroni's multiple comparison test was used to compare the titres of neutralizing antibodies against different strains in (f). ***p < 0.001, ****p < 0.0001, ns: not statistically significant. No multiple comparison adjustment has been done
The four variants showed similar trends with much lower GMTs (p < 0.0001). The peak GMTs of the four variants were reached on the 21st day, 52.5, 78.4, 46.0 and 24.0, respectively. At 3 months after the booster shot, the GMTs decreased significantly to 20.4, 37.5, 22.9 and 6.6; the seropositivity rates decreased to 80.6%, 92.9%, 83.9% and 42.6% for Beta, Gamma, Delta and Omicron variants, respectively (Figure 3b–e and Table S1). We used Bonferroni's multiple comparison test to compare the neutralizing titres of the variants. Among the four variants, the GMT of the Gamma variant was the highest (p < 0.0001), and the GMT of the Omicron variant was the lowest (p < 0.0001). There were no significant differences observed in GMTs between Beta and Delta variants (p > 0.9999) (Figure 3f).
SARS‐CoV‐2 RBD‐specific IgG subclasses after 2‐dose primary immunization and booster shot
ELISA analysis revealed that IgG1 and IgG3 were the most abundant subclasses (Figure 4). At 1 month after the 2‐dose primary immunization, the mean levels of IgG1 and IgG3 were 110.3 and 139.4, respectively. At 3 months, both IgG1 and IgG3 levels decreased rapidly. After that, the level of IgG1 reached the plateau phase, and the level of IgG3 gradually decreased. At 12 months, the mean levels of IgG1 and IgG3 decreased to 21.7 and 29.5, respectively. On the 3rd day after the booster shot, the mean levels of IgG1 and IgG3 were comparable to that of 12 months after the primary immunization. Subsequently, IgG1 and IgG3 levels increased rapidly, especially that of IgG1. The mean levels of IgG1 and IgG3 reached a peak on the 21st days after the booster shot (458.4 and 241.8, respectively) and decreased at 3 months (361.3 and 162.2, respectively).
FIGURE 4.
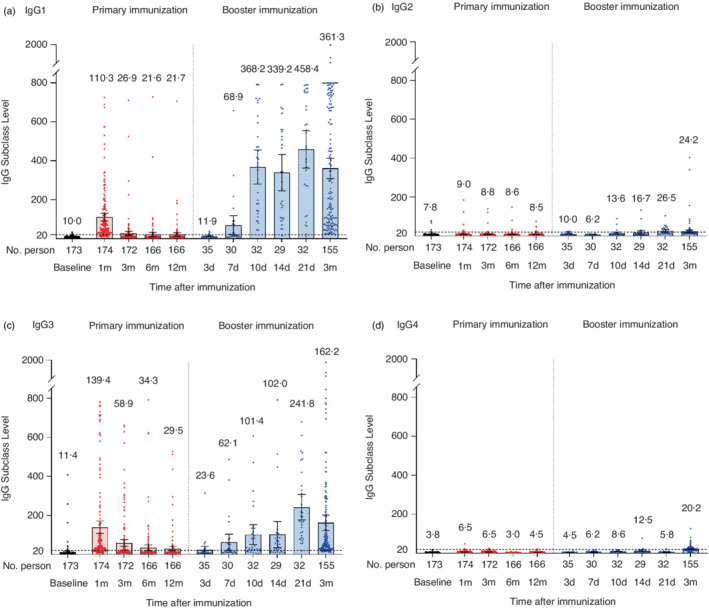
IgG subclasses at the different time points after 2‐dose primary immunization and the booster shot. The levels of IgG1 (a), IgG2 (b), IgG3 (c) and IgG4 (d) after 2‐dose primary immunization and the booster shot. Each dot represents the IgG subclass level of an individual. The numbers above the bars are the mean levels, and the error bars indicate the 95% CIs. The dotted horizontal line represents the seropositive threshold of 20
Relationship between IgG1, IgG3 subclasses and neutralizing antibodies
There was no correlation observed between the titres of the neutralizing antibodies and level of IgG1 or IgG3 individually (Table S2). We divided the participants into groups based on their neutralizing titres, that is, ≤8, 8–16, 16–32, 32–64, etc. The titres of the neutralizing antibodies showed a positive correlation with IgG1/IgG3 ratio in general. After the primary immunization, although the correlation was not linear, the correlation coefficients reached 0.8216 (p = 0.1784), 0.794 (p = 0.206), 0.824 (p = 0.176) and 0.7293 (p = 0.2707) at 1, 3, 6 and 12 months, respectively (Figure 5). After the booster shot, the titres of the neutralizing antibodies exhibited a strong linear relationship with the IgG/IgG3 ratio. At 1 month, the correlation coefficient was 0.776 (p = 0.0236) (Figure 6). At 3 months, the correlation coefficient was 0.9782 (p = 0.0001) (Figure 7a). As for the Beta, Gamma, Delta and Omicron variants, the correlation coefficients were 0.3296 (p = 0.4703), 0.6825 (p = 0.0911), 0.3069 (p = 0.5031) and −0.0110 (p = 0.9842) at 3 months after the booster shot, respectively (Figure 7b–d).
FIGURE 5.
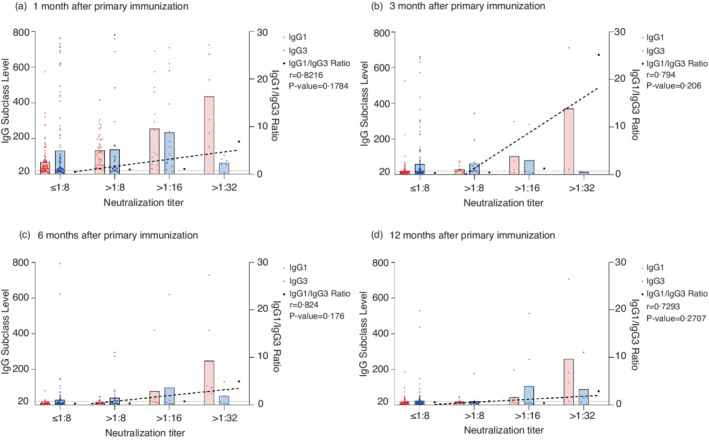
IgG1 and IgG3 distribution after 2‐dose primary immunization. IgG1, IgG3 and IgG1/IgG3 ratio was grouped by titres of the neutralizing antibodies at 1 month (a), 3 months (b), 6 months (c) and 12 months (d) after 2‐dose primary immunization. The dotted line represents the linear trend between the IgG1/IgG3 ratio and titres of the neutralizing antibodies. Based on each individual's neutralizing antibody titre, the seropositive threshold of 1:8, and the serial dilution multiplier of 2 in the neutralizing assay, the participants were divided into several mutually exclusive groups
FIGURE 6.
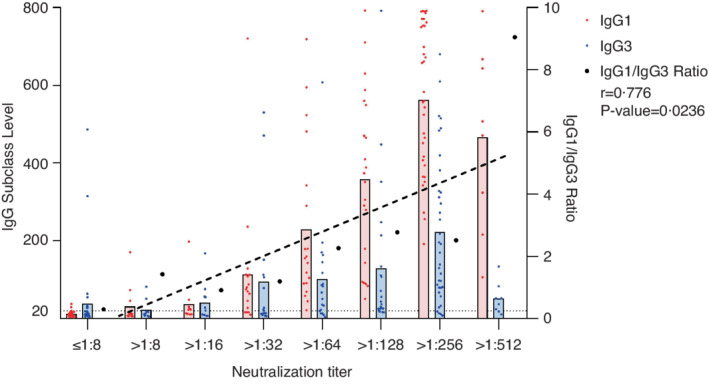
IgG1 and IgG3 distribution after the booster shot within 1 month. IgG1, IgG3 and IgG1/IgG3 ratio grouped by titres of the different neutralizing antibodies after the booster shot within 1 month. The dotted line reprints the linear trend between the IgG1/IgG3 ratio and titres of neutralizing antibodies. The grouping rationale similar to Figure 5 also applies here
FIGURE 7.
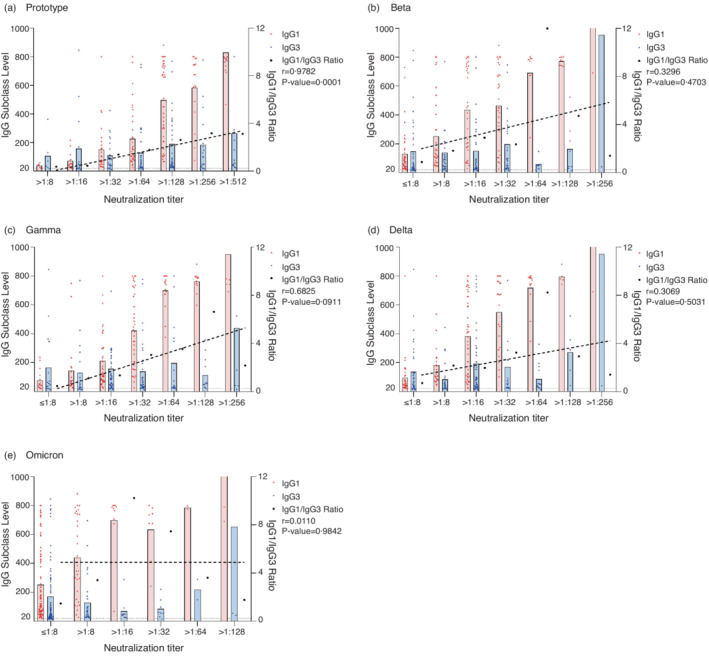
The association between IgG subclasses and neutralizing antibodies against the wild‐type strain and variants at 3 months after the booster shot. IgG1, IgG3 and IgG1/IgG3 ratio grouped by the titres of neutralizing antibodies against the wild‐type strain (a), Beta (b), Gamma (c), Delta (d) and Omicron(e) variants. The dotted line represents the linear trend between the IgG1/IgG3 ratio and the titres of different neutralizing antibodies. The grouping rationale similar to Figure 5 applies here
DISCUSSION
In the present study, we showed the kinetics of neutralizing antibodies against the wild‐type strain and variants after 2‐dose primary immunization and a booster shot of inactivated vaccine. After the primary immunization, the GMT of neutralizing antibodies exhibited a moderate increase and reached the peak at 1 month. However, the GMT against the wild‐type strain did not reach the threshold of 8 since most of the individuals had low titres of antibodies. The peak seroconversion rate was only 50.6% at 1 month. GMTs of the neutralizing antibodies against the variants were even lower. Therefore, regarding immunogenicity, the 2‐dose primary immunization only achieved low neutralizing ability. After the booster shot, the GMT rapidly increased, and the seroconversion rate reached 100% within 10 days, for the wild‐type strain, Beta, Gamma and Delta variants. At 3 months after the booster shot, the GMT against the wild‐type strain was about 20.2 times the GMT at 1 month after the primary immunization (Table S3). This pattern of rapid immune response and persistence of antibodies was consistent with the characteristics of immune memory. This finding can largely dispel the concerns that inactivated vaccines cannot induce significant cell‐mediated immune response, and that they can only result in weak immunogenicity with deficiency of long‐time immune memory. Furthermore, efficacy of inactivated vaccine against the variants has been demonstrated indirectly due to increased immunogenicity after the booster shot.
This study showed that IgG1 and IgG3 were the most abundant IgG subclasses at all time points. This observation was consistent with Fraley et al.'s finding for mRNA vaccines [21] and Suthar et al.'s finding in patients naturally infected with SARS‐CoV‐2 [22]. More specifically, in this study IgG1 and IgG3 levels were similar after primary immunization, whereas IgG1 level was much higher than IgG3 level after the booster shot. It has been reported that the humoral immune response in individuals recovered from the natural infections of COVID‐19 was primarily dominated by IgG1‐producing memory B cells whereas the amount of IgG3‐producing memory B cells was relatively low [23, 24]. Whether the humoral immune response after the booster immunization with SARS‐CoV‐2 inactivated vaccines might be directly related to the presence of memory B cells requires further verification.
Interestingly, the GMT and seropositivity rate of the neutralizing antibodies at 6 months after the primary immunization were higher than that at 3 months. Several previous studies have indicated that IgG subclass switching may substantially impact the titre of the neutralizing antibodies [25, 26]. It has been found that in infections with rubella and measles viruses, the affinity of IgG1 was higher than that of IgG3, and the affinity maturation time of IgG1 was later than that of IgG3 [27, 28]. We examined whether the relative amounts of IgG1 and IgG3 was correlated with the GMT of the neutralizing antibodies at different time points, and we noticed that a higher percentage of individuals with IgG1 > IgG3 is correlated with a higher GMT of the neutralizing antibody (Pearson correlation coefficient = 0.7718, p‐value = 0.0089, Figure S3). More specifically, at 1 month after primary immunization, the percentage of individuals with IgG1/IgG3 > 2 is 39.7%, and the GMT of the neutralizing antibodies was relatively high. At 3 months, the percentage of individuals with IgG1/IgG3 > 2 decreased to 8.7%, and the GMT of the neutralizing antibodies decreased as well. At 6 months, the percentage of individuals with IgG1/IgG3 > 2 increased to 12.0%, and the GMTs of the neutralizing antibodies also increased (Table S3, Table S4, and Figure S1). Therefore, we speculate that IgG1 affinity maturation might have occurred between 3 to 6 months after the primary immunization with SARS‐CoV‐2 inactivated vaccines.
A positive correlation was observed between the ratio of IgG1/IgG3 and GMT of the neutralizing antibodies after we grouped the participants by their neutralizing titre. Individually, when the IgG1/IgG3 ratio was less than 5, the GMT of the neutralizing antibodies increased concomitantly with the IgG1/IgG3 ratio. When the IgG1/IgG3 ratio was 5 or higher, the GMT reached a plateau phase (Figure S2).
The neutralization assay was conducted in vitro to evaluate the potential inhibitory effect of antibodies against the virus. The inhibitory effect primarily depends on the selective binding of the Fab segment to the viral epitope, while the function of the Fc segment remains elusive. Three different types of Fc receptors (FcγRI, FcγRII and FcγRIII) can interact with IgG. In humans, the FcγRI is expressed on the surface of monocytes, macrophages and dendritic cells, and can effectively bind to the monomeric IgG1 and IgG3 with high affinity [29]. Yates et al. have shown that the spike‐specific IgG subclasses may contribute to COVID‐19 disease severity through regulating potent Fc‐mediated effector functions [30]. In addition, the different subclasses of vaccine‐elicited antibodies may differentially recruit and activate innate immune effector cells expressing various IgG receptors on their surface, thereby significantly affecting the vaccine efficacy [31].
The present study has many strengths. First, we constructed a prospective cohort which covered a large sample size, a long follow‐up time, and multiple time points. Second, the correlation between IgG1/IgG3 ratio and the GMT of the neutralizing antibodies is a novel discovery.
However, due to limited detection reagents, we were only able to investigate the RBD‐specific IgG subclasses for the wild‐type strain. Therefore, we cannot confidently draw the practical conclusions regarding the Beta, Gamma, Delta and Omicron variants. Further studies will examine IgG subclasses specific to variant strains.
In conclusion, the findings of this study have indicated that the 2‐dose primary immunization of COVID‐19 inactivated vaccine only achieved low neutralization ability. After administration of the booster shot, the neutralization ability against the wild‐type strain and all variants significantly improved. IgG subclass switching affected neutralizing ability in people receiving COVID‐19 inactivated vaccines. The ratio of IgG1/IgG3 was positively correlated with the titre of neutralizing antibody. The underlying mechanism is not yet clear, and requires further research.
AUTHOR CONTRIBUTIONS
Jiang Wu, Weixin Chen, Qun Zheng and Wei Zhao conceived the project. Jiang Wu, Shuang Bai, Juan Li, Bing Zhang and Meng Chen coordinated and performed the cohort study. Weixin Chen, Yali Wang and Bing Zhang performed the experimental measurements. Weixin Chen, Lichi Zhang and Jiang Wu analysed the data and wrote the manuscript with inputs from all authors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary Figure S1 Percentage of the participants with IgG1/IgG3 ratio > 2 at different time points against the wild‐type strain
Supplementary Figure S2 Best‐fit curve of neutralizing titre versus IgG1/IgG3 ratio after the booster shot against the wild‐type strain. Each dot represents an individual
Supplementary Figure S3 Linear regression line with 95% CI of neutralizing titre versus the percentage of participants with IgG1/IgG3 ratio > 1 at each time point. Each dot represents one specific time point
Supplementary Table S1 The neutralizing antibody positivity rate and GMT at different time points for the prototype and variants
Supplementary Table S2 The correlation* between IgG subclass and neutralizing titre at different time point for the prototype
Supplementary Table S3 Comparison* of GMT at different time points for the prototype and variants
Supplementary Table S4 Number and percentage of participants with IgG1/IgG3 ratio > 2 at different time points for the prototype
ACKNOWLEDGEMENTS
We would like to thank all the participants who attended this study. In addition, we would like to thank Dr. Ying Sun (School of Basic Medical Science, Capital Medical University, Beijing, China) for discussion. This study was supported by the Beijing Municipal Science and Technology Commission (Z211100002521014).
Chen W, Zhang L, Li J, Bai S, Wang Y, Zhang B, et al. The kinetics of IgG subclasses and contributions to neutralizing activity against SARS‐CoV‐2 wild‐type strain and variants in healthy adults immunized with inactivated vaccine. Immunology. 2022. 10.1111/imm.13531
Funding information Beijing Municipal Science and Technology Commission, Grant/Award Number: Z211100002521014
Contributor Information
Wei Zhao, Email: zw830424@163.com.
Jiang Wu, Email: wj81732@hotmail.com.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (2022)
- 2. UNICEF . COVID‐19 vaccine market dashboard (2022). Accessed 9 Feb 2022.
- 3. https://ourworldindata.org/covid-vaccinations (2022)
- 4. Thomas JK. Vaccines in Kuby immunology. 6th ed. England: W. H. Freeman and Company; 2006. p. 484. [Google Scholar]
- 5. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS‐CoV‐2 vaccine in Chile. N Engl J Med. 2021;385:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ranzani OT, Hitchings MDT, Dorion M, D'Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID‐19 in Brazil: test negative case‐control study. BMJ. 2021;374:n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang M, Yi Y, Li Y, Sun L, Deng A, Hu T, et al. Effectiveness of inactivated COVID‐19 vaccines against COVID‐19 pneumonia and severe illness caused by the B.1.617.2 (delta) variant: evidence from an outbreak in Guangdong, China. SSRN; published online Aug 5. 2021. https://ssrn.com/abstract=3895639
- 8. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (2022)
- 9. GISAID . https://www.gisaid.org/hcov19-variants/ (2022)
- 10. Li Q, Nie J, Wu J, Zhang L, Ding R, Wang H, et al. SARS‐CoV‐2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell. 2021;184:2362–71e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia‐Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS‐CoV‐2 variants escape neutralization by vaccine‐induced humoral immunity. Cell. 2021;184:2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bar‐On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against COVID‐19 in Israel. N Engl J Med. 2021;385:1393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abu‐Raddad LJ, Chemaitelly H, Butt AA, National Study Group for C‐V . Effectiveness of the BNT162b2 COVID‐19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two‐dose schedule, in healthy adults: interim results from two single‐centre, double‐blind, randomised, placebo‐controlled phase 2 clinical trials. Lancet Infect Dis. 2021;22:483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS‐CoV‐2 in convalescent individuals. Nature. 2020;584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J, et al. Rapid generation of durable B cell memory to SARS‐CoV‐2 spike and nucleocapsid proteins in COVID‐19 and convalescence. Sci Immunol. 2020;5:eabf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X, Li R, Pan Z, Qian C, Yang Y, You R, et al. Human monoclonal antibodies block the binding of SARS‐CoV‐2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17:647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schur PH. IgG subclasses—a review. Ann Allergy. 1987;58:89–96. [PubMed] [Google Scholar]
- 19. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy children and adolescents: a double‐blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fraley E, LeMaster C, Geanes E, Banerjee D, Khanal S, Grundberg E, et al. Humoral immune responses during SARS‐CoV‐2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021;19:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, et al. Rapid generation of neutralizing antibody responses in COVID‐19 patients. Cell Rep Med. 2020;1:100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Campos‐Mata L, Tejedor VS, Tacho‐Pinot R, Pinero J, Grasset EK, Arrieta AI, et al. SARS‐CoV‐2 sculpts the immune system to induce sustained virus‐specific naive‐like and memory B‐cell responses. Clin Transl Immunol. 2021;10:e1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O, et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6:eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo H, Jia T, Chen J, Zeng S, Qiu Z, Wu S, et al. The characterization of disease severity associated IgG subclasses response in COVID‐19 patients. Front Immunol. 2021;12:632814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stephens DS, McElrath MJ. COVID‐19 and the path to immunity. JAMA. 2020;324:1279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson KM, Di Camillo C, Doughty L, Dax EM. Humoral immune response to primary rubella virus infection. Clin Vaccine Immunol. 2006;13:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El Mubarak HS, Ibrahim SA, Vos HW, Mukhtar MM, Mustafa OA, Wild TF, et al. Measles virus protein‐specific IgM, IgA, and IgG subclass responses during the acute and convalescent phase of infection. J Med Virol. 2004;72:290–8. [DOI] [PubMed] [Google Scholar]
- 29. Neidich SD, Fong Y, Li SS, Geraghty DE, Williamson BD, Young WC, et al. Antibody Fc effector functions and IgG3 associate with decreased HIV‐1 risk. J Clin Invest. 2019;129:4838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yates JL, Ehrbar DJ, Hunt DT, Girardin RC, Dupuis AP 2nd, Payne AF, et al. Serological analysis reveals an imbalanced IgG subclass composition associated with COVID‐19 disease severity. Cell Rep Med. 2021;2:100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nimmerjahn F, Ravetch JV. Antibody‐mediated modulation of immune responses. Immunol Rev. 2010;236:265–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Percentage of the participants with IgG1/IgG3 ratio > 2 at different time points against the wild‐type strain
Supplementary Figure S2 Best‐fit curve of neutralizing titre versus IgG1/IgG3 ratio after the booster shot against the wild‐type strain. Each dot represents an individual
Supplementary Figure S3 Linear regression line with 95% CI of neutralizing titre versus the percentage of participants with IgG1/IgG3 ratio > 1 at each time point. Each dot represents one specific time point
Supplementary Table S1 The neutralizing antibody positivity rate and GMT at different time points for the prototype and variants
Supplementary Table S2 The correlation* between IgG subclass and neutralizing titre at different time point for the prototype
Supplementary Table S3 Comparison* of GMT at different time points for the prototype and variants
Supplementary Table S4 Number and percentage of participants with IgG1/IgG3 ratio > 2 at different time points for the prototype
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


