Abstract
Background and purpose
Recent findings document a blunted humoral response to SARS‐CoV‐2 vaccination in patients on anti‐CD20 treatment. Although most patients develop a cellular response, it is still important to identify predictors of seroconversion to optimize vaccine responses.
Methods
We determined antibody responses after SARS‐CoV‐2 vaccination in a real‐world cohort of multiple sclerosis patients (n = 94) treated with anti‐CD20, mainly rituximab, with variable treatment duration (median = 2.9, range = 0.4–9.6 years) and time from last anti‐CD20 infusion to vaccination (median = 190, range = 60–1032 days).
Results
We find that presence of B cells and/or rituximab in blood predict seroconversion better than time since last infusion. Using multiple logistic regression, presence of >0.5% B cells increased probability of seroconversion with an odds ratio (OR) of 5.0 (95% confidence interval [CI] = 1.0–28.1, p = 0.055), whereas the corresponding OR for ≥6 months since last infusion was 1.45 (95% CI = 0.20–10.15, p = 0.705). In contrast, detectable rituximab levels were negatively associated with seroconversion (OR = 0.05, 95% CI = 0.002–0.392, p = 0.012). Furthermore, naïve and memory IgG+ B cells correlated with antibody levels. Although retreatment with rituximab at 4 weeks or more after booster depleted spike‐specific B cells, it did not noticeably affect the rate of decline in antibody titers. Interferon‐γ and/or interleukin‐13 T‐cell responses to the spike S1 domain were observed in most patients, but with no correlation to spike antibody levels.
Conclusions
These findings are relevant for providing individualized guidance to patients and planning of vaccination schemes, in turn optimizing benefit–risk with anti‐CD20.
Keywords: B‐cell depletion, multiple sclerosis, rituximab, SARS‐CoV‐2, vaccination
A blunted humoral response to SARS‐CoV‐2 vaccination has been reported in patients on anti‐CD20 treatment; thus, it is important to identify predictors of seroconversion to optimize vaccine responses. In this study, B‐cell and plasma rituximab levels are shown to be better predictors of seroconversion than time between treatment dose and vaccination.
INTRODUCTION
B‐cell‐depleting therapies (BCDTs), in particular anti‐CD20 monoclonal antibodies, are commonly administered for a broad spectrum of autoimmune disorders, including multiple sclerosis (MS) [1, 2]. With the outbreak of the COVID‐19 pandemic, concerns were raised regarding the impact of BCDTs on humoral and cellular immune response to SARS‐CoV‐2 infection and vaccination [3]. Since then, several studies have reported a blunted antibody response, albeit a largely intact T‐cell response, in persons with MS (pwMS) treated with BCDTs [4, 5, 6, 7, 8, 9, 10, 12]. Failure to seroconvert has in particular been observed in pwMS who received an anti‐CD20 infusion close to the time of vaccination [4, 13]. This observation has led to discussions regarding whether there is a need to extend dosing intervals before SARS‐CoV‐2 vaccination [14, 15]. However, the few studies that report on the relation between time since last infusion and vaccine efficacy included patient cohorts that had received anti‐CD20 treatment with the standard dosing regimen, and with limited treatment duration [4, 13, 16]. Although a correlation between time since last dose and B‐cell repletion exists at the group level, variable total treatment duration and individual differences may impact on B‐cell repopulation dynamics [17]. Therefore, an ideal dosing interval to optimize vaccine response that fits all patients seems unlikely, and there is a need for robust biomarkers that can predict vaccine efficacy while maintaining the effect of anti‐CD20 treatment on relapse rates [10, 12].
The main objective of this study was to determine the impact of time since last anti‐CD20 infusion, B‐cell levels, and rituximab (RTX) concentration in plasma on the seroconversion rate after SARS‐CoV‐2 mRNA vaccination. Notably, we had access to a cohort of pwMS on BCDTs where extended anti‐CD20 dosing intervals had already been implemented before the start of the pandemic, resulting in a large time span since last infusion, thereby providing more robust information on possible effects of BCDTs on vaccination responses.
MATERIALS AND METHODS
Study design and subjects
The study design is shown in Figure 1a. The anti‐CD20‐treated cohort comprised 94 pwMS at the Academic Specialist Clinic, Stockholm, Sweden participating in the prospective COMBAT‐MS study (COMparison Between All immunoTherapies for Multiple Sclerosis; Clinicaltrials.gov identifier: NCT03193866, EudraCT 2016–003587‐39). All pwMS in this study had received BCDT before inclusion with either RTX (Mabthera or Rixathon; n = 82), ocrelizumab (OCR; n = 10), or ofatumumab (OFA; n = 2) and had received two doses with BNT162b2 (Comirnaty) or mRNA‐1273 (Moderna). Peripheral blood mononuclear cells (PBMCs) and plasma were collected for detection of SARS‐CoV‐2 specific humoral and cellular responses before (n = 10) and/or 4 (n = 94) and/or 12 (n = 25) weeks after the second dose (booster) of SARS‐CoV‐2 vaccine. In addition, 11 healthy individuals were included and sampled at 4 (n = 11) and 12 (n = 1) weeks after booster. See Table 2 for cohort characteristics. PBMCs were freshly isolated from sodium citrate‐containing cell preparation tubes (BD Biosciences). All isolated PBMCs were cryopreserved in freezing media containing 10% dimethyl sulfoxide (Sigma‐Aldrich) and stored at −180°C. Plasma were prepared from EDTA tubes and stored at −80°C.
FIGURE 1.
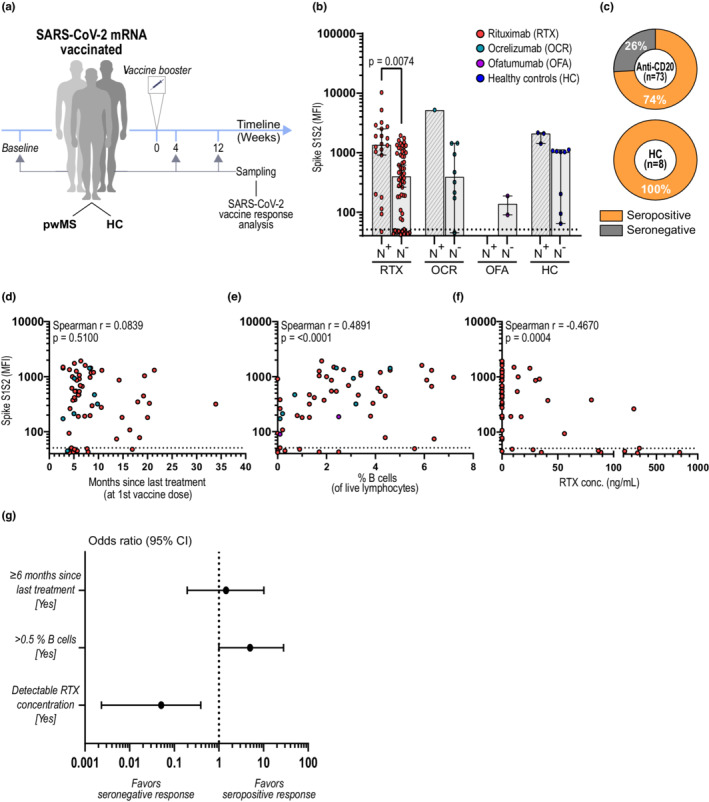
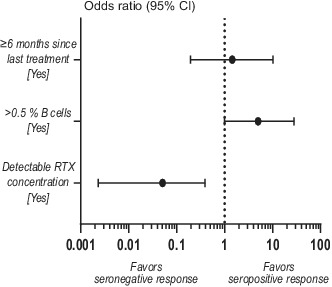
B‐cell and plasma rituximab (RTX) levels are better predictors of humoral response after SARS‐CoV‐2 vaccination than time since last anti‐CD20 treatment. (a) Study design. SARS‐CoV‐2 mRNA‐vaccinated individuals were sampled before (baseline) and/or 4 and/or 12 weeks after booster and analyzed for both SARS‐CoV‐2‐specific humoral and cellular responses. (b) Spike S1S2 antibody levels measured as median fluorescent intensity (MFI) 4 weeks after booster. Spike S1S2 antibody levels in COVID‐19‐recovered (N+) and naïvely vaccinated (N−) individuals are shown: RTX N+ (n = 20), N− (n = 62); OCR N+ (n = 1), N− (n = 9); OFA N+ (n = 0), N− (n = 2); HC N+ (n = 3), N− (n = 8). (c) Proportion of seropositivity 4 weeks after booster in naïvely vaccinated persons with multiple sclerosis (pwMS) on anti‐CD20 treatment (n = 73) and in HC (n = 8). (d–f) Correlations between antibody levels and (d) months since last treatment (at first vaccine dose), (e) percentages of B cells (of live lymphocytes), and (f) RTX concentration in plasma, all measured at 4 weeks after booster. (g) Forest plot showing the effect of time since last treatment, percentages of B cells, and detectable RTX concentration in plasma on seroconversion after SARS‐CoV‐2 mRNA vaccination in pwMS treated with RTX (n = 55). Dots represent individual data points. Box plots represent median and 95% confidence interval (CI). Dotted lines indicate cutoff value for antibody positivity. Kruskal–Wallis test with Dunn post hoc multiple comparison test was used for statistical analysis, and p‐values < 0.05 were considered significant. Spearman r and p‐values are shown. Multiple logistic regression analysis was used for prediction of seroconversion
TABLE 2.
Characteristics of the study population
| Study participants | n | Age, years, mean ± SD | Female, n (%) | EDSS, median (minimum; maximum) | Treatment duration, years, median (minimum; maximum) | COVID‐19‐recovered, n (%) | Likely naïvely vaccinated, n (%) |
|---|---|---|---|---|---|---|---|
| HC | 11 | 38.5 ± 11.9 | 6 (54.5%) | ‐ | ‐ | 3 (27.3%) | 8 (72.7%) |
| MS | 94 | 40.7 ± 9.9 | 63 (67.0%) | 1.5 (0; 7) | 2.9 (0.4; 9.6) | 21 (22.3%) | 73 (77.7%) |
| Rituximab | 82 | 41.1 ± 9.9 | 56 (68.3%) | 1.5 (0; 7) | 2.6 (0.4; 9.6) | 20 (24.4%) | 62 (75.6%) |
| Ocrelizumab | 10 | 35.9 ± 8.7 | 5 (50.0%) | 1 (0; 3) | 4.4 (0.4; 8.8) | 1 (10.0%) | 9 (90.0%) |
| Ofatumumab | 2 | 48.5 ± 3.5 | 2 (100%) | 0.5 (0; 1) | 3.1 (1.8; 4.4) | 0 (0%) | 2 (100%) |
Abbreviations: EDSS, Expanded Disability Status Scale; HC, healthy controls; MS, multiple sclerosis.
Study procedures were conducted under the following ethical permits approved by the Swedish ethical review authority: COMBAT‐MS, 2017/32–31/4; STOPMSII, 2009/2107–31/2, 2020–00052, with written informed consent from participants.
Data collection
Patient baseline characteristics were extracted from the Swedish Multiple Sclerosis Register (SMSReg) [18]. Clinical relapse events [19] were also extracted from the SMSReg, where they are recorded at every annual visit and upon acute contacts.
Serological analyses
Detection of SARS‐CoV‐2 IgG in plasma was performed using a multiplex antigen‐bead array assay as previously described [20]. The assay measured IgG reactivity toward the full‐length spike glycoprotein (spike S1S2 foldon), the spike S1 ectodomain produced in HEK cells, and the nucleocapsid protein C‐terminal domain produced in Escherichia coli (nucleocapsid C). Median fluorescent intensity (MFI) and bead count were determined for each antigen. A cutoff of reactivity was defined for the spike protein variants as the mean MFI + 6 SD, and for the nucleocapsid protein as the mean MFI + 12 SD of a set of 12 negative controls included in each analysis. Samples were regarded as seropositive when reactive to at least two viral antigens. Seven samples collected 4 weeks after booster in the pwMS cohort displayed an MFI value above the cutoff exclusively for the spike S1S2 foldon and were hence considered to be seronegative in the final assessment. Those samples were excluded for all analyses involving MFI values but included in categorical analyses involving serostatus.
Anti‐RTX enzyme‐linked immunosorbent assay
RTX concentrations were measured in plasma of patients treated with Mabthera or Rixathon by enzyme‐linked immunosorbent assay (ELISA). As a standard curve, Mabthera or Rixathon (10 mg/ml diluted in NaCl) was diluted in a threefold step into eight standard points (range = 0.14–300 ng/ml) for each treatment respectively. Hispec Assay Diluent (BUF049A, Bio‐Rad) was used to dilute the standard and plasma samples (dilution 1:50). A sandwich ELISA was performed in 96 well MaxiSorp plates (Thermo Fisher Scientific) using 1 μg/ml anti‐RTX (HCA186, Bio‐Rad) in phosphate‐buffered saline (PBS) as capture antibody, PBS containing 1% bovine serum albumin (Cell Signaling technology, #9982) as blocking solution, and horseradish peroxidase‐conjugated rat anti‐RTX antibody (MB2A4, Bio‐Rad) diluted 1:5000 in Hispec Assay Diluent as detection antibody. RTX concentrations for both Mabthera and Rixathon were grouped together in all analyses.
B‐ and T‐cell characterization of PBMCs
PBMCs were thawed in complete RPMI (cRPMI; R8758, Sigma‐Aldrich) containing 10% heat‐inactivated fetal bovine serum (F7524, Sigma‐Aldrich), 100 U/ml penicillin, and 100 μg/ml streptomycin (P4458, Sigma‐Aldrich), washed and stained with fluorochrome‐conjugated antibodies (Table 1) in the presence of Live Dead (Invitrogen) for 15–30 min at room temperature.
TABLE 1.
List of anti‐human antibodies used for lymphocyte immunophenotyping by flow cytometry
| Brand | Antigen | Fluorochrome | Clone |
|---|---|---|---|
| BD Horizon | CCR6/CD196 | BV605 | 11A9 |
| eBioscience | CCR7/CD197 | PerCP‐Cy5.5 | 3D12 |
| eBioscience | CD10 | AF647 | SN5c |
| BD Horizon | CD127 | BV480 | HIL‐7R‐M21 |
| BioLegend | CD19 | SN685 | HIB19 |
| BD Horizon | CD20 | BV711 | 2H7 |
| BioLegend | CD25 | PE | BC96 |
| BD Horizon | CD27 | BV650 | M‐T271 |
| BioLegend | CD3 | SB550 | SK7 |
| BioLegend | CD38 | AF488 | HIT2 |
| BD Horizon | CD4 | BV510 | SK3 |
| BioLegend | CD45RO | PerCP | UCHL1 |
| BD Pharmingen | CD8 | AF700 | RPA‐T8 |
| BioLegend | CXCR3/CD183 | PE‐Cy7 | G025H7 |
| BioLegend | CXCR5/CD185 | BV421 | J252D4 |
| BD Horizon | HLA‐DR | BV480 | G46‐6 |
| BioLegend | HLA‐DR | APC‐Cy7 | L243 |
| BioLegend | IgG | APC‐Cy7 | M1310G05 |
| BD Horizon | IgG | BV510 | G18‐145 |
| BioLegend | IgM | BV570 | MHM‐88 |
| BioLegend | PD‐1/CD279 | PE‐TxRed | EH12.2H7 |
For the measurement of spike‐specific B cells, B cells were enriched from PBMCs using magnetic‐bead negative selection (17963, StemCell) and then incubated with biotin‐conjugated spike recombinant protein, both streptavidin‐PE and streptavidin‐PE‐Vio770, and antibodies using the SARS‐CoV‐2 Spike B Cell Analysis Kit (130–128‐022, Miltenyi Biotec). Measurements were performed on an Aurora spectral cytometer (Cytek), and data were analyzed with FlowJo v10 (Tree Star).
FluoroSpot
Precoated human interferon‐γ (IFN‐γ)/interleukin‐13 (IL‐13) plates (FSP‐0104, Mabtech) were washed with sterile PBS (Sigma‐Aldrich) and blocked for 30 min with cRPMI. For the peptide stimulation, overlapping peptides spanning the nucleocapsid phosphoprotein (Prot_N) and the N‐terminal S1 domain of the spike glycoprotein (Prot_S1; PepTivator, Miltenyi Biotec) were used and processed as previously described [5]. Data are presented as delta‐spot forming units (ΔSFUs) calculated as the mean SFUs for each condition duplicate minus the mean SFUs from the negative controls. Positive T‐cell responses were defined as ≥12.5 ΔSFUs/2.5 × 105 cells. Nine of the 49 samples displayed a positive IFN‐γ T‐cell response and/or an antibody response to the nucleocapsid protein and were categorized as COVID‐19‐recovered individuals and excluded from further analyses.
Statistical analysis
Statistical analyses were performed with GraphPad Prism software v9.0.0. Group analyses were tested with a two‐sided Mann–Whitney test or Kruskal–Wallis with Dunn or Šídák post hoc multiple comparison test. Correlation was calculated using Spearman rank correlation test. A multiple logistic regression model was used for prediction of seroconversion.
RESULTS
Study cohort characteristics
The patient demographics and study design are shown in Table 2 and Figure 1a, respectively. The treatment duration and the interval between the last anti‐CD20 infusion and the first vaccine dose displayed a range of 0.4–9.6 years and 60–1032 days, respectively, thus covering a wide time span. Although 52% of patients did not receive treatment for >6 months, no relapses had been recorded.
Time since last dose, B‐cell levels, and RTX concentration as determinants of seroconversion
The specific SARS‐CoV‐2 antibody response was analyzed 4 weeks after booster in healthy controls (HC) and pwMS on BCDTs. Positive antibody levels and/or T‐cell responses to the nucleocapsid protein were used to separate between naïvely vaccinated and COVID‐19‐recovered individuals who received the vaccine. COVID‐19‐recovered pwMS treated with RTX displayed higher levels of antibody to the spike S1S2 domain compared to naïvely vaccinated pwMS (p = 0.0074), with a similar tendency for OCR‐treated subjects and HC (Figure 1b). In the naïvely vaccinated cohort, seroconversion occurred to a higher degree in HC compared to pwMS (100% and 74%, respectively), without significant differences in antibody levels (Figure 1b,c). In line with previous studies [4], seroconversion was more frequent in patients who received the booster ≥6 months after the last anti‐CD20 dose (odds ratio [OR] = 6.9, p = 0.0067), and the OR for B cells > 0.5% and detectable RTX was 15.0 (p = <0.0001) and 0.02 (p = <0.0001), respectively. However, time since last treatment did not correlate with levels of antibody to the spike S1S2 domain (r = 0.0839, p = 0.5100; Figure 1d). Instead, B‐cell levels and RTX concentration both displayed a strong correlation to antibody levels compared to time since last infusion (r = 0.4891, p = <0.0001; r = −0.4670, p = <0.0004, respectively; Figure 2e,f). When combined in a multiple logistic regression model, both presence of >0.5% B cells (OR = 5.0, 95% CI = 1.0–28.1, p = 0.055) and RTX concentration (OR = 0.05, 95% CI = 0.002–0.392, p = 0.012) were stronger predictors of seroconversion than time since last infusion (OR = 1.45, 95% CI = 0.20–10.15, p = 0.705; Figure 1g).
FIGURE 2.
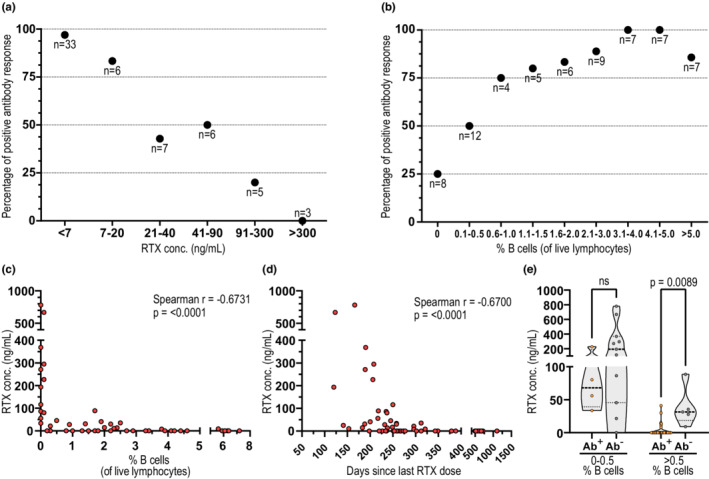
Dose‐dependent influence of rituximab (RTX) and B‐cell levels on seroconversion after SARS‐CoV‐2 mRNA vaccination. (a, b) Percentage of positive antibody responses in persons with multiple sclerosis (pwMS) on RTX in relation to (a) RTX concentration in plasma (n = 60) and (b) percentage of B cells (of live lymphocytes) in peripheral blood (n = 65). (c) Correlation between RTX concentration and percentage of B cells (of live lymphocytes) among pwMS on RTX (n = 55). (d) Correlation between RTX concentration and days since last RTX infusion (n = 60). (e) Levels of RTX concentration in plasma per serostatus and percentage of B cells. Dots represent individual data points. Kruskal–Wallis test with Dunn post hoc multiple comparison test was used for statistical analysis, and p‐values < 0.05 were considered significant. Spearman r and p‐values are shown. Ab, antibody; ns, not significant
Influence of RTX concentration and B‐cell levels on seroconversion
To assess whether RTX concentrations impact seroconversion in a dose‐dependent matter, pwMS on RTX were categorized according to their plasma level of RTX at 4 weeks after booster. We here observed a seemingly linear decrease in seroconversion with increased RTX concentrations, where 97.0% of individuals with an RTX concentration below the detection limit (<7 ng/ml) successfully seroconverted. In contrast, for individuals with the highest levels of RTX (≥91 ng/ml), the seroconversion rate was only 12.5% (Figure 2a). A similar tendency was observed when classifying individuals according to percentages of B cells at 4 weeks after booster (Figure 2b). As expected, percentages of B cells correlated well with RTX concentration (r = −0.6731, p = <0.0001), although detectable levels of RTX did not necessarily result in complete depletion of B cells in all individuals (Figure 2c). Interestingly, levels of RTX above the detection limit could be observed up to 321 days after last dose, although waning in concentration strongly correlated with time (r = −0.6700, p = <0.0001) and became undetectable approximately 8 months after last dose in 71.9% of the individuals (Figure 2d).
Among individuals with ≤0.5% B cells, 14 of 15 (93.3%) had detectable RTX in plasma, and only 4 of 15 (26.7%) had seroconverted. However, among the individuals with >0.5% B cells and RTX below detection limit, all (28 of 28) seroconverted. Moreover, among the seronegative individuals with >0.5% B cells, all (five of five) had detectable levels of RTX in plasma, whereas only six of 34 (17.6%) seropositive individuals also had detectable levels of RTX (p = 0.0089; Figure 2e).
Correlation of B‐ and/or T‐cell subpopulations with the humoral response
To further characterize the effect of lymphocyte subpopulations on seroconversion after vaccination, we performed a detailed immunophenotyping of both B and T cells (Figure 3a). We found that antibody titers correlated with the percentages of naïve and memory IgG+ B cells (Figure 3b,c), and inversely correlated with percentages of CD27+ and total memory B cells. In contrast, there was no association between antibody titers and percentages of T‐cell subpopulations except for CD8+ T cells expressing CXCR5.
FIGURE 3.
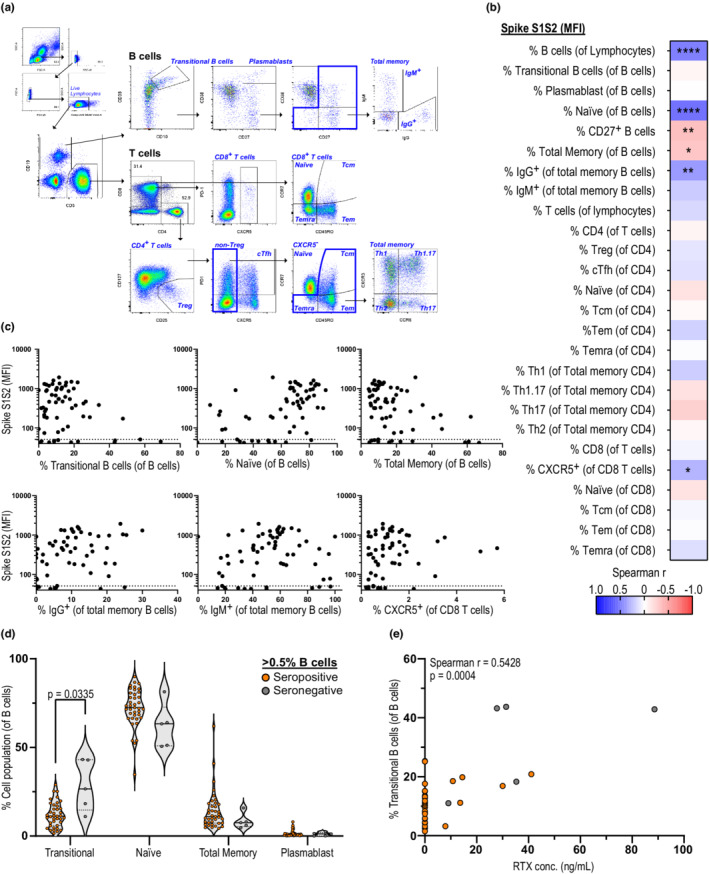
SARS‐CoV‐2 antibody levels correlate with percentages of B‐cell subpopulations. (a) Exemplar gating strategy for flow cytometry staining of B‐ and T‐cell populations. (b) Correlation matrix with Spearman r between spike S1S2 antibody levels (median fluorescent intensity [MFI]) and different B‐ and T‐cell subpopulations in persons with multiple sclerosis (pwMS) on anti‐CD20 4 weeks after booster (n = 62). (c) Individual correlations of spike S1S2 antibody levels and B‐ and T‐cell subpopulations of interest, as in b. (d) Percentages of B‐cell subpopulations (of total B cells) in pwMS with B‐cell levels > 0.5% 4 weeks after booster in seropositive (n = 34) and seronegative (n = 5) individuals. (e) Correlation of transitional B cells with rituximab (RTX) concentration in pwMS 4 weeks after booster (n = 39). Dots represent individual data points. Dotted lines indicate cutoff value for antibody positivity. Correlations analyzed using Spearman r and p‐values are shown: *p < 0.05, **p < 0.01, ****p < 0.0001. Mann–Whitney test with Holm–Šídák adjustments was used for statistical analysis between seropositive and seronegative donors, and adjusted p‐values < 0.05 were considered significant. cTfh, circulating T follicular helper cells; Tcm, central memory T cells; Tem, effector memory T cells; Temra, terminally differentiated effector memory T cells; Th, T helper cells; Treg, regulatory T cells
In line with these results, seronegative individuals with B cells > 0.5% displayed higher percentages of immature transitional B cells (p = 0.0335) and lower levels of naïve B cells (Figure 3d), whereas transitional B‐cell levels correlated with RTX concentrations (Figure 3e). These findings suggest that in addition to the levels of B cells, their maturation stage after repopulation might also impact the ability to mount a humoral response following vaccination.
Effect of RTX dosing on humoral response when given 4–6 weeks after booster
To determine the effect of BCDTs on pre‐existing antibody levels, we compared SARS‐CoV‐2 antibody titers 4 and 12 weeks after the booster in pwMS who did or did not receive an RTX infusion between the two time points. A trend of waning antibody titers was observed in all subgroups, regardless of whether they were treated between sample time points or previous COVID‐19 infection (Figure 4a). In addition, the fold‐change in spike S1S2 antibody levels between 4 and 12 weeks correlated well with the initial antibody levels at 4 weeks (r = −0.5925, p < 0.0001), suggesting antibody half‐life kinetics, rather than retreatment with anti‐CD20, to be the major cause of waning antibody titers (Figure 4b).
FIGURE 4.
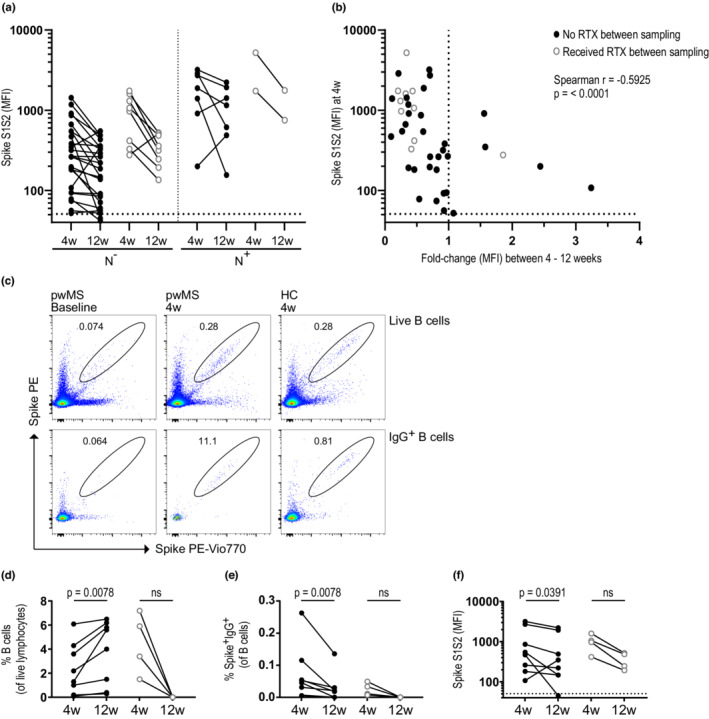
Rituximab (RTX) treatment between 4 (4w) and 12 weeks (12w) postvaccination does not impact SARS‐CoV‐2‐specific antibody levels. (a) Spike S1S2 antibody levels among seroconverted persons with multiple sclerosis (pwMS) on anti‐CD20 between 4 and 12 weeks after booster. Individuals with a likely previous COVID‐19 infection (N+) were separated from naïvely vaccinated (N−) subjects as in b. Further subdivision is shown between individuals who received treatment between the sample time points (open gray circles; n [N−] = 8, n [N+] = 2) and those who did not receive treatment (black circles; n [N−] = 25, n [N+] = 7). (b) Correlation between the spike S1S2 antibody levels at 4 weeks and the fold‐change in antibody levels between 4 and 12 weeks after booster in pwMS on anti‐CD20 treatment who were seroconverted at 4 weeks. (c) Representative dot plots for detection of spike‐specific B cells in pwMS and healthy controls (HC) at baseline and 4 weeks after booster. (d, e) Percentages of B cells of live lymphocytes (d) and percentages of spike‐specific IgG+ B cells (e) 4 and 12 weeks after booster in a subset of pwMS on RTX who did not receive (black circles, n = 8) or did receive (open gray circles, n = 4) RTX between these time points. (f) Spike S1S2 antibody levels 4 and 12 weeks after booster in the same pwMS cohort as in d and e. Dots represent individual data points. Dotted lines indicate cutoff value for antibody positivity. Wilcoxon matched‐pairs signed rank test was used for statistical analysis, and p‐values < 0.05 were considered significant. Spearman r and p‐values are shown. MFI, median fluorescent intensity; ns, not significant; PE, phycoerythrin
The decline in SARS‐CoV‐2 spike antibodies was also accompanied by a decrease in spike‐specific IgG+ B cells (Figure 4c‐f), whereas the total B‐cell levels instead increased in these patients. As expected, RTX treatment after Week 4 postvaccination led to the complete depletion of both total and spike‐specific B cells at Week 12 (Figure 4d,e).
SARS‐CoV‐2 specific T‐cell responses after vaccination
We assessed SARS‐CoV‐2‐specific T‐cell responses in an IFN‐γ/IL‐13 FluoroSpot assay for a subset of pwMS (n = 49). For naïvely vaccinated individuals, an exclusive IFN‐γ and/or IL‐13 T‐cell reactivity toward the spike protein (S1), but not to the nucleocapsid protein (N), was observed 4 weeks after booster (Figure 5a).
FIGURE 5.
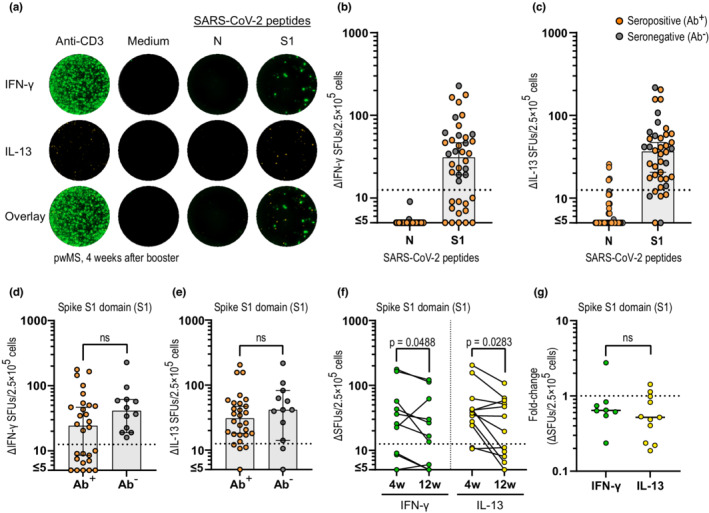
Development of SARS‐CoV‐2 specific T‐cell response in anti‐CD20‐treated persons with multiple sclerosis (pwMS) after vaccination. (a) Representative images of the interferon‐γ (IFN‐γ)/interleukin‐13 (IL‐13) FluoroSpot after stimulation with the positive control (anti‐CD3), negative control (medium only), and SARS‐CoV‐2‐specific peptides (N and S1) in one naïvely vaccinated pwMS 4 weeks after booster (4w). (b, c) Serology status and IFN‐γ (b) and IL‐13 (c) response after stimulation with SARS‐CoV‐2‐specific peptides (N and S1) 4 weeks after booster in naïvely vaccinated pwMS (n = 40). (d, e) Comparison of the IFN‐γ (d) and IL‐13 (e) response between seropositive (Ab+, n = 28) and seronegative (Ab−, n = 12) individuals 4 weeks after booster. (f) Number of IFN‐γ and IL‐13 delta‐spot forming units (ΔSFUs) 4 and 12 weeks after booster (12w) in pwMS on anti‐CD20 (n = 12). (g) Fold‐change in ΔSFUs between 4 and 12 weeks in pwMS with a response to IFN‐γ (n = 7) and IL‐13 (n = 10) above cutoff at 4 weeks after booster. Dots represent individual data points. Box plots represent median and 95% confidence interval. Dotted lines indicate cutoff value for T‐cell‐positive samples. Mann–Whitney and Wilcoxon matched‐pairs signed rank test was used for statistical analyses, and p‐values < 0.05 were considered significant. 4w, 4 weeks after booster; 12w, 12 weeks after booster; Ab, antibody; ns, not significant
Seventy percent of the naïvely vaccinated pwMS developed a positive IFN‐γ T‐cell response 4 weeks after booster. The same proportion (70%) were seropositive at that time, but populations did not overlap and all seronegative pwMS had a positive IFN‐γ T‐cell response (Figure 5b). Interestingly, a higher proportion of individuals (34/40, 85%) displayed a positive IL‐13 response (Figure 5c). Only three patients had no response to either IFN‐γ or IL‐13, resulting in a positive IFN‐γ and/or IL‐13 response of 92.5% in this subgroup. When comparing the number of spike S1‐specific T‐cell responses in seropositive and seronegative individuals, no significant differences were observed for either IFN‐γ (Figure 5d) or IL‐13 (Figure 5e) at 4 weeks after booster. Similar to the waning antibody titers, there was a modest but significantly weaker T‐cell response for both IFN‐γ and IL‐13 (p = 0.0488 and p = 0.0283, respectively) at 12 weeks compared to 4 weeks after booster, with no difference in the decline rate between IFN‐γ and IL‐13 (p = 0.4173; Figure 5f,g). No correlation between IFN‐γ or IL‐13 SFUs toward the spike protein (S1) and spike S1S2 antibody levels 4 weeks after booster were observed (r = −0.1267, p = 0.4359; r = 0.1105, p = 0.4971, respectively).
DISCUSSION
In this study, we evaluated humoral and cellular SARS‐CoV‐2 immune responses following mRNA‐based vaccination in a subcohort of participants in an observational MS drug trial (COMBAT‐MS) receiving anti‐CD20 treatment, mainly RTX. This cohort is characterized by a high variability in anti‐CD20 treatment duration and interval between the last anti‐CD20 infusion and vaccination and is therefore suitable for finding more robust predictors of seroconversion. We find that B‐cell levels are a better predictor of seroconversion than time since last infusion and that presence of RTX in blood is an even stronger indicator of an impaired humoral response after vaccination.
Several studies have reported an association between seroconversion after SARS‐CoV‐2 vaccination and time since the last anti‐CD20 treatment [7, 21, 22]. However, even among patients with extended dosing intervals, unsuccessful seroconversion occurs, suggesting that individual differences in B‐cell repopulation makes time since last treatment an uncertain predictor [10]. Instead, B‐cell count at the time of first vaccination has been suggested to be a better predictor of vaccine response [4, 21]. We found that B‐cell levels 4 weeks after booster not only correlated better with SARS‐CoV‐2‐specific antibody titers, but were also a better predictor of seroconversion than time since last infusion. Similarly, plasma RTX levels also correlated to antibody titers and predicted seroconversion. Further analyses indicated that the rate of seroconversion increased progressively with higher B‐cell levels and lower RTX concentrations. When all three parameters were analyzed together, presence of RTX was a better predictor than both time since last infusion and B‐cell levels. To be noted, all 28 pwMS with both >0.5% B cells and RTX below detection limit seroconverted, and all five pwMS with >0.5% B cells who did not seroconvert had detectable RTX levels. Taken together, these data indicate that the combination of B‐cell levels and RTX concentration might increase prediction of vaccine efficacy for anti‐CD20‐treated individuals. Because the main cohort of this study was treated with RTX, OCR and OFA concentrations were not measured in the few patients included. The pharmacokinetics and dosing regimens of these three anti‐CD20 agents differ from each other [23, 24], making it even more difficult to use time since last treatment as a general predictor of vaccine efficacy. The predictive value of drug concentration and B‐cell levels should be evaluated for OCR and OFA in future studies to determine whether drug concentration can be used as a general predictive marker for all three anti‐CD20 agents.
Looking further into the role of B‐ and T‐cell subsets on seroconversion, we observed a strong positive correlation between naïve B cells and antibody levels. This is in line with a recent study by Schulz et al. [25, 26] and the importance of a pre‐existing naïve B‐cell pool for successful seroconversion after SARS‐CoV‐2 vaccination. In addition, five of 11 pwMS with B‐cell repopulation (>0.5% B cells of live lymphocytes) and detectable levels of RTX did not develop a humoral response to the vaccine. As the samples were collected 4 weeks after the booster and contained a high percentage of immature B cells, it is possible that early repopulation explains the failure to seroconvert in these patients. When limiting the analysis to the pwMS with >0.5% B cells, the seronegative individuals displayed a higher proportion of transitional B cells compared to the seropositive individuals. Percentages of transitional B cells also correlated to RTX concentration, further supporting the concept of a more recent B‐cell reconstitution among seronegative pwMS [27, 28].
Contrary to our previous results on the immune response to COVID‐19 natural infection among pwMS, we did not observe a correlation between antibody titers and levels of circulating T follicular helper (Tfh) cells [5]. However, we found a positive correlation between antibody levels and CXCR5+CD8+ T cells, a subset of T cells that in vitro have been shown to have Tfh‐like characteristics in assisting B cells in antibody production and supporting B‐cell survival [29, 30].
In agreement with previous studies, we observed a decline in antibody levels over time in all groups [31, 32]. Interestingly, treatment with RTX >4 weeks after vaccine boost did not impact on the decline rate of spike‐specific antibodies. Thus, the decline in antibody levels observed probably results from the half‐life of spike‐specific IgG, which is estimated to be approximately 30 days [33]. However, the depletion of spike‐specific memory B cells by RTX redosing after vaccination needs to be characterized in more detail, as it could have clinical consequences in the case of reinfection.
Determination of specific T‐cell responses after SARS‐CoV‐2 vaccination confirm previous studies, namely that specific T‐cell responses characterized by the release of IFN‐γ arise even in the absence of detectable antibodies in naïvely vaccinated pwMS on BCDTs [4, 5, 6, 22]. Interestingly, in most pwMS we also observed a specific IL‐13 response at similar levels as IFN‐γ. For both cytokines, we observed a slight decline in SFUs between 4 and 12 weeks after booster, which is in line with previous studies that have reported waning of IFN‐γ‐producing T cells both after natural infection [34] and after mRNA vaccination [35]. IL‐13 can be produced by many immune cell types, but is particularly associated with CD4 T helper 2 (Th2) cells [36]. However, although studies have shown that SARS‐CoV‐2 mRNA vaccination induces a biased Th1 response and undetectable or low Th2 responses [37, 38], a recent study by Liu et al. [39] showed an increased frequency of IL‐13‐expressing CD4+ T cells 2 weeks after vaccination. No correlation between IFN‐γ or IL‐13 T‐cell responses to the spike S1 domain and spike S1S2 antibody levels was observed.
This study is not without limitations. For instance, to evaluate vaccine effectiveness, we differentiated naïvely vaccinated from COVID‐19‐recovered individuals using the presence of nucleocapsid‐specific antibodies and/or T‐cell responses as a marker. This could have led to an overestimation of the naïvely vaccinated group, because nucleocapsid‐specific antibodies decline rapidly and T‐cell responses were only assessed in a subset of pwMS [40, 41, 42]. Another limitation is that we analyzed B‐cell levels and RTX concentrations approximately 1 month after the second vaccine dose and hence cannot approximate the levels of B cells and RTX at the actual time of vaccination. However, given the nature of B‐cell repopulation kinetics and RTX pharmacokinetics, this limitation would not affect most of the analyses.
In conclusion, our results confirm the role of B‐cell reconstitution levels after anti‐CD20 treatment as a predictor of humoral response after vaccination and highlight the use of RTX plasma measurement as a better predictor than time since last infusion. These findings provide a tool for more precise individualized assessments of predictors of vaccine responses in anti‐CD20‐treated pwMS, which may lead to improved benefit–risk with this class of MS disease modulatory therapy.
AUTHOR CONTRIBUTIONS
Klara Asplund Högelin: Formal analysis (equal); investigation (lead); methodology (lead); writing – original draft (lead). Nicolas Ruffin: Formal analysis (equal); investigation (lead); methodology (equal); writing – original draft (equal). Elisa Pin: Investigation (equal); methodology (equal); writing – review and editing (equal). Sophia Hober: Investigation (equal); methodology (equal); writing – review and editing (equal). Peter Nilsson: Methodology (equal); writing – review and editing (equal). Chiara Starvaggi Cucuzza: Investigation (equal); writing – review and editing (equal). Mohsen Khademi: Resources (lead); writing – review and editing (equal). Tomas Olsson: Conceptualization (equal); funding acquisition (equal); supervision (equal); writing – review and editing (equal). Fredrik Piehl: Conceptualization (lead); funding acquisition (lead); project administration (equal); supervision (equal); writing – review and editing (equal). Faiez Al Nimer: Conceptualization (lead); funding acquisition (lead); project administration (equal); supervision (lead); writing – original draft (lead).
CONFLICT OF INTEREST
T.O. has received unrestricted grants for extended multiple sclerosis studies in relation to COVID‐19 from Biogen and Merck. Not related to this article, T.O. has received unrestricted grants and compensation for advisory board/lectures from Biogen, Merck, Novartis, and Sanofi. F.P. has received research grants from Janssen, Merck, and UCB, and fees for serving on data monitoring committees in clinical trials with Lundbeck, Parexel, and Roche. C.S.C. has received a travel grant from Sanofi‐Genzyme. All other authors declare no competing interests.
ACKNOWLEDGMENTS
We would like to thank the participating persons with MS, and the staff of Academic Specialist Center for help with sample collection. The COMBAT‐MS study is funded through a Patient‐Centered Outcomes Research Institute (PCORI) award (MS‐1511‐33196), modified to include the substudy reported herein. The views in this work are solely the responsibility of the authors and do not necessarily represent the views of PCORI, or its board of governors or methodology committee. The experimental procedures were funded by a grant from the Knut and Alice Wallenberg foundation (KAW 2020.0299_VC‐2020‐0040), with additional funding from the Swedish Medical Research council (grants 2017‐03054 and 2020‐02700), Swedish Brain Foundation, NEURO Sweden, Region Stockholm, and Erling‐Persson Family Foundation. C.S.C. is supported by the Blanceflor Boncompagni Ludovisi, née Bildt Foundation.
Asplund Högelin K, Ruffin N, Pin E, et al. B‐cell repopulation dynamics and drug pharmacokinetics impact SARS‐CoV‐2 vaccine efficacy in anti‐CD20‐treated multiple sclerosis patients. Eur J Neurol. 2022;00:1‐12. doi: 10.1111/ene.15492
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.
REFERENCES
- 1. Myhr KM, Torkildsen O, Lossius A, Bo L, Holmoy T. B cell depletion in the treatment of multiple sclerosis. Expert Opin Biol Ther. 2019;19(3):261‐271. [DOI] [PubMed] [Google Scholar]
- 2. Bar‐Or A, O'Brien SM, Sweeney ML, Fox EJ, Cohen JA. Clinical perspectives on the molecular and pharmacological attributes of anti‐CD20 therapies for multiple sclerosis. CNS Drugs. 2021;35(9):985‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker D, Roberts CAK, Pryce G, et al. COVID‐19 vaccine‐readiness for anti‐CD20‐depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202:149‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS‐CoV‐2 mRNA vaccination in patients with multiple sclerosis on anti‐CD20 therapy. Nat Med. 2021;27:1990‐2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asplund Hogelin K, Ruffin N, Pin E, et al. Development of humoral and cellular immunological memory against SARS‐CoV‐2 despite B cell depleting treatment in multiple sclerosis. iScience. 2021;24(9):103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brill L, Rechtman A, Zveik O, et al. Humoral and T‐cell response to SARS‐CoV‐2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78(12):1510‐1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Disanto G, Sacco R, Bernasconi E, et al. Association of disease‐modifying treatment and anti‐CD20 infusion timing with humoral response to 2 SARS‐CoV‐2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021;78(12):1529‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moor MB, Suter‐Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS‐CoV‐2 in patients with a history of CD20 B‐cell‐depleting therapy (RituxiVac): an investigator‐initiated, single‐Centre, open‐label study. Lancet Rheumatol. 2021;3(11):e789‐e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Novak F, Nilsson AC, Nielsen C, et al. Humoral immune response following SARS‐CoV‐2 mRNA vaccination concomitant to anti‐CD20 therapy in multiple sclerosis. Mult Scler Relat Disord. 2021;56:103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rico A, Ninove L, Maarouf A, et al. Determining the best window for BNT162b2 mRNA vaccination for SARS‐CoV‐2 in patients with multiple sclerosis receiving anti‐CD20 therapy. Mult Scler J Exp Transl Clin. 2021;7(4):20552173211062142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabatino JJ Jr, Mittl K, Rowles WM, et al. Multiple sclerosis therapies differentially affect SARS‐CoV‐2 vaccine‐induced antibody and T cell immunity and function. JCI Insight. 2022;7(4).e156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Kempen ZLE, Wieske L, Stalman EW, et al. Longitudinal humoral response after SARS‐CoV‐2 vaccination in ocrelizumab treated MS patients: to wait and repopulate? Mult Scler Relat Disord. 2021;57:103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS‐CoV‐2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baker D, MacDougall A, Kang AS, Schmierer K, Giovannoni G, Dobson R. Seroconversion following COVID‐19 vaccination: can we optimize protective response in CD20‐treated individuals? Clin Exp Immunol. 2021;207:263‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Kempen ZL, Toorop AA, Sellebjerg F, Giovannoni G, Killestein J. Extended dosing of monoclonal antibodies in multiple sclerosis. Mult Scler. 2021;13524585211065711. [DOI] [PubMed] [Google Scholar]
- 16. Bar‐Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999‐e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nissimov N, Hajiyeva Z, Torke S, et al. B cells reappear less mature and more activated after their anti‐CD20‐mediated depletion in multiple sclerosis. Proc Natl Acad Sci U S A. 2020;117(41):25690‐25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murley C, Friberg E, Hillert J, Alexanderson K, Yang F. Validation of multiple sclerosis diagnoses in the Swedish National Patient Register. Eur J Epidemiol. 2019;34(12):1161‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162‐173. [DOI] [PubMed] [Google Scholar]
- 20. Hober S, Hellstrom C, Olofsson J, et al. Systematic evaluation of SARS‐CoV‐2 antigens enables a highly specific and sensitive multiplex serological COVID‐19 assay. Clin Transl Immunology. 2021;10(7):e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali A, Dwyer D, Wu Q, et al. Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine. 2021;39(41):6111‐6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tallantyre EC, Vickaryous N, Anderson V, et al. COVID‐19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022;91(1):89‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gibiansky E, Petry C, Mercier F, et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br J Clin Pharmacol. 2021;87(6):2511‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu H, Graham G, David OJ, et al. Population pharmacokinetic‐B cell modeling for ofatumumab in patients with relapsing multiple sclerosis. CNS Drugs. 2022;36(3):283‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brewer RC, Ramadoss NS, Lahey LJ, Jahanbani S, Robinson WH, Lanz TV. BNT162b2 vaccine induces divergent B cell responses to SARS‐CoV‐2 S1 and S2. Nat Immunol. 2022;23(1):33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schulz E, Hodl I, Forstner P, et al. CD19+IgD+CD27‐ naive B cells as predictors of humoral response to COVID 19 mRNA vaccination in immunocompromised patients. Front Immunol. 2021;12:803742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colucci M, Carsetti R, Cascioli S, et al. B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol. 2016;27(6):1811‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roll P, Dorner T, Tony HP. Anti‐CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum. 2008;58(6):1566‐1575. [DOI] [PubMed] [Google Scholar]
- 29. Shen J, Luo X, Wu Q, et al. A subset of CXCR5(+)CD8(+) T cells in the germinal centers from human tonsils and lymph nodes help B cells produce immunoglobulins. Front Immunol. 2018;9:2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tyllis TS, Fenix KA, Norton TS, et al. CXCR5(+)CD8(+) T cells shape antibody responses in vivo following protein immunisation and peripheral viral infection. Front Immunol. 2021;12:626199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bayart JL, Douxfils J, Gillot C, et al. Waning of IgG, Total and neutralizing antibodies 6 months post‐vaccination with BNT162b2 in healthcare workers. Vaccines (Basel). 2021;9(10):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS‐CoV‐2 and variants of concern. Science. 2021;374(6572):abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murugesan K, Jagannathan P, Pham TD, et al. Interferon‐gamma release assay for accurate detection of severe acute respiratory syndrome coronavirus 2 T‐cell response. Clin Infect Dis. 2021;73(9):e3130‐e3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Almendro‐Vazquez P, Laguna‐Goya R, Ruiz‐Ruigomez M, et al. Longitudinal dynamics of SARS‐CoV‐2‐specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLoS Pathog. 2021;17(12):e1010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao K, Reinhardt RL. The differential expression of IL‐4 and IL‐13 and its impact on type‐2 immunity. Cytokine. 2015;75(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA‐1273 vaccine against SARS‐CoV‐2 in nonhuman primates. N Engl J Med. 2020;383(16):1544‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med. 2020;383(20):1920‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime‐boost schedules with an adenoviral vectored and mRNA COVID‐19 vaccine (com‐COV): a single‐blind, randomised, non‐inferiority trial. Lancet. 2021;398(10303):856‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krutikov M, Palmer T, Tut G, et al. Prevalence and duration of detectable SARS‐CoV‐2 nucleocapsid antibodies in staff and residents of long‐term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev. 2022;3(1):e13‐e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Elslande J, Oyaert M, Ailliet S, et al. Longitudinal follow‐up of IgG anti‐nucleocapsid antibodies in SARS‐CoV‐2 infected patients up to eight months after infection. J Clin Virol. 2021;136:104765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alfego D, Sullivan A, Poirier B, Williams J, Adcock D, Letovsky S. A population‐based analysis of the longevity of SARS‐CoV‐2 antibody seropositivity in the United States. EClinicalMedicine. 2021;36:100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.


