Abstract
Aim
This survey was conducted to evaluate COVID‐19 vaccination status in patients with autoimmune rheumatic diseases (AIRDs). Our objectives were to study vaccine hesitancy, adverse effects, breakthrough infections and flare of underlying disease in this population subgroup.
Methods
This was a multi‐center, cross‐sectional, interview‐based survey done at 6 tertiary care centers across Tamil Nadu, in the southern part of India from September 15, 2021 to October 14, 2021. The survey questionnaire was filled up by AIRD patients attending their clinics. The survey questionnaire comprised a set of 14 questions, distributed between patient characteristics, vaccines taken, their characteristics and COVID‐19 infection.
Results
There were 2092 participants, with a mean age of 47.5 ± 13.17 years. Among them, 1293 (61.81%) were vaccinated, of which 837 (64.73%) were fully vaccinated. Two‐thirds of our subjects were vaccinated with ChAdOx1 nCov‐19 (COVISHIELD) (77.64%) and 21.57% with BBV 152 (COVAXIN). Age, gender, education and comorbidities had no association with vaccine hesitancy. The commonest (421; 52.69%) reason for vaccine hesitancy was fear of side effects. The incidence (n = 72) of breakthrough infections was similar in both the vaccine groups, of which 58 (80.55%) were partially vaccinated and 14 (19.44%) were fully vaccinated. Thirty‐two patients had a flare of pre‐existing rheumatic disease.
Conclusion
ChAdOx1 nCov‐19 and BBV 152 were found to be safe in patients with rheumatic diseases. Fear of side effects was the major cause of vaccine hesitancy. All adverse effects were minor and self‐limiting. Breakthrough infections and disease flares occurred only in a small subset of our cohort.
Keywords: ChAdOx1 nCoV 19, COVID 19 vaccines, COVID‐19 breakthrough infections, side effects, vaccine hesitancy
1. INTRODUCTION
Severe acute respiratory syndrome corona virus (SARS‐CoV‐2) infection had changed the lives of people all over the world for the past 2 years including patients with autoimmune rheumatic diseases (AIRDs). SARS‐CoV‐2 as of 14 September 2021, has infected over 224 million globally with cumulative deaths of over 4.6 million. 1
Patients with AIRDs are considered to be at high risk for SARS‐CoV‐2 infection possibly due to immune dysregulation, active disease, immunosuppressive drugs and co‐existence of multiple comorbidities. 2 , 3 Vaccines had an immediate impact in mitigating COVID‐19 infection‐related hospital admissions, oxygen requirements and mortality, but its role in protection against infection is limited. 4
Vaccinating a huge population like the one we have in India has its own challenges (availability of vaccines to triaging the needed ones for early vaccination) and the scenario may not be dissimilar in other Asia Pacific League of Association for Rheumatology (APLAR) countries. The Indian vaccination policy initially was built on Oxford Astra Zeneca ChAdOx1 nCov‐19 (COVISHIELD, recombinant vaccine) and India’s indigenous BBV 152 (COVAXIN, inactivated vaccine), with Sputnik made available much later. 5 The vaccination program in India was started on January 16 2021 and the timeline of the COVID vaccination program in India is depicted in Figure 1.
FIGURE 1.
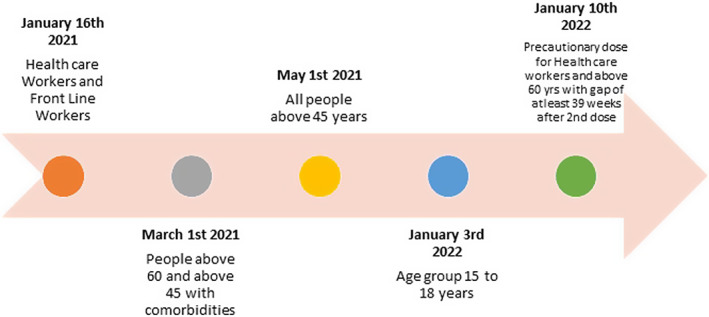
Timeline of covid vaccination in India
According to the APLAR consensus statement, all patients with AIRDs should receive COVID‐19 vaccine as soon as possible in accordance with the national or international guidelines and based on its availability. 6 However, vaccine hesitancy seems to be a major problem among the general population with AIRD patients being no exception. 7 In the initial trials of ChAdOx1 nCov‐19 and BBV 152 vaccines, patients with AIRDs on immunosuppressants were excluded. In view of limited data on the safety, efficacy and hesitancy of COVID‐19 vaccine in patients with AIRDs, in particular from the APLAR countries, we did this survey to explore the real word data. 8 , 9 , 10
The primary objectives were to study the COVID‐19 vaccination status, vaccine hesitancy and its causes in patients with AIRDs. The secondary objectives were to assess the breakthrough infections, adverse effects and flare of underlying rheumatic disease.
2. METHODS
This was an interview‐based, cross‐sectional, multi‐center survey carried out across 6 tertiary care centers. The study was conducted between September 15, 2021 and October 14, 2021. All consecutive out‐patients above 18 years of age and with a confirmed diagnosis of AIRD were included in the study. Other diseases such as osteoarthritis, soft tissue rheumatism and degenerative disc disease were excluded. The concerned rheumatologists asked the survey questions (using a structured questionnaire [Annexure 1]) to their respective out‐patients. The questionnaire was in English and the interviewer translated the questions in regional language as and when required. Informed consent was obtained from all the patients. The investigators (authors 1 and 2) did a pilot study on 30 patients with the survey questionnaire (Annexure 1). The questionnaire was revised and finalized by all the authors. A formal validation with an expert panel was not done. Vaccination status of individuals, while studying breakthrough infections, was categorized into partially vaccinated or fully vaccinated as per Centre for Disease Control and Prevention definition.
Partially vaccinated – infection occurring ≥14 days after first dose to <14 days after second dose.
Fully vaccinated – infection occurring ≥14 days after second dose.
The questionnaire had a total of 14 questions: 6 questions pertaining to the clinical characteristics of AIRD, 2 questions were based on COVID infection and the remaining 6 on COVID vaccination.
We studied the COVID‐19 infections during the first and second wave. During the second wave, the breakthrough infections (infections post‐vaccination) were separately analyzed.
Although India officially had its first case in January 2020, substantial increases in cases happened from March 2020; the first wave peaked in September 2020 and started to reduce in October 2020. The second wave where delta variant was dominant started from March, 2021. 11 Hence, in this study we considered all cases before March 2021 as first wave and after that as second wave.
2.1. Ethics committee approval
Institutional Ethics Committee for Biomedical Health and Research, Gleneagles Global Health City (EC/NEW/INST) – BMHR/2021/017 was obtained.
2.2. Statistical analysis
For normally distributed continuous data, mean and standard deviation was used; for non‐normal, median and interquartile range (IQR) were used and for categorical data, percentage and proportion were calculated. Chi‐square analysis was used to analyze categorical variables and Mann‐Whitney test for non‐parametric continuous variables. Statistical analysis was performed using SPSS software (version 25).
3. RESULTS
There were 2092 patients, with majority of them being female. The female to male ratio was 3.7:1.0. Rheumatoid arthritis followed by systemic lupus erythematosus (SLE) were the common AIRDs in our study population. The most frequent disease‐modifying agent used was methotrexate (51%) and 47% of those studied were on oral steroids. Diabetes mellitus was the most common comorbidity observed in our study. All the basic demographic characteristics of the study population are discussed in Table 1.
TABLE 1.
Basic demographics of the study population
| Characteristics | Results n (%) |
|---|---|
| Total no. study subjects | 2092 |
| Mean age ± SD | 47.50 ± 13.17 |
| Gender | |
| Male | 436 (20.84%) |
| Female | 1656 (79.16%) |
| Education | |
| None | 222 (10.61%) |
| Primary | 689 (32.93%) |
| Secondary | 528 (25.24%) |
| Graduate | 653 (31.21%) |
| Autoimmune rheumatic disease | |
| Rheumatoid arthritis | 1480 (70.74) |
| Systemic lupus erythematosus | 238 (11.37%) |
| Axial and peripheral spondyloarthritis | 99 (4.7%) |
| Psoriatic arthritis | 77 (3.7%) |
| Mixed connective tissue disease | 45 (2.1%) |
| Sjögren’s syndrome | 32 (1.5%) |
| Gout | 30 (1.4%) |
| Systemic sclerosis | 26 (1.24%) |
| Undifferentiated connective tissue disease | 26 (1.24%) |
| Vasculitis | 18 (0.8%) |
| Juvenile idiopathic arthritis | 8 (0.38%) |
| Sarcoidosis | 7 (0.33%) |
| Anti‐phospholipid antibody syndrome | 6 (0.28%) |
| Median disease duration (range) y | 3 (0‐33) |
| Current drugs | |
| Methotrexate | 1085 (51.84%) |
| Steroids | 995 (47.56%) |
| Hydroxychloroquine | 584 (27.91%) |
| Sulfasalazine | 434 (20.74%) |
| Leflunomide | 205 (9.79%) |
| Mycophenolate mofetil | 115 (5.4%) |
| Biologics | 85 (4.06%) |
| Iguratimod | 65 (3.1%) |
| Azathioprine | 64 (3%) |
| Janus‐activated kinase inhibitors | 14 (0.66%) |
| Allopurinol | 2 (0.09%) |
| Cyclosporine | 1 (0.04%) |
| Comorbidities | |
| Diabetes mellitus | 357 (17.06%) |
| Thyroid disorders | 318 (15.20%) |
| Systemic hypertension | 290 (13.86%) |
| Coronary artery disease | 85 (4.06%) |
Among the 2092 patients, 1293 (61.81%) were vaccinated; of which 837 (64.73%) had both their doses of vaccine. Among the vaccinated, 1004 (77.64%) were vaccinated with ChAdOx1 nCov‐19 followed by BBV 152 in 279 (21.57%). There were 799 patients who did not take the vaccine, and more than half of them (52.69%) were worried about vaccine‐related side effects. In the cohort, 849 (65.66%) patients discussed with their rheumatologist before taking the vaccine. A total of 209 patients were infected with COVID‐19, of which 137 were unvaccinated and 72 were vaccinated. Vaccine‐related adverse effects were reported in 435 (33.64%) patients. Body pain or myalgia was seen in 328 (25.37%) patients followed by fever (n = 258, 19.95%), injection site pain (n = 240, 18.56%), joint pain (n = 117, 9.04%) and others. Disease flare post‐vaccination was seen in 2.47% (n = 32) of patients, although none were major enough to require hospitalization. All the COVID infection and vaccine characteristics of the study population have been discussed in detail in Table 2.
TABLE 2.
COVID vaccine and infection characteristics of the study population
| Vaccinated | |
| Yes | 1293 (61.81%) |
| No | 799 (38.19%) |
| Doses | |
| One | 456 (35.27%) |
| Two | 837 (64.73%) |
| Type of vaccine | |
| ChAdOx1 nCov‐19 (Covishield) | 1004 (77.64%) |
| BBV 152 (COVAXIN) | 279 (21.57%) |
| SPUTNIK | 4 (0.31%) |
| PFIZER | 3 (0.23%) |
| SINOPHARM | 1 (0.08%) |
| ASTRAZENECA | 1 (0.08%) |
| SINOVAX | 1 (0.08%) |
| Reason for not taking vaccine, 799/2092; 38.19% | |
| Worried about vaccine side effects | 421 (52.69%) |
| Might worsen rheumatic disease | 195 (24.40%) |
| Do not feel it is needed | 98 (12.26%) |
| Fear due to comorbidities | 86 (10.76%) |
| Interaction with current drugs | 81 (10.13%) |
| Vaccine not available | 29 (3.62%) |
| Affordability | 5 (0.6%) |
| Others | |
| No time | 42 (5.25%) |
| Physically challenged | 2 (0.2%) |
| Advised not to take | 1 (0.1%) |
| Surgery | 1 (0.1%) |
| Walking difficulty | 1 (0.1%) |
| On chemotherapy | 1 (0.1%) |
| COVID epidemic has settled | 1 (0.1%) |
| Due to anemia | 1 (0.1%) |
| Previous arm pain | 1 (0.1%) |
| Vaccine‐related side effects, n = 435/1293 | |
| Body pain/myalgia | 328 (25.37%) |
| Fever | 258 (19.95%) |
| Injection site pain | 240 (18.56%) |
| Joint pain, self‐limiting, lasting for 1 to 3 d | 117 (9.04%) |
| Worsening of existing rheumatic disease | 32 (2.47%) |
| Minor flare | 20 (1.54%) |
| Flare requiring stepping up of drugs | 12 (<1%) |
| Major flare requiring hospitalization | 0 |
| Others | |
| Headache | 3 (0.2%) |
| Fatigue | 2 (0.14%) |
| Vomiting and loose stools | 1 (0.07%) |
| Weight gain | 1 (0.07%) |
| Worsened cutaneous psoriasis | 1 (0.07%) |
| New onset cutaneous vasculitis | 1 (0.07%) |
| Discussed with rheumatologist before taking vaccine, n = 1293 | |
| Yes | 849/1293 (65.66%) |
| No | 444/1293 (34.34%) |
| Rheumatologist asked to stop rheumatic drugs | |
| Yes | 489/849 (57.60%) |
| No | 360/849 (42.40%) |
| When to stop vaccine | |
| Before 1 wk | 2 (0.4%) |
| After 1 wk | 477 (97.54%) |
| Before and after 1 wk | 10 (2%) |
| Infected in unvaccinated | n = 137 |
| 1st wave | 65 (47.44%) |
| 2nd wave | 52 (37.96%) |
| Both waves | 20 (14.6%) |
| Treated at | |
| Home | 73 (53.28%) |
| Hospital | 64 (46.72%) |
| Post‐vaccination breakthrough infection | 72 |
| 1st wave | ‐ |
| 2nd wave | 72 (100%) |
| Treated at | |
| Home | 48 (66.66%) |
| Hospital, ward/intensive care unit | 24 (33.33%) |
| Infection after taking vaccine | 72 |
| Partially vaccinated | 58 (80.55%) |
| Fully vaccinated | 14 (19.44%) |
In Table 3, the breakthrough infections with regard to the type of vaccine and the status of vaccination have been discussed. The percentage of patients who were infected after being partially and fully vaccinated with 2 doses of vaccine in ChAdOx1 nCov‐19 group was 4.68% and 0.8% respectively and in BBV 152 group it was 3.94% and 1.79% respectively. The difference was not statistically significant. We tried to analyze the influence of age (P = .342), gender (P = .428), education (P = .142) and comorbidities (systemic hypertension [P = .872], diabetes mellitus [.843], coronary artery disease [P = .903] and hypothyroidism [P = .412]) with vaccine hesitancy but none of them had any significant association.
TABLE 3.
Breakthrough infections and the type of COVID vaccine
| Type of vaccine | Infected | Not infected | |
|---|---|---|---|
| Partially vaccinated | Fully vaccinated | ||
| ChAdOx1 nCov‐19, N = 1004 | 47 (4.68%) | 9 (0.89%) | 949 (94.52%) |
| BBV 152, N = 279 | 11 (3.94%) | 5 (1.79%) | 263 (94.27%) |
| SPUTNIK, N = 4 | 0 | 0 | 4 (100%) |
| PFIZER, N = 3 | 0 | 0 | 3 (100%) |
| SINOPHARM, N = 1 | 0 | 0 | 1 (100%) |
| SINOVAX, N = 1 | 0 | 0 | 1 (100%) |
| Total, N = 1293 | 58 (4.50%) | 14 (1.08%) | 1221 (94.43%) |
The Indian vaccination policy was initially built on ChAdOx1 nCov‐19 and BBV 152. Hence, the vaccine‐related side effects were compared between these 2 major groups (Table 4). Among them, the incidence of fever in the COVAXIN group was found to be statistically significant.
TABLE 4.
Type of COVID vaccine and adverse effects
| Adverse effects | BBV 152 (279) | ChAdOx1 nCov‐19 (1004) | P value |
|---|---|---|---|
| Fever | 68 (24.37%) | 189 (18.82%) | <.041 a |
| Body pain | 66 (23.65%) | 261 (25.99%) | .428 |
| Joint pain | 18 (6.45%) | 97 (9.66%) | .097 |
| Worsening of existing disease | 4 (1.43%) | 28 (2.78%) | .199 |
P < .05 (statistically significant).
4. DISCUSSION
This real‐world survey included 2092 patients, with a female to male ratio of 3.8:1.0 and a mean age of 47.5 years. The commonest disease was rheumatoid arthritis followed by SLE and others. In our study, 61.81% of the patients were vaccinated of which 64.73% had both their doses of vaccine. Among the unvaccinated, more than half were worried about the side effects. Our cohort had either ChAdOx1 nCov‐19 or BBV 152 vaccines and the incidence of breakthrough infections were similar in both the groups.
An international study on vaccines against COVID infection (VAXICOV) surveyed 1531 participants with SLE being the major AIRD subgroup (38.9%). The percent of patients who were willing to get vaccinated was 54.2%, 13.6% were unwilling and the remaining 32.2% were uncertain. 12 The COVAX study was a physician‐reported registry which had 5121 participants from over 30 countries with 68% female and a mean age of 60.5 years. The majority (78%) had messenger RNA based COVID‐19 vaccines and 17% had the Oxford Astra Zeneca vaccine. The majority (58%) of their cohort had inflammatory joint disease of which 38% had rheumatoid arthritis. 13
The biggest challenge any adult vaccination program comes across is vaccine hesitancy. A previous study from India done among 280 patients with AIRDs reported a vaccine hesitancy of 46% with the most common reason being indecision. 9 In the study by Fragoulis et al 12 vaccine hesitancy was reported in 21.39% with fear of side effects and disease flare being primary concerns of the unvaccinated group. In the VAXICOV study, 45% of the cohort had vaccine hesitancy. The participants had expressed similar concerns which were significantly more in the AIRD group when compared to healthcare workers. 12 The most common reason for not getting vaccinated in our cohort was the fear of side effects (52%) and worsening of pre‐existing rheumatic disease (24%). In a recent study from Hong Kong that used a questionnaire‐based survey, only 30% of their AIRD patient cohort was vaccinated. Fear of side effects and worsening of the underlying rheumatic disease were the 2 most frequent reasons for vaccine hesitancy in that cohort, similar to ours. 10 Preliminary reports from the COVAD study, an ongoing patient self‐report electronic survey, also suggest fear of vaccination‐related side effects and awaiting for better data as the major reasons of vaccination hesitancy. 15 Our study did not find any correlation between education status and vaccine hesitancy unlike the study by Gaur et al and the study by Fragoulis et al in which educated individuals significantly had higher vaccine acceptability. 9 , 14
In our study, 33.64% reported vaccine‐related adverse effects with the common side effects being myalgia (25%) and fever (20%). Incidence of fever following vaccination was higher with the BBV 152 group. All the adverse effects were mild and self‐limiting and none required hospitalization. In the study by Cherian et al, at least one adverse effect was reported in 70% of their cohort. 8 The most common side effects were pain in injection site (25%), fatigue (18%), fever (18%) and myalgia (9%). The rate of adverse events between patients with AIRDs and non‐AIRDs neither differed nor was there any difference between the ChAdOx1 nCov‐19 and BBV 152 groups.
In the COVAX study, around 70% were vaccinated with Pfizer/BioNTech, and only 16% of the AIRD patients received AstraZeneca/Oxford vaccine. 13 The overall vaccine‐related adverse events were reported as 37% which was slightly higher than our study. The majority had an early (within 7 days from vaccination) adverse event with pain at injection site reported in 19%; our study had a similar incidence. Myalgia, fever and joint pains were observed in 7%, 7% and 4% respectively, all of which were less in comparison to our study. 13
The disease flares following vaccination were seen in 2.47% of the patients in our study. Most of them were minor and managed conservatively. In the COVAX registry the flares were reported in 4.4% of patients. Mild to moderate flares were seen in 3.6% and severe flares in 0.6%. They reported a possible association between higher disease activity and severity of flares. They had reported that the incidence of flares was higher in inflammatory joint diseases in comparison to connective tissue diseases or vasculitis. 13
In the C19‐GRA (COVID‐19 Global Rheumatology Alliance) vaccine survey, patient‐reported adverse events post‐vaccination were similar to the general population and rheumatic disease flares requiring treatment modification were seen only in 4.6%. 16
In the study by Ahmed et al, breakthrough infections were seen in 7.4% (n = 47) of patients, of which 63.8% (n = 30) were vaccinated with ChAdOx1 nCov‐19 and the remaining 36.2% (n = 17) with BBV 152 vaccine. They studied the post‐vaccination antibody titers in patients with autoimmune rheumatic diseases and concluded that the breakthrough infections were strongly associated with absent or poor antibody response. 17
In our cohort, breakthrough infections were observed in 5.5%, of which 80.55% were partially vaccinated and 19.44% were fully vaccinated. The incidence of breakthrough infection did not differ statistically between the 2 major vaccine types used. In the COVAX registry, the incidence of breakthrough infections in fully vaccinated individuals with rheumatic and musculoskeletal disorders was 0.7% which was numerically less when compared to fully vaccinated immunocompetent individuals (1.1%). There was no significant difference between the type of vaccination and breakthrough infections.13 In another cross‐sectional study, the incidence of breakthrough infections was 8.4% in vaccinated individuals in comparison to 16.4% in unvaccinated individuals. 14
In our study, 33.3% of the patients with breakthrough infections were hospitalized, of which 8.3% required supplemental oxygen, none required mechanical ventilation and none died. In a recent study by Cook et al on vaccination in AIRD patients, the rate of breakthrough infections was (n = 16/340; 4.7%). Among the 16 patients, 6 required hospitalization, 4 required oxygen, 1 required mechanical ventilation and 2 died. 18
In a recent study, Singh et al observed that patients with rheumatic diseases had increased risk for breakthrough infections and it may also vary with the type of underlying rheumatic disease. 19 In the preliminary data from the C19‐GRA vaccine survey, the majority of patients with breakthrough infections were on B‐cell depleting therapy or anti‐metabolites like methotrexate. 16
Our study is real‐world data with a large patient population, suggesting that vaccinations do work in immunocompromised individuals as evidenced by low numbers of breakthrough infections. This data would be really useful while vaccinating AIRD individuals with ChAdOx1 nCov‐19 or BBV 152, since initial trials of these vaccines excluded immunocompromised individuals. This study adds on to the emphasis of the APLAR consensus statement on vaccination in AIRDs –“there is no real reason that vaccination in AIRDs should be unsafe or less effective in this subgroup of patients.” 6 The detailed evaluation of causes for vaccine hesitancy among patients with AIRDs would be helpful in improving the vaccine coverage by addressing those concerns.
The major limitation of this study was the lack of a control group. An immunocompetent control group population could have provided further insights into the study. Being a retrospective study there is a possibility of recall bias, although it may be negated to some extent due to the large sample size and the survey questions being asked by the treating rheumatologist. We did not quantify the severity of adverse effects, although none of them were serious enough to require hospitalization. We did not calculate the exact time duration between vaccination and infections while assessing for breakthrough infections, although we classified them as infections in partially and fully vaccinated individuals.
5. CONCLUSION
Our study further emphasizes that immunosuppressed people tolerate vaccines with no major vaccine‐related side effects. The fear of vaccine‐related side effects and flare up of pre‐existing rheumatic disease were some of the major factors for vaccine hesitancy among this group. No major differences in side effects and breakthrough infections were observed between the 2 major vaccine subgroups. Region‐specific guidelines and studies may assist in negating the vaccine hesitancy in this population subgroup and help in achieving vaccination goals.
AUTHOR CONTRIBUTIONS
Conception or design of the work: KM, SS. Data collection: KM, SS, RN, HM, TN, BR, SN. Data analysis and interpretation: KM, SS. Drafting the article: KM, SS. Critical revision of the article: KM, SS, RN, HM, TN, BR, SN. Final approval of the version to be published: KM, SS, RN, HM, TN, BR, SN.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGEMENT
Nil.
Mohanasundaram K, Santhanam S, Natarajan R, et al. Covid‐19 vaccination in autoimmune rheumatic diseases: A multi‐center survey from southern India. Int J Rheum Dis. 2022;25:1046‐1052. doi: 10.1111/1756-185X.14378
REFERENCES
- 1. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19. Accessed September 14, 2021.
- 2. Bakasis AD, Mavragani CP, Boki KA, et al. COVID‐19 infection among autoimmune rheumatic disease patients: data from an observational study and literature review. J Autoimmun. 2021;123:102687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Annamalai SV, Santhanam S, Mohanasundaram K, et al. COVID‐19 and rheumatic diseases in Tamil Nadu – A multicenter retrospective observational study. Indian J Rheumatol. 2021;16:441‐446. [Google Scholar]
- 4. Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on coronavirus disease 2019 (COVID‐19) outbreaks in the United States. Clin Infect Dis. 2021;73(12):2257‐2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar VM, Pandi‐Perumal SR, Trakht I, Thyagarajan SP. Strategy for COVID‐19 vaccination in India: the country with the second highest population and number of cases. NPJ Vaccines. 2021;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tam L‐S, Tanaka Y, Handa R, et al. Updated APLAR consensus statements on care for patients with rheumatic diseases during the COVID‐19 pandemic. Int J Rheum Dis. 2021;24:733‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yasmin F, Najeeb H, Moeed A, et al. COVID‐19 vaccine hesitancy in the United States: a systematic review. Front Public Health. 2021;23(9):770985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cherian S, Paul A, Ahmed S, et al. Safety of the ChAdOx1 nCoV‐19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post‐vaccination cross‐sectional survey. Rheumatol Int. 2021;41(8):1441‐1445. doi: 10.1007/s00296-021-04917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaur P, Agrawat H, Shukla A. COVID‐19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview‐based survey. Rheumatol Int. 2021;41(9):1601‐1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li YK, Lui MPK, Yam LL, et al. COVID‐19 vaccination in patients with rheumatic diseases: vaccination rates, patient perspectives, and side effects. ImmunInflamm Dis. 2022;10:e589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarkar A, Chakrabarti AK, Dutta S. Covid‐19 infection in india: a comparative analysis of the second wave with the first wave. Pathogens. 2021;10(9):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felten R, Dubois M, Ugarte‐Gil MF, et al. Vaccination against COVID‐19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):e243‐e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Machado PM, Lawson‐Tovey S, Strangfeld A, et al. Safety of vaccination against SARS‐CoV‐2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician‐reported registry. Ann Rheum Dis. 2021;81(5):695‐709. doi: 10.1136/annrheumdis-2021-221490 [DOI] [PubMed] [Google Scholar]
- 14. Fragoulis GE, Bournia VK, Mavrea E, et al. COVID‐19 vaccine safety and nocebo‐prone associated hesitancy in patients with systemic rheumatic diseases: a cross‐sectional study. Rheumatol Int. 2022;42(1):31‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sen P, Lilleker JB, Agarwal V, Kardes S, Milchert M, Gheita T et al. Vaccine hesitancy in patients with autoimmune diseases: data from the coronavirus disease‐2019 vaccination in autoimmune diseases study. Indian J Rheumatol. 2022;17(2):188. [Cited May 22, 2022]. [Google Scholar]
- 16. Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID‐19 vaccination in adults with systemic rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7(3):e001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed S, Mehta P, Paul A, et al. Postvaccination antibody titres predict protection against COVID‐19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis. 2022;81:868‐874. [DOI] [PubMed] [Google Scholar]
- 18. Cook C, Patel NJ, D’Silva KM, et al. Clinical characteristics and outcomes of COVID‐19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81(2):289‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh J, Singh N, Anzalone A, et al. Breakthrough COVID‐19 infections post‐vaccination among immunocompromised patients with autoimmune or inflammatory rheumatic diseases: a retrospective cohort analysis from U.S Nationally‐sampled electronic medical record data repository [abstract]. Arthritis. Rheumatology. 2021;73(suppl 10). https://acrabstracts.org/abstract/breakthrough‐covid‐19‐infections‐post‐vaccination‐among‐immunocompromised‐patients‐with‐autoimmune‐or‐inflammatory‐rheumatic‐diseases‐a‐retrospective‐cohort‐analysis‐from‐a‐u‐s‐nationally‐sampled‐el/. Accessed June 29, 2022. [Google Scholar]


