Abstract
Background and Objectives
The neuromuscular system is responsible for performing adequate muscle activities to maintain postural balance. Since COVID‐19 can cause damage to this system, long‐term sequelae might alter control of postural stability. This study aimed to evaluate the postural balance of patients with post‐acute COVID‐19 syndrome (PCS) who were not hospitalized and to evaluate the correlations of changes in postural balance with general fatigue, muscle strength, and quality of life (QoL).
Methods
This was a cross‐sectional study in which 40 patients with PCS and 40 controls underwent balance assessment through the Berg Balance Scale (BBS) and Tinetti Balance Scale (TBS). They were evaluated for general fatigue by the Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F) scale, handgrip strength (HGS), and quality of life (QoL) by the Short Form‐36 (SF‐36).
Results
When compared to controls, patients with PCS had lower BBS and TBS scores (p = 0.001 for both). The FACIT‐F score was lower in PCS patients (p = 0.0001). HGS was slightly lower in the PCS patients, but not statistically significant (p = 0.09). Regarding QoL, PCS patients showed worse evaluations in five dimensions of the SF‐36 (physical functioning, physical role limitations, bodily pain, general health perceptions, and mental health). Both the BBS and TBS scores had statistically significant positive correlations with the FACIT‐F score, HGS, and two SF‐36 dimensions (physical role limitations and emotional role limitations).
Conclusions
Patients with PCS show worse postural balance than controls, which is associated with general fatigue, lower HGS, and poor QoL. Postural balance assessment should be considered in the follow‐up and rehabilitation of PCS.
Keywords: COVID‐19, general fatigue, muscle strength, postural balance, quality of life
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has had an unprecedented effect on global health, both in the acute phase of the disease and later. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) enters human cells through a receptor, angiotensin‐converting enzyme 2 (ACE2). The high binding affinity of the virus for ACE2 is seen even on the surface of the spinal cord, which makes the central nervous system (CNS) vulnerable to its effects (Divani et al., 2020; Pergolizzi Jr et al., 2021). In addition to direct damage to the blood–brain barrier, SARS‐CoV‐2 causes an influx of cytokines in different sites of the CNS, triggering intense neuroinflammation (De Felice et al., 2020). As a possible late consequence of damage to the nervous system, many neuromuscular manifestations extend beyond the acute phase (Divani et al., 2020; Gervasoni et al., 2022; Pergolizzi Jr et al., 2021). These changes can potentially impair postural stability control and affect safe mobility, even in those who had a mild form of the disease (Augustin et al., 2021).
Postural balance refers to the ability to maintain the vertical projection of the body's centre of mass—centre of gravity—in the limits of stability through the interrelationship of the various forces acting on the body, including gravity, muscle strength, and inertia (Baracat & de Sá Ferreira, 2013; Feldman, 2016). In this context, control of postural stability can be defined as the ability to control the body's centre of mass in relation to the support base, which is the area of the body that is in contact with the support surface (Baracat & de Sá Ferreira, 2013). For the body to stay balanced effectively, it must integrate information from multiple subsystems of the body, such as the somatosensory, vestibular, and visual systems, so it must activate an appropriate neuromuscular synergy that upholds its posture in all situations (Horak, 2006). Functional goals of the balance system include maintaining postural alignment (such as sitting or standing) and facilitating balance recovery reactions to external disturbances (such as tripping, slipping, or pushing) (Mancini & Horak, 2010).
Using the hunova robotic device, a recent study showed that regardless of disease severity, post‐acute COVID‐19 syndrome (PCS) causes patients to perform worse on the elastic balance test when the individual integrates vision, somatosensory information, and vestibular information (Gervasoni et al., 2022). These data suggest a new mechanism of PCS that deserves further investigation because of its potential impact on activities of daily living (ADL). Such investigation should especially be done by using simpler tools to assess postural balance and in patients without obvious neurological sequelae associated with PCS.
The identification of balance disorders in patients with PCS is important for the assessment of the risk of falls and the need for balance rehabilitation. In this scenario, no studies have evaluated the postural balance of individuals after acute COVID‐19 infection using tools routinely used in clinical practice, such as the Berg Balance Scale (BBS) and Tinetti Balance Scale (TBS) (Berg et al., 1992; Tinetti, 1986). Our hypothesis is that the neuromuscular alterations caused by SARS‐CoV‐2 are capable of altering postural balance even in patients with PCS who had mild acute illness without the need for hospitalization, and that these alterations are related to worse muscle function and quality of life (QoL). Thus, this study aimed to evaluate the postural balance of patients with PCS who were not hospitalized using simple and easy‐to‐apply instruments and to evaluate the correlations of changes in postural balance with general fatigue, muscle strength, and QoL.
2. METHODS
2.1. Study design, participants and ethics
This was a cross‐sectional observational study on 40 patients (out of 45 eligible) treated at basic health units of Sousa, Cajazeiras, and Santa Cruz, all located in the state of Paraíba, Brazil, between February and May 2021. The study participants came from a convenience sample. Patients were eligible if they were ≥18 years old, had a previous diagnosis of COVID‐19 confirmed by reverse transcription–real‐time quantitative polymerase chain‐reaction (RT‐qPCR) and diagnosis of PCS, and did not require hospitalization or intensive care unit (ICU) admission. PCS was characterized by a set of clinical findings that appear during or after a SARS‐CoV‐2 infection that continue for more than 12 weeks and are not explained by any alternative diagnosis (Ayoubkhani et al., 2021). All patients were unvaccinated against SARS‐CoV‐2. Patients with positive RT‐qPCR at the time of inclusion in the study and those with neurological or musculoskeletal disease before COVID‐19 were excluded.
We also evaluated a control group of 40 subjects aged ≥18 years who did not have COVID‐19. This group consisted of people living in the same cities where the patients were recruited. This group was composed of asymptomatic individuals residing in the community and invited to attend the basic health units to carry out the RT‐qPCR test and study measurements. These subjects had no previous neurological or orthopaedic disease and, moreover, did not have any difficulty walking.
The project was previously approved by the National Research Ethics Committee of Brazil under the number CAAE‐30135320.0.0000.5259 and followed the principles of the Declaration of Helsinki. All participants signed an informed consent form.
2.2. Measurements
Standardized data collection procedures for clinical use were taken to assess postural balance, peripheral muscle strength, general fatigue, and QoL (Berg et al., 1992; Ciconelli et al., 1999; Crosby et al., 1994; Mosher & Duhamel, 2012; Tinetti, 1986).
We used the Short Form‐36 (SF‐36) to evaluate QoL. It is a multidimensional and self‐applied tool composed of 36 items grouped into eight dimensions: physical functioning, physical role limitations, bodily pain, general health perceptions, vitality, social functioning, emotional role limitations, and mental health (Ciconelli et al., 1999). Their result is a score from 0 to 100 obtained from a list of questions regarding various QoL aspects. The higher the score, the better the QoL.
The BBS assesses the subject's functional balance based on 14 items common to daily life. It is a simple, safe, and easy‐to‐apply test. Each item consists of a five‐point ordinal scale ranging from 0 to 4 points, with a maximum score of 56. The points are based on how long the subject can maintain a position, the distance the arm can reach in front of the body, and the time to complete each task. In the BBS, the lower the score the subject achieves, the greater the risk of falls (Berg et al., 1992). Scores between 53 and 46 indicate a low to moderate risk of falls, and scores below 46 indicate a high risk of falls (Woollacott & Shumway‐Cook, 2010).
The TBS consists of several tasks representing ADLs; the examiner evaluates the tasks through observation. This test consists of two parts, one that evaluates balance and the other that evaluates gait. The TBS classifies the aspects of gait of speed, step distance, symmetry, and balance while standing, turning, and changing position with eyes closed. The score for each exercise ranges from 0 to 1 or 0 to 2, a lower score indicating poorer physical ability. The total score is the sum of body balance and gait balance scores. The maximum score is 28, with a score between 19 and 24 indicating a moderate risk of falls and a score below 19 indicating a high risk of falls (Tinetti, 1986).
To evaluate general fatigue, we used the Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F) scale, which has 13 questions scored from 0 to 4. The higher the scores, the lower the fatigue. The score ranges from 0 to 52. The FACIT‐F scale is an easy‐to‐apply, specific fatigue assessment scale that has good representativeness of the subject (Lima et al., 2019; Mosher & Duhamel, 2012).
To measure the handgrip strength (HGS) we used a manual digital dynamometer (SH5001, Saehan Corporation, Korea), which displays handgrip strength (HGS) in kilogrammes. We evaluated HGS with the participants sitting in an armless chair, with 90° elbow flexion, the forearms in a neutral position, and the wrist in extension from 0° to 30° (Crosby et al., 1994). This study assessed the maximum force after a sustained contraction of 3 s in the dominant hand and used the highest value of three attempts at 1‐min intervals for the analysis.
2.3. Statistical analysis
The normality of the data was assessed using the Shapiro–Wilk test; in this analysis, the anthropometric variables for the two groups showed a Gaussian distribution while the postural balance, general fatigue, HGS, and QoL variables showed a non‐Gaussian distribution. The results were expressed as the mean ± standard deviation or median (interquartile ranges) based on the Gaussian or non‐Gaussian distribution of each variable, respectively. Anthropometric variables between the 2 groups were compared using Student's t test for independent samples (parametric). Postural balance, general fatigue, HGS, and QoL between the 2 groups were compared using the Mann–Whitney test (non‐parametric). Comparisons between sexes were made using the chi‐squared test. The associations between the body balance scales (BBS and TBS) and the other variables was analysed by Spearman's correlation coefficient (non‐parametric). The significance level adopted was 5%. Statistical analysis was performed using IBM SPSS Statistics version 23.0 software (IBM Corp.).
3. RESULTS
Among the 45 patients who were evaluated for inclusion in the study, five were excluded for the following reasons: two patients had a report of neurological disease before COVID‐19, two patients had a report of musculoskeletal disease before COVID‐19, and one patient had a positive RT‐qPCR test. Concerning controls, there were no exclusions after the initial evaluation.
Twenty‐four (60%) patients were women, with a mean age was 35 ± 7.2 years. The median time after diagnosis of COVID‐19 was 8 (5–12) months. Nine (22.5%) patients had a BBS lower than 46 points, while seven (17.5%) patients had a TBS lower than 19 points. When compared to controls, patients with PCS had lower scores on the BBS [53 (46–56) vs. 56 (56–56), p = 0.001] and TBS [27 (22–28) vs. 28 (27–28), p = 0.001]. The FACIT‐F scale score was also significantly lower in PCS patients [39.5 (28–46) versus 48.5 (41–52), p = 0.0001]. HGS was lower in the PCS patients than in the controls, but not significantly [26.7 (22–36) vs. 28.5 (23–36), p = 0.09]. The anthropometric data, postural balance, and functionality of patients and controls are shown in Table 1.
TABLE 1.
Anthropometric data, postural balance, general fatigue, and handgrip strength of the post‐acute COVID syndrome and control groups
| Post‐acute COVID‐19 syndrome group (n = 40) | Control group (n = 40) | p Value | |
|---|---|---|---|
| Anthropometric data | |||
| Male/female | 16/24 | 17/23 | 0.82 a |
| Age (years) | 35 ± 7.2 | 34 ± 9.7 | 0.58 b |
| Weight (kg) | 76.8 ± 13.9 | 72.7 ± 12.6 | 0.17 b |
| Height (m) | 1.66 ± 0.09 | 1.68 ± 0.09 | 0.10 b |
| BMI (kg/m2) | 27.2 ± 3.9 | 25.3 ± 3.8 | 0.07 b |
| BBS (Points) | 53 (46–56) | 56 (56–56) | 0.001 c |
| TBS (points) | 27 (22–28) | 28 (27–28) | 0.001 c |
| FACIT‐F scale (points) | 39.5 (28–46) | 48.5 (41–52) | 0.0001 c |
| HGS (kgf) | 26.7 (22–36) | 28.5 (23–36) | 0.09 c |
Note: The values shown are mean ± SD or median (interquartile range). Bold type indicates significant differences.
Abbreviations: BBS, Berg Balance Scale; BMI, body mass index; FACIT‐F, Functional Assessment of Chronic Illness Therapy‐Fatigue; HGS, handgrip strength; TBS, Tinetti Balance Scale.
The p value was calculated using the chi‐squared test.
The p value was calculated using the Student's t test for independent samples.
The p value was calculated using the Mann–Whitney test.
Regarding the QoL measured by the SF‐36, the PCS patients showed worse evaluations in the following dimensions: physical functioning [90 (66–100) vs. 100 (86–100), p = 0.032]; physical role limitations [87.5 (50–100) vs. 100 (100–100), p = 0.003]; bodily pain [52 (41–82) vs. 84 (74–84), p = 0.0004]; general health perceptions [60 (52–67) vs. 66 (52–80), p = 0.044]; and mental health [70 (49–92) vs. 84 (81–92), p = 0.009]. The QoL data of patients and controls are shown in Table 2.
TABLE 2.
Quality of life assessed by the Short Form‐36 of the post‐acute COVID‐19 syndrome and control groups
| Post‐acute COVID‐19 syndrome group (n = 40) | Control group (n = 40) | p Value | |
|---|---|---|---|
| Physical functioning | 90 (66–100) | 100 (86–100) | 0.032 a |
| Physical role limitations | 87.5 (50–100) | 100 (100–100) | 0.003 a |
| Bodily pain | 52 (41–82) | 84 (74–84) | 0.0004 a |
| General health perceptions | 60 (52–67) | 66 (52–80) | 0.044 a |
| Vitality | 62.5 (50–85) | 65 (55–75) | 0.33 a |
| Social functioning | 62.5 (50–88) | 68.8 (63–97) | 0.25 a |
| Emotional role limitations | 90 (33–100) | 100 (67–100) | 0.096 a |
| Mental health | 70 (49–92) | 84 (81–92) | 0.009 a |
Note: The values shown are median (interquartile range). Bold type indicates significant differences.
The p value was calculated using the Mann–Whitney test.
Table 3 shows the associations between postural balance measures (BBS and TBS) and measurements of functionality and QoL in the group of patients with PCS. BBS score correlated significantly with the FACIT‐F score (r s = 0.482, p = 0.002) and HGS (r s = 0.602, p < 0.0001) (Figure 1) and with the SF‐36 dimensions measuring physical role limitations (r s = 0.480, p = 0.002) and emotional role limitations (r s = 0.487, p = 0.001). TBS score correlated significantly with the FACIT‐F score (r s = 0.376, p = 0.016) and HGS (r s = 0.462, p = 0.02) (Figure 1) and with the SF‐36 dimensions measuring physical role limitations (r s = 0.539, p = 0.0003) and emotional role limitations (r s = 0.531, p = 0.0004). The BBS and TBS scores were correlated with each other (r s = 0.740, p < 0.0001).
TABLE 3.
Spearman's correlation coefficients between postural balance measures, functionality measures, and quality of life among subjects with post‐acute COVID‐19 syndrome
| Postural balance | ||||
|---|---|---|---|---|
| BBS | TBS | |||
| r s | p Value | r s | p Value | |
| FACIT‐F scale | 0.482 | 0.002 | 0.376 | 0.016 |
| HGS | 0.602 | <0.0001 | 0.462 | 0.002 |
| Physical functioning | 0.280 | 0.08 | 0.228 | 0.16 |
| Physical role limitations | 0.480 | 0.002 | 0.539 | 0.0003 |
| Bodily pain | 0.179 | 0.27 | 0.086 | 0.60 |
| General health perceptions | 0.081 | 0.62 | 0.055 | 0.74 |
| Vitality | 0.251 | 0.12 | 0.120 | 0.46 |
| Social functioning | 0.035 | 0.83 | 0.068 | 0.68 |
| Emotional role limitations | 0.487 | 0.001 | 0.531 | 0.0004 |
| Mental health | 0.158 | 0.33 | 0.140 | 0.39 |
Note: Bold type indicates significant correlations.
Abbreviations: BBS, Berg Balance Scale; FACIT‐F, Functional Assessment of Chronic Illness Therapy‐Fatigue; HGS, handgrip strength; TBS, Tinetti Balance Scale.
FIGURE 1.
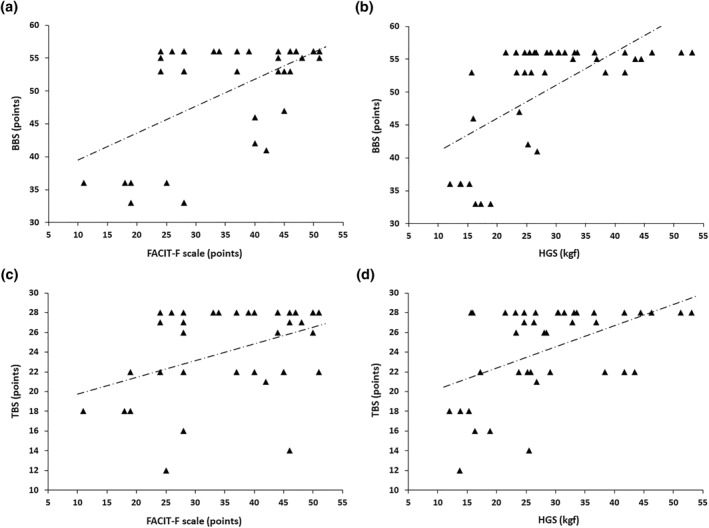
Relationships of Berg Balance Scale (BBS) score with the Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F) score (r s = 0.482, p = 0.002) (a) and handgrip strength (HGS) (r s = 0.602, p ˂ 0.0001) (b). Relationships of Tinetti Balance Scale (TBS) score with FACIT‐F score (r s = 0.376, p = 0.016) (c) and HGS (r s = 0.462, p = 0.002) (d)
To provide context for interpreting the null findings, a post hoc analysis was performed using G*Power software. Based on the comparisons made with Wilcoxon–Mann–Whitney test, in a balanced design (1:1: ratio of patients:control), and pre‐specified type‐I error of 5% (two‐tailed), the achieved power was 94%, 99%, 98%, and 6% for BBS, TBS, FACIT‐F, and HGS, respectively.
4. DISCUSSION
Understanding the deficiencies allows directing rehabilitation to the real needs of patients with PCS. Thus, a comprehensive clinical assessment of postural balance is important for both diagnostic and therapeutic reasons in clinical practice. The main finding of the present study was that patients with PCS had worse postural balance than controls. These patients had greater general fatigue and worse QoL in the form of bodily pain, physical role limitations, and mental health. In addition, their postural balance was correlated with general fatigue, HGS, and QoL (emotional role limitations and physical role limitations). To the best of our knowledge, this is the first study to evaluate postural balance in patients with PCS who were not hospitalized using simple tests that are easily applicable in routine clinical practice.
An intact postural balance is necessary not only to maintain postural stability, but also to ensure safe ADL‐related tasks such as standing while performing manual tasks, rising from a chair, walking, and turning (Mancini & Horak, 2010). In the present study, almost a quarter of patients had BBS and/or TBS scores suggesting a high risk of falls. The various balance tests show distinct particularities and limitations, so it is reasonable to apply more than one instrument to ensure better assessments (Postigo‐Martin et al., 2021). Regarding the psychometric characteristics of the scales used in our study, both the BBS and the TBS assess the individual's balance in representative situations of ADLs (Karuka et al., 2011). However, it is important to note that the BBS has poor sensitivity and the TBS has poor specificity, and both scales have a ceiling effect for younger people with balance deficits (Mancini & Horak, 2010).
To quantify the balance deficit and proprioception related to PCS, Gervasoni et al. (2022) analysed the data of 66 post‐COVID‐19 outpatients using a hunova robotic device. The hospitalized group performed worse than the non‐hospitalized group both in the oscillation trajectory and in the oscillation range, their worst performance being with their eyes closed. These authors speculated that neurotropism, the neuroinflammatory component of COVID‐19 infection, and the myopathic changes induced by SARS‐CoV‐2 may have contributed to this multisensory integration deficit, although psychological or psychiatric aspects may also have played a role. Using a stabilometric platform, Giardini et al. (2022) observed poor dynamic balance and increased sway during quite stance in patients who had a severe form in the acute phase of COVID‐19; however, hospitalization itself can result in balance problems and muscle weakness that are not necessarily related to COVID‐19.
In our study, patients with PCS had worse general fatigue than controls, with a large difference in the FACIT‐F score between groups. Although they used other self‐assessment scales to measure perceived fatigue, Ortelli et al. (2021) found significantly worse scores in post‐COVID‐19 patients than in healthy controls. Interestingly, we observed significant correlations between general fatigue (assessed by the FACIT‐F scale) and postural balance (assessed by both BBS and TBS). In COVID‐19, the combination of the cytokine storm and the entry of the virus into the CNS can cause neuroinflammation that can lead to prolonged generalized symptoms, including general fatigue and worse postural balance. However, there are other hypotheses that focus more on neurological issues secondary to vascular rupture than the direct penetration of the virus into neurons (Malik et al., 2022). An imbalance between the neural circuits of GABAergic and dopaminergic transmission has been postulated to explain the general fatigue (Ortelli et al., 2021), and it may explain, at least in part, the relationship between general fatigue and postural imbalance.
It is worth noting the significant associations that we observed between postural balance scores and HGS, since HGS is suggested to have a relationship with post‐COVID‐19 health status, including overall muscle performance (Tanriverdi et al., 2021). Unlike our study, which showed no significant differences in HGS between patients with PCS and controls, Tanriverdi et al. (2021) observed reduced HGS in 39.6% of the participants when they were evaluated 3 months after the diagnosis of COVID‐19. Comparing institutionalized older adults at two moments of the COVID‐19 pandemic, Greco et al. (2021) found a 19% greater deterioration in HGS in the COVID‐19 patients than the controls. A possible explanation for the non‐significant difference we found in HGS is that we did not include patients who required hospitalization or ICU admission. In fact, PCS survivors who are hospitalized or critically ill in the ICU may develop significant muscle loss with impaired muscle function (Greve et al., 2020; Postigo‐Martin et al., 2021).
PCS is associated with a low QoL that can negatively impact the functionality and difficulty of participation in the social life, including the inability to work (Lemhöfer et al., 2021). We observed reductions in several dimensions of the SF‐36 in patients with PCS compared with healthy controls, especially in physical functioning, physical role limitations, bodily pain, general health perceptions, and mental health. Evaluating 1027 patients who had mild or moderate COVID‐19, Lemhöfer et al. (2021) also observed low QoL in several dimensions of the SF‐36. This may be related to the findings that mental disorders are common in patients after SARS‐CoV‐2 infection (Malik et al., 2022; Townsend et al., 2020). Furthermore, a worse QoL in these patients may be associated with worsening dyspnoea, neuropsychological disorders, and worse mental health (Malik et al., 2022; Yardley & Redfern, 2001), which may reflect a worsening of postural balance, as shown in our results.
4.1. Study limitations
The strength of this study is that it evaluated postural balance in patients with PCS and its associations with extrapulmonary manifestations, as compared with control subjects matched by anthropometric variables, and both were from multiple centres. However, we should point out some of the limitations of the study. First, this was a cross‐sectional observational study, which impairs a cause‐and‐effect analysis; thus, the lack of pre‐COVID‐19 measures hinders the interpretation and generalization of our results. Second, we also did not use more objective tools to evaluate postural balance, although our aim was to evaluate more accessible tests in clinical practice. Third, the use of scales can help to assess the risk of falls, but it does not differentiate between types of balance deficits (Mancini & Horak, 2010). Finally, we did not recruit patients with PCS who had recovered from severe or critical illness, although this choice allowed us to focus more on the late effects of SARS‐CoV‐2 on postural balance, reducing confounding factors. Despite these limitations, this study may serve as a starting point for randomized controlled trials of more patients who can be subjected to more sophisticated methods for the evaluation of postural balance in PCS.
5. CONCLUSION AND IMPLICATIONS FOR PHYSIOTHERAPY PRACTICE
Using simple tests and tools, we evaluated changes in postural balance in patients with PCS who were not hospitalized and analysed how the changes in postural balance were correlated with general fatigue, muscle strength, and QoL. Our results indicate that survivors of SARS‐CoV‐2 infection have worse postural balance than controls, which points to a greater risk of falls. PCS patients had more general fatigue and worse QoL. Postural balance was correlated with general fatigue, HGS, and QoL, suggesting a possible link between balance damage and muscle dysfunction in patients with PCS. Thus, the routine assessment of postural balance should be considered in the follow‐up of patients with PCS. This may allow the early detection of changes in postural control and the introduction of rehabilitation strategies, including balance and mobility physiotherapy, even in non‐severe patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the National Research Ethics Committee of Brazil under the number CAAE‐30135320.0.0000.5259.
PATIENT CONSENT STATEMENT
All participants signed an informed consent form.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
STUDY REGISTRATION
Not applicable.
ACKNOWLEDGEMENTS
The authors wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico [grant number #302215/2019‐0], Brazil, the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (grant numbers #E‐26/211.024/2019, #E‐26/211.187/2021, #E‐26/202.177/2021, and #E‐26/200.929/2022), Brazil, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Finance Code 001], Brazil.
de Sousa, K. C. A. , Gardel, D. G. , & Lopes, A. J. (2022). Postural balance and its association with functionality and quality of life in non‐hospitalized patients with post‐acute COVID‐19 syndrome. Physiotherapy Research International, 27(4), e1967. 10.1002/pri.1967
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Augustin, M. , Schommers, P. , Stecher, M. , Dewald, F. , Gieselmann, L. , Gruell, H. , Horn, C. , Vanshylla, K. , Cristanziano, V. D. , Osebold, L. , Roventa, M. , Riaz, T. , Tschernoster, N. , Altmueller, J. , Rose, L. , Salomon, S. , Priesner, V. , Luers, J. C. , Albus, C. , … Lehmann, C. (2021). Post‐COVID syndrome in non‐hospitalised patients with COVID‐19: A longitudinal prospective cohort study. The Lancet Regional Health Europe, 6, 100122. 10.1016/j.lanepe.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubkhani, D. , Khunti, K. , Nafilyan, V. , Maddox, T. , Humberstone, B. , Diamond, I. , & Banerjee, A. (2021). Post‐covid syndrome in individuals admitted to hospital with covid‐19: Retrospective cohort study. British Medical Journal, 372, n693. 10.1136/bmj.n693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracat, P. J. , & de Sá Ferreira, A. (2013). Postural tasks are associated with center of pressure spatial patterns of three‐dimensional statokinesigrams in young and elderly healthy subjects. Human Movement Science, 32(6), 1325–1338. 10.1016/j.humov.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Berg, K. O. , Wood‐Dauphinee, S. L. , Willians, J. I. , & Maki, B. (1992). Measuring balance in the elderly: Validation of an instrument. Canadian Journal of Public Health, 83(Suppl. 2), S7–S11. [PubMed] [Google Scholar]
- Ciconelli, R. M. , Ferraz, M. B. , Santos, W. , Meinão, I. , & Quaresma, M. R. (1999). Brazilian‐Portuguese version of the SF‐36. A reliable and valid quality of life outcome measure. Revista Brasileira de Reumatologia, 39(3), 143–150. [Google Scholar]
- Crosby, C. A. , Wehbe, M. A. , & Mawr, B. (1994). Hand strength: Normative values. The Journal of Hand Surgery, 19(4), 665–670. 10.1016/0363-5023(94)90280-1 [DOI] [PubMed] [Google Scholar]
- De Felice, F. G. , Tovar‐Moll, F. , Moll, J. , Munoz, D. P. , & Ferreira, S. T. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the central nervous system. Trends in Neurosciences, 43(6), 355–357. 10.1016/j.tins.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divani, A. A. , Andalib, S. , Biller, J. , Di Napoli, M. , Moghimi, N. , Rubinos, C. A. , Nobleza, C. O. , Sylaja, P. N. , Toledano, M. , Lattanzi, S. , McCullough, L. D. , Cruz‐Flores, S. , Torbey, M. , & Azarpazhooh, M. R. (2020). Central nervous system manifestations associated with COVID‐19. Current Neurology and Neuroscience Reports, 20(12), 60. 10.1007/s11910-020-01079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, A. G. (2016). The relationship between postural and movement stability. Advances In Experimental Medicine and Biology, 957, 105–120. 10.1007/978-3-319-47313-0_6 [DOI] [PubMed] [Google Scholar]
- Gervasoni, F. , LoMauro, A. , Ricci, V. , Salce, G. , Andreoli, A. , Visconti, A. , & Pantoni, L. (2022). Balance and visual reliance in post‐COVID syndrome patients assessed with a robotic system: A multi‐sensory integration deficit. Neurological Sciences, 43(1), 85–88. 10.1007/s10072-021-05647-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardini, M. , Arcolin, I. , Guglielmetti, S. , Godi, M. , Capelli, A. , & Corna, S. (2022). Balance performance in patients with post‐acute COVID‐19 compared to patients with an acute exacerbation of chronic obstructive pulmonary disease and healthy subjects. International Journal of Rehabilitation Research, 45(1), 47–52. 10.1097/MRR.0000000000000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco, G. I. , Noale, M. , Trevisan, C. , Zatti, G. , Pozza, M. D. , Lazzarin, M. , Haxhiaj, L. , Ramon, R. , Imoscopi, A. , Bellon, S. , Maggi, S. , Sergi, G. , & Sergi, S. (2021). Increase in frailty in nursing home survivors of coronavirus disease 2019: Comparison with noninfected residents. Journal of the American Medical Directors Association, 22(5), 943–947. 10.1016/j.jamda.2021.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve, J. M. D. , Brech, G. C. , Quintana, M. , Soares, A. L. S. , & Alonso, A. C. (2020). Impacts of COVID‐19 on the immune, neuromuscular, and musculoskeletal systems and rehabilitation. Revista Brasileira de Medicina do Esporte, 26(4), 285–288. 10.1590/1517-869220202604ESP002 [DOI] [Google Scholar]
- Horak, F. B. (2006). Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age and Ageing, 35(Suppl 2), ii7–ii11. 10.1093/ageing/afl077 [DOI] [PubMed] [Google Scholar]
- Karuka, A. H. , Silva, J. A. M. G. , & Navega, M. T. (2011). Analysis of agreement of assessment tools of body balance in the elderly. Revista Brasileira de Fisioterapia, 15(6), 460–466. 10.1590/s1413-35552011000600006 [DOI] [PubMed] [Google Scholar]
- Lemhöfer, C. , Sturm, C. , Loudovici‐Krug, D. , Best, N. , & Gutenbrunner, C. (2021). The impact of Post‐COVID‐Syndrome on functioning ‐ Results from a community survey in patients after mild and moderate SARS‐CoV‐2‐infections in Germany. Journal of Occupational Medicine and Toxicology, 16(1), 45. 10.1186/s12995-021-00337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, T. R. L. , Kasuki, L. , Gadelha, M. , & Lopes, A. J. (2019). Physical exercise improves functional capacity and quality of life in patients with acromegaly: A 12‐week follow‐up study. Endocrine, 66(2), 301–309. 10.1007/s12020-019-02011-x [DOI] [PubMed] [Google Scholar]
- Malik, P. , Patel, K. , Pinto, C. , Jaiswal, R. , Tirupathi, R. , Pillai, S. , & Patel, U. (2022). Post‐acute COVID‐19 syndrome (PCS) and health‐related quality of life (HRQoL)‐A systematic review and meta‐analysis. Journal of Medical Virology, 94(1), 253–262. 10.1002/jmv.27309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini, M. , & Horak, F. B. (2010). The relevance of clinical balance assessment tools to differentiate balance deficits. European Journal of Physical and Rehabilitation Medicine, 46(2), 239–248. [PMC free article] [PubMed] [Google Scholar]
- Mosher, C. E. , & Duhamel, K. N. (2012). An examination of distress, sleep, and fatigue in metastatic breast cancer patients. Psycho‐Oncology, 21(1), 100–107. 10.1002/pon.1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli, P. , Ferrazzoli, D. , Sebastianelli, L. , Engl, M. , Romanello, R. , Nardone, R. , Bonini, I. , Koch, G. , Saltuari, L. , Quartarone, A. , Oliviero, A. , Kofler, M. , & Versace, V. (2021). Neuropsychological and neurophysiological correlates of fatigue in post‐acute patients with neurological manifestations of COVID‐19: Insights into a challenging symptom. Journal of the Neurological Sciences, 420, 117271. 10.1016/j.jns.2020.117271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi, J. V., Jr , Raffa, R. B. , Varrassi, G. , Magnusson, P. , LeQuang, J. A. , Paladini, A. , Taylor, R. , Wollmuth, C. , Breve, F. , Chopra, M. , Nalamasu, R. , & Christo, P. J. (2021). Potential neurological manifestations of COVID‐19: A narrative review. Postgraduate Medicine, 134(4), 395–405. 10.1080/00325481.2020.1837503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo‐Martin, P. , Cantarero‐Villanueva, I. , Lista‐Paz, A. , Castro‐Martín, E. , Arroyo‐Morales, M. , & Seco‐Calvo, J. (2021). A COVID‐19 rehabilitation prospective surveillance model for use by physiotherapists. Journal of Clinical Medicine, 10(8), 1691. 10.3390/jcm10081691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi, A. , Savci, S. , Kahraman, B. O. , & Ozpelit, E. (2021). Extrapulmonary features of post‐COVID‐19 patients: Muscle function, physical activity, mood, and sleep quality. Irish Journal of Medical Science, 191(3), 969–975. 10.1007/s11845-021-02667-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti, M. E. (1986). Performance‐oriented assessment of mobility problems in elderly patients. Journal of the American Geriatrics Society, 34(2), 119–126. 10.1111/j.1532-5415.1986.tb05480.x [DOI] [PubMed] [Google Scholar]
- Townsend, L. , Dyer, A. H. , Jones, K. , Dunne, J. , Mooney, A. , Gaffney, F. , O'Connor, L. , Leavy, D. , O'Brien, K. , Dowds, J. , Sugrue, J. A. , Hopkins, D. , Martin‐Loeches, I. , Ni Cheallaigh, C. , Nadarajan, P. , McLaughlin, A. M. , Bourke, N. M. , Bergin, C. , O'Farrelly, C. , … Conlon, N. (2020). Persistent fatigue following SARS‐CoV‐2 infection is common and independent of severity of initial infection. PLoS One, 15(11), e0240784. 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott, M. H. , & Shumway‐Cook, A. (2010). Controle motor: teoria e aplicações práticas (3rd ed.). Manole. [Google Scholar]
- Yardley, L. , & Redfern, M. S. (2001). Psychological factors influencing recovery from balance disorders. Journal of Anxiety Disorders, 15(1–2), 107–119. 10.1016/s0887-6185(00)00045-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


