Abstract
Introduction
Responses to SARS‐CoV‐2 vaccination in patients with MS (pwMS) varies by disease‐modifying therapies (DMTs). We perform a meta‐analysis and systematic review of immune response to SARS‐CoV‐2 vaccines in pwMS.
Methods
Two independent reviewers searched PubMed, Google Scholar, and Embase from January 1, 2019‐December 31, 2021, excluding prior SARS‐CoV‐2 infections. The meta‐analysis of observational studies in epidemiology (MOOSE) guidelines were applied. The data were pooled using a fixed‐effects model.
Results
Eight‐hundred sixty‐four healthy controls and 2203 pwMS from 31 studies were included. Antibodies were detected in 93% healthy controls (HCs), and 77% pwMS, with >93% responses in all DMTs (interferon‐beta, glatiramer acetate, cladribine, natalizumab, dimethyl fumarate, alemtuzumab, and teriflunomide) except for 72% sphingosine‐1‐phosphate modulators (S1PM) and 44% anti‐CD20 monoclonal antibodies (mAbs). T‐cell responses were detected in most anti‐CD20 and decreased in S1PM. Higher antibody response was observed in mRNA vaccines (99.7% HCs) versus non‐mRNA vaccines (HCs: 72% inactivated virus; pwMS: 86% vector, 59% inactivated virus). A multivariate logistic regression model to predict vaccine response demonstrated that mRNA versus non‐mRNA vaccines had a 3.4 odds ratio (OR) for developing immunity in anti‐CD20 (p = 0.0052) and 7.9 OR in pwMS on S1PM or CD20 mAbs (p < 0.0001). Antibody testing timing did not affect antibody detection.
Conclusion
Antibody responses are decreased in S1PM and anti‐CD20; however, cellular responses were positive in most anti‐CD20 with decreased T cell responses in S1PM. mRNA vaccines had increased seroconversion rates compared to non‐RNA vaccines. Further investigation in how DMTs affect vaccine immunity are needed.
Introduction
Disease‐modifying therapies (DMTs) and immune reconstitution therapies (IRTs) are used to treat patients with MS (pwMS) to decrease relapses, or new MRI lesions in the brain and spine, and to slow disability progression. 1 The coronavirus disease 2019 (COVID‐19) pandemic is concerning in pwMS due to possible increased susceptibility to COVID‐19, considering many potential interactions with the immune system, neurological complications associated with SARS‐CoV‐2 infection and the immunosuppressive effects of DMTs. 2 , 3 These complications include an increased risk of death due to COVID‐19 in pwMS who have significant neurological disability. 2 Expert consensus has recommended vaccines in pwMS 4 and vaccination is critical as developing immunity to COVID‐19 is protective against serious consequences. 5
Multiple observational studies have shown a variable response to SARS‐CoV‐2 vaccines in pwMS, and several concerns were raised in regard to the immunogenicity of these vaccines in this patient population, particularly on treatment with high efficacy DMTs. This systematic review and meta‐analysis aim to assess rates of immune response, including antibody and T‐cell mediated, to SARS‐CoV‐2 vaccines in pwMS on different DMTs and IRTs.
Methods
A systematic review was conducted for manuscripts from January 1, 2019, until December 31, 2021, by two independent reviewers who are neurologists (M.D. and G.G.). MeSH terms in PubMed, Google Scholar, and Embase included “multiple sclerosis,” “SARS‐CoV‐2”, “Coronavirus‐19”, “vaccines”, and “vaccinations.” Publications reporting on antibody or cellular immunity data related to vaccination response in pwMS were included. Due to limited data available from larger studies, we also included case reports and case series. Antibody and cellular response cut‐off values were as defined by the manufacturer's assays performed in each study. Preprint publications not yet peer‐reviewed and immune response to prior COVID infections were excluded as this was not the focus of this study. The authors were not contacted for additional information. Duplicates and non‐primary articles were also excluded (Fig. 1). The Newcastle‐Ottawa Scale was used to measure the strength of each study 6 (Table S1).
Figure 1.
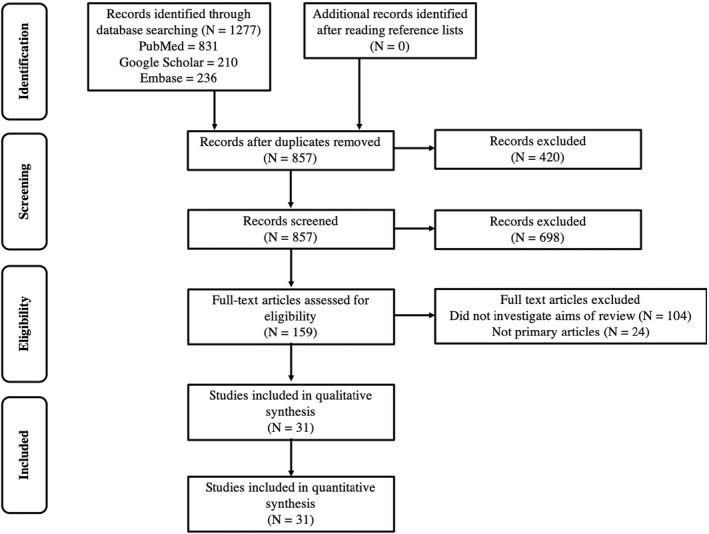
Flow chart of systematic review of SARS‐CoV2 vaccination responses in patients with multiple sclerosis.
Measurements included age, sex, type of MS treatment, positive/negative antibody detection, and T cell responses to SARS‐CoV‐2. Time after vaccination was completed (defined as after second dose after mRNA vaccines or after single dose for other vaccines) and time of anti‐CD20 monoclonal antibody treatment were collected after the initial analysis as these could be confounders for vaccine response (Figs. 2 and 3).
Figure 2.
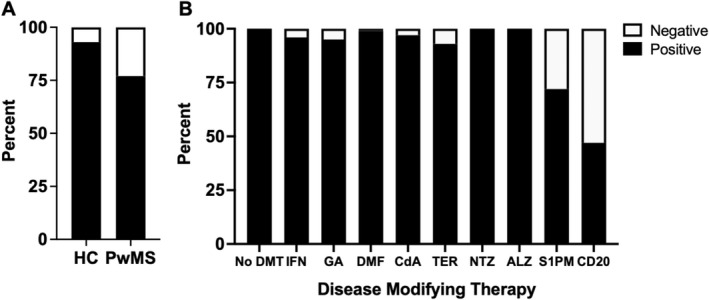
(A) Proportions of positive SARS‐CoV2 antibodies after vaccination in healthy controls (HC) as compared to patients with MS (PwMS). (B) Proportions of positive SARS‐CoV2 antibodies after vaccination in PwMS by type of DMT (disease‐modifying therapy), including interferon‐beta (IFN), glatiramer acetate (GA), dimethyl fumarate/diroximel fumarate (DMF), cladribine (CdA), teriflunomide (TER), natalizumab (NTZ), alemtuzumab (ALZ), sphingosine‐1 phosphate modulators (S1PM), and anti‐CD20 monoclonal antibodies (CD20).
Figure 3.
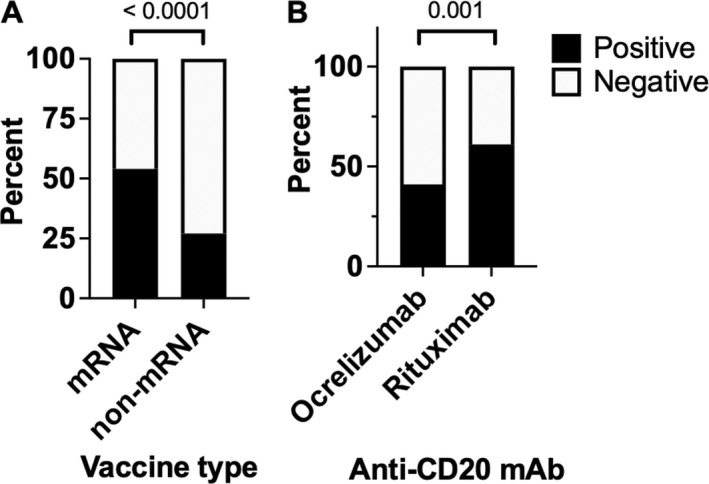
(A) Proportion of patients on anti‐CD20 monoclonal antibodies (mAbs) and sphingosine‐1‐phosphate modulators with positive SARS‐CoV‐2 antibodies by vaccine type (mRNA vaccine versus non‐mRNA vaccine). (B) Proportion of patients who were positive for SARS‐CoV‐2 antibodies post‐vaccination by anti‐CD20 mAbs.
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC), Descriptive statistics were reported. Proportions were compared using either chi‐square tests or Fischer's exact test, and continuous variables were compared using Student's t test or Wilcoxon rank sum test when appropriate. A multivariate logistic regression model was developed predicting SARS‐CoV‐2 antibody positivity by adjusting for anti‐CD20 versus S1PM, time after vaccination was completed, the timing of anti‐CD20 monoclonal antibodies (mAbs), and vaccine type. Sensitivity analyses were performed by applying different times of CD20 administration with vaccine timing.
Results
We identified 1277 articles and screened 857 after 420 duplicates were removed. We excluded 698 articles and then assessed 159 articles for eligibility by removing 104 articles that did not investigate the primary aims of this review and 24 were not primary articles. Thirty‐one articles were included in the analysis (Fig. 1). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35
Antibody response to SARS‐CoV‐2 vaccination
Data from 864 healthy controls and 2203 pwMS were included. Healthy controls were included from the same studies as the pwMSs although some studies did not include controls. Antibody responses to SARS‐CoV‐2 were detected in 93% of healthy controls (803/864) and 77% (1687/2203) of pwMS. Positive antibodies were found in 100% pwMS who were not on treatment (215/215). PwMS on DMTs except for anti‐CD20 or S1PM (sphingosine 1‐phosphate modulators) developed >93% positive antibody responses: 96% pwMS on beta‐interferons (179/187), 95% pwMS on glatiramer acetate (76/80), 99% on dimethyl fumarate/diroximel fumarate (200/203), 100% natalizumab (189/189), 100% alemtuzumab (20/20), 97% cladribrine (173/178), and 93% teriflunomide (111/120). Seventy‐two percent of S1PM (197/274) and 44% (327/737) of pwMS on anti‐CD20 mAbs had positive antibody responses (Fig. 2).
SARS‐CoV‐2 vaccination type
Vaccinations in healthy controls included mRNA (73.5%, 650/864), inactivated virus (24.8%, 635/8640), vector in 0.2% (2/864), and unknown in 1.55% (13/864). For healthy controls, mRNA vaccinations resulted in 99.7% (633/635) and 72.4% (155/214) of inactivated virus with positive antibodies. For pwMS, mRNA was the most common vaccine administered (90.0%, 1982/2203), followed by inactivated virus (6.8%, 150/2203), unknown (2.6%, 57/2203), and vector (0.6%, 14/2203). PwMS who received mRNA vaccines had the highest rates of COVID‐19 seropositivity 78.2% (1549/1982), with 85.7% (12/14) in vector vaccines, and 58.7% (88/150) for the inactivated viral vaccine (p < 0.0001). Similar trends were seen in the DMTs where nearly all pwMS who received mRNA vaccines had seropositivity except for pwMS on CD20 or S1P modulators, with lower seropositivity noted with vector or inactivated viral vaccines (Table 1).
Table 1.
Grouped data from all 31 publications.
| Patient population | SARS‐Cov‐2 antibody positive/Total, % (N) | ||||
|---|---|---|---|---|---|
| ALL | Vaccine type | ||||
| mRNA | Inactivated viral | Vector | Unknown | ||
| Healthy controls | 92.9 (803/864) | 99.7 (633/635) | 72.4 (155/214) | 100 (2/2) | 100 (13/13) |
| Multiple sclerosis (Total) | 76.6 (1687/2203) | 78.2 (1549/1982) | 58.7 (88/150) | 85.7 (12/14) | 66.7 (38/57) |
| Untreated | 100 (215/215) | 100 (206/206) | – | – | 100 (9/9) |
| Interferon‐beta | 95.7 (179/187) | 99.4 (158/159) | 75.0 (21/28) | – | – |
| Glatiramer acetate | 95.0 (76/80) | 100 (65/65) | 73.3 (11/15) | – | – |
| Dimethyl fumarate/Diroximel fumarate | 98.5 (200/203) | 100 (176/176) | 88.9 (24/27) | – | – |
| Cladribine | 97.2 (173/178) | 99.4 (164/165) | 42.9 (3/7) | 100 (6/6) | – |
| Teriflunomide | 92.5 (111/120) | 100 (99/99) | 57.1 (12/21) | – | – |
| Natalizumab | 100 (189/189) | 100 (183/183) | – | – | 100 (6/6) |
| Alemtuzumab | 100 (20/20) | 100 (19/19) | 100 (1/1) | – | – |
| Sphingosine‐1 phosphate modulators (Total) | 71.9 (197/274) | 75.5 (179/237) | 36.4 (8/22) | 75.0 (6/8) | 57.1 (4/7) |
| Fingolimod | 72.7 (160/220) | 76.8 (152/198) | 36.4 (8/22) | 75.0 (6/8) | – |
| Unspecified | 68.5 (37/54) | 71.1 (32/45) | – | – | 57.1 (4/7) |
| Anti‐CD20 mAb (Total) | 44.4 (327/737) | 44.6 (300/673) | 27.6 (8/29) | – | 54.3 (19/35) |
| Ocrelizumab | 40.6 (226/556) | 39.5 (211/534) | – | – | 68.2 (15/22) |
| Rituximab | 60.5 (46/76) | 66.7 (42/63) | – | – | 30.8 (4/13) |
| Unspecified | 61.8 (47/76) | 61.8 (47/76) | 27.6 (8/29) | – | – |
Proportion of subjects who had positive antibody responses after COVID‐19 vaccination, listed by diagnosis and treatment, and vaccine type. Bolded titles are patient groupings.
pwMS, patients with multiple sclerosis; mAb, monoclonal antibody.
Antibody assays in anti‐CD20 monoclonal antibody treatments
We also compared immune responses to two different anti‐CD20 mAbs, rituximab, and ocrelizumab. Ocrelizumab is a fully humanized mAb and rituximab is a chimeric (mouse/human) mAb. Out of 737 patients on anti‐CD20, 63 patients were on rituximab and 534 patients were on ocrelizumab. We then performed subgroup analyses within the anti‐CD20 mAb cohort and compared the proportions of patients who had a positive antibody response. However, pwMS on anti‐CD20 mAbs who received the mRNA vaccines had 44.6% (300/673) positive antibodies as compared to 27.6% (8/29) who received the inactivated viral vaccine (chi‐square test, p = 0.071). Time when antibody testing was performed after the second vaccine dose did not affect response.
When we compared pwMS on ocrelizumab versus rituximab, 60.5% (46/76) of rituximab patients had a positive antibody response as compared to 40.6% (226/556) of ocrelizumab patients (chi‐square test, p = 0.001, Fig. 3). We then performed a multivariate logistic regression model to determine whether vaccine response was affected by anti‐CD20 type (ocrelizumab versus rituximab) and vaccine type (mRNA versus non‐mRNA vaccines) with controlling for test time. In this logistic regression model, the specific CD20 immunotherapy did affect the probability of developing a protective humoral response with an odds ratio (OR) of 3.068 in rituximab as compared to ocrelizumab (95% confidence interval (CI): 1.639, 5.745; p = 0.0005) and time when antibodies were tested after immunization did not affect odds of positive antibodies (OR 1.142, 95% CI 0.585–2.229, p = 0.6983). Moreover, receiving the mRNA vaccine versus a non‐mRNA vaccine resulted in a 3.437 OR for developing immunity (95% CI: 1.447, 8.166; p = 0.0052).
Antibody assays in anti‐CD20 monoclonal antibodies and fingolimod treated pwMS
We then compared antibody responses in anti‐CD20 mAbs (N = 579) versus fingolimod or siponimod (N = 180). The other S1PM was excluded (ozanimod) due to not knowing which S1PM were included or which vaccine was administered. When examining vaccine response in pwMS on CD20 mAbs and fingolimod by type of vaccine, higher rates of antibody responses were noted in mRNA vaccines (54%, 330/612) as compared to non‐mRNA vaccines (27%,16/59, chi‐square p < 0.0001). No differences were observed in mRNA vaccine type with 58% (66/113) in mRNA‐1273 vaccines as compared to 53% (264/499) who received BNT162b2 mRNA vaccine (chi‐square p‐value = 0.2894). Timing of antibody assay after the second vaccination did not affect the rates of antibody positivity, with 54% (216/398) and 53% (108/217) in the combined anti‐CD20 and S1PM group had detectable antibodies when tested at <1 month and ≥1 month, respectively (chi‐square, p = 0.0895).
We then performed a logistic regression model predicting vaccine response with CD20 mAb versus fingolimod, mRNA vaccine versus non‐mRNA vaccine, mRNA vaccine type, and test time as predictor variables. PwMS on S1PM were more likely to develop an antibody response with an OR of 7.86 (95% CI 4.984–12.389, p < 0.0001) as compared to CD20 mAb. The mRNA vaccine increased odds of response 5.472 (95%CI 2.701–11.088, p < 0.0001) as compared to non‐mRNA vaccines with no differences observed between mRNA‐2173 versus BNT 162b2 (1.250, 95%CI 0.827–1.890, p = 0.2900). Time after vaccination for antibody testing did not affect probability of antibody detection (OR 1.028, 95% CI 0.795–1.328, p = 0.8354). Sensitivity analysis including the timing of anti‐CD20 administration in relation to the timing of vaccination was included and the model of mRNA versus non‐mRNA vaccine response remained robust (OR 2.406 to 3.962).
For fingolimod, 88% (137/156) of mRNA vaccines had seropositivity as compared to 8/22 (36%) of non‐mRNA vaccines (p < 0.0001). In a logistic regression model, mRNA vaccination resulted in a 6.5 OR for seropositivity (95% CI 1.949, 21.680, p = 0.0023) as compared to non‐mRNA vaccines. No differences within mRNA vaccine type were observed (96%, 25/26 mRNA‐1273 as compared to 82% (112/130) BNT162b2 mRNA vaccines–Fisher's exact test, p = 0.1547). The mRNA vaccine type and test time to predict vaccine response were examined in this model but the results were not significant (Table 2).
Table 2.
Logistic regression models in patients with MS on anti‐CD20 monoclonal antibodies or fingolimod.
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| Anti‐CD20 mAb and fingolimod | |||
| DMT | |||
| CD20 mAb | Reference | Reference | Reference |
| Fingolimod | 7.86 | 4.984, 12.389 | <0.0001 |
| Vaccine type | |||
| Non‐mRNA | Reference | Reference | Reference |
| mRNA | 5.472 | 2.701, 11.088 | <0.0001 |
| BNT162b2 | Reference | Reference | Reference |
| mRNA‐1273 | 1.250 | 0.827, 1.890 | 0.2900 |
| Test time* | |||
| 30 days or less | Reference | Reference | Reference |
| More than 30 days | 1.028 | 0.795, 1.328 | 0.8354 |
| Anti‐CD20 mAb | |||
| Ocrelizumab | Reference | Reference | Reference |
| Rituximab | 3.068 | 1.639, 5.745 | 0.0005 |
| Vaccine type | |||
| Non‐mRNA | Reference | Reference | Reference |
| mRNA | 3.437 | 1.447, 8.166 | 0.0052 |
| Test time* | |||
| 30 days or less | Reference | Reference | Reference |
| More than 30 days | 1.142 | 0.585, 2.229 | 0.6983 |
| Fingolimod | |||
| Vaccine type | |||
| Non‐mRNA | Reference | Reference | Reference |
| mRNA | 6.500 | 1.949, 21.680 | 0.0023 |
| Test time* | |||
| 30 days or less | Reference | Reference | Reference |
| More than 30 days | 1.398 | 0.902 (2.1660 | 0.1337 |
Bolded titles are each patient grouped by DMTs.
OR, odds ratio; CI, confidence interval; mAb, monoclonal antibody; DMT, disease‐modifying therapy.
Test time: timing of antibody testing after second vaccine dose.
Neutralizing assays were performed in four studies. One study found that 17% (4/24) of patients on fingolimod had neutralizing antibodies as compared to healthy controls (100%, 69/69). 7 The neutralizing antibodies correlated with anti‐RBD antibody titers (p = 0.0024) but ρ = 0.591 which is a low correlation coefficient. A case report of a patient on teriflunomide had a good neutralization response. 8 Another study looking at neutralization found that 100% of controls (5/5) and untreated pwMS (5/5) had neutralizing antibodies, with decreased neutralization in CD20 and S1PM. This study also showed an association between neutralization and RBD antibodies with a significant but low correlation coefficient (ρ = 0.5604 and 0.0156). 9 Neutralization antibodies were decreased CD20 compared to controls. 10
Cellular response to SARS‐CoV‐2 vaccination
Six studies 7 , 9 , 10 , 11 , 12 , 36 and one case report 13 examined T‐cell responses in pwMS on DMTs. Three studies used interferon‐ɣ (IFNg) release assays, two used activation‐induced markers (AIM), and one used the T‐Detect assay. For the IFN‐ɣ release assays, 100% (93/93) of controls and 91% (50/55) of pwMS on CD20 mAbs developed positive responses. 7 , 11 , 13 As for other DMTs, when measuring cellular responses through IFNg release, 89% (25/28) IFN‐β, 70% (14/20) Cladribine, and lowest in fingolimod with 14% (5/35). 7
One study by Apostolidis et al. 10 using AIM found that 100% of CD20 patients had CD4 and CD8 T cell responses after vaccination in all pwMS on anti‐CD20 therapy, even in those who failed to generate anti‐receptor binding domain (RBD) IgG. 10 Another study by Sabatino et al. 9 using AIM had different results. This study examined CD4+ and CD8+ T cells pre‐ and post‐vaccination in healthy controls and pwMS (untreated, glatiramer acetate, dimethyl fumarate, natalizumab, S1P modulators, rituximab, and ocrelizumab). 9 CD4+ T cells increased in all groups including controls and pwMS post‐vaccination as compared to pre‐vaccination CD4+ T cells, except for S1P modulators which had no difference pre and post‐vaccination. For spike tetramer‐positive CD8+ T cells, about 40% of controls and 27% to 56% of MS (untreated, glatiramer acetate, dimethyl fumarate, natalizumab, S1P modulators, rituximab, and ocrelizumab), patients developed post‐vaccination CD8+ T cells with the lowest in the S1P group. 9 In addition, T cells are capable of recognizing mutant SARS‐CoV‐2 variants that partially escape humoral‐based immunity. 10 Another assay to detect SARS‐CoV‐2 specific T cells is the T‐Detect assay, which sequences T‐cell receptors (TCR) to identify SARS‐CoV‐2 TCR sequences. When used in ocrelizumab patients who had negative SARS‐CoV‐2 antibodies, 100% (27/27) had positive SARS‐CoV‐2 T cells. 36
Discussion
MS is a chronic inflammatory, demyelinating, and neurodegenerative disease affecting the central nervous system. The SARS‐CoV‐2 pandemic has presented a clinical concern for patients with multiple sclerosis (pwMS) as treatments may increase adverse outcomes in SARS‐CoV‐2 infection. 37
Vaccine‐mediated immunity
Immunological studies have shown a coordinated interaction between T and B lymphocytes of the adaptive immune system for immunological memory and production of neutralizing antibodies following recognition of vaccine antigens by innate immune cells. 38 The different types of SARS‐CoV‐2 vaccines include mRNA vaccines (BNT162b2 or mRNA‐1273), vector‐mediated, and inactivated virus. These vaccines induce robust humoral and cellular immune responses against the SARS‐CoV‐2 spike protein. 39 , 40 Quantitative measurement of SARS‐CoV‐2 antibodies is used to approximate a protective antibody response, as receptor‐binding domains antibodies have been shown to be neutralizing against infection. 41 The mRNA vaccines were more likely to produce an antibody response in controls and pwMS as compared to other vaccines, especially the inactivated viral vaccine. The majority of patients with measured responses to the inactivated viral vaccine were from a single study 14 that also used a different antibody detection assay than the other studies, so the differences observed could be either the vaccine itself or the assay performed. However, the assay has been validated, so the differences are most likely due to the vaccine type.
Vaccine immunity involves both B‐cell‐mediated antibody responses and T‐cell responses. 15 T cells are critical to generate antibody‐producing plasma cells, long‐lived memory cells, and to eliminate virus‐infected cells. Early and robust T‐cell responses have been associated with mild or asymptomatic COVID‐19 infection even when antibodies are absent. 42 , 43 , 44 , 45 , 46 Additionally, T cells can recognize mutant SARS‐CoV‐2 variants that partially escape humoral‐based immunity. 10 It is still unclear which combination of the immune responses is responsible for the best immunity to SARS‐CoV‐2, both in healthy subjects and in patients on B and T cell‐depleting therapies. Protective humoral responses vary by vaccine type, 47 but this highlights the need for vaccination for any potential protective immunity. Factors that may affect antibody responses including DMT, lymphopenia, and age, but the majority of older patients, including 85 years old or older will develop an immune response, so age is less likely to be a factor.
DMTs can affect immune responses to SARS‐CoV‐2 vaccines. DMTs that do not seem to affect vaccine responses are beta‐interferons, glatiramer acetate, teriflunomide, dimethyl/diroximel fumarate, and natalizumab. Interestingly, decreased antibody response to the influenza vaccine has been observed in natalizumab, 48 whereas 100% of pwMS on natalizumab in this meta‐analysis demonstrated seropositivity to SARS‐CoV‐2 vaccination. PwMS on immune reconstitution therapies such as alemtuzumab and cladribine also had SARS‐CoV‐2 antibody responses, although 93% of cladribine patients had positive antibodies, with 99% response to mRNA vaccines. The most profound effect observed was associated with anti‐CD20 mAbs and S1P1 modulators, with decreased detection of SARS‐CoV‐2 antibodies. However, T cell responses were observed in the majority of patients on CD20 mAbs despite blunted humoral responses. Since anti‐CD20 mAbs and S1P1 modulators had the most significant impact on antibody response to SARS‐CoV2 vaccinations, we focus on mechanisms of vaccine responses in these two DMT classes.
Anti‐CD20 mAbs
Anti‐CD20 mAbs had the greatest effect on antibody response in all the DMTs. This observation may be logical since anti‐CD20 mAbs deplete B cells and B cells produce antibodies. However, antibodies are primarily produced by long‐lived plasma cells. Plasma cells do not express CD20 and persist despite prolonged anti‐CD20 mAb administration. 49 Interestingly, even though anti‐CD20 mAbs do not eliminate plasma cells, anti‐CD20 mAbs affect antibody production including IgG by CD20‐negative long‐lived plasma cells that are produced prior to any anti‐CD20 administration and should not be affected by anti‐CD20 medications. Some studies report that the timing of vaccination versus infusion affects the likelihood of seropositivity, whereas others do not. The presence of CD19+ and/or CD20+ cells were associated with an increased likelihood of antibody response in some studies 9 , 10 , 11 , 12 , 16 , 17 but not in others 18 , 19 , 36 ; the presence of CD19+/CD20+ cells is somewhat correlated with timing 11 , 15 , 17 , 19 , 50 but was not consistently in all patients. 16
This observation highlights the mechanism by which anti‐CD20 mAbs decrease the antibody response to vaccines. Naïve B cells express CD20 and are activated upon encounter with specific antigen. They then differentiate to mature B cells through multiple steps, including proliferation, which may or may not require T cell help. After proliferation, B cells then differentiate into short‐lived plasma cells, germinal center (GC) B cells, and/or memory B cells. The short‐lived plasma cells remain in peripheral lymphoid tissues and can give rise to CD20‐negative long‐lived plasma cells. GC B cells can also give rise to long‐lived plasma cells. 51 While CD20 mAbs reduce circulating CD20+ B cells, CD20 mAbs may not be able to efficiently clear the B cells that reside in secondary lymphoid organs and tissues. 49 Long‐lived plasma cells also reside in the bone marrow and pathogenic antigen‐secreting cells may continue to arise from GC B cells and autoreactive memory B cells. 51 , 52 , 53 , 54 Thus, it may be GC or memory B cells that are being targeted by anti‐CD20 mAbs and reducing inflammation in MS.
We observed that a higher proportion of patients developed positive antibody responses in rituximab versus ocrelizumab. Rituximab is a chimeric monoclonal antibody whereas ocrelizumab is a humanized monoclonal antibody. 55 As compared to rituximab, ocrelizumab binds to an alternate but overlapping epitope and in vitro studies have reported that ocrelizumab has enhanced antibody‐dependent cell‐mediated cytotoxicity and less complement‐dependent cytotoxicity than rituximab. 56 , 57 Thus, one possible explanation for the increased serologic response in rituximab could be that ocrelizumab is potentially more effective at depleting B cells. However, in pwMS on anti‐CD20 mAbs who received mRNA vaccines, a higher proportion of patients on rituximab (27%) received the mRNA‐1273 vaccine as compared to ocrelizumab (8%) (Fischer's exact test, p = 0.009) and the type of CD20 mAb did not correlate with positive antibody responses in the multivariate logistic regression model. The type of mRNA vaccine may explain the differences in antibody response in rituximab versus ocrelizumab.
Other factors such as timing of vaccines related to anti‐CD20 mAb infusions and lymphocyte counts likely affect both serological and cell‐mediated responses to vaccinations, which were not included in the analyses due to limited data. This is a limitation of our analysis. However, some studies associated higher vaccine antibody levels with higher lymphocyte 15 or B cell counts, 10 , 15 , 17 whereas other studies found no correlation with B cell counts or lymphocyte counts with seropositivity. 20 , 21 Additionally, timing of infusions related to vaccination affected antibody response in that a longer time that had passed from the last infusion to vaccination was more likely to result in positive antibody response. 22 , 23 For example, antibodies may be more likely to be detected farther away from vaccination such as 143 days after vaccination, 15 suggestive of a delayed immune response. 10 However, since our analysis did not support this hypothesis, it may the timing of infusion that is affecting this response. While the timing of infusion in relationship with vaccination was not available for most patients, sensitivity analysis showed in our model with vaccine type that infusion timing did not affect antibody response. Also, despite B cell depletion, some pwMS still had detectable antibodies in ocrelizumab 24 and in other DMTs, such as alemtuzumab. 25
However, while B‐cell‐depleting therapies may reduce anti‐SARS‐CoV‐2 antibodies and memory B cell responses, in contrast, the majority of patients had evidence of vaccine‐generated antigen‐specific CD4+ and CD8+ T‐cell responses following vaccination with mRNA vaccines, due to T cell priming. 10 , 11
Sphingosine‐1 phosphate modulators
S1PM also reduced antibody responses to SARS‐CoV‐2 vaccination in pwMS. Decreased antibody response to other vaccines has been reported in influenza 48 although as high as 85% response in pwMS on fingolimod. 58 Lower rates of antibody response to tetanus toxoid vaccine as compared to influenza vaccine has also been noted in pwMS on fingolimod 58 and decreasing varicella‐zoster viral antibodies after starting fingolimod. 59 S1PM decrease S1P receptor expression on lymphocytes, which prevents their egress from lymph nodes. Therefore, lymphocytes do not circulate to the central nervous system and inhibit inflammatory responses. 60 S1PM has shown to decrease antibody‐ssecreting cells and immunoglobulins potentially by its effects on dendritic cells (DCs), follicular T help cells (Tfh), and T helper (Th) cell subsets, including Th1, Th2, and Th17, in the spleen. Fingolimod has been shown to decrease the migration of DCs to the spleen. DCs are important antigen‐presenting cells that interact with B cells and CD4+ naïve T cells to initiate Tfh. Tfh, Th2, and Th17 cells to generate humoral responses. By reducing DCs, Tfh, and Th cells, B cells are less likely to produce antibodies. 61
Other vaccine studies in siponimod demonstrated that antibodies to influenza vaccination and to pneumococcal polysaccharide vaccine, T‐cell independent processes, were comparable to placebo. 62 Further studies to compare the differences in SARS‐CoV‐2 vaccines versus other vaccines are needed to understand which components promote immune response and then improve vaccination efficacy. Additionally, lymphopenia may also affect antibody titers in S1PM although this was not included in this analysis due to insufficient data. 15 Limited studies are available on how S1PM affects the cellular response to COVID vaccination; however, T‐cell responses to the influenza vaccine were detected in pwMS on fingolimod. 63
Conclusion
Additional studies are needed to further understand the mechanisms of how vaccines induce immunity in order to improve immunity in those on DMTs. The studies included in this meta‐analysis were cohorts, case series, and case studies. Publication may affect the results although pwMS on anti‐CD20 with both positive and negative antibody responses were published. PwMS on anti‐CD20 therapy or an S1P‐modulator may have blunted, but not absent, antibody responses to the COVID‐19 vaccines. Nevertheless, they likely benefit from protective or at least partially protective T‐cell responses. 10 This immunity may be sufficient to prevent symptomatic infection, severe disease or death from COVID‐19. Anti‐CD20 treatment may result in a worse clinical outcome, but this could also be complicated by data suggesting that pwMS with higher disability have worse clinical outcomes. Time interval from the last CD20 mAb administration likely affects vaccine‐mediated antibody responses and thus optimal timing of CD20 mAb administration should be assessed in larger studies. For pwMS, vaccination to SARS‐CoV‐2 should occur as soon as possible prior to starting treatment, especially if CD20 mAb or S1PM are being considered. However, the balance of delaying DMTs and IRTs for vaccination must be weighed against the risk for disease activity. 38 , 64 Vaccinations are recommended and are safe in pwMS. 65 , 66 Further investigation is needed to assess how vaccine types affect the immunogenic response in pwMS.
Funding Information
This work was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 and KL2TR002381.
Author Contributions
G.G. Conceived and designed the analysis; Collected the data; Contributed data or analysis tools; Performed the analysis; Wrote the paper. M.D. contributed to study design, data collection, and manuscript writing and editing. W.T. contributed to study design, analysis, and major manuscript editing.
Conflict of Interest
M.D. and W.T. have nothing to declare. G.G. receives part‐time salary support from the Centers for Disease Control and Prevention for acute flaccid myelitis surveillance.
Supporting information
Table S1 The list of studies included in this meta‐analysis and review, including the Newcastle Ottawa Scale (NOS).
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1TR002378 and KL2TR002381.
Annals of Clinical and Translational Neurology 2022;9(8): 1321–1331
Funding Statement
This work was funded by National Institutes of Health grants KL2TR002381 and UL1TR002378; National Center for Advancing Translational Sciences .
References
- 1. Filippi M, Danesi R, Derfuss T, et al. Early and unrestricted access to high‐efficacy disease‐modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol. 2021. doi: 10.1007/s00415-021-10836-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prosperini L, Tortorella C, Haggiag S, Ruggieri S, Galgani S, Gasperini C. Increased risk of death from COVID‐19 in multiple sclerosis: a pooled analysis of observational studies. J Neurol. 2021. doi: 10.1007/s00415-021-10803-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sormani MP, De Rossi N, Schiavetti I, et al. Disease‐modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780‐789. doi: 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centonze D, Rocca MA, Gasperini C, et al. Disease‐modifying therapies and SARS‐CoV‐2 vaccination in multiple sclerosis: an expert consensus. J Neurol. 2021;268(11):3961‐3968. doi: 10.1007/s00415-021-10545-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heudel P, Favier B, Assaad S, Zrounba P, Blay JY. Reduced SARS‐CoV‐2 infection and death after two doses of COVID‐19 vaccines in a series of 1503 cancer patients. Ann Oncol. 2021. doi: 10.1016/j.annonc.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 7. Tortorella C, Aiello A, Gasperini C, et al. Humoral‐ and T‐cell‐specific immune responses to SARS‐CoV‐2 mRNA vaccination in patients with MS using different disease‐modifying therapies. Neurology. 2022;98(5):e541‐e554. doi: 10.1212/WNL.0000000000013108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michiels Y, Houhou‐Fidouh N, Collin G, Berger J, Kohli E. Humoral response induced by prime‐boost vaccination with the chadox1 ncov‐19 and mrna bnt162b2 vaccines in a teriflunomide‐treated multiple sclerosis patient. Vaccines (Basel). 2021;9(10). doi: 10.3390/vaccines9101140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabatino JJ Jr, Mittl K, Rowles WM, et al. Multiple sclerosis therapies differentially affect SARS‐CoV‐2 vaccine‐induced antibody and T cell immunity and function. JCI Insight. 2022;7(4). doi: 10.1172/jci.insight.156978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS‐CoV‐2 mRNA vaccination in patients with multiple sclerosis on anti‐CD20 therapy. Nat Med. 2021. doi: 10.1038/s41591-021-01507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brill L, Rechtman A, Zveik O, et al. Humoral and T‐cell response to SARS‐CoV‐2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021. doi: 10.1001/jamaneurol.2021.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madelon N, Lauper K, Breville G, et al. Robust T cell responses in anti‐CD20 treated patients following COVID‐19 vaccination: a prospective cohort study. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferguson J, Murugesan K, Banaei N, Liu A. Interferon‐gamma release assay testing to assess COVID‐19 vaccination response in a SARS‐CoV‐2 seronegative patient on rituximab: a case report. Int J Infect Dis. 2021;110:229‐231. doi: 10.1016/j.ijid.2021.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Etemadifar M, Sedaghat N, Nouri H, et al. SARS‐CoV‐2 serology among people with multiple sclerosis on disease‐modifying therapies after BBIBP‐CorV (Sinopharm) inactivated virus vaccination: same story, different vaccine. Mult Scler Relat Disord. 2022;57:103417. doi: 10.1016/j.msard.2021.103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS‐CoV‐2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;103581. doi: 10.1016/j.ebiom.2021.103581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Kempen ZLE, Wieske L, Stalman EW, et al. Longitudinal humoral response after SARS‐CoV‐2 vaccination in ocrelizumab treated MS patients: to wait and repopulate? Mult Scler Relat Disord. 2022;57:103416. doi: 10.1016/j.msard.2021.103416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Disanto G, Sacco R, Bernasconi E, et al. Association of disease‐modifying treatment and anti‐CD20 infusion timing with humoral response to 2 SARS‐CoV‐2 vaccines in patients with multiple sclerosis. JAMA Neurol. 2021. doi: 10.1001/jamaneurol.2021.3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rico A, Ninove L, Maarouf A, et al. Determining the best window for BNT162b2 mRNA vaccination for SARS‐CoV‐2 in patients with multiple sclerosis receiving anti‐CD20 therapy. Mult Scler J Exp Transl Clin. 2021;7(4):20552173211062142. doi: 10.1177/20552173211062142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Capone F, Lucchini M, Ferraro E, et al. Immunogenicity and safety of mRNA COVID‐19 vaccines in people with multiple sclerosis treated with different disease‐modifying therapies. Neurotherapeutics. 2022;19(1):325‐333. doi: 10.1007/s13311-021-01165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Achiron A, Mandel M, Dreyer‐Alster S, et al. Humoral immune response to COVID‐19 mRNA vaccine in patients with multiple sclerosis treated with high‐efficacy disease‐modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012835. doi: 10.1177/17562864211012835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerrieri S, Lazzarin S, Zanetta C, Nozzolillo A, Filippi M, Moiola L. Serological response to SARS‐CoV‐2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real‐life experience. J Neurol. 2021. doi: 10.1007/s00415-021-10663-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buttari F, Bruno A, Dolcetti E, et al. COVID‐19 vaccines in multiple sclerosis treated with cladribine or ocrelizumab. Mult Scler Relat Disord. 2021;52:102983. doi: 10.1016/j.msard.2021.102983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khayat‐Khoei M, Conway S, Rubinson DA, Jarolim P, Houtchens MK. Negative anti‐SARS‐CoV‐2 S antibody response following Pfizer SARS‐CoV‐2 vaccination in a patient on ocrelizumab. J Neurol. 2021;268(10):3592‐3594. doi: 10.1007/s00415-021-10463-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mado H, Kubicka‐Baczyk K, Adamczyk‐Sowa M. Anti‐severe acute respiratory syndrome coronavirus‐2 antibody responses following Pfizer‐BioNTech vaccination in a patient with multiple sclerosis treated with ocrelizumab: a case report. J Int Med Res. 2021;49(9):3000605211044378. doi: 10.1177/03000605211044378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drulovic J, Ivanovic J, Martinovic V, et al. Humoral response to SARS‐CoV‐2 COVID‐19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult Scler Relat Disord. 2021;54:103150. doi: 10.1016/j.msard.2021.103150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bigaut K, Kremer L, Fabacher T, et al. Impact of disease‐modifying treatments of multiple sclerosis on anti‐SARS‐CoV‐2 antibodies: an observational study. Neurol Neuroimmunol Neuroinflamm. 2021;8(5). doi: 10.1212/NXI.0000000000001055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Capuano R, Donnarumma G, Bisecco A, et al. Humoral response to SARS‐CoV‐2 mRNA vaccine in patients with multiple sclerosis treated with natalizumab. Ther Adv Neurol Disord. 2021;14:17562864211038111. doi: 10.1177/17562864211038111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallo A, Capuano R, Donnarumma G, et al. Preliminary evidence of blunted humoral response to SARS‐CoV‐2 mRNA vaccine in multiple sclerosis patients treated with ocrelizumab. Neurol Sci. 2021;42(9):3523‐3526. doi: 10.1007/s10072-021-05397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rommer PS, Bsteh G, Berger T, Zettl UK. SARS‐CoV‐2 antibodies in multiple sclerosis patients depending on the vaccine mode of action? Mult Scler. 2021;13524585211039128. doi: 10.1177/13524585211039128 [DOI] [PubMed] [Google Scholar]
- 30. Krbot Skoric M, Rogic D, Lapic I, Segulja D, Habek M. Humoral immune response to COVID‐19 vaccines in people with secondary progressive multiple sclerosis treated with siponimod. Mult Scler Relat Disord. 2022;57:103435. doi: 10.1016/j.msard.2021.103435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Novak F, Nilsson AC, Nielsen C, et al. Humoral immune response following SARS‐CoV‐2 mRNA vaccination concomitant to anti‐CD20 therapy in multiple sclerosis. Mult Scler Relat Disord. 2021;56:103251. doi: 10.1016/j.msard.2021.103251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maniscalco GT, Manzo V, Ferrara AL, et al. Interferon Beta‐1a treatment promotes SARS‐CoV‐2 mRNA vaccine response in multiple sclerosis subjects. Mult Scler Relat Disord. 2022;58:103455. doi: 10.1016/j.msard.2021.103455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grothe C, Steffen F, Bittner S. Humoral immune response and lymphocyte levels after complete vaccination against COVID‐19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J Cent Nerv Syst Dis. 2021;13:11795735211060118. doi: 10.1177/11795735211060118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giossi R, Consonni A, Torri Clerici V, et al. Anti‐spike IgG in multiple sclerosis patients after BNT162b2 vaccine: an exploratory case‐control study in Italy. Mult Scler Relat Disord. 2022;58:103415. doi: 10.1016/j.msard.2021.103415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brill L, Rechtman A, Zveik O, et al. Effect of cladribine on COVID‐19 serology responses following two doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;57:103343. doi: 10.1016/j.msard.2021.103343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katz JD, Bouley AJ, Jungquist RM, Douglas EA, O'Shea IL, Lathi ES. Humoral and T‐cell responses to SARS‐CoV‐2 vaccination in multiple sclerosis patients treated with ocrelizumab. Mult Scler Relat Disord. 2022;57:103382. doi: 10.1016/j.msard.2021.103382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS‐CoV‐2 infection in a north American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699‐708. doi: 10.1001/jamaneurol.2021.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamout BI, Zakaria M, Inshasi J, et al. MENACTRIMS practice guideline for COVID‐19 vaccination in patients with multiple sclerosis. Mult Scler Relat Disord. 2021;56:103225. doi: 10.1016/j.msard.2021.103225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sahin U, Muik A, Derhovanessian E, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594‐599. doi: 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 40. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS‐CoV‐2 infection. Nature. 2020;584(7819):115‐119. doi: 10.1038/s41586-020-2380-z [DOI] [PubMed] [Google Scholar]
- 42. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158‐168 e14. doi: 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Le Bert N, Tan AT, Kunasegaran K, et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature. 2020;584(7821):457‐462. doi: 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- 44. Nelde A, Bilich T, Heitmann JS, et al. SARS‐CoV‐2‐derived peptides define heterologous and COVID‐19‐induced T cell recognition. Nat Immunol. 2021;22(1):74‐85. doi: 10.1038/s41590-020-00808-x [DOI] [PubMed] [Google Scholar]
- 45. Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS‐CoV‐2‐specific T cells associates with rapid viral clearance and mild disease in COVID‐19 patients. Cell Rep. 2021;34(6):108728. doi: 10.1016/j.celrep.2021.108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gallais F, Velay A, Nazon C, et al. Intrafamilial exposure to SARS‐CoV‐2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27(1). doi: 10.3201/eid2701.203611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bar‐Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999‐e2008. doi: 10.1212/WNL.0000000000010380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olberg HK, Eide GE, Cox RJ, et al. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur J Neurol. 2018;25(3):527‐534. doi: 10.1111/ene.13537 [DOI] [PubMed] [Google Scholar]
- 49. Hale M, Rawlings DJ, Jackson SW. The long and the short of it: insights into the cellular source of autoantibodies as revealed by B cell depletion therapy. Curr Opin Immunol. 2018;55:81‐88. doi: 10.1016/j.coi.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ali A, Dwyer D, Wu Q, et al. Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine. 2021;39(41):6111‐6116. doi: 10.1016/j.vaccine.2021.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cyster JG, Allen CDC. B cell responses: cell interaction dynamics and decisions. Cell. 2019;177(3):524‐540. doi: 10.1016/j.cell.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baker D, Roberts CAK, Pryce G, et al. COVID‐19 vaccine‐readiness for anti‐CD20‐depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149‐161. doi: 10.1111/cei.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Md Yusof MY, Vital EM, Buch MH. B cell therapies, approved and emerging: a review of infectious risk and prevention during use. Curr Rheumatol Rep. 2015;17(10):65. doi: 10.1007/s11926-015-0539-7 [DOI] [PubMed] [Google Scholar]
- 54. Kado R, Sanders G, McCune WJ. Suppression of normal immune responses after treatment with rituximab. Curr Opin Rheumatol. 2016;28(3):251‐258. doi: 10.1097/BOR.0000000000000272 [DOI] [PubMed] [Google Scholar]
- 55. Sorensen PS, Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord. 2016;9(1):44‐52. doi: 10.1177/1756285615601933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van der Kolk LE, Grillo‐Lopez AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side‐effects of rituximab treatment. Br J Haematol. 2001;115(4):807‐811. doi: 10.1046/j.1365-2141.2001.03166.x [DOI] [PubMed] [Google Scholar]
- 57. Ocrelizumab [data on file]. Genentech; 2003. [Google Scholar]
- 58. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod‐treated patients with multiple sclerosis. Neurology. 2015;84(9):872‐879. doi: 10.1212/WNL.0000000000001302 [DOI] [PubMed] [Google Scholar]
- 59. Signoriello E, Bonavita S, Sinisi L, et al. Is antibody titer useful to verify the immunization after VZV vaccine in MS patients treated with fingolimod? A case series. Mult Scler Relat Disord. 2020;40:101963. doi: 10.1016/j.msard.2020.101963 [DOI] [PubMed] [Google Scholar]
- 60. McGinley MP, Cohen JA. Sphingosine 1‐phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021;398(10306):1184‐1194. doi: 10.1016/S0140-6736(21)00244-0 [DOI] [PubMed] [Google Scholar]
- 61. Liu Y, Yang CL, Yang B, et al. Prophylactic administration of fingolimod (FTY720) ameliorated experimental autoimmune myasthenia gravis by reducing the number of dendritic cells, follicular T helper cells and antibody‐secreting cells. Int Immunopharmacol. 2021;96:107511. doi: 10.1016/j.intimp.2021.107511 [DOI] [PubMed] [Google Scholar]
- 62. Ufer M, Shakeri‐Nejad K, Gardin A, et al. Impact of siponimod on vaccination response in a randomized, placebo‐controlled study. Neurol Neuroimmunol Neuroinflamm. 2017;4(6):e398. doi: 10.1212/NXI.0000000000000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mehling M, Hilbert P, Fritz S, et al. Antigen‐specific adaptive immune responses in fingolimod‐treated multiple sclerosis patients. Ann Neurol. 2011;69(2):408‐413. doi: 10.1002/ana.22352 [DOI] [PubMed] [Google Scholar]
- 64. Cabreira V, Abreu P, Soares‐Dos‐Reis R, Guimaraes J, Sa MJ. Multiple sclerosis, disease‐modifying therapies and COVID‐19: a systematic review on immune response and vaccination recommendations. Vaccines (Basel). 2021;9(7). doi: 10.3390/vaccines9070773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kelly H, Sokola B, Abboud H. Safety and efficacy of COVID‐19 vaccines in multiple sclerosis patients. J Neuroimmunol. 2021;356:577599. doi: 10.1016/j.jneuroim.2021.577599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Briggs FBS, Mateen FJ, Schmidt H, et al. COVID‐19 vaccination reactogenicity in persons with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2022;9(1). doi: 10.1212/NXI.0000000000001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The list of studies included in this meta‐analysis and review, including the Newcastle Ottawa Scale (NOS).


