Abstract
Background
The healthcare industry's efforts to immunize the global community against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) have been unprecedented. Given the fast‐tracking of the novel vaccine, its short‐ and long‐term medical implications remain largely to‐be‐determined in most patient populations. This study aims to analyze 90‐day post‐operative outcomes in microsurgical patients, who have received or not received SARS‐CoV‐2‐vaccination, using a continuously updated federated electronic medical record network (TriNetX Inc, Cambridge, MA).
Methods
After screening 70 million de‐identified records, 16,799 microsurgery patients aged 18–99 meeting medical coding criteria were allocated into two cohorts. Cohort One received SARS‐CoV‐2‐vaccination prior to undergoing microsurgery whereas Cohort Two did not. Two equally sized cohorts, totaling 818 patients were created after propensity score matching for characteristics including: age, race, ethnicity, smoking, hypertension, heart disease, diabetes, obesity, chronic obstructive pulmonary disease, and history of SARS‐CoV‐2 exposure. Postoperative outcomes within 30‐, 60‐, and 90‐days of microsurgery were analyzed.
Results
Patients who were SARS‐CoV‐2‐immunized experienced significantly lower (p < .01) surgical site infections (Absolute Risk Reduction (ARR)[95%CI]) = (3.79%–5.36% [0.84–8.54]) ICU admission (9.47%–9.82%[5.45–13.88]), generalized infections (7.68%–9.92%[3.15–14.64]), and hospitalizations (28.48%–32.57%[20.99–40.13]) within 30‐, 60‐, and 90‐days of microsurgery. Additionally, SARS‐CoV‐2‐vaccinated patients also experienced significantly less flap failure (2.49%[0.97–4.02]) and death (2.46%[0.96–3.97]) within 30‐ and 60‐days post‐operatively.
Conclusion
Our analysis examines the potential protective effect of SARS‐CoV‐2‐vaccination in microsurgical patients. Limitations include the retrospective nature of this analysis and the inherent reliance on medical coding. Future prospective studies are warranted to better understand if in fact pre‐operative SARS‐CoV‐2‐vaccination has the potential to protect against post‐operative microsurgery outcomes.
1. INTRODUCTION
Since the first reported case in December of 2019, Severe Acute Respiratory Syndrome‐Coronavirus‐2 (SARS‐CoV‐2) has been highly destructive and disruptive (Ioannidis, 2020; Kolahchi et al., 2021). As of December 2021, SARS‐CoV‐2 has infected over 270 million people with a mortality exceeding five million, thereby drastically altering the course of daily life and day‐to‐day operations of global healthcare systems (WHO, 2021). More specifically, COVID‐19 has affected both elective and emergent surgery procedures, across all specialties (Collaborative CO, 2020a; Moletta et al., 2020). From stringent personal protective equipment (PPE) protocols to pre‐admission and discharge screening measures which have frequently led to the postponement of procedures and increased length of hospital stay respectively, SARS‐CoV‐2 has truly altered surgical life as we knew it (Miller et al., 2020; Patel et al., 2020; Prasad et al., 2021; Thione et al., 2021; Toro et al., 2021; Yuan & Jiang, 2020).
Aside from its impact on pre‐operative management and procedure selection, SARS‐CoV‐2 may also negatively impact surgical outcomes (Katsiampoura et al., 2020; Phelps et al., 2020). Previous studies have shown that 30‐day mortality and complications, including thrombosis, are significantly increased in patients who have undergone surgery while infected with COVID‐19 (Carrier et al., 2021; Doglietto et al., 2020). More specifically, prior studies have shown that SARS‐CoV‐2 may increase risk for thrombotic events via induction of a pro‐coagulable state which, when combined with the pro‐inflammatory environment induced by surgery, can contribute to formation of deep vein thrombi, pulmonary emboli, and large vessel strokes (Abou‐Ismail et al., 2020; Janardhan et al., 2020; Mucha et al., 2020; Zapata et al., 2020). In addition to the proposed increased risk for thrombotic events, the resulting damage and inflammation from viral invasion of endothelial cells may predispose SARS‐CoV‐2‐positive patients to acute respiratory distress syndrome (ARDS) and hypoxia (Siddiqi et al., 2021).
Microsurgical cases are particularly vulnerable to potential vascular and thrombotic complications. Recently, several studies have examined SARS‐CoV‐2 manifestations in patients requiring either microvascular free tissue transfer or amputations, noting increased risk of vasculitis and microvascular thrombi (Ilonzo et al., 2021; Mazzeffi et al., 2021; Morales‐Perez et al., 2021). Notably, the patients in these studies underwent surgery in March 2020, well before the development of any of the SARS‐CoV‐2 vaccines (Ilonzo et al., 2021; Morales‐Perez et al., 2021). Further investigations have suggested that the risk of complications may be worse in middle‐aged and elderly patients with pre‐existing endothelial dysfunction (Roncati et al., 2020). While scant data regarding post‐operative outcomes in COVID‐19‐vaccinated patients exists, vaccination modeling has suggested that COVID‐19 immunization may improve SARS‐CoV‐2‐associated post‐operative morbidity and mortality (Covidsurg Collaborative GC, 2021). To our knowledge, no cohort studies have been performed to examine the effects of COVID‐19 vaccination specifically on microsurgical outcomes. Therefore, this study retrospectively analyzes 30‐, 60‐, and 90‐day‐post‐operative outcomes of COVID‐19‐vaccinated microsurgical patients with the aim of characterizing any potential protective effects in this population.
2. METHODS
De‐identified records of 70,632,709 patients aged 18 to 99 were screened retrospectively in June 2021 using the TriNetX (TriNetX Inc, Cambridge, MA, USA) database. TriNetX is a Health Insurance Portability and Accountability Act (HIPAA) compliant, globally‐federated health research network that aggregates the data of over 70 million de‐identified electronic medical records (EMRs) from 51 healthcare organizations (HCOs) worldwide (Topaloglu & Palchuk, 2018).
Current Procedural Terminology (CPT) codes were used to identify 16,799 patients aged 18–99 who had undergone any microvascular procedures (Table 1). Of these individuals, only patients undergoing microsurgery from January 2020 to June 2021 were included in the analysis. Patients in Cohort One included those that received at least one dose of either Pfizer (91300), Moderna (91301), Oxford University‐AstraZeneca (91302), or Johnson & Johnson (91303) SARS‐CoV‐2 vaccines 0–6 months prior to undergoing microsurgery. Patients in Cohort Two excluded patients that received at least one dose of SARS‐CoV‐2 Vaccination 0–6 months prior to undergoing microsurgery. After balancing, a total of 818 patients remained, with 409 SARS‐CoV‐2‐vaccinated microsurgery patients and a matched cohort of the 409 most similar microsurgery patients who had not received SARS‐CoV‐2 vaccination within 6 months of undergoing microsurgery (Figure 1). CPT codes were also used to capture patients who had undergone microsurgery within 6 months of receiving the SARS‐CoV‐2 vaccines. This 6‐month timeframe was utilized because evidence has suggested vaccination‐elicited antibody persistence through 6 months following second dose of the vaccine (Doria‐Rose et al., 2021). Patients outside of the 18‐ to 99‐year‐old age group, those who did not undergo microsurgery within 6 months of SARS‐CoV‐2 vaccination, or those who did not meet the study criteria on or before June 2021 were excluded. Additionally, each adverse outcome analysis excluded subjects that experienced the individual adverse outcome prior to the time window of interest (i.e., experienced the adverse outcome on the same day as surgery).
TABLE 1.
CPT codes used for patients having undergone microsurgery within six‐months of receiving or not receiving COVID‐19 immunization with the Pfizer, Moderna, Oxford University‐AstraZeneca, or Johnson & Johnson vaccines
| Microsurgical procedures with CPT codes | Non COVID‐19 vaccinated | COVID‐19 vaccinated |
|---|---|---|
| 20972—Free osteocutaneous flap with microvascular anastomosis, metatarsal | 0.2% (1) | 0% (0) |
| 15842—Graft for facial nerve paralysis; free muscle flap by microsurgical technique | 1.0% (4) | 2.0% (8) |
| 20970—Free osteocutaneous flap with microvascular anastomosis; iliac crest | 0.5% (2) | 0% (0) |
| 64822—Sympathectomy; ulnar artery | 1.2% (5) | 2.0% (8) |
| 20956—Bone graft with microvascular anastomosis; Iliac crest | 0.5% (2) | 2.0% (8) |
| 20955—Bone graft with microvascular anastomosis; Fibula | 0.2% (1) | 2.0% (8) |
| 19364—Breast reconstruction with free flap | 26.4% (108) | 28.9% (118) |
| 20970—Free osteocutaneous flap with microvascular anastomosis; iliac crest | 0.2% (1) | 0% (0) |
| 20969—Free osteocutaneous flap with microvascular anastomosis; other than iliac crest, metatarsal, or great toe | 12.7% (52) | 11.0% (45) |
| 20962—Bone graft with microvascular anastomosis; other than fibular, iliac crest, or metatarsal | 1.0% (4) | 2.0% (8) |
| 26556—Transfer, free toe joint, with microvascular anastomosis | 0.2% (1) | 0% (0) |
| 20973—Free osteocutaneous flap with microvascular anastomosis; great toe with web space | 0.2% (1) | 0% (0) |
| 64821—Sympathectomy; radial artery | 1.5% (6) | 2.0% (8) |
| 64820—Sympathectomy; digital arteries, each digit | 1.5% (6) | 2.0% (8) |
| 15756—Free muscle or myocutaneous flap with microvascular anastomosis | 20.3% (83) | 18.8% (77) |
| 15757—Free skin flap with microvascular anastomosis | 24.4% (100) | 17.8% (73) |
| 64823—Sympathectomy; superficial palmar arch | 1.0% (4) | 2.0% (8) |
| 15758—Free facial flap with microvascular anastomosis | 6.8% (28) | 7.8% (32) |
FIGURE 1.
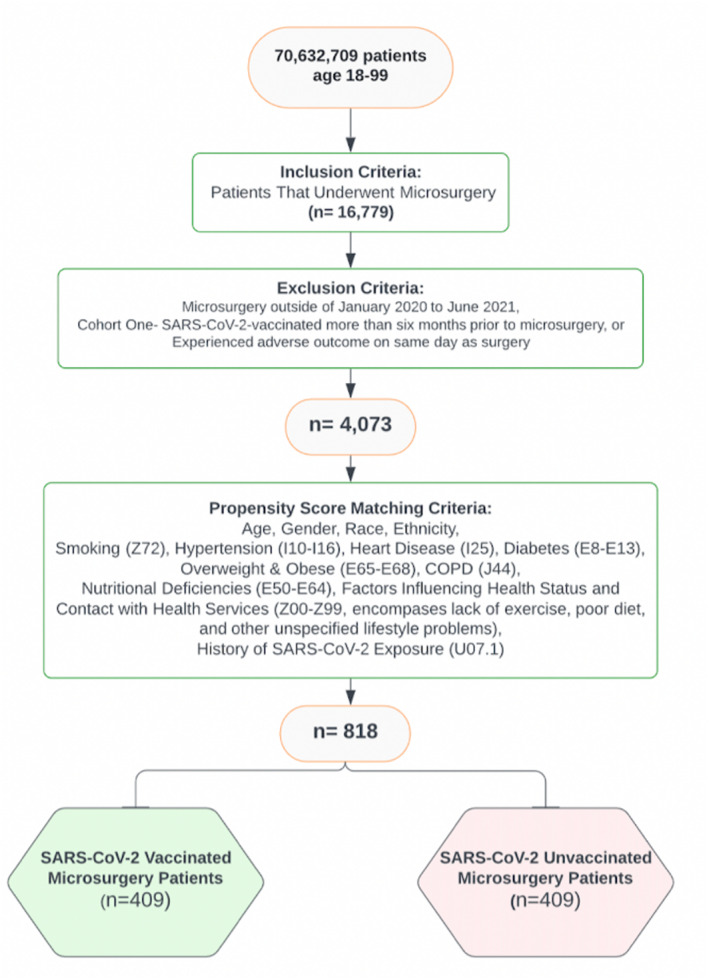
Inclusion and exclusion criteria used to narrow down the EMRs from initial 70,632,709 patients into 16,779 total microsurgery patients, and ultimately forming two propensity score matched 409‐patient cohorts (N = 818) for direct comparison of adverse outcomes within 30, 60, & 90 days of undergoing microsurgery with or without COVID‐vaccination January 2020–June 2021
2.1. Cohort balancing
Following application of inclusion and exclusion criteria, two cohorts of microsurgery patients were created, one of which had received SARS‐CoV‐2‐vaccination within 6 months of surgery and one that had not. Propensity score matching prior to outcome assessment was conducted for various factors including age, race, gender, ethnicity, smoking (Z72.0), hypertension (I10‐I16), heart disease (I25), diabetes (E08‐E13), overweight/obese body habitus (E65‐E68), chronic obstructive pulmonary disease (J44), nutritional deficiencies(E50‐E64), factors influencing health status and contact with health services (Z00‐Z99), and history of SARS‐CoV‐2 exposure (U07.1).
Propensity score matching was carried out in order to reduce confounding bias and maximize external validity (Kane et al., 2020). 1:1 propensity score balancing was completed via logistic regression utilizing version 3.7 of Python Software Foundation's Scikit‐Learn package (Python Software Foundation, Delaware, USA). A greedy nearest neighbor matching algorithm approach was used, setting standard differences to a value of less than 0.1 to indicate appropriate matching. To eliminate record order bias, randomization of the record order in a covariate matrix was performed before matching. Baseline characteristics with a standardized mean difference between cohorts lower than 0.1 were used and considered well‐balanced.
Following propensity score matching, two equally sized cohorts, totaling 818 patients, were created. Cohort One received pre‐operative Pfizer, Moderna, Oxford University‐AstraZeneca, or Johnson & Johnson SARS‐CoV‐2 vaccination within 6 months prior to surgery, while Cohort Two did not. Post‐operative outcomes were compared between cohorts within 30‐, 60‐, and 90‐days following surgery. International Classification of Diseases (ICD‐10) codes were used to assess adverse outcomes including sepsis (A41.9, T81.44), deep vein thrombosis (DVT) (I82.22, I82.40‐I82.89, I82.19), stroke (I63), acute respiratory distress syndrome (ARDS) (J80), pulmonary embolism (PE) (I26), surgical site infection (SSI) (T81.41, T81.42, T81.49), flap failure (T86.821), generalized infections (L00‐L08), gangrene (I96), dehiscence (T81.30, T81.31), hematoma (L76.32), seroma (L76.34), hospitalizations (1013659), intensive care unit (ICU) admissions (1013729), and death.
2.2. Data analysis
Statistical analysis and logistical regression were performed using the Analytics function of the TriNetX platform by comparing indices and absolute risks of post‐operative complications after successful cohort matching with a p‐value greater than .05. Outcomes for all measures were calculated using 95% confidence intervals (CIs). All p‐values were two‐sided with an alpha level of .05. Absolute risk reduction (ARR) was defined in this study as the difference in risk of adverse post‐operative outcomes between the SARS‐CoV‐2‐vaccinated group and non‐vaccinated group. ARR was calculated for each adverse outcome. ARR should be interpreted in the context of reduction of baseline risk and its reciprocal was calculated as number needed to treat, operationalized henceforth as number needed to vaccinate (NNV), which can assess benefit to patient (Ranganathan et al., 2016). NNV enabled evaluation of potential SARS‐CoV‐2 immunization benefit through calculation of the average number of microsurgery patients needed to vaccinate pre‐operatively to prevent one of the aforementioned outcomes (Hashim et al., 2015). Kaplan–Meier curves, used to show probability of survival over a given length of time, were created for ED visit, ICU admission, and hospitalization 0–90 days post‐operatively (Goel et al., 2010).
3. RESULTS
SARS‐CoV‐2‐immunized patients undergoing microsurgery experienced significantly decreased risks of SSIs (Absolute Risk Reduction(ARR): 3.79%–5.36% 95% CI: 0.836–8.535, NNV: 18.7–26.3, p < .01), ICU admission (ARR: 9.47%–9.82%, 95%CI: 5.452–13.88, NNV: 10.2–10.6, p < .0001), generalized infections (ARR: 7.68–9.92, 95% CI: 3.153–14.638, NNV: 10.1–13.0, p < .003), and utilization of hospital inpatient services (ARR: 28.48%–32.57%, 95%CI: 20.994–40.13, NNV: 3.1–3.5, p < .0001) at 30, 60, and 90 days‐post‐operatively. Additionally, SARS‐CoV‐2‐immunized patients also experienced significantly less flap failure (ARR: 2.49%, 95% CI: 0.968–4.02, NNV: 40.1, p = .0019) and death (ARR:2.46%, 95% CI: 0.955–3.971, NNV: 40.6, p = .0014) at 30 and 60 days‐post‐operatively. SARS‐CoV‐2‐immunized patients were found to have a decreased risk for dehiscence (ARR: 4.035%–4.257%, 95%CI: 0.818–7.253, NNV: 23.5–24.8, p < .02) at 60‐ and 90‐days following microsurgery (Table 2). Lastly, SARS‐CoV‐2‐vaccinated patients were found to have decreased ED Visits (ARR: 7.447%, 95%CI: 2.346, 12.548, NNV: 13.4, p < .01) at 90 days post‐operatively (Figures 2 and 3, Table 2).
TABLE 2.
Statistical analysis of post‐operative adverse outcomes within 30, 60, & 90 days of microsurgery
| 30 Days (N = 818) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No vaccine | Vaccine | Risk difference | 95% CI | p‐value | Risk ratio | 95% CI | Odds ratio | 95% CI | NNV | |
| *Death | 2.463% | 0.000% | 2.463% | (0.955, 3.971) | .0014 | — | — | — | — | 40.6 |
| *Flap failure | 2.494% | 0.000% | 2.494% | (0.968, 4.02) | .0019 | — | — | — | — | 40.1 |
| *Hospital admission | 35.833% | 7.353% | 28.480% | (20.994, 35.967) | <.0001 | 4.873 | (2.621, 9.06) | 7.036 | (3.509, 14.111) | 3.5 |
| *ICU admission | 12.754% | 3.226% | 9.528% | (5.496, 13.56) | <.0001 | 3.954 | (2.025, 7.721) | 4.385 | (2.167, 8.875) | 10.5 |
| *Infection | 12.226% | 4.545% | 7.681% | (3.153, 12.208) | .0023 | 2.690 | (1.372, 5.273) | 2.925 | (1.427, 5.994) | 13.0 |
| *SSI | 6.494% | 2.695% | 3.799% | (0.836, 6.76) | .013 | 2.409 | (1.173, 4.946) | 2.507 | (1.187, 5.295) | 26.3 |
| Dehiscence | 4.905% | 3.049% | 1.856% | (−1.033, 4.744) | .2142 | 1.609 | (0.753, 3.435) | 1.640 | (0.746, 3.606) | |
| DVT | 2.538% | 2.639% | −0.101% | (−2.34, 2.139) | .9299 | 0.962 | (0.405, 2.285) | 0.961 | (0.395, 2.336) | |
| ED visit | 7.631% | 5.181% | 2.450% | (−2.095, 6.994) | .3023 | 1.473 | (0.701, 3.094) | 1.512 | (0.686, 3.331) | |
| Gangrene | 2.618% | 2.660% | −0.042% | (−2.324, 2.24) | .9714 | 0.984 | (0.414, 2.337) | 0.984 | (0.405, 2.392) | |
| Hematoma | 2.469% | 2.519% | −0.050% | (−2.209, 2.109) | .9640 | 0.980 | (0.413, 2.329) | 0.980 | (0.403, 2.38) | |
| PE | 2.494% | 2.538% | −0.044% | (−2.222, 2.133) | .9682 | 0.983 | (0.414, 2.335) | 0.982 | (0.404, 2.386) | |
| Sepsis | 2.577% | 2.778% | −0.201% | (−2.517, 2.116) | .8652 | 0.928 | (0.391, 2.203) | 0.926 | (0.381, 2.251) | |
| Seroma | 2.488% | 2.577% | −0.089% | (−2.282, 2.102) | .9360 | 0.965 | (0.406, 2.293) | 0.964 | (0.397, 2.343) | |
| Stroke | 0.000% | 0.000% | 0.000% | — | — | — | — | — | — | |
| 60 Days (N = 818) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No vaccine | Vaccine | Risk difference | 95% CI | p‐value | Risk ratio | 95% CI | Odds ratio | 95% CI | NNV | |
| *Death | 2.463% | 0.000% | 2.463% | (0.955, 3.971) | .0014 | — | — | — | — | 40.6 |
| *Flap failure | 2.494% | 0.000% | 2.494% | (0.968, 4.02) | .0019 | — | — | — | — | 40.1 |
| *Hospital admission | 35.833% | 7.353% | 28.480% | (20.994, 35.967) | <.0001 | 4.873 | (2.621, 9.06) | 7.036 | (3.509, 14.111) | 3.5 |
| *ICU admission | 13.043% | 3.226% | 9.817% | (5.756, 13.879) | <.0001 | 4.043 | (2.074, 7.885) | 4.500 | (2.226, 9.095) | 10.2 |
| *Infection | 14.420% | 4.545% | 9.875% | (5.138, 14.611) | .0002 | 3.172 | (1.636, 6.15) | 3.538 | (1.745, 7.177) | 10.1 |
| *SSI | 8.052% | 2.695% | 5.357% | (2.178, 8.535) | .0012 | 2.987 | (1.486, 6.006) | 3.161 | (1.527, 6.545) | 18.7 |
| *Dehiscence | 7.084% | 3.049% | 4.035% | (0.818, 7.253) | .0166 | 2.324 | (1.138, 4.745) | 2.425 | (1.151, 5.108) | 24.8 |
| DVT | 2.538% | 2.639% | −0.101% | (−2.34, 2.139) | .9299 | 0.962 | (0.405, 2.285) | 0.961 | (0.395, 2.336) | |
| ED visit | 9.237% | 5.181% | 4.056% | (−0.71, 8.821) | .1076 | 1.783 | (0.869, 3.656) | 1.862 | (0.864, 4.013) | |
| Gangrene | 2.618% | 2.660% | −0.042% | (−2.324, 2.24) | .9714 | 0.984 | (0.414, 2.337) | 0.984 | (0.405, 2.392) | |
| Hematoma | 2.469% | 2.519% | −0.050% | (−2.209, 2.109) | .9640 | 0.980 | (0.413, 2.329) | 0.980 | (0.403, 2.38) | |
| PE | 2.494% | 2.538% | −0.044% | (−2.222, 2.133) | .9682 | 0.983 | (0.414, 2.335) | 0.982 | (0.404, 2.386) | |
| Sepsis | 2.577% | 2.778% | −0.201% | (−2.517, 2.116) | .8652 | 0.928 | (0.391, 2.203) | 0.926 | (0.381, 2.251) | |
| Seroma | 2.488% | 2.577% | −0.089% | (−2.282, 2.102) | .9360 | 0.965 | (0.406, 2.293) | 0.964 | (0.397, 2.343) | |
| Stroke | 0.000% | 0.000% | 0.000% | — | — | — | — | — | — | |
| 90 Days (N = 818) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No vaccine | Vaccine | Risk difference | 95% CI | p‐value | Risk ratio | 95% CI | Odds ratio | 95% CI | NNV | |
| *Dehiscence | 7.297% | 3.040% | 4.257% | (1.023, 7.493) | .0121 | 2.401 | (1.18, 4.884) | 2.511 | (1.196, 5.27) | 23.5 |
| *ED visit | 12.549% | 5.102% | 7.447% | (2.346, 12.548) | .0070 | 2.46 | (1.24, 4.88) | 2.669 | (1.278, 5.573) | 13.4 |
| *Hospital admission | 39.918% | 7.353% | 32.565% | (25.004, 40.125) | <.0001 | 5.429 | (2.932, 10.054) | 8.371 | (4.185, 16.744) | 3.1 |
| *ICU admission | 12.680% | 3.215% | 9.465% | (5.452, 13.477) | <.0001 | 3.944 | (2.019, 7.702) | 4.371 | (2.16, 8.845) | 10.6 |
| *Infection | 14.420% | 4.505% | 9.915% | (5.193, 14.638) | .0002 | 3.201 | (1.651, 6.207) | 3.572 | (1.761, 7.244) | 10.1 |
| *SSI | 7.772% | 2.695% | 5.077% | (1.938, 8.215) | .0018 | 2.883 | (1.43, 5.814) | 3.042 | (1.465, 6.316) | 19.7 |
| Death | 2.445% | 2.451% | −0.006% | (−2.125, 2.113) | .9956 | 0.998 | (0.42, 2.371) | 0.997 | (0.411, 2.423) | |
| DVT | 2.513% | 2.632% | −0.119% | (−2.345, 2.107) | .9165 | 0.955 | (0.402, 2.268) | 0.954 | (0.392, 2.318) | |
| Flap failure | 2.519% | 2.611% | −0.092% | (−2.312, 2.128) | .9352 | 0.965 | (0.406, 2.292) | 0.964 | (0.397, 2.342) | |
| Gangrene | 2.591% | 2.653% | −0.062% | (−2.33, 2.206) | .9574 | 0.977 | (0.411, 2.32) | 0.976 | (0.402, 2.373) | |
| Hematoma | 2.488% | 2.513% | −0.025% | (−2.189, 2.139) | .9819 | 0.990 | (0.417, 2.353) | 0.990 | (0.407, 2.405) | |
| PE | 2.519% | 2.532% | −0.013% | (−2.198, 2.173) | .9909 | 0.995 | (0.419, 2.364) | 0.995 | (0.409, 2.417) | |
| Sepsis | 2.584% | 2.770% | −0.186% | (−2.502, 2.13) | .8747 | 0.933 | (0.393, 2.215) | 0.931 | (0.383, 2.264) | |
| Seroma | 2.488% | 2.571% | −0.083% | (−2.272, 2.106) | .9406 | 0.968 | (0.407, 2.299) | 0.967 | (0.398, 2.349) | |
| Stroke | 0.000% | 0.000% | 0.000% | — | — | — | — | — | — | |
Note: Each analysis excluded subjects that experienced each individual adverse outcome prior to the time window of interest (i.e., experienced the adverse outcome on the same day as surgery), thus some cohorts may be less than the full 409 subjects for each adverse outcome of interest.
Statistically significant (p < .05).
FIGURE 2.
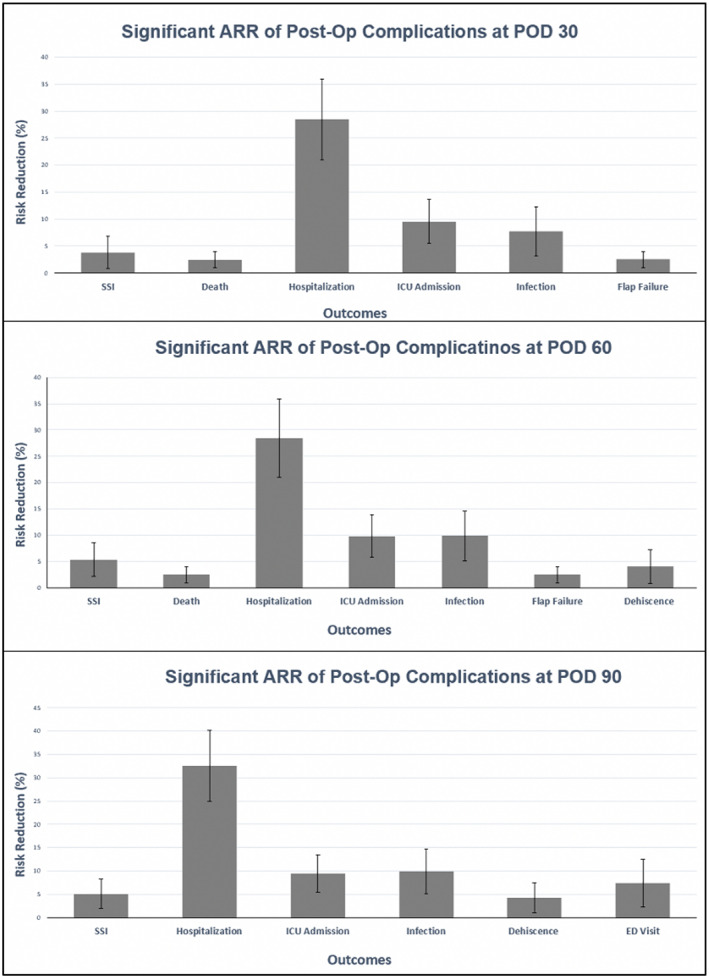
Significant absolute risk reductions (ARRs) between COVID‐19 vaccinated and non‐COVID‐19 vaccinated patients by 30, 60, and 90 days post‐operatively (p < .05). Error bars display 95% confidence interval
FIGURE 3.
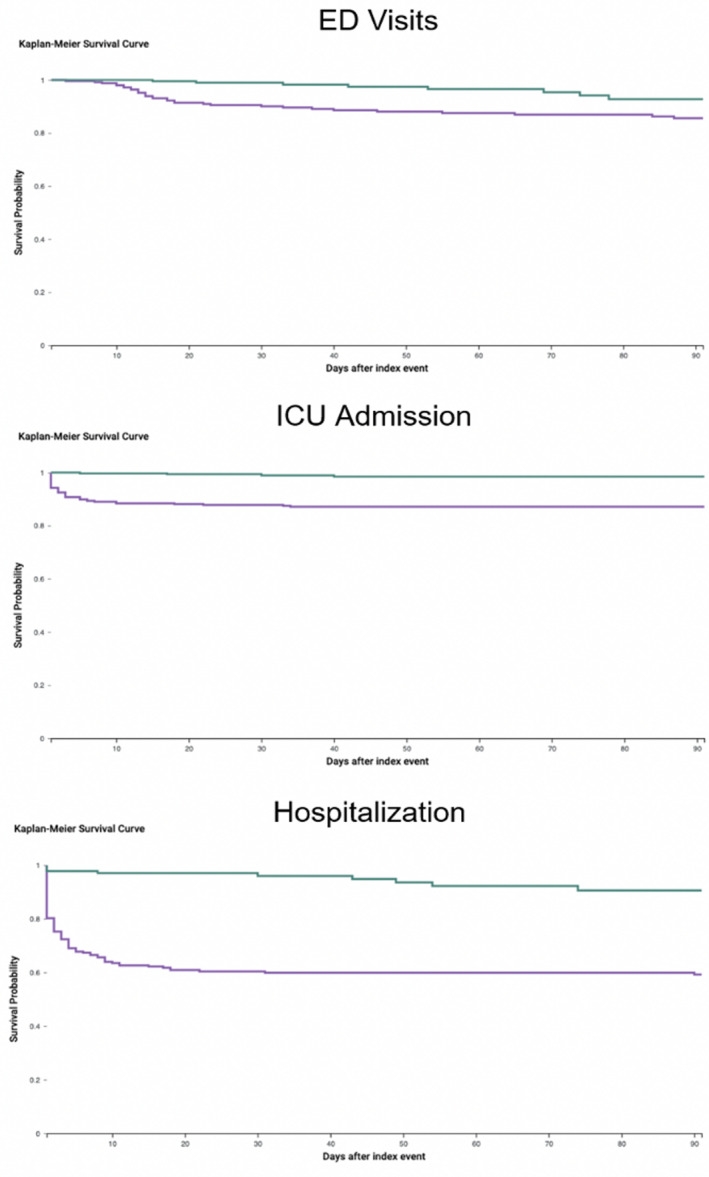
Kaplan–Meier survival curves for patients with ED visits, ICU admission, & hospitalization 0–90 days post‐operatively (p < .05) (green = COVID‐19 vaccinated, purple = not COVID‐19 vaccinated)
There were no significant differences between SARS‐CoV‐2‐vaccinated and non‐vaccinated patients for the remaining adverse outcomes including sepsis, DVT, stroke, ARDS, PE, gangrene, hematoma, and seroma within the 30‐, 60‐, or 90‐day post‐operative time points (Table 2). In addition, no significant differences were found between the two cohorts for flap failure and death at 90 days.
4. DISCUSSION
To the best of our knowledge, this study is the first to investigate the potential protective effects of pre‐operative SARS‐CoV‐2‐vaccination on post‐operative outcomes following microsurgery. Utilizing a live‐updated, federated EMR network comprised of 51 global HCOs, over 70 million de‐identified patient charts were analyzed for post‐operative adverse events in both SARS‐CoV‐2‐vaccinated and non‐vaccinated microsurgical patients within 30, 60, and 90 days. These time windows were selected in order to capture the majority of adverse events at key benchmarks of the acute post‐operative period (Thompson et al., 2003).
Outside of microsurgery, SARS‐CoV‐2‐postive surgical patients have been found to be at increased risk for morbidity and mortality in the post‐operative period relative to SARS‐CoV‐2‐negative patients (Carrier et al., 2021; Collaborative CO, 2020b; Covidsurg Collaborative GC, 2021; Doglietto et al., 2020; El‐Boghdadly et al., 2021; Haffner et al., 2021; Katsiampoura et al., 2020; Phelps et al., 2020; Prasad et al., 2021). Specifically, the international COVIDSurg Collaborative demonstrated that pre‐operative SARS‐CoV‐2‐vaccination led to reduced risk of both post‐operative morbidity and mortality in SARS‐CoV‐2‐positive surgical patients (Collaborative CO, 2020b). Several recent reports have suggested that microsurgery patients may be particularly at risk for adverse outcomes (Ilonzo et al., 2021; Mazzeffi et al., 2021; Morales‐Perez et al., 2021). Although additional work is needed, this finding may possibly be attributed to the ability of SARS‐CoV‐2 to infiltrate patient vasculature, thereby manifesting as hypercoagulability, endotheliitis, and vasculitis (Ilonzo et al., 2021; Morales‐Perez et al., 2021).
Our investigation found significantly decreased risks of SSIs, ICU admission, generalized infections, and hospitalizations within 30, 60, and 90 days after microsurgery for patients vaccinated against SARS‐CoV‐2 when compared to propensity score matched, non‐SARS‐CoV‐2‐vaccinated counterparts (Table 2, Figures 2 and 3). Although this is the first study of its kind to focus specifically on the microsurgical patient population, several of these findings are supported by examinations of the post‐operative implications of SARS‐CoV‐2 infection in the recent literature. For example, both El‐Boghdadly et al. and the COVIDSurg Collaborative found that peri‐operative SARS‐CoV‐2 infection led to increased risk of hospitalization and ICU admission (El‐Boghdadly et al., 2021; Haffner et al., 2021). This analysis also observed a significantly decreased risk of death at 30 and 60 days, a finding echoed by the work of El‐Boghdadly, Haffner et al., and the COVIDSurg Collaborative, as well as significantly decreased risk of dehiscence at 60 and 90 days (Table 2) (El‐Boghdadly et al., 2021; Haffner et al., 2021; Thompson et al., 2003).
Conversely, this analysis did not demonstrate any potential protective benefit of SARS‐CoV‐2 vaccination against outcomes including PE, DVT, ARDS, sepsis, hematoma, gangrene, dehiscence, seroma, or stroke at any of the time points included in the analysis (Table 2). The lack of an observed PE benefit is supported by a recent multicenter prospective study of 30‐day general surgery outcomes by Prasad et al. which found no significant difference in 30‐day post‐operative PE complication rates between SARS‐CoV‐2‐positive patients and their propensity score matched SARS‐CoV‐2‐negative counterparts (Prasad et al., 2021).
At 30 and 60 days post‐operatively, flap failure risk was found to be significantly reduced in SARS‐CoV‐2‐vaccinated microsurgery patients (Table 2). This finding is particularly notable given that flap procedure protocols have been impacted by the current pandemic with recommendations deferring reconstruction for SARS‐CoV‐2‐positive patients and advocating for the use of less complex closures (Das De et al., 2020; Thacoor et al., 2021). Although this study propensity score matched and balanced for numerous factors including age, race, diabetes, BMI, smoking, and pre‐operative SARS‐CoV‐2 status, the increased likelihood of non‐vaccinated microsurgery patients developing flap failure may simply have been due to an increased risk of contracting pro‐inflammatory SARS‐CoV‐2 during the acute post‐operative period. That being said, additional underlying mechanisms may be at play as the impact of novel SARS‐CoV‐2 vaccinations upon the body's inflammatory response to both viral and non‐viral stressors continues to be characterized (Hotez et al., 2020). Regardless of mechanism, the stringency of operative protocols for flap procedures during the pandemic combined with the potential protective effect observed in this study suggest that preoperative advocacy for SARS‐CoV‐2 immunization for all patients undergoing microsurgery may merit further consideration.
By calculating NNV, the COVIDSurg Collaborative concluded that vaccination of hundreds to a few thousand patients against SARS‐CoV‐2, depending upon type of surgical procedure performed, may protect against post‐operative death (Covidsurg Collaborative GC, 2021). In our uniquely microsurgical study population, significant findings included NNV: 18.7–26.3 for SSIs, NNV: 10.2–10.6 for ICU admission, NNV: 10.1–13.0 for generalized infections, and NNV: 3.1–3.5 for increased utilization of hospital inpatient services at 30, 60, and 90 days‐post‐operatively. SARS‐CoV‐2‐immunized patients demonstrated NNV: 40.1 for flap failure and NNV: 40.6 for death at 30 and 60 days‐post‐operatively. Lastly, SARS‐CoV‐2‐immunized patients were found to have an NNV: 23.5–24.8 for dehiscence at 60 and 90 days and an NNV: 13.4 for ED Visits following microsurgery (Table 2, Figure 3).
In light of the over 276 million confirmed SARS‐CoV‐2‐positive cases worldwide, the use of NNV calculations allows for a deeper appreciation of the potential benefit of pre‐operative SARS‐CoV‐2 vaccination for patients undergoing microsurgery (WHO, 2021). When considered on a global scale, the Kaplan–Meier curves and NNVs calculated in this study may serve to benefit not only patients requiring microsurgery, but also the finances and resources of the health systems responsible should patients suffer these adverse outcomes (Table 2, Figure 3).
The strengths of this study include its large sample size and rigorously balanced cohorts which allow for maximization of external validity. However, it is not without limitations, including its retrospective nature which prohibits a lack of detailed, individualized historical patient data. Moreover, this study is limited by its reliance on the accuracy of medical coding. While the use of the federated EMR network allows for analysis of over 70 million patient charts, conclusions lend themselves only to measures of association, not causation. Additionally, the fact that the EMR data is completely de‐identified for the sake of patient privacy precludes the ability to evaluate individual records for subsequent detailed analysis of significant findings. Lastly, the combining of four of the leading, approved SARS‐CoV‐2 vaccines currently in use, prohibits a more granular examination of whether vaccines based upon mRNA mechanisms of action, including the Moderna and Pfizer products, have any appreciable difference on post‐operative outcomes relative to the viral vector‐based Oxford University‐AstraZeneca or Johnson & Johnson immunizations (Moore, 2021). Although this study focused on SARS‐CoV‐2 vaccination status, immunization with other vaccines, such as the influenza vaccine which has previously been shown to potentially protect against severe adverse outcomes of COVID‐19 in non‐surgical patients, was not accounted for and may benefit from future examination in the microsurgical patient population (Conlon et al., 2021; Taghioff et al., 2021; Yang et al., 2021).
Our findings highlight the ability of federated EMR networks to analyze exceptionally large sample sizes with rapid turnaround times, an invaluable tool that may be critical during pandemics. While the conclusions drawn in this study are certainly not causative and do not allow for subsequent analysis of significant findings secondary to the de‐identified nature of the EMR network, the cohort balancing, sample size, and propensity score matching do afford a great deal of external validity that may guide future directions. Thus, additional prospective investigations, such as randomized controlled trials, are warranted to confirm and better characterize the observed potential protective effect of SARS‐CoV‐2 vaccination against adverse post‐operative outcomes following microsurgery.
5. CONCLUSION
This investigation examines the potential protective effect of a history of at least one dose of SARS‐CoV‐2 vaccination within 6 months of undergoing microsurgery. Awareness of this data may influence the surgeon, as well as the patient, who may benefit from pre‐operative SARS‐CoV‐2 vaccination if medically indicated. Limitations include this study's retrospective nature and reliance on the accuracy of medical coding. Thus, future prospective studies, such as randomized control trials, are warranted to better characterize and understand these findings in hopes of minimizing adverse microsurgical outcomes in the age of the current pandemic.
DISCLOSURES
Dr. Holton serves as a consultant for KCI/3M and Stryker. Dr. Singh, Mr. Narasimman, Dr. Taghioff, Dr. G. Samaha, Dr. M. Samaha, and Dr. Slavin have no relevant disclosures. The authors have not received any consulting fees, stock options, research funding, capital equipment, or educational grants from TriNetX.
Taghioff, S. M. , Slavin, B. R. , Narasimman, M. , Samaha, G. , Samaha, M. , Holton, T. , & Singh, D. (2022). The influence of SARS‐CoV‐2 vaccination on post‐operative outcomes in microsurgery patients. Microsurgery, 42(7), 685–695. 10.1002/micr.30940
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abou‐Ismail, M. Y. , Diamond, A. , Kapoor, S. , Arafah, Y. , & Nayak, L. (2020). The hypercoagulable state in COVID‐19: Incidence, pathophysiology, and management. Thrombosis Research, 194, 101–115. 10.1016/j.thromres.2020.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier, F. M. , Amzallag, E. , Lecluyse, V. , Côté, G. , Couture, É. J. , D'Aragon, F. , Kandelman, S. , Turgeon, A. F. , Deschamps, A. , Nitulescu, R. , Djade, C. D. , Girard, M. , Beaulieu, P. , & Richebé, P. (2021). Postoperative outcomes in surgical COVID‐19 patients: A multicenter cohort study. BMC Anesthesiology, 21(1), 15. 10.1186/s12871-021-01233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative CO . (2020a). Global guidance for surgical care during the COVID‐19 pandemic. The British Journal of Surgery, 107(9), 1097–1103. 10.1002/bjs.11646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative CO . (2020b). Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: An international cohort study. Lancet, 396(10243), 27–38. 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon, A. , Ashur, C. , Washer, L. , Eagle, K. A. , & Hofmann Bowman, M. A. (2021). Impact of the influenza vaccine on COVID‐19 infection rates and severity. American Journal of Infection Control, 49(6), 694–700. 10.1016/j.ajic.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covidsurg Collaborative GC . (2021). SARS‐CoV‐2 vaccination modelling for safe surgery to save lives: Data from an international prospective cohort study. British Journal of Surgery, 108(9), 1056–1063. 10.1093/bjs/znab101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das De, S. , Liang, Z. C. , Cheah, A. E. , Puhaindran, M. E. , Lee, E. Y. , Lim, A. Y. T. , & Chong, A. K. S. (2020). Emergency hand and reconstructive microsurgery in the COVID‐19‐positive patient. The Journal of Hand Surgery, 45(9), 869–875. 10.1016/j.jhsa.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doglietto, F. , Vezzoli, M. , Gheza, F. , Lussardi, G. L. , Domenicucci, M. , Vecchiarelli, L. , Zanin, L. , Saraceno, G. , Signorini, L. , Panciani, P. P. , Castelli, F. , Maroldi, R. , Rasulo, F. A. , Benvenuti, M. R. , Portolani, N. , Bonardelli, S. , Milano, G. , Casiraghi, A. , Calza, S. , & Fontanella, M. M. (2020). Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID‐19) in Italy. JAMA Surgery, 155(8), 691–702. 10.1001/jamasurg.2020.2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria‐Rose, N. , Suthar, M. S. , Makowski, M. , O'Connell, S. , McDermott, A. , Flach, B. , Ledgerwood, J. E. , Mascola, J. R. , Graham, B. S. , Lin, B. C. , O'Dell, S. , Schmidt, S. D. , Widge, A. T. , Edara, V. V. , Anderson, E. J. , Lai, L. , Floyd, K. , Rouphael, N. G. , Zarnitsyna, V. , … mRNA‐1273 Study Group . (2021). Antibody persistence through 6 months after the second dose of mRNA‐1273 vaccine for Covid‐19. New England Jounal of Medicine, 384(23), 2259–2261. 10.1056/NEJMc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Boghdadly, K. , Cook, T. M. , Goodacre, T. , Kua, J. , Blake, L. , Denmark, S. , McNally, S. , Mercer, N. , Moonesinghe, S. R. , & Summerton, D. J. (2021). SARS‐CoV‐2 infection, COVID‐19 and timing of elective surgery: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri‐operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. Anaesthesia, 76(7), 940–946. 10.1111/anae.15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel, M. K. , Khanna, P. , & Kishore, J. (2010). Understanding survival analysis: Kaplan‐Meier estimate. International Journal of Ayurveda Research, 1(4), 274–278. 10.4103/0974-7788.76794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner, M. R. , Le, H. V. , Saiz, A. M., Jr. , Han, G. , Fine, J. , Wolinsky, P. , & Klineberg, E. O. (2021). Postoperative in‐hospital morbidity and mortality of patients with COVID‐19 infection compared with patients without COVID‐19 infection. JAMA Network Open, 4(4), e215697. 10.1001/jamanetworkopen.2021.5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim, A. , Dang, V. , Bolotin, S. , & Crowcroft, N. S. (2015). How and why researchers use the number needed to vaccinate to inform decision making—A systematic review. Vaccine, 33(6), 753–758. 10.1016/j.vaccine.2014.12.033 [DOI] [PubMed] [Google Scholar]
- Hotez, P. J. , Bottazzi, M. E. , & Corry, D. B. (2020). The potential role of Th17 immune responses in coronavirus immunopathology and vaccine‐induced immune enhancement. Microbes and Infection, 22(4–5), 165–167. 10.1016/j.micinf.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilonzo, N. , Kumar, S. , Borazan, N. , Hansen, T. , Rao, A. , Lantis, J. , Faries, P. , & Ting, W. (2021). Endotheliitis in coronavirus disease 2019‐positive patients after extremity amputation for acute thrombotic events. Annals of Vascular Surgery, 72, 209–215. 10.1016/j.avsg.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis, J. P. A. (2020). Global perspective of COVID‐19 epidemiology for a full‐cycle pandemic. European Journal of Clinical Investigation, 50(12), e13423. 10.1111/eci.13423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhan, V. , Janardhan, V. , & Kalousek, V. (2020). COVID‐19 as a blood clotting disorder masquerading as a respiratory illness: A cerebrovascular perspective and therapeutic implications for stroke thrombectomy. Journal of Neuroimaging, 30(5), 555–561. 10.1111/jon.12770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, L. T. , Fang, T. , Galetta, M. S. , Goyal, D. K. C. , Nicholson, K. J. , Kepler, C. K. , Vaccaro, A. R. , & Schroeder, G. D. (2020). Propensity score matching: A statistical method. Clinical Spine Surgery, 33(3), 120–122. 10.1097/BSD.0000000000000932 [DOI] [PubMed] [Google Scholar]
- Katsiampoura, A. , Perozo, C. , Varkaris, A. , Vellayappan, S. , Tam, M. Z. , Vellayappan, U. , Agnihotri, A. , & Tam, S. (2020). Covid‐19 positivity affects outcome of cardiac surgical patients. Journal of Cardiac Surgery, 35(12), 3650–3652. 10.1111/jocs.14982 [DOI] [PubMed] [Google Scholar]
- Kolahchi, Z. , De Domenico, M. , Uddin, L. Q. , Cauda, V. , Grossmann, I. , Lacasa, L. , Grancini, G. , Mahmoudi, M. , & Rezaei, N. (2021). COVID‐19 and its global economic impact. Advances in Experimental Medicine and Biology, 1318, 825–837. 10.1007/978-3-030-63761-3_46 [DOI] [PubMed] [Google Scholar]
- Mazzeffi, M. A. , Chow, J. H. , & Tanaka, K. (2021). COVID‐19 associated hypercoagulability: Manifestations, mechanisms, and management. Shock, 55(4), 465–471. 10.1097/SHK.0000000000001660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, L. E. , Shaye, D. A. , & Lee, L. N. (2020). Considerations for the otolaryngologist in the era of COVID‐19: A review of the literature. Current Opinion in Otolaryngology & Head and Neck Surgery, 28(4), 228–234. 10.1097/MOO.0000000000000632 [DOI] [PubMed] [Google Scholar]
- Moletta, L. , Pierobon, E. S. , Capovilla, G. , Costantini, M. , Salvador, R. , Merigliano, S. , & Valmasoni, M. (2020). International guidelines and recommendations for surgery during Covid‐19 pandemic: A systematic review. International Journal of Surgery, 79, 180–188. 10.1016/j.ijsu.2020.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. P. (2021). Approaches for optimal use of different COVID‐19 vaccines: Issues of viral variants and vaccine efficacy. JAMA, 325(13), 1251–1252. 10.1001/jama.2021.3465 [DOI] [PubMed] [Google Scholar]
- Morales‐Perez, M. J. , Gallardo‐Calero, I. , Rivas‐Nicolls, D. , Gelabert Mestre, S. , Garcia Forcada, I. , & Soldado, F. (2021). Reconstruction of COVID‐19 vasculitis‐related thumb necrosis with a microsurgical free flap. Microsurgery, 41(4), 393–395. 10.1002/micr.30719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha, S. R. , Dugar, S. , McCrae, K. , Joseph, D. , Bartholomew, J. , Sacha, G. L. , & Militello, M. (2020). Coagulopathy in COVID‐19: Manifestations and management. Cleveland Clinic Journal of Medicine, 87(8), 461–468. 10.3949/ccjm.87a.ccc024 [DOI] [PubMed] [Google Scholar]
- Patel, R. J. , Kejner, A. , & McMullen, C. (2020). Early institutional head and neck oncologic and microvascular surgery practice patterns across the United States during the SARS‐CoV‐2 (COVID19) pandemic. Head & Neck, 42(6), 1168–1172. 10.1002/hed.26189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, D. L. , Saso, S. , & Ghaem‐Maghami, S. (2020). Analysis of worldwide surgical outcomes in COVID‐19‐infected patients: A gynecological oncology perspective. Future Science OA, 6(10), FS0629. 10.2144/fsoa-2020-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, N. K. , Lake, R. , Englum, B. R. , Turner, D. J. , Siddiqui, T. , Mayorga‐Carlin, M. , Sorkin, J. D. , & Lal, B. K. (2021). Increased complications in patients who test COVID‐19 positive after elective surgery and implications for pre and postoperative screening. American Journal of Surgery, 223(2), 380–387. 10.1016/j.amjsurg.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan, P. , Pramesh, C. S. , & Aggarwal, R. (2016). Common pitfalls in statistical analysis: Absolute risk reduction, relative risk reduction, and number needed to treat. Perspectives in Clinical Research, 7(1), 51–53. 10.4103/2229-3485.173773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncati, L. , Ligabue, G. , Fabbiani, L. , Malagoli, C. , Gallo, G. , Lusenti, B. , Nasillo, V. , Manenti, A. , & Maiorana, A. (2020). Type 3 hypersensitivity in COVID‐19 vasculitis. Clinical Immunology, 217, 108487. 10.1016/j.clim.2020.108487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi, H. K. , Libby, P. , & Ridker, P. M. (2021). COVID‐19 – A vascular disease. Trends in Cardiovascular Medicine, 31(1), 1–5. 10.1016/j.tcm.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghioff, S. M. , Slavin, B. R. , Holton, T. , & Singh, D. (2021). Examining the potential benefits of the influenza vaccine against SARS‐CoV‐2: A retrospective cohort analysis of 74,754 patients. PLoS One, 16(8), e0255541. 10.1371/journal.pone.0255541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacoor, A. , Sofos, S. S. , Miranda, B. H. , Thiruchelvam, J. , Perera, E. H. K. , Randive, N. , Tzafetta, K. , & Ahmad, F. (2021). Outcomes of major head and neck reconstruction during the COVID‐19 pandemic: The St. Andrew's centre experience. Journal of Plastic, Reconstructive & Aesthetic Surgery, 74, 2133–2140. 10.1016/j.bjps.2020.12.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thione, A. , Sanchez‐Garcia, A. , Perez‐Garcia, A. , Garcia‐Vilarino, E. , Salmeron‐Gonzalez, E. , & Balaguer‐Cambra, J. (2021). A protocol for performing reconstructive microsurgery on patients with COVID‐19. Plastic Surgical Nursing, 41(1), 36–39. 10.1097/PSN.0000000000000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. S. , Baxter, B. T. , Allison, J. G. , Johnson, F. E. , Lee, K. K. , & Park, W. Y. (2003). Temporal patterns of postoperative complications. Archives of Surgery, 138(6), 596–602; discussion 602‐3. 10.1001/archsurg.138.6.596 [DOI] [PubMed] [Google Scholar]
- Topaloglu, U. , & Palchuk, M. B. (2018). Using a federated network of real‐world data to optimize clinical trials operations. JCO Clinical Cancer Informatics, 2, 1–10. 10.1200/CCI.17.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro, M. D. , Brezin, A. P. , Burdon, M. , Cummings, A. B. , Kemer, O. E. , Malyugin, B. E. , Prieto, I. , Teus, M. A. , Tognetto, D. , Törnblom, R. , Posarelli, C. , Chorągiewicz, T. , & Rejdak, R. (2021). Early impact of COVID‐19 outbreak on eye care: Insights from EUROCOVCAT group. European Journal of Ophthalmology, 31(1), 5–9. 10.1177/1120672120960339 [DOI] [PubMed] [Google Scholar]
- WHO . (2021). WHO Coronavirus (COVID‐19) Dashboard. World Health Organization. https://covid19.who.int/ [Google Scholar]
- Yang, M. J. , Rooks, B. J. , Le, T. T. , Santiago, I. O., 3rd , Diamond, J. , Dorsey, N. L. , & Mainous, A. G., 3rd . (2021). Influenza vaccination and hospitalizations among COVID‐19 infected adults. Journal of American Board of Family Medicine, 34(Suppl), S179–S182. 10.3122/jabfm.2021.S1.200528 [DOI] [PubMed] [Google Scholar]
- Yuan, J. T. , & Jiang, S. I. B. (2020). Urgent safety considerations for dermatologic surgeons in the COVID‐19 pandemic. Dermatology Online Journal, 26(8), 13030/qt2qr3w771. [PubMed] [Google Scholar]
- Zapata, M. A. , Banderas Garcia, S. , Sanchez‐Moltalva, A. , Falcó, A. , Otero‐Romero, S. , Arcos, G. , Velazquez‐Villoria, D. , & García‐Arumí, J. (2020). Retinal microvascular abnormalities in patients after COVID‐19 depending on disease severity. British Journal of Ophthalmology, 106, 559–563. 10.1136/bjophthalmol-2020-317953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


