Abstract
In many bacteria, including the enteric species Salmonella typhimurium and Escherichia coli, heme is synthesized starting from glutamate by a pathway in which the first committed step is catalyzed by the hemA gene product, glutamyl-tRNA reductase (HemA). We have demonstrated previously that when heme limitation is imposed on cultures of S. typhimurium, HemA enzyme activity is increased 10- to 25-fold. Western (immunoblot) analysis with monoclonal antibodies reactive with HemA revealed that heme limitation results in a corresponding increase in the abundance of the enzyme. Similar regulation was also observed for E. coli. The near absence of regulation of hemA-lac operon fusions suggested a posttranscriptional control. We report here the results of pulse-labeling and immunoprecipitation studies of this regulation. The principal mechanism that contributes to elevated HemA abundance is protein stabilization. The half-life of HemA protein is ≃20 min in unrestricted cells but increases to >300 min in heme-limited cells. Similar regulation was observed for a HemA-LacZ hybrid protein containing almost all of the HemA protein (416 residues). Sodium azide prevents HemA turnover in vivo, suggesting a role for energy-dependent proteolysis. This was confirmed by the finding that HemA turnover is completely blocked in a lon clpP double mutant of E. coli. Each single mutant shows only a small effect. The ClpA chaperone, but not ClpX, is required for ClpP-dependent HemA turnover. A hybrid HemA-LacZ protein containing just 18 amino acids from HemA is also stabilized in the lon clpP double mutant, but this shorter fusion protein is not correctly regulated by heme limitation. We suggest that the 18 N-terminal amino acids of HemA may constitute a degradation tag, whose function is conditional and modified by the remainder of the protein in a heme-dependent way. Several models are discussed to explain why the turnover of HemA is promoted by Lon-ClpAP proteolysis only when sufficient heme is available.
Salmonella typhimurium and the other enteric bacteria, including Escherichia coli, are nutritionally versatile organisms. For example, S. typhimurium can use any one of at least 73 different compounds as a sole carbon and energy source (20). Many of these carbon sources are known or predicted to be nonfermentable: they are metabolized by oxidative pathways that utilize a terminal electron acceptor and require the participation of respiratory chains with heme-containing cytochromes. At the same time, heme can be dispensable for growth. Null mutants completely defective in heme biosynthesis grow normally under anaerobic conditions by using a fermentable carbon source such as glucose, so long as cysteine is provided (11, 30). The level of heme is accordingly high during aerobic growth, especially on nonfermentable carbon sources, and low during fermentative growth. An important unsolved problem is to understand how heme synthesis is regulated in the enteric bacteria.
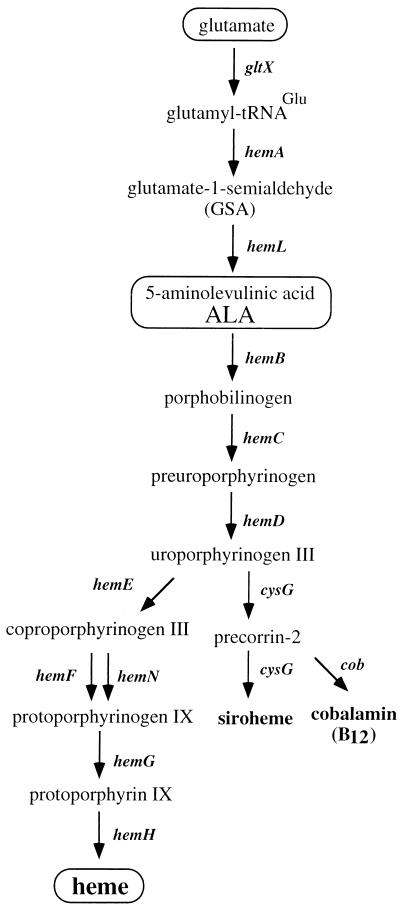
The first segment of the heme pathway involves the formation of 5-aminolevulinic acid (ALA). In the enteric bacteria, this occurs by a C5 mechanism. Glutamate, which has first been activated by esterification to tRNAGlu, is reduced by the hemA-encoded glutamyl-tRNA reductase (HemA) to form glutamate-1-semialdehyde, which is then converted to ALA by the hemL-encoded enzyme, glutamate-semialdehyde aminotransferase (HemL).
Previous work (reviewed in references 5, 8, and 29) has provided suggestive evidence regarding modes of heme regulation including the following possibilities: (i) that the formation of ALA is either mainly or partially rate limiting, (ii) that HemA activity might be feedback inhibited by heme, and (iii) that late oxidative enzymes in the pathway (HemF, HemN, and HemG in Fig. 1) might control heme synthesis by virtue of the coupling of their activity to respiratory capacity. Transcriptional control is conspicuously absent from proposed models. No evidence has been found for substantial control of expression of the hem genes, which are scattered on the genetic map (8, 30).
FIG. 1.
Heme biosynthesis. The heme biosynthetic pathway consists of 10 reactions by which glutamate is converted to heme; minor branches lead to siroheme and cobalamin. Glutamyl-tRNA reductase (HemA) is considered the first committed enzyme in the heme pathway since the vast majority (>99%) of charged tRNAGlu is used for protein synthesis. Mutants defective in either hemA or hemL require either ALA or both heme and cysteine supplementation for wild-type growth. In contrast to hemA mutants, hemL strains are leaky auxotrophs and can adapt to growth in the absence of supplementation, as described previously (29).
Our recent development of a panel of monoclonal antibodies (MAbs) reactive with HemA, together with use of a specific enzyme assay, led to the first direct demonstration of regulation of heme biosynthesis in the enteric bacteria (29). In that study, the levels of HemA enzyme activity and protein were shown to rise in concert by 10- to 25-fold after limitation of growing S. typhimurium and E. coli cultures for heme. One method by which this was accomplished was to adapt hemL mutants, which are leaky ALA and heme auxotrophs (bradytrophs), to growth in the absence of any supplementation. Here we explore the mechanism of this regulation further. We show that the main way in which HemA is regulated by heme limitation is through conditional proteolysis. This proteolysis, which is active in normally growing but not in heme-limited cells, depends on the Lon and ClpAP proteases in vivo. Models for the molecular mechanisms that might regulate HemA turnover are presented in the Discussion.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. All S. typhimurium strains are otherwise isogenic with the wild-type strain LT-2 except for the indicated markers; similarly, except for the indicated markers all E. coli strains are isogenic with either the wild-type strain MG1655 or the standard lac deletion strain MC4100 (SG20250 in Table 1). The S. typhimurium hemL mutant strain TE472 is a deletion lacking nearly all of the hemL gene; reference 11 contains a deletion map of hemL showing the extent of this and the hemL376 deletion, also used in this work. The hemA60 mutant strain TE719 carries a point mutation that maps to the C terminus of hemA. Strain TE3739 carries a DNA fragment encoding Kanr inserted at the NheI site at codon 161 of hemA (9); this insertion is polar on prfA, an essential gene. The hemA::Kan insertion strain also carries the plasmid pTE367 to provide prfA function (12). Fusions of hemA to lac were constructed and placed in single copy in the S. typhimurium chromosome, by a method described previously (10). These constructs are present at the put locus. Details of the lac fusion to codon 18 of hemA (TE2685 and its derivatives) have been given previously (4, 10). The lac fusion at codon 416 of hemA was constructed in exactly the same way as the hemA-prfA-lac fusion described in reference 10. Fusions were transferred to F′ plasmids (10) and introduced into E. coli by conjugation by using the intermediate strain HMS174 as shown in Table 1. Because the F′ hemA-lac plasmids and the clpX and clpA mutant E. coli strains all carry Kanr, a Camr was added to the F plasmid as the selective marker in strain TE7137 and its derivatives.
TABLE 1.
Bacterial strains
| Strain | Genotype | Source and/or reference |
|---|---|---|
| Salmonella typhimurium | ||
| LT-2 | Wild type | Lab collection |
| TE299-1 | ΔhemL376 | TT12009 (11) |
| TE472 | DEL854 [zae-1868*Mud-J*hemL332] | TT12006 (11) |
| TE565 | proAB47/F+zzf-1854::Tn10d-Cam | Lab collection |
| TE719 | hemA60 | TT11991 (11) |
| TE2470 | araC1 DUP[(hemA702::Kan cob-4)*Tn10*(zdd-1852)] | 9 |
| TE2685 | putPA1303::Kanr-hemA-lac [pr]a (codon 18) | 4 |
| TE2713 | ΔhemL376 putPA1303::Kanr-hemA-lac [pr] (codon 18) | P22.TE2685 × TE299-1 |
| TE3413 | putPA1303::Kanr-hemA-lac [pr] (codon 416) | This study |
| TE3726 | LT-2/pTE367 (Ampr; E. coli prfA+) | 12 |
| TE3739 | hemA702::Kan/pTE367 (Ampr; E. coli prfA+) | P22.TE2470 × TE3726 |
| TE4351 | pyrD121 Δput(PA)521/F+zzf-6807::Tn10d-putA1302::Cam | 10 |
| TE4377 | pyrD121 Δput(PA)521/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 18) | P22.TE2685 × TE4351 |
| TE6595 | ΔhemL376 putPA1303::Kanr-hemA-lac [pr] (codon 416) | P22.TE3413 × TE299-1 |
| TE6920 | pyrD121 Δput(PA)521/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 416) | P22.TE3413 × TE4351 |
| TE7137 | pyrD121 Δput(PA)521/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 416) zzf-1854::Tn10d-Cam | P22.TE565 × TE6920 |
| TE7160 | ΔhemL376 his::Tn10d-Cam (hisB or hisH) | This study |
| Escherichia coli | ||
| MG1655 | K-12 F−λ−prototroph | D. Biek |
| HMS174 | K-12 F−hsdR recA Rifr | W. F. Studier |
| SG12047 | C600 lon-146::ΔTn10 | S. Gottesman (15) |
| SG22007 | MC4100 clpP1::Cam | S. Gottesman (24) |
| SG20250 | MC4100 = K-12 F− λ−araD139 Δ(lacIpoZYA argF)U169 flb5301 relA1 rpsL150 deoC1 ptsF25 rbsR | S. Gottesman |
| SG22099 | MC4100 clpA::Kan | S. Gottesman (23) |
| TE5301 | MG1655 ΔlacX74 | 3 |
| TE6905 | MG1655 lon-146::ΔTn10 | P1.SG12047 × MG1655 |
| TE6906 | MG1655 clpP1::Cam | P1.SG22007 × MG1655 |
| TE6907 | MG1655 clpP1::Cam lon-146::ΔTn10 | P1.SG12047 × TE6906 |
| TE7023 | MG4100 clpP1::Cam lon-146::ΔTn10 | P1.SG12047 × SG22007 |
| TE7028 | MC4100 clpP1::Cam lon-146::ΔTn10/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 18) | TE4377 cb HMS174 c TE7023 |
| TE7029 | MC4100 clpP1::Cam lon-146::ΔTn10/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 416) | TE6920 c HMS174 c TE7023 |
| TE7031 | MC4100 clpP1::Cam/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 416) | TE6920 c HMS174 c SG22007 |
| TE7033 | MC4100 lon-146::ΔTn10/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 416) | TE6920 c HMS174 c TE7034 |
| TE7034 | MC4100 lon-146::ΔTn10 | P1.SG12047 × SG22007 |
| TE7091 | MC4100/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 416) | TE6920 c HMS174 c SG20250 |
| TE7121 | MC4100 clpA::Kan lon-146::ΔTn10 | P1.SG12047 × SG22099 |
| TE7151 | MC4100 clpA::Kan lon-146::ΔTn10/F+zzf-6807::Tn10d-putPA1303::KanrhemA-lac [pr] (codon 416) zzf-1854::Tn10d-Cam | TE7137 c HMS174 c TE7121 |
| TE7254 | MG1655 recD1903::mini-Tet clpX::Kan | This study |
| TE7276 | MG1655 ΔlacX74 clpX::Kan | P1.TE7254 × TE5301 |
| TE7282 | MG1655 ΔlacX74 clpX::Kan lon-146::ΔTn10 | P1.SG12047 × TE7276 |
| TE7290 | MG1655 ΔlacX74 clpX::Kan lon-146::ΔTn10/F+zzf-6807::Tn10d-putPA1303::Kanr-hemA-lac [pr] (codon 416) zzf-1854::Tn10d-Cam | TE7137 c HMS174 c TE7282 |
| TE7315 | MG1655 ΔlacX74 lon-146::ΔTn10 | P1.SG12047 × TE7276 |
| TE7319 | MG1655 ΔlacX74 lon-146::ΔTn10/F+zzf-6807::Tn10d-putPA1303:Kanr-hemA-lac [pr] (codon 416) zzf-1854::Tn10d-Cam | TE7137 c HMS174 c TE7315 |
All fusions used in this work are protein fusions [pr].
c, conjugal transfer.
We constructed a Kanr insertion mutant in E. coli clpX for this work, because we were unable to construct certain strains with the existing mutation for unknown reasons. To make this construct, plasmid pWPC9 (clpP+ clpX+) was digested with BglII and a BamHI fragment from pUC4K encoding Kanr was inserted, disrupting clpX at codon 294. Digestion with BamHI and linear transformation of a recD mutant of MG1655 (10) gave TE7254. After a backcross to MG1655 ΔlacX74, the mutation showed >95% linkage in transduction with P1 donor phage grown on SG12047 (lon-146::ΔTn10). We were unable to transfer this mutation into SG20250; hence, tests of clpX function were carried out in the MG1655 background.
Growth of cultures.
All cultures were grown at 37°C in either Luria-Bertani medium (27) or minimal MOPS (morpholinepropanesulfonic acid) medium (25) as modified (7) containing 0.2% glycerol as the carbon source. Plates were prepared with nutrient agar (Difco) with 5 g of NaCl per liter or with NCE medium (6) with 0.2% glycerol as the carbon source. ALA was used at 2 μM in minimal medium (11). Antibiotics were added to rich medium to final concentrations as follows: 100 μg of sodium ampicillin per ml, 20 μg of chloramphenicol per ml, 50 μg of kanamycin sulfate per ml, 20 μg of tetracycline hydrochloride per ml, and 200 μg of streptomycin sulfate per ml. For strains with F′ plasmids grown in minimal medium, final antibiotic concentrations were 10 μg of chloramphenicol per ml and 100 μg of kanamycin sulfate per ml.
Adaptation of hemL mutant strains of S. typhimurium and E. coli was carried out according to the method in reference 29. Cells were first grown overnight in minimal MOPS glycerol medium with 2 μM ALA and then diluted 1:50 into the same medium and grown to an optical density at 600 nm (OD600) of 0.4 before growth was stopped by rapidly chilling the flask in ice-water. A 2.5-ml aliquot of the culture was centrifuged and resuspended in 10 volumes of minimal MOPS glycerol medium and grown to an OD600 of 0.4 (adaptation). This culture was also chilled and held overnight. Each culture was diluted 1/10 into the appropriate medium and grown to an OD600 of 0.4 for labeling.
For testing the specificity of HemA induction, strain TE7160 (ΔhemL his::Tn10d-Cam) was grown in minimal MOPS glycerol medium under the following conditions: (a) unlimited growth in medium with 10 mM NH4Cl and containing 2 μM ALA and 0.1 mM l-histidine; (b) heme-limited growth in medium containing NH4Cl and l-histidine but with adaptation to lack of ALA as described above; (c) histidine-limited growth in medium containing 50 μg of l-histidinol per ml as the source of histidine, containing ALA and with NH4Cl as the nitrogen source; and (d) nitrogen-limited growth in medium containing ALA, with 0.1 mM l-histidine and 5 mM l-arginine as sources of nitrogen.
Labeling and immunoprecipitation.
The rates of synthesis and turnover of native HemA and HemA-LacZ hybrid proteins were examined by labeling or pulse-chase protocols with immunoprecipitation with anti-HemA MAb H17 of the γ1 isotype (29) and/or anti-LacZ (β-galactosidase) antibody (Promega). Strains were grown to an OD600 of 0.4 in minimal MOPS medium containing 0.2% glycerol, with or without 2 μM ALA, and with antibiotics as necessary. Tran35S-label l-[35S]methionine and l[35S]cysteine; ICN) was added to a 1-ml sample of each culture at 100 μCi/ml, and after 5 min, unlabeled l-methionine and l-cystine were added to final concentrations of 1.3 and 0.6 mM, respectively. For the pulse-chase protocol, all amounts were scaled up to provide 1 ml of labeled culture corresponding to each sampling point. Trichloroacetic acid precipitation, immunoprecipitation, and adsorption onto protein A-Sepharose and subsequent processing were all exactly as described and referenced elsewhere (4, 9, 21). After processing, samples totaled 35 μl, of which 15 μl was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. For the anti-HemA MAb, a secondary antibody was used (monoclonal anti-mouse γ1a of the immunoglobulin G2a [IgG2a] isotype, [American Type Culture Collection]).
Detection of proteins by Western blotting.
Techniques for Western blotting (immunoblotting) have been described in detail elsewhere (29). The primary antibody was a mouse anti-HemA MAb of the γ1 isotype (H23), which was detected by sequential application of biotin-conjugated goat anti-mouse IgG1, followed by streptavidin-conjugated horseradish peroxidase (Southern Biotechnology), and finally visualized by enhanced chemiluminescence (Amersham).
RESULTS
Pulse-labeling and immunoprecipitation of HemA protein.
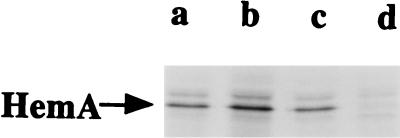
In order to establish the mechanism by which HemA abundance is regulated during heme-limited growth, we compared the rates of synthesis and turnover of the HemA protein in an adapted (heme-limited) S. typhimurium culture with those for cells grown in medium supplemented with ALA and thus not limited for heme. To do this, a MAb reactive with HemA was employed to immunoprecipitate the protein from cultures that had been pulse-labeled for 5 min with a mixture of 35S-labeled methionine and cysteine. In a preliminary experiment to establish the specificity of the antibody (Fig. 2), a band of the correct size to be HemA (46 kDa) was observed in immunoprecipitates of labeled wild-type cells (lane c) and hemL mutant cells (lanes a and b), but the HemA band was not seen in a hemA::Kan insertion mutant (lane d). A minor species migrating slower than HemA can be seen in Fig. 2 (more prominent in lane b) and in subsequent figures; it is a gel artifact caused by the large amount of unlabeled IgG heavy chain (data not shown), and its intensity depends on the amount of labeled HemA protein on the gel. Although HemA protein synthesis was apparently somewhat greater in heme-limited cells (compare lanes a and b in Fig. 2), this difference cannot account for the ≈20-fold induction observed by Western blot analysis (29).
FIG. 2.
Pulse-labeling and immunoprecipitation of HemA. HemA protein was analyzed by pulse-labeling of a hemL deletion mutant of S. typhimurium (TE2713) grown in MOPS glycerol medium in the presence of 2 μM ALA (lane a) or adapted to growth in the same medium without ALA (lane b). Also analyzed were the wild-type strain LT-2 (lane c) and a hemA::Kan insertion mutant (lane d), both grown in MOPS glycerol medium in the presence of ALA, and these two strains also carried plasmid pTE367, which provides the essential function of prfA to the hemA::Kan insertion mutant (9, 12). One milliliter of each culture was pulse-labeled (OD600 of 0.4) with 100 μCi of Tran35S-label for 5 min and then chased with unlabeled l-methionine (1.3 mM) and l-cystine (0.6 mM) for 2 min. Protein extracts were prepared, immunoprecipitated with anti-HemA MAb H17, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The position of the HemA protein is indicated by an arrow.
Proteolysis regulates HemA abundance.
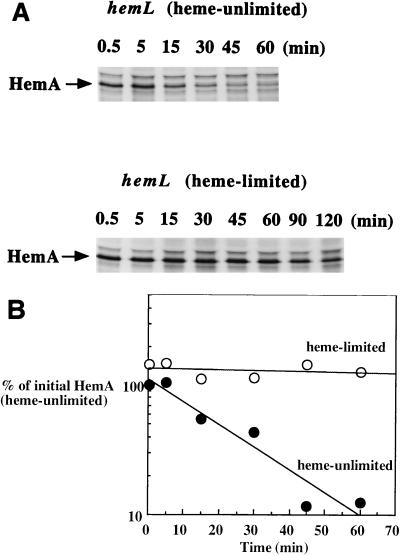
These initial observations suggested that a change in the rate of protein turnover might be the primary means by which HemA abundance is increased during heme limitation. A pulse-chase analysis confirmed that this inference was correct (Fig. 3). In a time course comparison of the amount of HemA protein seen in adapted hemL mutant cells (heme limited, bottom of Fig. 3A) with that in cells grown with ALA supplementation (top of Fig. 3A), HemA protein was much more stable in heme-limited cells. Identical gels were also analyzed by PhosphorImager analysis (Fig. 3B). The amount of HemA protein remaining after various times of the chase was quantitated, and each data point is plotted as a percentage of the initial amount of labeled HemA present in cells not limited for heme. Heme limitation results in only a small increase in the rate of HemA synthesis (≈2-fold or less; compare values at time zero). In contrast, the half-life of HemA was calculated to be ≈20 min in unlimited hemL mutant cells and in wild-type S. typhimurium (data not shown), while the half-life was more than 10 times longer (>300 min) in heme-limited cells. HemA turnover is therefore conditional, rapid in normally growing cells but inhibited in heme-limited cells, thereby resulting in an elevated level of the enzyme.
FIG. 3.
Pulse-chase analysis of HemA turnover in adapted hemL mutant cells. A hemL deletion mutant of S. typhimurium (TE472) was grown in MOPS glycerol medium to an OD600 of 0.4 in the presence of 2 μM ALA (top panel in panel A; heme unlimited) or adapted to growth in the same medium but without ALA and grown to an OD600 of 0.4 (bottom panel in panel A; heme limited). Cultures were labeled and analyzed as described in the legend to Fig. 2, except that the chase with unlabeled methionine and cystine was extended as shown above each lane. Identical gels (not treated with fluor) were analyzed by using a PhosphorImager and its ImageQuant software to produce the data plotted (B). The calculated half-life of HemA protein in unlimited cells is ≈20 min (closed circles), compared with a half-life estimated to be in excess of 300 min in adapted, heme-limited cells (open circles).
HemA turnover by energy-dependent proteases.
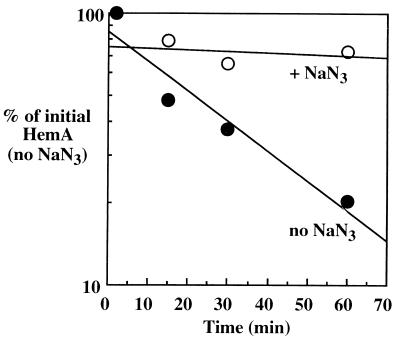
We expect HemA to be a cytoplasmic enzyme based on the lack of a signal sequence and its use of glutamyl-tRNA as substrate and NADPH as a cofactor. Cytoplasmic proteolysis is almost entirely due to energy-dependent proteases (16, 17). A standard test of energy dependence is to measure the rate of protein turnover after cultures have been treated with sodium azide (reviewed in reference 13); this treatment poisons respiration and ATP generation among other processes (26). Addition of sodium azide to pulse-labeled cultures of S. typhimurium prevented the turnover of HemA protein (Fig. 4).
FIG. 4.
HemA turnover is sensitive to azide. The wild-type S. typhimurium strain LT-2 was grown in MOPS glycerol medium to an OD600 of 0.4; duplicate samples were then pulse-labeled with Tran35S-label, chased for various times, and analyzed by immunoprecipitation with anti-HemA MAb H17. One sample (open circles) received 5 mM NaN3 at 2 min after the addition of unlabeled amino acids; the second sample was untreated (closed circles). Data were obtained by using a PhosphorImager and ImageQuant software.
We wished to determine which proteases are responsible for HemA proteolysis. To do this, we analyzed HemA turnover in E. coli, because of the existence of a large set of mutants defective in energy-dependent proteases (reviewed in reference 16). Also, a lon clpP double mutant of S. typhimurium grows very poorly, a phenotype which is not seen with E. coli mutants. We were encouraged to use E. coli because Western blot analysis had shown regulation of HemA by heme limitation in E. coli similar to that in S. typhimurium (29). This study confirms and extends that result (see below).
We tested mutations affecting the proteases Lon, ClpP, and ClpQ, as well as the ClpP chaperones ClpA and ClpX (Table 1; the mutant strains were generously provided by S. Gottesman). Pulse-chase and immunoprecipitation experiments established that HemA protein is completely stabilized in a lon clpP double mutant (Fig. 5). Either a lon or a clpP single mutation, by itself, stabilized HemA by only a small amount (two- to threefold increase in half-life [data not shown]). The stability of HemA protein was not further enhanced in a clpQ lon double mutant compared to the otherwise isogenic lon mutant. These results indicate that both Lon and ClpP have roughly equal abilities to degrade HemA and that contributions from other enzymes are probably minimal.
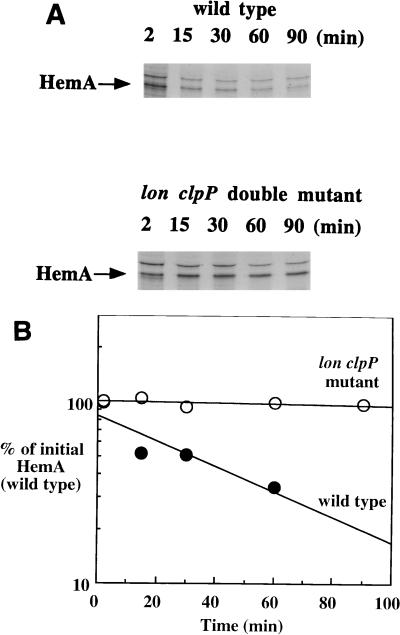
FIG. 5.
Proteases Lon and ClpP are both involved in HemA turnover. The genetic requirements for proteolysis of HemA in vivo were determined in E. coli because of the poor growth of lon clpP double mutants of S. typhimurium. The wild-type E. coli strain MG1655 and its lon::Tn10 clpP::Cam double mutant derivative (TE6907) were analyzed for HemA turnover by the same methods as those used for previous experiments. (A) Top, pulse-chase analysis of wild type; bottom, pulse-chase analysis of the double mutant; (B) data obtained from PhosphorImager analysis of duplicate gels. HemA was unstable in the wild-type strain (half-life of ≈30 min), while it was stable (half-life of >300 min) in the lon clpP double mutant.
Heme limitation also regulates a full-length HemA-LacZ hybrid protein.
The experiments described above were extended by determining the stability of two HemA-LacZ hybrid proteins in a pulse-chase protocol followed by immunoprecipitation. Results with the full-length fusion protein (HemA1–416-LacZ) recapitulate those found with native HemA. This large protein also gives a stronger signal, especially in E. coli, and confirms the specificity of the antibodies used. We first determined that HemA1–416-LacZ is correctly regulated by heme limitation in an S. typhimurium hemL deletion mutant (Fig. 6). In this strain, the half-life of HemA1–416-LacZ was increased more than 15-fold by heme limitation. In contrast, a fusion protein including only the first 18 amino acids of HemA (HemA1–18-LacZ) was unstable, but its short half-life was not conditional on heme limitation (Fig. 7). For both HemA1–18-LacZ and HemA1–416-LacZ, turnover was blocked in a lon clpP double mutant of E. coli (data for HemA1–416-LacZ shown in Fig. 8; for HemA1–18-LacZ, the data are not shown). Because the same two proteases are needed for turnover of both HemA-LacZ fusion proteins and native HemA (see also reference 4), the N-terminal 18 amino acids or a subset of them may constitute a degradation tag which confers sensitivity to proteolysis (see the Discussion).
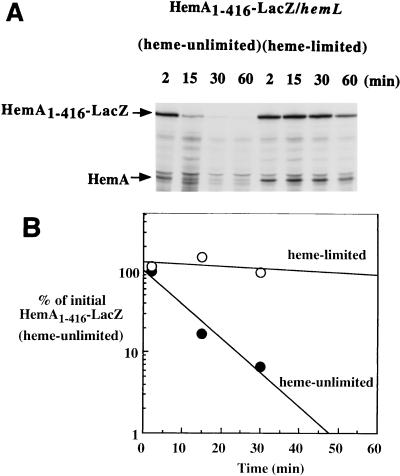
FIG. 6.
Turnover of a HemA-LacZ hybrid protein is correctly regulated by heme limitation. A fusion construct that expresses the HemA1–416-LacZ hybrid protein was introduced into an S. typhimurium hemL mutant background (TE6595). Two cultures of this strain (either adapted to heme limitation or not heme limited) were grown and analyzed as described in the legend to Fig. 3, except that a mixture of anti-HemA MAb and anti-LacZ MAb (Promega) was used for the immunoprecipitation. Both native HemA and HemA1–416-LacZ were detected and are indicated by arrows (A). The half-life of HemA1–416-LacZ was increased more than 15-fold by heme limitation (B) (open circles) compared to growth in the presence of ALA (closed circles).
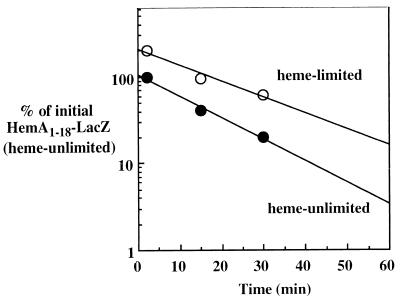
FIG. 7.
Turnover of HemA1–18-LacZ is not regulated by heme limitation. A fusion construct that expresses the HemA1–18-LacZ hybrid protein was introduced into an S. typhimurium hemL mutant background (TE2713). Two cultures of this strain (either adapted to heme limitation or not heme limited) were grown and analyzed as described in the legends to Fig. 3 and 6.
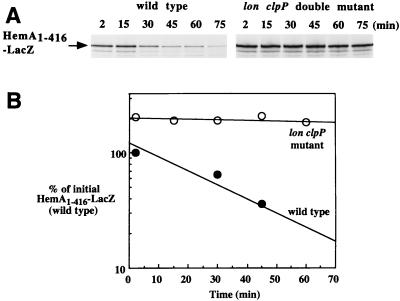
FIG. 8.
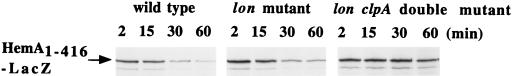
The HemA1–416-LacZ hybrid protein is degraded by both Lon and ClpP proteases in E. coli. An F′ plasmid encoding HemA1–416-LacZ was introduced into E. coli MC4100 derivatives, either wild type (TE7091) or a lon clpP double mutant (TE7029), and grown in MOPS glycerol medium with kanamycin to select for the plasmid. A pulse-chase protocol was employed, with the anti-HemA MAb H17 used for immunoprecipitation (A). HemA1–416-LacZ was >10-fold more stable in the double mutant (B) (open circles) than in the wild type (closed circles). The half-life of HemA1–416-LacZ in MC4100 was similar to that of native HemA.
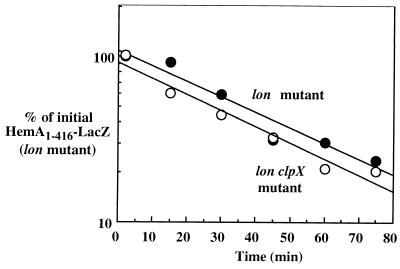
ClpA chaperone but not ClpX chaperone is required for ClpP-directed HemA turnover.
Using HemA1–416-LacZ as a model substrate, we examined the contribution of the two known ClpP chaperones, ClpA and ClpX, to ClpP-directed turnover of HemA in vivo. These two proteins are jointly required with ClpP for all ClpP-dependent proteolysis in E. coli. We found that HemA1–416-LacZ was significantly more stable in an E. coli lon clpA double mutant than in the lon single mutant (Fig. 9), whereas in a similar experiment the addition of a clpX allele to the lon mutant did not further increase the stability of HemA protein (Fig. 10). These results indicate that Lon and ClpAP, but not ClpXP or any other energy-dependent protease, are the main enzymes responsible for HemA turnover under the conditions examined (37°C and minimal glycerol medium).
FIG. 9.
Proteolysis of HemA1–416-LacZ requires the ClpA chaperone. The stability of HemA1–416-LacZ was examined in the wild-type strain in an experiment like that shown in Fig. 8 and is here compared with a lon single mutant and a lon clpA double mutant. The lon mutation does not alter HemA1–416-LacZ stability very much by itself; in the lon clpA double mutant, HemA1–416-LacZ is nearly as stable as it is in the lon clpP double mutant (Fig. 8).
FIG. 10.
Proteolysis of HemA1–416-LacZ is not affected by the lack of ClpX chaperone. The stability of HemA1–416-LacZ was examined in a lon clpX strain in an experiment like that shown in Fig. 8 and is here compared with that in a lon single mutant.
HemA induction is not a general consequence of growth limitation.
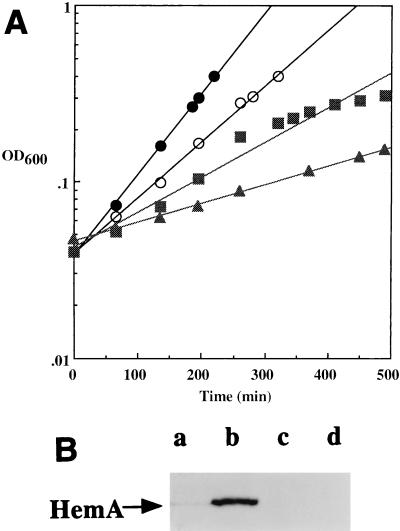
We used Western (immunoblot) analysis to determine the specificity of HemA induction by heme limitation (Fig. 11). We compared the amounts of HemA protein observed with a single strain (ΔhemL his::Tn10d-Cam) grown under conditions where the growth rate was (i) limited by available nitrogen (280-min doubling time), (ii) limited by available histidine, with histidinol as the source of histidine (154-min doubling time [2] and (iii) limited by available heme (95-min doubling time) or with unlimited growth (68-min doubling time). The only condition in which HemA abundance was elevated was growth under heme limitation. Other experiments indicate that the abundance of HemA is not markedly different in cultures grown with glucose, pyruvate, or acetate rather than glycerol as the sole carbon and energy source (unpublished observations). Together, these findings suggest that the induction of HemA by heme limitation is a specific response, rather than a result of a lower growth rate.
FIG. 11.
Specificity of HemA induction by heme limitation. A hisD hemL double mutant of S. typhimurium (TE7160) was grown in MOPS glycerol medium, supplemented to achieve limitation for different nutrients as shown in panel A: unlimited growth in medium containing 2 μM ALA, 0.1 mM histidine, and NH4 as the nitrogen source (closed circles); heme-limited growth in medium containing histidine and NH4 but without ALA (open circles); histidine-limited growth in medium containing 50 μg of histidinol per ml, with ALA and containing NH4 as the nitrogen source (squares); and nitrogen-limited growth in medium containing ALA, with histidine and arginine as sources of nitrogen (triangles). Samples taken from these cultures were resuspended directly in protein gel sample buffer and analyzed for HemA protein level by Western blotting with anti-HemA MAb H23, exactly as described previously (29); results are shown in panel B. Lanes a, b, c, and d in panel B correspond to closed circles, open circles, squares, and triangles, respectively, in panel A.
DISCUSSION
HemA catalyzes the first committed step in the heme pathway (Fig. 1) and is thus expected to be a target of regulation. In our previous work, analysis by Western blotting (immunoblotting) showed that the level of the HemA protein is elevated 10- to 20-fold during heme limitation, an increase that accounts for a similar rise in the enzyme activity as assayed in vitro (29). Pulse-chase and immunoprecipitation experiments reported here establish that regulation is achieved by an unusual mechanism: HemA is conditionally stable in a manner that is promoted by heme limitation. Instability of full-length proteins is rare in enteric bacteria, and regulated stability is very rare. The only known examples are the sigma factors RpoH and RpoS, the repressor LexA (reviewed in reference 17), and possibly the chromosomal addiction system antidote, MazE (1).
HemA turnover in S. typhimurium was found to be sensitive to azide, indicating the involvement of energy-dependent enzymes. This was confirmed by experiments with E. coli mutants. Single and double mutants of lon, clpP, clpA, clpQ, and clpX were examined. HemA is completely stable in a lon clpP double mutant but only slightly stabilized by either a lon or a clpP single mutation alone. We also tested the stability of two hybrid proteins: HemA1–18-LacZ and HemA1–416-LacZ. The nearly full-length fusion protein HemA1–416-LacZ is regulated by heme in a manner similar to that of native HemA and is also stabilized in cells mutant for both Lon and ClpP. In contrast, turnover of the short fusion protein HemA1–18-LacZ is insensitive to heme limitation, although it is stabilized in the same lon clpP double mutant. Extrapolating from results with HemA1–416-LacZ, we infer that HemA is a substrate for ClpAP but not for ClpXP. Instability of HemA may explain the difficulty that several groups including our own have encountered in attempts to overproduce the enzyme for biochemical studies.
The amino acid sequence that marks HemA as a substrate for ClpAP and Lon, the degradation tag, may be N terminal since transplant of just the first 18 amino acids from HemA to LacZ makes HemA1–18-LacZ a target for the Lon and ClpAP proteases. Because proteolysis by Lon and ClpP is processive, only one such tag or protease-sensitive site may be necessary. However, this tag does not confer correct regulation by heme.
Regulation of HemA during heme limitation is not a general property of media that restrict the growth rate of S. typhimurium: the level of HemA protein is not elevated during growth limited by a poor nitrogen source or when a low concentration of histidinol is used to satisfy a requirement for histidine (Fig. 11). Western blot analysis also showed no noticeable increase in HemA abundance during growth on carbon sources such as pyruvate or acetate which give a lower growth rate than does glycerol. This regulatory mechanism responds to an artificially limiting level of heme, achieved through a genetic defect, but in wild-type cells there is no discrimination between growth in the presence of excess ALA and growth in the absence of supplementation. In this respect, conditional stability of HemA is logically similar to the role played by the attenuator in histidine biosynthesis (for example), where control of enzyme level is exerted only during starvation for the end product. An unresolved question is the value of such a regulatory system to wild-type cells, in which it is presumably selected. One possible use would be to respond transiently to starvation for the end product during a shift in growth conditions (as suggested in reference 14); alternatively, the mechanism may normally operate subtly, at much less than the maximum effect. Still other regulatory controls such as feedback inhibition of HemA, or regulation of later oxidative steps in the pathway, may also be important to vary the rate of heme synthesis during normal growth or in the presence of excess heme.
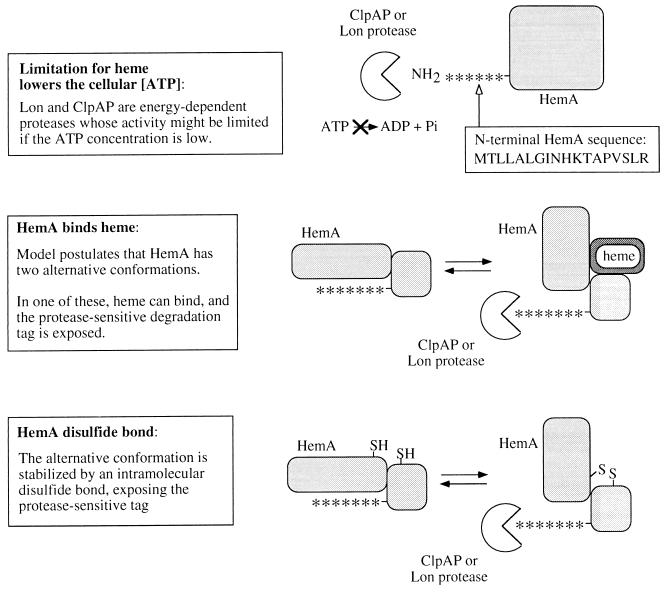
The molecule or process whose lack is ultimately sensed is not known. In principle, it might be heme or a modified derivative, or even a process affected by limited respiration or elevated H2O2 (29). Here, we consider three models for HemA regulation. These very simple models do not invoke unknown proteins or new activities of known proteins.
In the first model, the ATP concentration in vivo is postulated to decrease during heme-limited growth to a point that ATP becomes limiting for energy-dependent proteolysis (or at least proteolysis of HemA). This general possibility has previously been judged unlikely because the ATP concentration measured in cells is much higher than the Km measured in vitro for those substrates examined so far (see reference 19). Several factors may be relevant in the case of HemA. First, ADP is a competitive inhibitor of ATP for the Lon protease (reviewed in references 19 and 24a); thus, the energy charge rather than ATP level per se may be important. Second, when the Lon and ClpAP proteases act on HemA, the Km for ATP might be higher than that with other substrates. It is thought that ATP hydrolysis by the chaperone subunit or domain facilitates unfolding of the substrate to allow access to the protease active site: perhaps HemA is particularly resistant to unfolding. If one or more ClpAP or Lon substrates were shown to be stabilized in parallel with HemA, the model would be supported. The fact that the short fusion protein, HemA1–18-LacZ, is not regulated normally by heme limitation does not contradict the model, since HemA contributes only 18 residues to be unfolded in this protein, and also, it is not certain that this sequence is the one first recognized in the native HemA protein.
In the other two models, HemA is proposed to alternate between protease-sensitive and protease-resistant conformations (Fig. 12). For example, the degradation tag may be sequestered in the resistant state but accessible in the sensitive state. In model 2, the protease-sensitive conformation is stabilized by direct binding of heme to the HemA protein. This model is suggested by the finding of heme in a partially purified preparation of a HemA homolog from barley (28) and the sensitivity of HemA to inhibition by heme in crude extracts of E. coli (22), as well as feedback inhibition of purified enzyme from other organisms. In model 3, the protease-sensitive conformation is stabilized by formation of a disulfide bond, which is favored when the cell has excess oxidation capacity. The potential for disulfide bond formation in HemA has not been tested yet, but the protein contains three cysteines, two of which are conserved in homologs from other organisms. Tests of these models are in progress.
FIG. 12.
Three models for regulation of HemA turnover by heme limitation. These models are discussed in the text: control by ATP level, control by direct binding of HemA to heme, and control subsequent to formation of a disulfide bond in the HemA protein.
ACKNOWLEDGMENTS
We are grateful to the individuals listed in Table 1 for providing bacterial strains.
This work was supported by Public Health Service grant GM40403.
REFERENCES
- 1.Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia coli chromosomal “addiction module” regulated by guanosine-3′,5′-bispyrophospate: a model for programmed bacterial cell death. Proc Natl Acad Sci USA. 1996;93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames B N, Martin R G, Garry B J. The first step of histidine biosynthesis. J Biol Chem. 1961;236:2019–2026. [PubMed] [Google Scholar]
- 3.Archer C D, Elliott T. Transcriptional control of the nuo operon which encodes the energy-conserving NADH dehydrogenase of Salmonella typhimurium. J Bacteriol. 1995;177:2335–2342. doi: 10.1128/jb.177.9.2335-2342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer C D, Wang X, Elliott T. Mutants defective in the energy-conserving NADH dehydrogenase of Salmonella typhimurium identified by a decrease in energy-dependent proteolysis after carbon starvation. Proc Natl Acad Sci USA. 1993;90:9877–9881. doi: 10.1073/pnas.90.21.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beale S I. Biosynthesis of hemes. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 731–748. [Google Scholar]
- 6.Berkowitz D, Hushon J M, Whitfield H J, Roth J, Ames B N. Procedure for identifying nonsense mutations. J Bacteriol. 1968;96:215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochner B R, Ames B N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982;257:9759–9769. [PubMed] [Google Scholar]
- 8.Choi P, Wang L, Archer C D, Elliott T. Transcription of the glutamyl-tRNA reductase (hemA) gene in Salmonella typhimurium and Escherichia coli: role of the hemA P1 promoter and the arcA gene product. J Bacteriol. 1996;178:638–646. doi: 10.1128/jb.178.3.638-646.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989;171:3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott T. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J Bacteriol. 1992;174:245–253. doi: 10.1128/jb.174.1.245-253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott T, Roth J R. Heme-deficient mutants of Salmonella typhimurium: two genes required for ALA synthesis. Mol Gen Genet. 1989;216:303–314. doi: 10.1007/BF00334369. [DOI] [PubMed] [Google Scholar]
- 12.Elliott T, Wang X. Salmonella typhimurium mutants defective in release factor 1. J Bacteriol. 1991;173:4144–4154. doi: 10.1128/jb.173.13.4144-4154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg A L, St. John A C. Intracellular protein degradation in mammalian and bacterial cells: part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- 14.Gorini L, Maas W K. The potential for the formation of a biosynthetic enzyme in Escherichia coli. Biochim Biophys Acta. 1957;25:208–209. doi: 10.1016/0006-3002(57)90450-x. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman S. Minimizing proteolysis in Escherichia coli: genetic solutions. Methods Enzymol. 1990;185:119–129. doi: 10.1016/0076-6879(90)85013-e. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 17.Gottesman S. Roles for energy-dependent proteases in regulatory cascades. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Co.; 1996. pp. 503–519. [Google Scholar]
- 18.Gottesman S, Clark W P, de Crecy-Lagard V, Maurizi M R. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1993;268:22618–22626. [PubMed] [Google Scholar]
- 19.Gottesman S, Maurizi M R. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol Rev. 1992;56:592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutnick D, Calvo J M, Klopotowski T, Ames B N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969;100:215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito K, Bassford P J, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981;24:707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- 22.Javor G T, Febre E F. Enzymatic basis of thiol-stimulated secretion of porphyrins by Escherichia coli. J Bacteriol. 1992;174:1072–1075. doi: 10.1128/jb.174.3.1072-1075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama Y, Gottesman S, Pumphrey J, Rudikoff S, Clark W P, Maurizi M R. The two-component, ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1988;263:15226–15236. [PubMed] [Google Scholar]
- 24.Maurizi M R, Clark W P, Katayama Y, Rudikoff S, Pumphrey J, Bowers B, Gottesman S. Sequence and structure of ClpP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990;265:12536–12545. [PubMed] [Google Scholar]
- 24a.Miller, C. G. Protein degradation and modification, p. 938–954. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 25.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver D B, Cabelli R J, Dolan K M, Jarosik G P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci USA. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 28.Vothknecht U C, Kannangara C G, von Wettstein D. Expression of catalytically active barley glutamyl tRNAGlu reductase in Escherichia coli as a fusion protein with glutathione S-transferase. Proc Natl Acad Sci USA. 1996;93:9287–9291. doi: 10.1073/pnas.93.17.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Brown L, Elliott M, Elliott T. Regulation of heme biosynthesis in Salmonella typhimurium: activity of glutamyl-tRNA reductase (HemA) is greatly elevated during heme limitation by a mechanism which increases abundance of the protein. J Bacteriol. 1997;179:2907–2914. doi: 10.1128/jb.179.9.2907-2914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu K, Delling J, Elliott T. The genes required for heme synthesis in Salmonella typhimurium include those encoding alternative functions for aerobic and anaerobic coproporphyrinogen oxidation. J Bacteriol. 1992;174:3953–3963. doi: 10.1128/jb.174.12.3953-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]