Abstract
Introduction/Aims
In this study we investigated COVID‐19 vaccination–related adverse events (ADEs) 7 days postvaccination in patients with idiopathic inflammatory myopathies (IIMs) and other systemic autoimmune and inflammatory disorders (SAIDs).
Methods
Seven‐day vaccine ADEs were collected in an international patient self‐reported e‐survey. Descriptive statistics were obtained and multivariable regression was performed.
Results
Ten thousand nine hundred respondents were analyzed (1227 IIM cases, 4640 SAID cases, and 5033 healthy controls [HCs]; median age, 42 [interquartile range, 30‐455] years; 74% female; 45% Caucasian; 69% completely vaccinated). Major ADEs were reported by 76.3% of the IIM patients and 4.6% reported major ADEs. Patients with active IIMs reported more frequent major (odds ratio [OR], 2.7; interquartile range [IQR], 1.04‐7.3) and minor (OR, 1.5; IQR, 1.1‐2.2) ADEs than patients with inactive IIMs. Rashes were more frequent in IIMs (OR, 2.3; IQR, 1.2‐4.2) than HCs. ADEs were not impacted by steroid dose, although hydroxychloroquine and intravenous/subcutaneous immunoglobulins were associated with a higher risk of minor ADEs (OR, 1.9; IQR, 1.1‐3.3; and OR, 2.2; IQR, 1.1‐4.3, respectively). Overall, ADEs were less frequent in inclusion‐body myositis (IBM) and BNT162b2 (Pfizer) vaccine recipients.
Discussion
Seven‐day postvaccination ADEs were comparable in patients with IIMs, SAIDs, and HCs, except for a higher risk of rash in IIMs. Patients with dermatomyositis with active disease may be at higher risk, and IBM patients may be at lower risk of specific ADEs. Overall, the benefit of preventing severe COVID‐19 through vaccination likely outweighs the risk of vaccine‐related ADEs. Our results may inform future guidelines regarding COVID‐19 vaccination in patients with SAIDs, specifically in those with IIMs. Studies to evaluate long‐term outcomes and disease flares are needed to shed more light on developing future COVID‐19 vaccination guidelines.
Keywords: COVID‐19, dermatomyositis, myositis, rheumatology, vaccination

Abbreviations
- ADE
adverse drug event
- ASSD
anti‐synthetase syndrome
- BLR
binary logistic regression
- CI
confidence interval
- CNI
calcineurin inhibitor
- DM
dermatomyositis
- HC
healthy control
- HCQ
hydroxychloroquine
- IBM
inclusion‐body myositis
- IIM
idiopathic Inflammatory myopathies
- IVIg
intravenous immunoglobulin
- JDM
juvenile dermatomyositis
- NAM
necrotizing myositis
- OM
overlap myositis
- NS
not significant
- OM
overlap myositis
- OR
odds ratio
- PM
polymyositis
- SAID
systemic autoimmune and inflammatory disorder
1. INTRODUCTION
The development of vaccines against the novel coronavirus has improved outcomes after COVID‐19 infection in the general population. Several reports have demonstrated the safety and efficacy of COVID‐19 vaccines in the general population, but there are significant gaps in vaccine safety and efficacy data in vulnerable populations, including patients with systemic autoimmune and inflammatory disorders (SAIDs), those on immunosuppressive medications, and in pregnant women. 1 , 2 , 3 The exclusion of patients with SAIDs and those on immunosuppressive medication from the initial vaccine safety trials has inevitably resulted in a paucity of safety and efficacy data of COVID‐19 vaccination in this vulnerable patient group. Although recent studies have included rheumatic disorders, significant gaps exist in understanding the safety of COVID‐19 vaccination in patients with rare diseases, such as idiopathic inflammatory myopathies (IIMs). 4 , 5 , 6 The study on vaccination‐related adverse events (ADEs) in dermatomyositis (DM) from the TrinetX (Cambridge, MA) database has looked at the overall 1‐, 30‐, and 60‐day ADEs after three vaccines (BNT162b2, mRNA‐1273, and Ad26.COV.2.S) used in USA. 7 The study included a total of 6104 vaccinated dermatomyositis (DM) patients from the USA. DM patients demonstrated a higher rate of ADEs compared with age‐matched healthy controls (HCs). The studies so far available on vaccine ADEs in SAIDs or IIMs are largely regional and small. 8 Studies claiming to be global have had an underrepresentation of various ethnic groups (blacks and Asians). 4
An interplay between underlying autoimmunity and dysregulated immune pathways compounded by the effect of immunosuppressive medications and potentially impacted by comorbid illness may predispose IIM patients to an increased risk of postvaccination ADEs, including allergic reactions, anaphylaxis, and disease flares. 9 , 10 Concerns have recently emerged that adjuvants and immune activators in vaccines induce autoimmune disease flares and de‐novo immune thrombotic and demyelinating events. 11 , 12 Poor characterization of postvaccination ADEs in IIM patients due to a lack of vaccine safety and efficacy study in this group may have contributed to vaccine hesitancy. 13 , 14 Therefore, there is a need for evidence‐based vaccine safety data with proper characterization of post‐vaccination ADEs to potentially improve vaccination rates in patients with IIM, a vulnerable patient group.
In this study, we evaluated the short‐term safety of COVID‐19 vaccination using a patient self‐reported global multicenter electronic survey.
2. METHODS
2.1. Study design
This is an international, online, cross‐sectional, multicenter survey, part of the COVID‐19 Vaccination in Autoimmune Diseases (COVAD) study. 15 Informed consent of the participants was obtained via a cover letter. Approval was obtained from the local institutional ethics committee as per local guidelines, and the Checklist for Reporting Results of the Internet E‐Surveys was adhered to when reporting results. 16 , 17
2.2. Data collection
A comprehensive patient‐self reporting electronic survey was developed, consisting of a questionnaire of 36 COVID‐19–related and SAID‐related questions, which included demographic details; SAID diagnosis; treatment details; current symptom status; COVID‐19 infection history, including symptoms, duration, and complications (hospitalization and need for oxygen therapy); COVID‐19 vaccination details; 7‐day short‐term postvaccination ADEs (based on criteria from the US Centers for Disease Control and Prevention); and patient‐reported outcome measures, as per the Patient Reported Outcomes Measurement Information System tool. 18 After vetting by international experts, pilot testing, revisions, validation, and translation into 18 languages, the survey was hosted on an online platform (surveymonkey.com) and circulated by the international COVAD Study Group (over 110 physicians) in health‐care centers in at least 94 countries (Supplementary Data), as well as through numerous social media platforms and online patient‐support groups. Patients with multiple overlapping autoimmune diseases were put into all the corresponding categories. Convenience sampling was used and all participants over 18 years of age were included. Electronic protocols were used to remove duplicate responses from a single respondent. Methods have been detailed at length in the published COVAD study protocol. 15
2.3. Data extraction
Data were retrieved on September 30, 2021. Patients who had not received even a single dose of any COVID‐19 vaccine at the time of survey completion, and who had not completed the survey in full, were excluded from the analysis (Figure 1). Multiple relevant variables were extracted from the survey responses of the included participants, including COVID‐19 infection history and 7‐day postvaccination ADEs.
FIGURE 1.
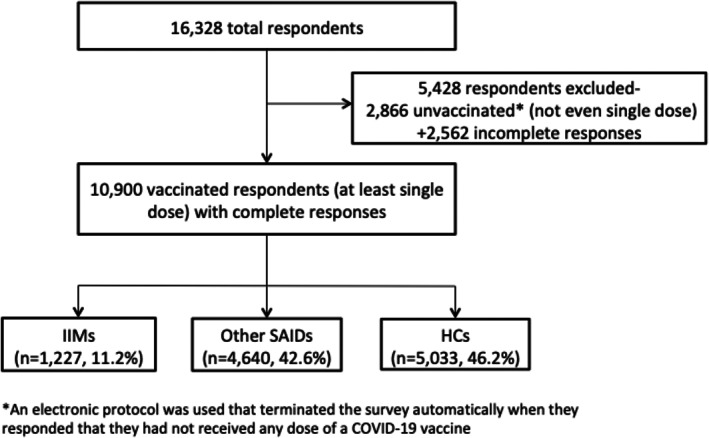
Data extraction.
2.4. Active and inactive disease
Active and inactive disease 4 weeks before vaccination were assessed by patients' response to questions about their symptoms before vaccination (eg rash, muscle weakness, joint pain, and swelling, etc) and need to step‐up immunosuppression (Supplementary Methods).
2.5. ADEs postvaccination
Seven‐day ADEs were categorized as injection‐site pain and reaction, minor ADEs, major ADEs, and hospitalizations. Minor ADEs included myalgia, body aches, fever, chills, nausea and vomiting, headache, rashes, fatigue, diarrhea, abdominal pain, high pulse rate or palpitations, rise in blood pressure, fainting, difficulty in breathing, dizziness, and chest pain. Major ADEs consisted of serious reactions to vaccination requiring urgent medical attention, including anaphylaxis, a marked difficulty in breathing, throat closure (choking), and severe rashes. 19 Other ADEs that were not listed were reported as “others” via an open‐ended question.
2.6. Statistical analysis
Chi‐square and Mann‐Whitney U tests were used for categorical and continuous variables, respectively. The variables expected to be independently significant between IIMs, SAIDs, and HCs, and between different IIM subtypes, after univariable analysis, underwent binary logistic regression analysis with adjustment for factors considered relevant based on evidence from current literature and clinical judgment, including the factors of age, gender, ethnicity, immunosuppressant use, and vaccine received, and stratified by country of origin. The results for continuous variables are expressed as median (interquartile range [IQR]). P < .05 was considered significant. The Bonferroni‐corrected P value for univariate analysis was taken as significant (P < 0.0125 for 2 × 2 chi‐square analysis). Nonparametric tests were used because the data were not normally distributed (by Kolmogorov‐Smirnov test and Shapiro‐Wilk test). Statistical analysis was performed using SPSS version 20 (IBM Corporation, Armonk, NY) and R software version 3.5.3 (R Core Team, Vienna, Austria).
3. RESULTS
3.1. Population characteristics
Of the 16 328 total respondents, 2866 had not received a single dose of any COVID‐19 vaccine at the time of survey completion and 2562 did not complete the survey in full and were thus excluded from further analysis (Figure 1). The 10 900 vaccinated respondents included in the analysis (74% female; median age, 42 [IQR, 30‐55] years; 46% Caucasian) were primarily from Turkey, Mexico, India, UK, and the USA, and consisted of 11.2% with IIMs, 42.6% with other SAIDs, and 46.2% HCs. The most common SAIDs reported in the cohort were rheumatoid arthritis, followed by IIM and hyper/hypothyroidism. Of the respondents, 69% had received both primary doses of the COVID‐19 vaccine. The largest number of respondents received the BNT162b2 (Pfizer) vaccine (39.8%), followed by theChadOx1 nCOV‐19 vaccine (Oxford/AstraZeneca). Of the IIM patients, 34% had dermatomyositis, 17% had polymyositis, and 23% had inclusion‐body myositis (IBM). Other population characteristics of the study cohort are provided in Tables S1 and S5, and Tables S1,S2, and S6.
TABLE 1.
Population characteristics
| Variable | Total (%) (n = 10 900) | IIMs (%) (n = 1227) | Other SAIDs (%) (n = 4640) | HCs (%) (N = 5033) |
|---|---|---|---|---|
| Age (range), in years | 42 (30‐55) | 49 (38‐61) | 47 (36‐57) | 33 (25‐46) |
| Gender M:F | 2432:8558 (1:2.9) | 283:782 (1:2.76) | 568:3249 (1:5.7) | 1491:2798 (1:1.8) |
| Ethnicity | ||||
| Caucasian | 4972 (46) | 882 (72) | 2303 (49) | 1787 (35) |
| African American or of African origin | 83 (0.7) | 34 (3) | 22 (0.5) | 27 (0.5) |
| Asian | 2018 (18) | 71 (6) | 781 (17) | 1166 (23) |
| Hispanic | 1193 (11) | 49 (4) | 399 (8.5) | 745 (15) |
| Native American/Indigenous/Pacific Islander | 342 (3) | 1 (0) | 18 (0.4) | 323 (6) |
| Do not wish to disclose | 449 (4) | 13 (1) | 191 (4) | 245 (5) |
| Other | 865 (8) | 21 (2) | 127 (3) | 717 (14) |
| Vaccine received | ||||
| BNT162b2 (Pfizer)‐BioNTech | 4333 (39) | 645 (53) | 2042 (44) | 1443 (28.7) |
| ChadOx1 nCOV‐19 (Oxford/AstraZeneca) | 1456 (13) | 124 (10) | 845 (18) | 487 (9.7) |
| JNJ‐78436735 (JOHNSON AND JOHNSON) | 95 (1) | 15 (1.2) | 42 (1) | 38 (0.8) |
| MRNA‐1273 (Moderna) | 910 (8) | 360 (29) | 387 (8) | 163 (3.2) |
| NVX‐CoV2373 (Novovax) | 14 (0.1) | 0 (0) | 10 (0.2) | 4 (0.1) |
| ChAdOx1 nCoV‐19 (Covishield Serum Institute India) | 1194 (11) | 43 (3.5) | 430 (9) | 721 (14) |
| BBV152 (Covaxin Bharat Biotech) | 248 (2) | 15 (1.2) | 111 (2) | 122 (2.4) |
| Gam‐COVID‐Vac (Sputnik) | 204 (2) | 4 (0.3) | 64 (1) | 136 (2.7) |
| BBIBP‐CorV (Sinopharm) | 1821 (17) | 4 (0.3) | 374 (8) | 1443 (28.7) |
| Not sure | 62 (0.5) | 0 (0) | 27 (0.5) | 35 (0.7) |
| Others | 563 (5) | 17 (1.4) | 309 (6) | 238 (4.7) |
| Discontinued medicines before vaccination | 773 (13) | 147 (12) | 626 (13) | ‐‐‐ |
| Duration of discontinuing medicines (days) | 13 (7‐21) | 14 (7‐21) | 12 (7‐21) | ‐‐‐ |
Abbreviations: HC, healthy control; IIM, idiopathic inflammatory myopathy; SAID, systemic autoimmune and inflammatory disease.
TABLE 5.
Effects of COVID‐19 vaccination in patients with IIMs vs other SAIDs and HCs
| IIMs a (n = 1227) | Other SAIDs a (n = 4640) | HCs a (n = 5033) | OR 1 (CI) | OR 2 (CI) | P1 | P2 | |
|---|---|---|---|---|---|---|---|
| Injection‐site pain | 784 (63) | 3036 (65) | 3138 (62) | .316 | .365 | ||
| Minor ADEs | |||||||
| Myalgia | 144 (12) | 777 (17) | 778 (15.5) | 0.6 (0.5‐0.8) | 0.7 (0.6‐0.8) | <.001 | <.001 |
| Body ache | 233 (19) | 1067 (23) | 1082 (21) | 0.8 (0.7‐0.9) | .003 | .055 | |
| Fever | 151 (12) | 863 (18) | 960 (19) | 0.6 (0.5‐0.7) | 0.6 (0.5‐0.7) | <.001 | <.001 |
| Chills | 176 (14) | 714 (15) | 631 (12.5) | .365 | .104 | ||
| Nausea and vomiting | 74 (6) | 311 (7) | 222 (4.4) | 1.3 (1‐1.8) | .398 | .021 | |
| Headache | 271 (22) | 1290 (28) | 1125 (22.4) | 0.7 (0.6‐0.8) | <.001 | .884 | |
| Rashes | 34 (3) | 91 (2) | 48 (1) | 2.9 (1.8‐4.5) | .081 | <.001 | |
| Fatigue | 348 (28) | 1511 (32) | 1359 (27) | 0.8 (0.7‐0.9) | <.001 | .395 | |
| Diarrhea | 29 (2.4) | 174 (4) | 120 (2.4) | 0.6 (0.4‐0.9) | .018 | .945 | |
| Abdominal pain | 27 (2) | 126 (3) | 72 (1.4) | .314 | .059 | ||
| High pulse rate or palpitations | 27 (2) | 166 (4) | 125 (2.5) | 0.6 (0.4‐0.9) | .016 | .527 | |
| Rise in blood pressure | 8 (0.6) | 65 (1) | 47 (0.9) | 0.5 (0.2‐0.9) | .035 | .328 | |
| Fainting | 4 (0.3) | 23 (0.5) | 16 (0.3) | .435 | .980 | ||
| Difficulty in breathing | 10 (0.2) | 59 (1) | 50 (1) | .187 | .543 | ||
| Dizziness | 58 (4.8) | 291 (6) | 229 (4.4) | 0.7 (0.5‐0.99) | .042 | .498 | |
| Chest pain | 17 (1.4) | 81 (2) | 60 (1.2) | .381 | .611 | ||
| Others | 77 (6) | 431 (9) | 270 (5) | .567 | .247 | ||
| Major ADEs | |||||||
| Anaphylaxis | 5 (0.4) | 6 (0.1) | 5 (0.1) | 5 (1.3‐19) | .060 | .070 | |
| Marked difficulty in breathing | 9 (0.7) | 27 (0.6) | 27 (0.5) | .545 | .430 | ||
| Throat closure | 4 (0.3) | 23 (0.5) | 4 (0.3) | .435 | .167 | ||
| Severe rashes | 10 (0.8) | 31 (0.7) | 15 (0.3) | 2.7 (1.2‐6) | .583 | .011 | |
| Others | 40 (3) | 149 (3) | 56 (1) | 2.9 (1.9‐4.4) | .945 | .042 | |
| Hospitalization | 7 (0.6) | 20 (0.4) | 11 (0.2) | 2.5 (1‐6.7) | .521 | .042 |
Notes: Bold values are statistically significant (P < .05). Chi‐square test used for categorical variables and Mann‐Whitney U test used for continuous variables.
Abbreviations: ADE, adverse drug event; CI, confidence interval; HC, healthy control; IIM, idiopathic inflammatory myopathy; OR, odds ratio; OR1, OR between IIMs and other SAIDs; OR2 OR between IIMs and HCs; P1 P value between IIMs and other SAIDs, P2 P value between IIMs and HCs; SAID, systemic autoimmune and inflammatory disorder.
Data expressed as number (%).
3.2. Post–COVID‐19 vaccination–associated ADEs in patients with IIM
Any ADE was seen in 76.5%, with minor ADEs in 76.3% and major ADEs in 4.6%. All‐cause hospitalization was seen in 0.6%. Minor ADEs most commonly seen were fatigue, myalgia, and fever. Severe rashes were statistically more frequent in IIM patients when compared with HCs (Table 5).
Of the 102 patients with DM who reported a rash in the 4 weeks prevaccination, 47 had a heliotrope rash, 66 had Gottron papules, 17 had Holster signs, 51 had a malar rash, 63 had a V sign, 42 had a forearm/arm rash, and 60 had “mechanic's hands.” Of the 22 patients who had a rash after vaccination, 31% had inactive disease before vaccination, 27% had a DM rash, 27% had muscle weakness, 22% had joint pain in the hands, and 4.5% had joint pain in other regions. The increase in postvaccination rashes could have represented a flare of a DM rash as 69% had active disease before vaccination.
3.3. Post–COVID‐19 vaccination–associated ADEs in patients with active and inactive IIM
Among the IIM patients, 855 had active IIM 4 weeks before vaccination and 352 had inactive disease. Any ADE after COVID‐19 vaccination was more frequent in active IIM cases. Any minor ADE, myalgia, body ache, headache fatigue, dizziness, and overall major ADE were more frequent in patients with active IIM compared with inactive disease before vaccination (Table 2 ).
TABLE 2.
Comparison of vaccination‐related ADEs among active and inactive IIM cases
| Active IIM a (n = 855) | Inactive IIM a (n = 352) | Univariate | Multivariable | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Any ADE | 681 (79.6) | 242 (68.8) | 1.7 (1.3‐2.3) | <.001 | 1.6 (1.1‐2.2) | .006 |
| Injection site pain | 557 (65) | 216 (61) | .213 | |||
| Minor ADEs | ||||||
| Any minor ADE | 679 (79.4) | 242 (68.8) | 1.7 (1.3‐2.3) | <.001 | 1.5 (1.1‐2.2) | .007 |
| Myalgia | 117 (13.7) | 25 (7) | 2 (1.3‐3.2) | .001 | 2.2 (1.3‐3.8) | .002 |
| Body ache | 178 (21) | 49 (14) | 1.6 (1.1‐2.2) | .005 | 1.5 (1.07‐2.3) | .020 |
| Fever | 112 (13) | 37 (10.5) | ‐‐‐ | .214 | ||
| Chills | 134 (15.7) | 38 (10.8) | 1.5 (1.04‐2.2) | .028 | 1.3 (0.8‐2.1) | .162 |
| Nausea and vomiting | 60 (7) | 12 (3.4) | 2.1 (1.1‐4) | .016 | 1.7 (0.8‐3.6) | .107 |
| Headache | 212 (25) | 55 (15.6) | 1.7 (1.2‐2.4) | <.001 | 1.5 (1.04‐2.1) | .028 |
| Rashes | 25 (2.9) | 9 (2.6) | .726 | |||
| Fatigue | 266 (31.1) | 73 (20.7) | 1.7 (1.2‐2.3) | <.001 | 1.5 (1.08‐2.1) | .015 |
| Diarrhea | 22 (2.6) | 5 (1.4) | .218 | |||
| Abdominal pain | 24 (2.8) | 3 (0.9) | .051 | |||
| High pulse rate or palpitations | 21 (2.5) | 6 (1.7) | .422 | |||
| Rise in blood pressure | 5 (0.6) | 3 (0.9) | .603 | |||
| Fainting | 2 (0.2) | 2 (0.6) | .358 | |||
| Difficulty in breathing | 9 (1.1) | 1 (0.3) | .181 | |||
| Dizziness | 51 (6) | 7 (2) | 3.1 (1.4‐6.9) | .003 | 2.5 (1.08‐5.9) | .031 |
| Chest pain | 14 (1.6) | 2 (0.6) | .140 | |||
| Major ADEs | ||||||
| Any major ADE | 49 (5.7) | 6 (1.7) | 3.5 (1.4‐8.2) | .002 | 2.7 (1.04‐7.3) | .040 |
| Anaphylaxis | 5 (0.6) | 0 (0) | .151 | |||
| Marked difficulty in breathing | 5 (0.6) | 3 (0.9) | .603 | |||
| Throat closure | 4 (0.5) | 0 (0) | .199 | |||
| Severe rashes | 7 (0.8) | 1 (0.3) | .298 | |||
| Hospitalization | 5 (0.6) | 2 (0.6) | .972 | |||
Notes: Chi‐square test was used for categorical variables and Mann‐Whitney U test used for continuous variables.
Factors adjusted in multivariable analysis (binary logistic regression) include age, gender, ethnicity, vaccine received, number of vaccine doses received, and immunosuppressants received.
Abbreviations: ADE, adverse drug event; CI, confidence interval; IIM, idiopathic inflammatory myopathy; OR, odds ratio.
Data expressed as number (%).
3.4. Post–COVID‐19 vaccination–associated ADEs in patients with IIM based on immunosuppression received
Although the wide variety of treatments used for IIMs results in a low frequency for each, an adjusted analysis shows that IIM patients who were on rituximab (n = 40, 3%) more frequently had chills (odds ratio [OR], 2.6; IQR, 1.2‐5.8; P = .012) and dizziness (OR, 3.9; IQR, 1.3‐11; P = .010) after vaccination. Among the intravenous/subcutaneous immunoglobulin recipients (n = 117, 9%), any minor ADE (OR, 2.2; IQR, 1.1‐4.3; P = .019) was more frequent and muscle pain (OR, 0.28; IQR, 0.1‐0.7; P =.01) was less frequent.
3.5. Post–COVID‐19 vaccination–associated ADEs between patients with different IIM subtypes
There was no significant difference for risk of overall minor ADEs between the different IIM subtypes. However, a higher risk of headache was observed in DM patients compared with other IIM subtypes in the adjusted analysis, although the absolute risk of rash was very low across IIM subtypes (0%‐5%). In contrast to their DM counterparts, patients with IBM appeared to be less affected by postvaccination ADEs, with a lower risk of myalgia compared with other IIM subtypes (Table 3 and Table S3). The risk of major ADEs and hospitalizations remained consistent across different IIM subtypes with a very small absolute risk (0%‐2%), but the numbers were too small (n = 0‐10) to draw firm conclusions.
TABLE 3.
COVID infection and COVID‐19 vaccination–associated ADEs in IIM subtypes a
| DM (n = 418) | PM (n = 207) | IBM (n = 284) | ASSD (n = 136) | NAM (n = 52) | OM (n = 116) | JDM (n = 14) | |
|---|---|---|---|---|---|---|---|
| Injection‐site pain | 298 (71) *** | 136 (65) | 145 (51) *** | 88 (65) | 31 (59) | 73 (63) | 11 (78) |
| Minor ADEs | |||||||
| Myalgia | 46 (11) | 35 (17)* | 15 (5) b*** | 22 (16) | 4 (7) | 16 (14) | 3 (21) |
| Body ache | 90 (21) | 42 (20) | 27 (9) *** | 33 (24) | 8 (15) | 30 (13) | 2 (14) |
| Fever | 64 (15) | 28 (13) | 19 (7) ** | 14 (10) | 5 (9) | 19 (16) | 2 (14) |
| Chills | 66 (16) | 29 (14) | 23 (8) ** | 26 (19) | 7 (13) | 22 (19) | 2 (14) |
| Nausea and vomiting | 32 (8) | 6 (3) | 7 (2.5)* | 9 (7) | 4 (7) | 14 (12) ** | 1 (7) |
| Headache | 123 (29) b*** | 37 (18) | 29 (10) *** | 32 (23) | 10 (19) | 36 (31)* | 2 (14) |
| Rashes | 21 (5) b ,*** | 2 (1) | 1 (0.4) * | 3 (2) | 1 (2) | 6 (5) | 0 (0) |
| Fatigue | 138 (33) ** | 63 (30) | 47 (16) *** | 40 (29) | 19 (36) | 34 (29) | 6 (43) |
| Diarrhea | 13 (3) | 2 (1) | 4 (1.4) | 2 (1.5) | 1 (2) | 7 (6) * | 0 (0) |
| Abdominal pain | 11 (3) | 3 (1) | 5 (2) | 3 (2) | 2 (4) | 3 (3) | 0 (0) |
| High pulse rate or palpitations | 8 (2) | 6 (3) | 2 (0.7) | 4 (3) | 1 (2) | 5 (4) | 1 (7) |
| Rise in blood pressure | 3 (0.7) | 1 (0.5) | 0 (0) | 1 (0.7) | 0 (0) | 3 (3)* | 0 (0) |
| Fainting | 4 (1) ** | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Difficulty in breathing | 6 (1) | 1 (0.5) | 1 (0.4) | 1 (0.7) | 1 (2) | 1 (1) | 0 (0) |
| Dizziness | 20 (5) | 11 (5) | 4 (1.4) ** | 7 (5) | 5 (10) | 11 (9) * | 2 (14) |
| Chest pain | 7 (2) | 0 (0) | 1 (0.4) | 4 (3) | 1 (2) | 3 (3) | 1 (7) |
| Major ADEs | |||||||
| Anaphylaxis | 5 (0.4) | 2 (1) | 2 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Marked difficulty in breathing | 9 (0.7) | 0 (0) | 1 (0.4) | 3 (2) | 0 (0) | 0 (0) | 0 (0) |
| Throat closure | 4 (0.3) | 1 (0.5) | 1 (0.4) | 1 (0.7) | 0 (0) | 0 (0) | 1 (7) |
| Severe rashes | 10 (0.8) | 1 (0.5) | 0 (0) | 0 (0) | 1 (2) | 1 (1) | 0 (0) |
| Hospitalization | 7 (0.6) | 2 (1) | 1 (0.4) | 1 (0.7) | 0 (0) | 1 (1) | 0 (0) |
Notes: Chi‐square test used for categorical variables and Mann‐Whitney U test used for scale variables. Comparisons are between each IIM subtype vs the rest of IIM subtypes. Bold indicates increased OR vs the others. Bold+underlined indicates decreased OR vs the others.
Abbreviations: ADE, adverse drug event; ASSD, anti‐synthetase syndrome; CI, confidence interval; DM, dermatomyositis; IBM, inclusion‐body myositis; IIM, idiopathic inflammatory myopathies; JDM, juvenile dermatomyositis; NAM, necrotizing myositis; OD, odds ratio; OM, overlap myositis; PM, polymyositis.
Data expressed as number (%).
Significant in BLR (binary logistic regression) adjusted for age, gender, ethnicity, immunosuppressant dose, and stratified by country.
* P < .05, ** P < .005, *** P < .001.
3.6. Comparison of post–COVID‐19 vaccination ADE among patients with IIM by vaccine type
After adjustment for baseline variables, IIM patients receiving the ChAdOx1 nCoV‐19 vaccine (Covishield Serum Institute India) were at a higher risk of myalgia and fever compared with the other vaccine recipients. There was a significantly higher overall risk of minor ADEs, as well as a higher individual risk of injection site pain, chills, rashes, and nausea and vomiting, observed in IIM patients receiving the MRNA‐1273 vaccine (Moderna). Similarly, IIM patients receiving the ChAdOx1 nCOV‐19 vaccine had a higher risk of myalgia, fever, chills, headache, abdominal pain, and tachycardia/palpitations, yet a lower risk of injection‐site pain compared with other vaccine recipients (Table 4 and Table S4). Conversely, IIM patients receiving the BNT162b2 vaccine had a lower overall risk (Table 4 and Table S4). The absolute risk in IIMs, SAIDs, and HCs, as well as in different IIM subtypes, was very small (<2% in most cases) across vaccine types (Table S5).
TABLE 4.
Vaccine ADEs based on COVID‐19 vaccine received among IIM patients (vs rest of vaccines)
| BNT162b2 (Pfizer) (n = 634) | ChadOx1 nCOV‐19 (Oxford/AstraZeneca) (n = 124) | MRNA‐1273 (Moderna) (n = 360) | ChAdOx1 nCoV‐19 (Covishield Serum Institute India) (n = 43) | |
|---|---|---|---|---|
| Injection‐site pain | 398 (62) | 69 (55) a * | 273 (76) a *** | 25 (58) |
| Minor ADEs | ||||
| Any minor ADE | 456 (71) a *** | 96 (77) | 303 (85) a *** | 35 (81) |
| Myalgia | 55 (8) a *** | 23 (18) a * | 44 (12) | 15 (35) a *** |
| Body ache | 99 (15) a *** | 35 (28) a * | 76 (21) | 12 (28) |
| Fever | 49 (7) a *** | 26 (21) a * | 54 (15) | 18 (42) a *** |
| Chills | 72 (11) a *** | 29 (23) a * | 65 (18) *, a | 4 (9) |
| Nausea and vomiting | 22 (3) a *** | 15 (12) * | 31 (8) *, a | 5 (11) |
| Headache | 120 (18) a *** | 41 (33) a ** | 91 (25) | 10 (23) |
| Rashes | 10 (1.5) a ** | 5 (4) | 21 (6) a *** | 1 (2) |
| Fatigue | 178 (27) | 37 (29) | 120 (33)* | 7 (16)* |
| Diarrhea | 14 (2) | 8 (6) * | 8 (2) | 1 (2) |
| Abdominal pain | 12 (2) | 9 (7) a *** | 7 (2) | 2 (4) |
| High pulse rate or palpitations | 12 (2) | 8 (6) a ** | 7 (2) | 2 (4) |
| Rise in blood pressure | 7 (1) | 3 (2) | 1 (0.2) | 1 (2) |
| Fainting | 5 (0.7) | 1 (1) | 1 (0.2) | 1 (2) |
| Difficulty in breathing | 7 (1) | 1 (1) | 4 (1) | 2 (4) |
| Dizziness | 21 (3) a ,* | 9 (7) | 25 (6)* | 3 (7) |
| Chest pain | 10 (1.5) | 2 (1.6) | 4 (1) | 2 (4) |
| Major ADEs | ||||
| Any major ADE | 22 (3.3) * | 11 (8.3) * | 17 (4.7) | 3 (7) |
| Anaphylaxis | 3 (0.4) | 3 (2) * | 1 (0.2) | 1 (2) |
| Marked difficulty in breathing | 6 (1) | 2 (1.6) | 4 (1) | 1 (2) |
| Throat closure | 1 (0.1) * | 2 (1.6) | 2 (0.5) | 1 (2) |
| Severe rashes | 6 (1) | 3 (2) | 2 (0.5) | 1 (2) |
| Hospitalization | 2 (0.2) | 1 (1) | 3 (0.8) | 1 (2) |
Notes: Chi‐square test used for categorical variables and Mann‐Whitney U test used for scale variables. Bold indicates increased odds ratio vs the remaining vaccines. Bold+underlined indicates decreased odds ratio vs remaining vaccines.
Abbreviations: ADE, adverse drug event; AID, autoimmune disease; HC, healthy control; IIM, idiopathic inflammatory myopathy.
*P < .05, ** P < .005, *** P < .001.
Significant according to binary logistic regression adjusted for age, gender, ethnicity, and immunosuppressant dose, and stratified by country.
3.7. Post–COVID‐19 vaccination–associated ADEs in patients with IIM compared with HCs
The incidence of injection site pain was similar in IIM patients (63%) and HCs (62%), with a very small absolute risk difference. Among minor ADEs, patients with IIM were at a higher risk of rashes compared with HCs, although the absolute risk of rash in both IIMs and HCs was very low (1%‐2%) (Table 5 and Table S3). The absolute risk of major ADEs and hospitalizations was low (0%–4%), and was similar between IIM patients and HCs (Table 5).
3.8. Post–COVID‐19 vaccination–associated ADEs in patients with IIM compared with other SAIDs
The incidence of injection site pain was similar in IIM patients (63%) and other SAID patients (65%). Although the risk of most minor ADEs was lower in IIM patients than in other SAID patients, the differences observed in uncontrolled univariable analysis did not attain significance after multivariable analysis with baseline adjustment. Regardless, the absolute risk in both IIM and SAID patients was very low (0.6%‐1%) (Table 4 and Table S3). Major ADEs and hospitalizations were rare in IIM and other SAID patients, with a low absolute risk (0%‐4%) and no significant differences between the two groups (Table 5).
4. DISCUSSION
Overall, COVID‐19 vaccination is safe in patients with IIMs and other SAIDs, and the majority of minor vaccine ADEs are easily manageable. COVID‐19 vaccination in DM may lead to a mild increase in some minor ADEs, mainly rash, without increasing either major ADEs or hospitalization rate. This could be due to flares of cutaneous disease postvaccination. Those with active disease before vaccination reported higher minor ADE, major ADE, and any ADE overall. Considering the potentially severe consequences of SARS‐CoV‐2 infection, our study adds to the growing body of evidence indicating that the benefit of preventing severe COVID‐19 through vaccination in SAIDs, especially in IIMs, likely outweighs the risk of postvaccination ADEs, and thus supports guidance statements by the American College of Rheumatology that encourage COVID‐19 vaccination in patients with rheumatic diseases. 19 Our results provide insights that may inform future guidelines regarding COVID‐19 vaccination in patients with SAIDs, specifically in IIM.
Although large‐scale studies regarding COVID‐19 vaccination safety in autoimmune diseases are lacking, the safety data gleaned from general population and small studies of immunocompromised patients are reassuring. In a small, single‐center cohort, Geisen et al demonstrated the safety and efficacy of SARS‐CoV‐2 mRNA vaccines, showing no considerable side effects. 20 Regarding IIM patients, the data thus far is even more limited. 21
Among IIM patients, those with active disease reported higher minor, major, and overall ADEs after vaccination. This is due to the cycle of autoimmunity triggering reactions and vice versa. Similar results have been demonstrated in other autoimmune diseases. 7 Among IIM subgroups, minor ADEs appear to be increased in DM and less frequent in IBM. However, it is important to note that subset analyses by vaccine type were limited by small numbers, preventing firm conclusions. Specifically, few respondents had major postvaccine ADEs and hospitalizations. The absolute risk in IIMs, SAIDs, and HCs, as well as in different IIM subtypes, was very small (<2% in most cases) across vaccine types. It is well known that skin rash in DM patients may be exacerbated by environmental insults, 22 and hence plausible that COVID‐19 vaccination could also induce a flare of pre‐existing rashes in these patients. Although the pathogenesis of IBM remains poorly understood, it has been shown to be an interplay between an autoimmune and degenerative disorder, yet antibody against cN‐1A (NT5‐c1A) has been identified. 23 Autoimmunity is thought to be an important part of IBM pathogenesis, but other factors appear to be at play, as manifest by the prominent degenerative features and mitochondrial dysfunction on muscle biopsy analysis. This may provide a possible explanation for the differences in minor ADEs between IBM and DM. The other possibility is that DM and overlap myositis patients are at an increased risk of rashes inherent in the disease phenotype, accounting for reporting bias in this patient‐reported e‐survey. Patients with active disease normally have rashes, and these may be misconstrued as vaccine ADEs. Whether this rash worsened was unfortunately not specifically queried by the survey. Notably, seven patients had inactive disease, and later developed rashes. Even if it is true that the possibility of a postvaccine flare cannot be substantiated, long‐term studies analyzing patients’ physical function and other organ involvement may provide further insight into the possibility of disease flares, as data at 7 days are insufficient to substantiate these speculations.
With respect to vaccine type, our data suggest that IIM patients have lower ADEs with BNT162b2 (Pfizer), ChadOx1 nCOV‐19 (Oxford/AstraZeneca), and MRNA‐1273 (Moderna) vaccines, when compared with other autoimmune disease patients and HCs. Within the IIM cohort, patients receiving the BNT162b2 vaccine were more protected from most minor ADEs when compared with MRNA‐1273, ChadOx1 nCOV‐19, and ChAdOx1 nCoV‐19 recipients. To explain these differences, several aspects need to be considered: different criteria, depending on the country or region for approval of vaccination; different treatment when vaccination took place; and different post‐manufacturing processes. The type of vaccine and adjuvants present, their interaction with the underlying immune dysfunction, and the interplay with the immunosuppressive medications taken by most of these patients may affect the efficacy and safety of these vaccines in patients with IIM and other SAIDs. Further studies are required to ascertain the safety profile of the various vaccines.
This study has some limitations. Our data are based on patient self‐reported information, which could not be verified by medical records. Our population also represented a convenience sample, where low‐income patients without internet access, those with severe disability, and those deceased were not represented. People of African and African‐American ethnicity were underrepresented in the cohort. Furthermore, we did not obtain information on the treatment required for the hospitalized patients after vaccination ADEs. Patients receiving immunosuppressive and biological drugs may have impaired humoral responses, although the role of clinical significance of this altered immune response is not yet clearly understood. 23 Furthermore, severe ADEs were rarely observed. Thus, limited events in each subgroup, such as major ADEs and hospitalization, would make it difficult to find any statistically significant correlations. Finally, the survey used in our study focused on short‐term ADEs, and long‐term outcomes and disease flares were not assessed.
5. CONCLUSION
In this study we have shown that COVID‐19 vaccination has a favorable short‐term safety profile in IIM patients as in healthy individuals and those with other SAIDs. Marginally higher ADRs, such as rashes, may have been related to disease phenotype and did not lead to an increased hospitalization rate. Those with active disease before vaccination reported higher ADEs. Among IIM patients, the DM patients may have been predisposed to specific ADEs, whereas those with IBM subgroup had fewer reported ADEs. Studies on long‐term outcomes and disease flares are needed to shed more light on developing future COVID‐19 vaccination guidelines.
ROLE OF THE FUNDING SOURCE
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
DECLARATIONS
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
ETHICAL APPROVAL STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ethical approval was obtained from the Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Raebareli Road, Lucknow, 226,014.
AUTHOR CONTRIBUTIONS
Conceptualization: A.G.V., N.R., P.S., A.N., A.S.O., L.G., V.A., and R.A.; data curation: all authors; formal analysis: A.G.V., N.R., L.G., and R.A.; investigation: A.G.V., N.R., A.S.O., P.S., L.G., and R.A.; methodology: A.G.V., N.R., P.S., L.G., J.B.L., H.C., and R.A.; software: L.G.; validation: V.A., R.A., J.B.L., H.C., and L.G.; visualization: R.A., V.A., and L.G.; writing of original draft: A.G.V., N.R., P.S., A.N., A.S.O., L.G., and R.A.; writing, review, and editing: all authors.
Disclaimer: No part of this manuscript is copied or published elsewhere in whole or in part.
CONFLICTS OF INTEREST
J.D. has received research funding from CSL, Ltd. A.L.T. has received honoraria for advisory boards and speaking for AbbVie, Gilead, Janssen, Lilly, Novartis, BNT162b2 (Pfizer), and UCB. E.N. has received speaker honoraria or participated on advisory boards for Celltrion, BNT162b2 (Pfizer), Sanofi, Gilead, Galapagos, AbbVie, and Lilly, and holds research grants from BNT162b2 (Pfizer) and Lilly. H.C. has received grant support from Eli Lilly and UCB, consulting fees from Novartis, Eli Lilly, Orphazyme, and AstraZeneca, and speaker fees from UCB and Biogen. I.P. has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, and Hoffmann‐La Roche. N.Z. has received speaker fees, advisory board fees, and research grants from BNT162b2 (Pfizer), Roche, AbbVie, Eli Lilly, NewBridge, Sanofi‐Aventis, Boehringer Ingelheim, Janssen, and Pierre Fabre (none related to this work). O.D. has/had consultancy relationship with and/or has received research funding from or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the past 3 years: AbbVie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, Baecon, Blade, Bayer, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, Novartis, Roche, Roivant, Sanofi, Serodapharm, Topadur, and UCB. O.D. has also issued a patent “mir‐29 for the Treatment of Systemic Sclerosis” (US8247389, EP2331143). R.A. has/had a consultancy relationship with and/or has received research funding from the following companies: Bristol Myers‐Squibb, BNT162b2 (Pfizer), Genentech, Octapharma, CSL Behring, Mallinckrodt, AstraZeneca, Corbus, Kezar, AbbVie, Janssen, Alexion, Argenx, Q32, EMD‐Serono, Boehringer Ingelheim, and Roivant. The remaining authors declare no conflicts of interest.
Supporting information
APPENDIX S1 Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to all respondents for completing their questionnaires. We also thank the Myositis Association, Myositis India, Myositis UK, Myositis Support and Understanding, the Myositis Global Network, Deutsche Gesellschaft für Muskelkranke e.V. (DGM), Dutch and Swedish Myositis patient support groups, Cure JM, Cure IBM, Sjögren's India Foundation, Patients Engage, Scleroderma India, Lupus UK, Lupus Sweden, the Emirates Arthritis Foundation, EULAR PARE, the ArLAR research group, the AAAA patient group, the APLAR myositis special interest group, the Thai Rheumatism Association, PANLAR, NRAS, the Anti‐Synthetase Syndrome support group, and various other patient support groups and organizations for their contributions in dissemination of this survey. Finally, we thank all members of the COVAD Study Group for their invaluable role in data collection. COVAD Study Group: Bhupen Barman, Yogesh Preet Singh, Rajiv Ranjan, Avinash Jain, Sapan C Pandya, Rakesh Kumar Pilania, Aman Sharma, Manesh Manoj M, Vikas Gupta, Chengappa G Kavadichanda, Pradeepta Sekhar Patro, Sajal Ajmani, Sanat Phatak, Rudra Prosad Goswami, Abhra Chandra Chowdhury, Ashish Jacob Mathew, Padnamabha Shenoy, Ajay Asranna, Keerthi Talari Bommakanti, Anuj Shukla, Arunkumar R Pande, Kunal Chandwar, Döndü Üsküdar Cansu, John D Pauling, Chris Wincup, Ashima Makol, Nicoletta Del Papa, Gianluca Sambataro, Atzeni Fabiola, Marcello Govoni, Simone Parisi, Elena Bartoloni Bocci, Gian Domenico Sebastiani, Enrico Fusaro, Marco Sebastiani, Luca Quartuccio, Franco Franceschini, Pier Paolo Sainaghi, Giovanni Orsolini, Rossella De Angelis, Maria Giovanna Danielli, Vincenzo Venerito, Lisa S Traboco, Suryo Anggoro Kusumo Wibowo, Jorge Rojas Serrano, Ignacio García‐De La Torre, Erick Adrian Zamora Tehozol, Jesús Loarce‐Martos, Sergio Prieto‐González, Akira Yoshida, Ran Nakashima, Shinji Sato, Naoki Kimura, Yuko Kaneko, Stylianos Tomaras, Margarita Aleksandrovna Gromova, Or Aharonov, Ihsane Hmamouchi, Leonardo Santos Hoff, Margherita Giannini, François Maurier, Julien Campagne, Alain Meyer, Melinda Nagy‐Vincze, Daman Langguth, Vidya Limaye, Merrilee Needham, Nilesh Srivastav, Marie Hudson, Océane Landon‐Cardinal, Syahrul Sazliyana Shaharir, Wilmer Gerardo Rojas Zuleta, José António Pereira Silva, João Eurico Fonseca, Olena Zimba.
Gil‐Vila A, Ravichandran N, Selva‐O'Callaghan A, et al. COVID‐19 Vaccination in Autoimmune Diseases (COVAD) study: Vaccine safety in idiopathic inflammatory myopathies. Muscle & Nerve. 2022;66(4):426‐437. doi: 10.1002/mus.27681
Albert Gil‐Vila and Parikshit Sen contributed equally to this article.
Rohit Aggarwal and Latika Gupta contributed equally to this article.
Funding information National Institution for Health Research Manchester Biomedical Research Centre (to H.C.).
DATA AVAILABILITY STATEMENT
All data is available as a manuscript in the supplementary material. Additional data is available on request.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tariq J, Gupta L. Safety and efficacy of COVID‐19 vaccines in pregnant women with rheumatic diseases: an immunologic perspective. Rheumatol Int. 2021;41:1545‐1547. doi: 10.1007/s00296-021-04918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wack S, Patton T, Ferris LK. COVID‐19 vaccine safety and efficacy in patients with immune‐mediated inflammatory disease: review of available evidence. J Am Acad Dermatol. 2021;85:1274‐1284. doi: 10.1016/j.jaad.2021.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID‐19 vaccination in adults with systemic rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7:e001814. doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medeiros‐Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744‐1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 6. Boekel L, Kummer LY, van Dam KPJ, et al. Adverse events after first COVID‐19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3:e542‐e545. doi: 10.1016/S2665-9913(21)00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pakhchanian H, Saud A, Raiker R, Kardes S, Aggarwal R, Gupta L. COVID‐19 vaccination outcomes among patients with dermatomyositis: a multicentered analysis. Clin Rheumatol. 2022;41:2257‐2260. doi: 10.1007/s10067-022-06081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan Y, Geng Y, Wang Y, et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: a large real‐world survey on inactivated COVID‐19 vaccines. Ann Rheum Dis. 2022;81:443‐445. doi: 10.1136/annrheumdis-2021-221736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS‐CoV‐2. Ann Rheum Dis. 2021;80(3):e42. [DOI] [PubMed]
- 10. Saud A, Naveen R, Aggarwal R, Gupta L. COVID‐19 and myositis: what we know so far. Curr Rheumatol Rep. 2021;23:63. doi: 10.1007/s11926-021-01023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng MP, Kozoriz MG, Ahmadi AA, Kelsall J, Paquette K, Onrot JM. Post‐vaccination myositis and myocarditis in a previously healthy male. Allergy Asthma Clin Immunol. 2016;12:6. doi: 10.1186/s13223-016-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watad A, De Marco G, Mahajna H, et al. Immune‐mediated disease flares or new‐onset disease in 27 subjects following mRNA/DNA SARS‐CoV‐2 vaccination. Vaccine. 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sen P, Lilleker JB, Agarwal V, et al. Vaccine hesitancy in patients with autoimmune diseases‐ data from the COVID‐19 vaccination in autoimmune diseases (COVAD) study. Ind . J Rheumatol. 2021;17:188‐191. doi: 10.4103/injr.injr_221_21 [DOI] [Google Scholar]
- 14. Khan H, Gasparyan AY, Gupta L. Lessons learned from publicizing and retracting an erroneous hypothesis on the mumps, measles, rubella (MMR) vaccination with unethical implications. J Korean Med Sci. 2021;36:e126. doi: 10.3346/jkms.2021.36.e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sen P, Gupta L, Lilleker JB, et al. COVID‐19 vaccination in autoimmune disease (COVAD) survey protocol. Rheumatol Int. 2022;42(1):23‐29. doi: 10.1007/s00296-021-05046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E‐surveys (CHERRIES). J Med Internet Res. 2004;6:e132. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaur PS, Zimba O, Agarwal V, Gupta L. Reporting survey based studies‐‐‐a primer for authors. J Korean Med Sci. 2020;35:e398. doi: 10.3346/jkms.2020.35.e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cella D, Yount S, Rothrock N, et al. PROMIS Cooperative Group. The patient‐reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3‐S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed]
- 19. US Centers for Disease Control and Prevention. Understanding adverse events and side effects. Vaccine safety. 2021. https://www.cdc.gov/vaccinesafety/ensuringsafety/sideeffects/index.html. Accessed January 7, 2022.
- 20. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti‐SARS‐CoV‐2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306‐1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shinjo SK, de Souza FHC, Borges IBP, et al. Systemic autoimmune myopathies: A prospective phase 4 controlled trial of an inactivated virus vaccine against SARS‐CoV‐2. Rheumatology (Oxford). 2022;61(8):3351‐3361. doi: 10.1093/rheumatology/keab773. [DOI] [PMC free article] [PubMed]
- 22. Mamyrova G, Rider LG, Ehrlich A, et al. Environmental factors associated with disease flare in juvenile and adult dermatomyositis. Rheumatology (Oxford). 2017;56:1342‐1347. doi: 10.1093/rheumatology/kex162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnold J, Winthrop K, Emery P. COVID‐19 vaccination and antirheumatic therapy. Rheumatology (Oxford). 2021;60:3496‐3502. doi: 10.1093/rheumatology/keab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1 Supporting Information
Data Availability Statement
All data is available as a manuscript in the supplementary material. Additional data is available on request.


