Abstract
Multiple myeloma (MM) and chronic lymphocytic leukemia (CLL) patients have increased morbidity and mortality rates of COVID‐19 due to immunosuppression associated with the disease and ongoing therapy. The same immune impairment accompanying CLL and MM also affects suboptimal vaccine response. The study assessed the effectiveness of the humoral and T cell‐mediated immunity following mRNA COVID‐19 vaccination (using either BNT162b2 or mRNA‐1273) in short‐term (2‐5 weeks after second dose) and long‐term follow‐up (12 weeks after vaccination). Between March and August 2021, blood samples were obtained from 62 CLL and 60 MM patients from eight different hematology departments in Poland. Total anti‐RBD antibodies were detected in 37% MM patients before vaccination, increased to 91% and 94% in short‐ and long‐term follow‐up, respectively. In CLL, serological responses were detectable in 21% of patients before vaccination and increased to 45% in the short‐term and 71% in long‐term observation. We detected a tendency to higher frequencies of specific CD8+ T cells against SARS‐CoV‐2 after vaccination compared to samples before vaccination in MM patients and no changes in frequencies of specific T cells in CLL patients. Our study provides novel insights into mRNA vaccination efficacy in immunocompromised MM and CLL patients, and our findings highlight that specific CD8+ T cells against SARS‐CoV‐2 might be induced by vaccination but do not correlate positively with serological responses.
Keywords: CLL, COVID‐19, MM, mRNA vaccine, SARS‐CoV‐2
What's new?
Multiple myeloma (MM) and chronic lymphocytic leukemia (CLL) patients are at high risk of life‐threatening complications associated with severe coronavirus disease 2019 (COVID‐19). MM and CLL patients further experience suboptimal immune responses against COVID‐19 vaccines. Our study shows that while serological responses to COVID‐19 vaccination are detectable in MM and CLL patients, the responses are not correlated with T cell‐mediated immunity. In particular, whereas a specific CD8+ T cell response against SARS‐CoV‐2 occurred following vaccination in MM patients, frequencies of specific T cells were unaltered in CLL patients. Hence, especially for CLL patients, sequential COVID‐19 immunization could prove invaluable.
Abbreviations
- BCMA
B‐cell maturation antigen
- CM
central memory
- ELISA
enzyme‐linked immunosorbent assay
- EM
effector memory
- FMOC
fluorescence minus one control
- HLA
human leukocyte antigen
- ISS
International Staging System
- MdFI
median fluorescence intensity
- MHC
major histocompatibility complex
- OD
optical density
- PBMCs
peripheral blood mononuclear cells
- RBD
receptor‐binding domain
- TCR
T cell receptor
- TEM
terminally differentiated effector memory
1. INTRODUCTION
The ongoing COVID‐19 pandemic is caused by the novel coronavirus—severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 The mortality rate of over 30% is observed among patients with lymphoproliferative diseases, including patients with multiple myeloma (MM) and patients with chronic lymphocytic leukemia (CLL). 2 , 3 , 4 , 5 , 6 Such a high rate of death in MM and CLL is associated with hypogammaglobulinemia, a reduced percentage and responses of neutrophils, T and B cells, and as well as immunosuppression associated with the ongoing therapy. 7 , 8 MM or CLL patients are particularly at risk of life‐threatening pneumonia and multiorgan failure associated with severe COVID‐19 infection. Moreover, a prolonged course of disease observed in immunocompromised patients favors creating viral reservoirs and the development of new virus mutations. The same immune impairment accompanying CLL and MM also affects a suboptimal vaccine response as exemplified by the low levels of antibodies after immunization by other vaccines. 9 The analyses of the effectiveness of the postvaccination response may lead to the optimization of vaccination. Notably, there are currently insufficient studies on the generation of short‐term and, above all, long‐term T‐cell immunity in these groups of patients.
2. MATERIALS AND METHODS
2.1. Patient samples
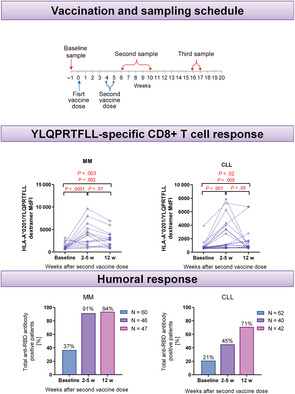
The study assessed the humoral and T cell‐mediated immunity following mRNA COVID‐19 vaccination using BNT162b2 or mRNA‐1273 in 60 MM as well as 62 CLL patients. Blood samples were collected at three time points: before vaccination, 2‐5 weeks after the second dose (short‐term follow‐up), and 12 weeks after the second dose of vaccine (long‐term follow‐up) from eight hematology departments in Poland.
2.2. Sample preparation
Peripheral blood mononuclear cells (PBMCs) were isolated by Biocoll (Biochrom) density gradient centrifugation. Cells were cryopreserved in RPMI 1640 medium (Biochrom) containing 20% fetal bovine serum (Biochrom) and 10% dimethyl sulfoxide (Sigma Aldrich), and stored in liquid nitrogen until the time of further analyses. Serum samples were collected by centrifuging at 2000 rpm for 10 min and immediately stored at −80°C.
2.3. Assessment of serological response
To evaluate serological response in prevaccination and postvaccination serum samples, total antibody SARS‐CoV‐2, receptor‐binding domain (RBD) test (Mabtech) according to manufacturer protocol was performed. 10 All diluted serums samples were screened for anti‐SARS‐CoV‐2 spike RBD antibodies (IgM, IgG and IgA). Positive samples cut‐off was set to 0.151 optical density (OD) value.
2.4. Assessment of T‐cell response
Specific cytotoxic T‐cell response after mRNA vaccination was evaluated in the HLA‐A*02‐positive CLL and MM samples. One million PBMCs were analyzed by flow cytometry using MHC‐dextramer staining with the most common and with the highest binding score SARS‐CoV‐2 specific epitope—HLA‐A*0201/YLQPRTFLL269‐277 (YLQ, Immudex), that is included in all vaccines sequence 11 (see Supplementary Materials for details).
2.5. Statistical analysis
Statistical analyses were performed using Graph Pad Prism_9 (La Jolla, Graph Pad Software). To evaluate the differences in values before and after vaccination, Wilcoxon matched‐pairs signed‐rank test was used. The Mann‐Whitney U‐test and Kruskal‐Wallis test were performed to evaluate the differences between the subgroups of CLL and MM patients. The correlations of variables were computed with the Spearman rank correlation coefficient. Results were presented as median values. Statistical significance was defined as P value of less than .05. Percentages of specific T cells subpopulations and median fluorescence intensities (MdFI) were described as medians.
3. RESULTS
3.1. Patient characteristics
Patients were vaccinated with two doses 3 weeks apart (±1 week) and compared to matched samples before vaccination (baseline). Ten CLL patients (16%) were untreated, 33 patients (55%) had received anti‐CLL therapy (15 patients—anti‐CD20 mAb) and 16 patients (26%) were in remission. Thirty‐nine MM patients (65%) received antimyeloma treatment, 9 MM patients (15%) were in remission, and 2 patients (3%) were untreated. Patient characteristics are summarized in Table 1, Supplementary Tables 1 and 2.
TABLE 1.
Patient characteristics
| Overall (n, %) | Multiple myeloma (n, %) | Chronic lymphocytic leukemia (n, %) | |
|---|---|---|---|
| Patients (n) | 122 | 60 | 62 |
| Gender | |||
| Female | 57 (47%) | 30 (50%) | 27 (44%) |
| Male | 65 (53%) | 30 (50%) | 35 (56%) |
| Median age (range), y | 64 (35‐81) | 64 (35‐81) | 64 (43‐81) |
| mRNA vaccine | |||
| BNT162b2 | 114 (93%) | 56 (93%) | 58 (94%) |
| mRNA‐1273 | 8 (7%) | 4 (7%) | 4 (6%) |
| ISS stage system for MM | |||
| ISS I | 30 (50%) | ||
| ISS II | 16 (27%) | ||
| ISS III | 8 (13%) | ||
| No data | 8 (13%) | ||
| Binet staging system for CLL | |||
| Binet A | 22 (35%) | ||
| Binet B | 26 (42%) | ||
| Binet C | 13 (21%) | ||
| No data | 1 (2%) | ||
| Disease status | |||
| Untreated | 12 (10%) | 2 (3%) | 10 (16%) |
| In treatment | 72 (59%) | 39 (65%) | 33 (55%) |
| In remission | 25 (20%) | 9 (15%) | 16 (26%) |
| No data | 13 (11%) | 10 (17%) | 3 (5%) |
| Steroids | 28 (23%) | 25 (42%) | 3 (5%) |
| Antibiotics | 12 (10%) | 4 (7%) | 8 (13%) |
| NSAID | 8 (7%) | 6 (10%) | 2 (3%) |
| Frequent infections | 15 (12%) | 5 (8%) | 10 (16%) |
| Anti‐SARS‐CoV‐2 antibodies at baseline | 35 (29%) | 22 (37%) | 13 (21%) |
| HLA‐A*02 positivity | 42 (34%) | 17 (28%) | 25 (40%) |
Abbreviations: ISS, International Staging System; NSAID, nonsteroidal antiinflammatory drugs.
3.2. Multiple myeloma patients
Total anti‐RBD antibodies were detected in 22/60 (37%) MM patients before vaccination. Following vaccination, this rate increased to 42/46 (91%) 2‐5 weeks after the second dose, which then remained stable with 44/47 (94%) positive patients 12 weeks after the second dose (Figure 1A). However, OD values were significantly higher 12 weeks after the second dose compared to 2‐5 weeks (median: 3.78 vs 1.54, P < .001).
FIGURE 1.
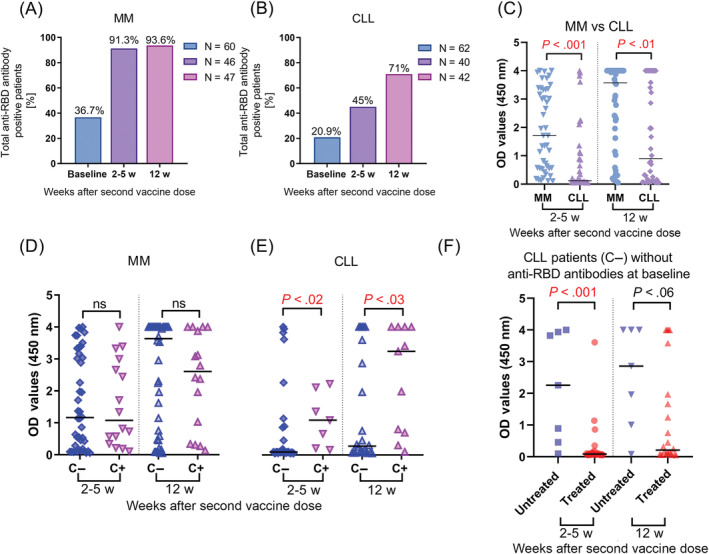
Total anti‐RBD anti‐SARS‐CoV‐2 spike receptor‐binding domain antibodies responses after mRNA vaccination. (A) Anti‐RBD antibody response were measured prior mRNA vaccination as a baseline, next 2‐5 weeks after second and 12 weeks second dose in multiple myeloma (MM) patients and (B) chronic lymphocytic leukemia (CLL). (C) Significantly higher response rates in MM patients in comparison with CLL measured 2‐5 weeks after second as well as 12 weeks after second dose evaluated by ELISA. (D) Differences in OD values between patients with the presence of anti‐RBD antibodies before vaccination (C+) and without (C−) in MM and CLL (E) in two‐time points after second vaccine dose. (F) Effect of antileukemic treatment in CLL patients without anti‐RBD antibodies before vaccination on antibody response, data for MM not showed due to an insufficient number of untreated patients. OD values were obtained with absorbance measured at 450 nm. The nonparametric two‐sided Mann‐Whitney U‐test was performed for pairwise comparisons of distributions between groups, P < .05 was considered as statistically significant [Color figure can be viewed at wileyonlinelibrary.com]
We compared OD values due to severity of the disease evaluated by the International Staging System (ISS) and we found higher OD values shortly after the second vaccine dose in MM patients in ISS stage I compared to the ISS III stage (median: 1.83 vs 0.56, P < .03) and no significant differences according to other stages 12 weeks after second dose. Age, gender, and history of infections were not related to the antibody response rate in MM patients. After vaccination, there were no significant differences in OD values between MM patients previously exposed to SARS‐CoV‐2 or not (Figure 1D). Next, we analyzed serological response according to administered therapy and found no differences in antibody levels in patients treated with steroids. Interestingly, we found a tendency to lower OD values in MM patients treated with antibiotics during vaccination in comparison with untreated in a short‐time observation after vaccine (median: 0.34 vs 1.719, P < .06), without differences in the long‐term.
It is previously reported, that the presence of CD8+ T cells was critical for hematological malignancies COVID‐19 patients survival even without existing antibodies. 12 In our study, we used flow cytometry analyses to evaluate frequencies of specific cytotoxic T cells directed against YLQPRTFLL269‐277—SARS‐CoV‐2 Spike epitope (Figure 2C) and we found a tendency to higher frequencies of YLQ‐specific CD8+ T cells in a short time after second dose as compared to baseline (median: 0.18 vs 0.11, P < .06), which might confirm induction of specific CD8+ T cells after vaccination. Moreover, we demonstrated higher MdFI levels of specific TCRs in samples obtained 2‐5 weeks (median: 1925 vs 668, P < .001) as well as after 12 weeks after second dose (median: 1021 vs 668, P < .01) compared to paired baseline samples (Figure 2A). Interestingly, we demonstrated a strong negative correlation between OD values and YLQPRTFLL‐specific CD8+ T cells frequencies that appeared 12 weeks after second dose of vaccine (r = −0.62, P < .03).
FIGURE 2.
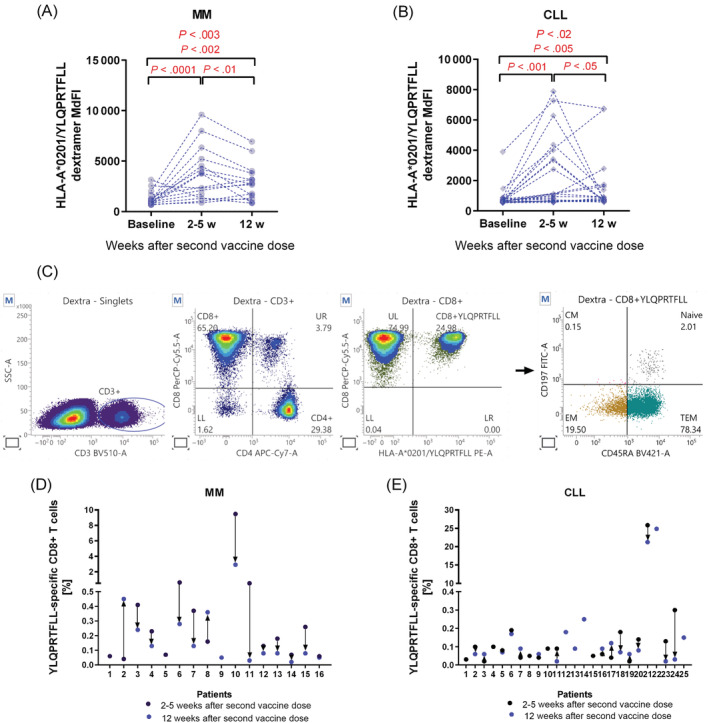
Existence of HLA‐A*0201 restricted YLQPRTFLL‐specific cytotoxic CD8+ T cells in MM and CLL patients. HLA‐A2 positive samples (1‐3 × 106) were incubated with 1 μL of Fixable Viability Stain 700 (BD Biosciences) at room temperature for 15 min. Afterwards, 10 μL of dextramer HLA‐A*0201/YLQPRTFLL‐specific‐PE (Immudex, Copenhagen, Denmark) conjugated complex was added to cells and incubated at room temperature for 10 min. Next, anti‐CD3 BV510, anti‐CD4 APC‐Cy7, anti‐CD8 Percp‐Cy 5.5, anti‐CD45RA BV421, anti‐CCR7 FITC, anti‐CD27 APC and anti‐CD28 Pe‐Cy7 (all BD Biosciences) were added to cells. Cells were analyzed by FACSuite on BD FACS Lyric Flow Cytometer (BD Biosciences, CA, USA). Increase in MdFI levels 2‐5 weeks after second vaccine dose and decrease 12 weeks after second dose in MM (A) and CLL (B) were observed. Statistical comparisons were performed using the Wilcoxon matched‐pairs signed‐rank test and the one‐way analysis of variance (ANOVA). (C) Results were compared to isotypic controls and fluorescence minus one control (FMOC), with the exclusion of dead cells to characterize YLQPRTFLL‐specific CD8+ naïve (CD45RA+CCR7+), central memory—CM (CD45RA‐CCR7+), effector memory—EM (CD45RA‐CCR7−) and terminally differentiated effector memory—TEM (CD45RA+CCR7−) phenotype of specific cytotoxic T‐cells. Distribution of frequencies of YQL‐specific CD8+ T cells in all HLA‐A*0201 positive MM (D) and CLL patients (E) in two‐time points after second vaccine dose [Color figure can be viewed at wileyonlinelibrary.com]
Flow cytometry analysis of specific cytotoxic T cells subpopulations revealed an increase of effector memory (EM) T cell frequencies after 2‐5 weeks after second vaccine dose‐related to paired baseline samples (median: 43% vs 33%, P < .02), and then the decrease after 12 weeks after second dose (median: 47% vs 36%, P < .03). Noteworthy, we observed increased frequencies of terminally‐differentiated effector memory (TEM) T cells after 12 weeks postvaccination compared to paired samples obtained 2‐5 weeks after second dose (median: 46% vs 37%, P < .04). A strong negative correlation between serological response reflected by OD values and YLQPRTFLL‐specific CD8+ naïve T cells 12 weeks after second dose of vaccine was observed (r = −0.63, P < .02).
3.3. Chronic lymphocytic leukemia patients
In the CLL cohort, total antibody responses were detectable in 13/62 (21%) of patients before vaccination, and numbers increased to 18/40 (45%) 2‐5 weeks after the second dose with an additional increase to 30/42 (71%) 12 weeks the after second dose (Figure 1B). We observed a significant increase of total antibody response measured 12 and 2‐5 weeks after the second dose in comparison with paired baseline samples (median: 0.90 vs 0.09; P < .001 and 0.76 vs 0.16; P < .01, respectively). Similarly, in the cohort without antibodies at baseline samples, we observed a significant increase of OD values 12 weeks after second dose in comparison with paired baseline samples (median: 0.45 vs 0.09, P < .001, Figure 1E).
The stage of the disease according to Binet classification, gender and age were not related with the level of humoral response measured in two‐time points after the second vaccine dose. However, COVID‐19 naïve patients treated for CLL demonstrated significantly lower OD values early after the second dose (median: 0.08 vs 2.2, P < .001) as well as in long‐term follow‐up (median: 0.21 vs 2.86, P < .05) than untreated COVID‐19 naïve patients (Figure 1F). Moreover, we analyzed results according to the type of anticancer treatment and found no differences between them in short‐term nor long‐term serological responses. mRNA COVID‐19 vaccines showed significantly higher efficacy in CLL patients exposed to SARS‐CoV‐2 before vaccination compared to patients without exposure 2‐5 weeks after second dose (median: 1.09 vs 0.09, P < .02) as well as 12 weeks after second dose (median: 3.23 vs 0.28, P < .03).
In 27 CLL patients, we were able to look for specific T‐cell responses. Four of these patients had detectable anti‐SARS‐CoV‐2 antibodies at baseline. We did not find any differences between frequencies of YLQPRTFLL‐specific CD8+ T cells in short‐term nor long‐term follow‐up after second dose compared to baseline samples in paired and grouped analyses. Interestingly, we observed higher MdFI levels of specific TCRs after 2‐5 weeks compared to 12 weeks after second vaccine dose in paired samples (median: 1084 vs 750, P < .04, Figure 2B).
We found a tendency to higher frequencies of YLQPRTFLL‐specific CD8+ T cells in the 4 SARS‐CoV‐2 exposed patients 12 weeks after second dose compared to those without antibodies (median: 0.18% vs 0.07%, P < .08) and respectively significantly higher MdFI levels of specific TCRs (median: 2777 vs 747, P < .05). There were no correlations between frequencies of YLQPRTFLL‐specific CD8+ T cells with antibody response rates in short‐term as well as long‐term follow‐up after vaccination. However, a weak positive correlation between MdFI and OD values 2‐5 weeks after second dose (r = 0.46, P < .05) was observed.
Higher EM frequencies were observed short‐time after second dose compared to baseline samples (median: 34.93% vs 26.32%, P < .05) and significantly decreased after 12 weeks after second dose (median: 34.93% vs 25.17%, P < .04). Individual results for MM and CLL patients are displayed in Figure 2D,E. Analyses of the subpopulation of YLQPRTFLL‐specific T cells revealed significantly lower frequencies of CM cells in patients previously exposed for SARS‐CoV‐2 compared to no exposure after 12 weeks after second dose (median: 1.32% vs 5.32%, P < .02). Age, gender, and stage of the disease were not related to the changes of CD8+ T cells populations in CLL samples.
3.4. Comparison of immune responses in CLL and MM
Interestingly, a comparison between the two chronic lymphoid malignancies MM and CLL with different mechanisms of immune deregulation showed a statistically significant increase in OD values in MM samples after first dose (median: 1.71 vs 0.12, P < .0001) and after second dose (median: 3.57 vs 0.90, P < .005) (Figure 1C). Similar to serological responses the frequency of specific CD8+ T cells was significantly higher in MM 2‐5 weeks after the second dose compared to CLL samples (median: 0.18% vs 0.09%, P < .03) at the same time, without differences in long‐term follow‐up samples.
4. DISCUSSION
Consistent with other studies concerning serological responses to anti‐SARS‐CoV‐2 in CLL and MM patients, we confirm mRNA vaccines are effective in inducing antibody production and boosting anti‐RBD antibody levels that persist for at least 3 months after the second dose in both diseases. 2 , 5 , 9 , 13 , 14 , 15 In MM, we detected high frequencies of serological responses: 91% in short‐term and 94% in a long‐term follow‐up that were higher compared to previous reports. 16 , 17 Higher results might be associated with the selection of more accurate tests or a high number of patients immunized before vaccination (37%).
In our study, most MM patients were in ongoing treatment (96%), enabling them to perform tests comparing patients according to treatment status. However, such high response rates in patients receiving antimyeloma therapy provide evidence for the high efficacy of mRNA‐based vaccines. We did not find any differences in seroconversion rates between groups divided by treatment agents, probably because of the high diversity in our group. Recent studies demonstrated significantly lower antibody levels in MM patients receiving anti‐myeloma therapy, particularly in patients treated with anti‐CD38 or BCMA targeted therapy. 17 , 18 Stampfer et al 19 reported lower antibody levels in patients who received steroids but we did not observe this association in our MM cohort. Antibody responses in CLL cohort were 41% shortly after second dose and increased to 71% after 12 weeks from second vaccine shot. The seroconversion rate in the CLL cohort was below this reported by Herishanu et al, 2 but equal with those observed by Chung et al 19 and Parry et al, 18 , 20 similarly with no correlations with age, disease stage, or sex. In line with other observations, CLL patients, who were not exposed to SARS‐CoV‐2, but received antileukemic treatment have impaired anti‐SARS‐CoV‐2 production. 13 , 21
In our study, we observed high frequencies of previously exposed CLL as well as MM patients which confirms high exposure to SARS‐CoV‐2 during the third wave of COVID‐19 in spring 2021 in Poland. Similar results were published for other patients with hematological malignancies in Poland as well as in the general population of 500 cases analyzed at a time corresponding to our experiment. 22 , 23 Budziar et al 23 identified 35.5% of IgG positive healthy volunteers who had never been diagnosed with COVID‐19 nor vaccinated against SARS‐CoV‐2 and 52.3% positive for IgA‐SARS‐CoV‐2‐specific without any symptoms of the disease.
T‐cell response against SARS‐CoV‐2 is a key factor in reducing mortality from COVID‐19 in hematological patients. 12 For the first time, we demonstrated anti‐SARS‐CoV‐2 specific cytotoxic T cells to assess cellular response after mRNA vaccines in patients with hematologic malignancies using flow cytometry technique. We found CD8+ T‐cell immune responses against the immunodominant SARS‐CoV‐2 spike epitope (YLQPRTFLL) in all HLA‐A*02 positive MM (n = 17) and CLL (n = 25) patients.
Interestingly, we discovered a strong negative correlation between YLQPRTFLL‐specific CD8+ cells and serological response in long‐term follow‐up. This might be explained by shorter half‐time of T‐cell responses or the fact that serological immune response might be skewed by ongoing antimyeloma treatment. 24 This phenomenon was limited to MM while in CLL we did not find significant differences between the frequencies of specific CD8+ T cells at any time‐point. However, the highest frequencies of YLQPRTFLL‐specific CD8+ cells in CLL, as well as MM patients, were found in those who were exposed to the virus before vaccination compared to those without exposure. Marasco et al 25 detected T‐cell response in 86% of seronegative patients using in vitro ELISA‐based test T‐helper cell type 1‐associated cytokine release in plasma samples of five groups of lymphoid malignancies. Malard et al 15 assessed T‐cell immune response in lymphoid and myeloid hematological malignances using the IFN‐γ ELISPOT assay. They reported a significant increase in T‐cell response against the Spike protein, with 53% of patients having an anti‐S IgG‐positive ELISPOT after the second vaccine dose. Our study confirmed the development of specific T cell‐mediated responses after mRNA vaccines in CLL patients. These data may suggest the occurrence of specific T‐cell response is independent of seroconversion level and treatment status.
We did not observe differences in median YLQPRTFLL‐specific CD8+ frequencies and their subpopulations in CLL patients with ongoing treatment, which in majority comprised anti‐CD20 mAbs, BCL2 antagonists, or BTK inhibitors compared to untreated patients, but results were obtained from small cohort of HLA‐A*02‐restricted patients. Interestingly, in both CLL and MM, we detected an increase in EM‐specific CD8+ T cells shortly after vaccination, then decreased after 12 weeks. It might confirm proliferation boost of EM specific CD8+ T cells after priming with virus peptides. Similarly, immune boost was shown in the study of HV after vaccination with BNT162b2. 26 We observed also higher TEM cell numbers after 12 weeks in MM patients who had generally better responses to vaccination compared to CLL patients. This mechanism mimicked a response on mRNA SARS‐CoV‐2 vaccines in HV.
In summary, we showed for the first‐time acceleration of spike‐specific T cell‐mediated immune response in CLL and MM patients after anti‐SARS‐CoV‐2 mRNA vaccination, which did not correlate positively with serological response in both diseases. The results might indicate the necessity of assessing both serological and T‐cell responses and need for sequential immunization, especially for CLL patients.
AUTHOR CONTRIBUTIONS
Krzysztof Giannopoulos designed the research project; Joanna Zaleska, Paulina Kwasnik, Magdalena Paziewska, Joanna Purkot, Aleksandra Szabelak, Mateusz Jurek, Natalia Masny, Izabela Dziatkiewicz and Bartosz Pronobis‐Szczylik, performed research; Agnieszka Piebiak, Agnieszka Szymczyk, Katarzyna Jarosz‐Chudzik, Lukasz Bolkun, Katarzyna Kozlowska, Jaroslaw Piszcz, Edyta Subocz, Janusz Halka, Michał Bator, Elzbieta Kalicinska, Tomasz Wrobel, Lidia Usnarska‐Zubkiewicz, Justyna Rybka, Izabela Deren‐Wagemann, Marta Szyca‐Smieszniak, Jaroslaw Dybko, Iwona Hus, Bartosz Pula, Edyta Cichocka, Marcin Rymko, Dorota Zdunczyk, Mateusz Ziarkiewicz, Grzegorz Wladyslaw Basak and Krzysztof Giannopoulos recruited the patients and provide clinical data; Joanna Zaleska, Paulina Kwasnik, Magdalena Paziewska, Joanna Purkot, Aleksandra Szabelak and Krzysztof Giannopoulos performed formal analysis; Joanna Zaleska, Paulina Kwasnik, Magdalena Paziewska, Lars Bullinger, and Krzysztof Giannopoulos wrote the manuscript. All the work reported in the paper has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
Our study was supported by research grants from the Medical University of Lublin PBsd 251 and DS 174.
CONFLICT OF INTEREST
Within the last 36 months, Lars Bullinger received honoraria from Seattle Genetics, Sanofi, Astellas, Amgen, consultancy fee from Gilead, Hexal, and Menarini, consultancy fee and Honoraria Abbvie, BMS/Celgene, Daiichi Sankyo, Janssen, Jazz Pharmaceuticals, Novartis and Pfizer, and research funding from Bayer and Jazz Pharmaceuticals, all not linked to the content of this article. Krzysztof Giannopoulos received research funding from Janssen, Abbvie, AstraZeneca, Roche and advisory honoraria from Janssen, Abbvie, AstraZeneca, Roche, Pfeizer, Sandoz. All other authors declare no competing financial interests with regard to our study.
ETHICS STATEMENT
The study was approved by the local Ethics Committee of the Medical University of Lublin (number KE‐0254/42/2021). Informed consent was obtained from each individual.
Supporting information
Appendix S1 Supporting Information.
Zaleska J, Kwasnik P, Paziewska M, et al. Response to anti‐SARS‐CoV‐2 mRNA vaccines in multiple myeloma and chronic lymphocytic leukemia patients. Int J Cancer. 2022;1‐8. doi: 10.1002/ijc.34209
Funding information Medical University of Lublin, Grant/Award Numbers: DS 174, PBsd 251
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available on request from the corresponding author.
REFERENCES
- 1. https://covid19.who.int/; 2021. Accessed December 5, 2021.
- 2. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165‐3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chari A, Samur MK, Martinez‐Lopez J, et al. Clinical features associated with COVID‐19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136:3033‐3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scarfò L, Chatzikonstantinou T, Rigolin GM, et al. COVID‐19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354‐2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig H, Sonneveld P, Facon T, et al. COVID‐19 vaccination in patients with multiple myeloma: a consensus of the European Myeloma Network. Lancet Haematol. 2021;8:e934‐e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573‐581. [DOI] [PubMed] [Google Scholar]
- 8. Guillerey C, Nakamura K, Vuckovic S, Hill GR, Smyth MJ. Immune responses in multiple myeloma: role of the natural immune surveillance and potential of immunotherapies. Cell Mol Life Sci. 2016;73:1569‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjamini O, Rokach L, Itchaki G, et al. Safety and efficacy of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2021;107:625‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Min L, Sun Q. Antibodies and vaccines target RBD of SARS‐CoV‐2. Front Mol Biosci. 2021;8:671633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baruah V, Bose S. Immunoinformatics‐aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019‐nCoV. J Med Virol. 2020;92:495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID‐19 and hematologic cancer. Nat Med. 2021;27:1280‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tadmor T, Benjamini O, Braester A, Rahav G, Rokach L. Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2727‐2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS‐CoV‐2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021;11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bagacean C, Letestu R, Al‐Nawakil C, et al. Humoral response to mRNA anti‐COVID‐19 vaccines BNT162b2 and mRNA‐1273 in patients with chronic lymphocytic leukemia. Blood Adv. 2021;138:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avivi I, Balaban R, Shragai T, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021;195:186‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Oekelen O, Gleason CR, Agte S, et al. Highly variable SARS‐CoV‐2 spike antibody responses to two doses of COVID‐19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39:1028‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chung DJ, Shah GL, Devlin SM, et al. Disease‐ and therapy‐specific impact on humoral immune responses to COVID‐19 vaccination in hematologic malignancies. Blood Cancer Discov. 2021;2:568‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stampfer SD, Goldwater M‐S, Jew S, et al. Response to mRNA vaccination for COVID‐19 among patients with multiple myeloma. Leukemia. 2021;35:3534‐3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid‐19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuneo A, Rigolin GM, Coscia M, et al. Management of chronic lymphocytic leukemia in Italy during a one year of the COVID‐19 pandemic and at the start of the vaccination program. A campus CLL report. Hematol Oncol. 2021;39:570‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Majcherek M, Matkowska‐Kocjan A, Szymczak D, et al. Two doses of BNT162b2 mRNA vaccine in patients after hematopoietic stem cell transplantation: humoral response and serological conversion predictors. Cancers (Basel). 2022;14:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Budziar W, Gembara K, Harhala M, et al. Hidden fraction of Polish population immune to SARS‐CoV‐2 in May 2021. PLoS One. 2022;17:e0253638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noonan K, Rudraraju L, Ferguson A, et al. Lenalidomide‐induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18:1426‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marasco V, Carniti C, Guidetti A, et al. T‐cell immune response after mRNA SARS‐CoV‐2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2021;196:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guerrera G, Picozza M, D'Orso S, et al. BNT162b2 vaccination induces durable SARS‐CoV‐2 specific T cells with a stem cell memory phenotype. Sci Immunol. 2021;6:eabl5344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the findings of our study are available on request from the corresponding author.


