Abstract
Pulmonary thromboembolic events have been linked to coronavirus disease 2019 (COVID‐19), but their incidence and long‐term sequelae remain unclear. We performed a systematic literature review to investigate the incidence of pulmonary embolism (PE), microthrombi, thrombosis in situ (thromboinflammatory disease), and chronic thromboembolic pulmonary hypertension (CTEPH) during and after COVID‐19. PubMed and the World Health Organization Global Research Database were searched on May 7, 2021. Hospital cohort and database studies reporting data for ≥1000 patients and autopsy studies reporting data for ≥20 patients were included. Results were summarized descriptively. We screened 1438 records and included 41 references (32 hospital/database studies and 9 autopsy studies). The hospital/database studies reported the incidence of PE but not CTEPH, microthrombi, or thromboinflammatory disease. PE incidence varied widely (0%–1.1% of outpatients, 0.9%–8.2% of hospitalized patients, and 1.8%–18.9% of patients in intensive care). One study reported PE events occurring within 45 days after hospital discharge (incidence in discharged patients: 0.2%). Segmental arteries were generally the most common location for PE. In autopsy studies, PE, thromboinflammatory disease, and microthrombi were reported in 6%–23%, 43%–100%, and 45%–84% of deceased patients, respectively. Overall, the included studies mostly focused on PE during the acute phase of COVID‐19. The results demonstrate the challenges of identifying and characterizing vascular abnormalities using current protocols (e.g., visual computed tomography reads). Further research is needed to detect subtle pulmonary vascular abnormalities, distinguish thromboinflammatory disease from PE, optimize treatment, and assess the incidence of long‐term sequelae after COVID‐19.
Keywords: incidence, inflammation, pulmonary embolism, pulmonary hypertension, SARS‐CoV‐2
INTRODUCTION
There is considerable evidence of a link between coronavirus disease 2019 (COVID‐19) and thromboembolic events (TE), but the incidence of TE in COVID‐19 remains unclear due to wide‐ranging study results. 1 Pulmonary TE may take the form of in situ thrombosis (due to a thromboinflammatory state in the pulmonary vasculature) rather than acute pulmonary embolism (PE) in many patients, which may have implications for treatment. 2 , 3 Emerging evidence indicates that pulmonary vascular sequelae of COVID‐19 are not restricted to the acute phase of the disease, 4 but the nature and scale of long‐term cardiopulmonary sequelae remain unclear. The true impact of TE on long‐term prognosis in survivors of COVID‐19 remains unknown and is difficult to predict without a clear understanding of the incidence of TE and long‐term sequelae.
We, therefore, performed a systematic literature review (SLR) to investigate the incidence of PE, microthrombi, and thrombosis in situ (thromboinflammatory disease) in large‐scale studies of adult patients with COVID‐19. In addition, evidence was sought describing the rate of clot resolution after COVID‐19‐associated PE and the incidence of chronic thromboembolic pulmonary hypertension (CTEPH) in COVID‐19 survivors.
METHODS
Data sources and search strategy
Electronic database searches were conducted on May 7, 2021 using PubMed and the World Health Organization (WHO) COVID‐19 Global Research Database. The searches were designed to capture studies reporting the incidence of pulmonary TE in patients with COVID‐19. Search terms are described in Supporting Information: Tables 1 and 2.
Screening
Search results were combined, and duplicates were removed using EndNote (Clarivate Analytics). Titles and abstracts were screened by a single reviewer to assess the potential relevance of the study according to prespecified eligibility criteria. For potentially relevant citations, full‐text articles were obtained and reviewed to confirm inclusion in the SLR. Incidence data were extracted into predefined tables by a single reviewer and checked by a second reviewer.
Eligibility criteria
Inclusion and exclusion criteria are described in Supporting Information: Table 3. Both retrospective and prospective studies were included, with data from the control arms of comparative studies of treatment efficacy or effectiveness also considered for inclusion. Uncontrolled studies of interventions not representing standard of care (at the time of the study) were not included.
Size thresholds were used to limit the review to the largest relevant studies. For studies in hospital cohorts and databases, a sample size of at least 1000 patients was required to select the studies providing the most robust incidence estimates. For autopsy studies, sample sizes were considered to be adequate if at least 35 patients were included (providing 95% confidence of prevalence estimates with a 10% margin of error and a 10% assumed prevalence). 5 , 6 However, because the number of large autopsy studies was limited, those with data for at least 20 patients were included in the review. Autopsy studies with 20–34 patients were marked as having an inadequate sample size in the quality assessment checklist and estimates of the prevalence of TE in these studies should be treated with caution.
Quality assessment
Study quality was assessed using a modified version of the Joanna Briggs Institute Checklist for prevalence studies (items 5 and 9, addressing coverage bias and response rate, respectively, were removed as they were not considered relevant to the present SLR). 6
RESULTS
Search results
The SLR process is shown in Figure 1. In total, 1692 records were identified; after removal of duplicates, 1438 were screened, and full‐text versions of 304 were assessed for eligibility. The final review included 41 references: hospital cohort and database studies and nine autopsy studies. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47
Figure 1.
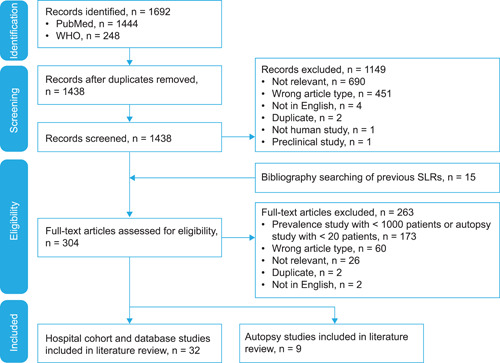
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses; SLR, systematic literature review; WHO, World Health Organization.
Quality assessment results
Quality assessment results are shown in Supporting Information: Tables 4 and 5. The quality of the included hospital cohort and database studies was generally moderate, with the most common issues being limited description of the overall population and, as expected for such studies, only a subset of patients having imaging results. The included autopsy studies were of generally good quality.
Summary of hospital cohort and database studies
In total, 32 hospital cohort and database studies with follow‐up ranging from 6.8 days (median duration of hospitalization) to 198 days (maximum follow‐up period for patients in hospital at the end of the enrollment period) were included in the literature review (Table 1). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 Of these, 26 studies described analyses of hospital (or multi‐hospital) records, and included outpatients (three studies), 8 , 9 , 10 patients treated in emergency departments (EDs; two studies) 11 , 12 and patients treated in hospital (22 studies) 8 , 10 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 ; six studies described cohorts treated in ICU. 10 , 15 , 17 , 24 , 26 , 32 A single study followed patients discharged after hospitalization for COVID‐19. 7
Table 1.
Hospital cohort and database studies
| Study (country) | Study type | Enrollment period | N (CTPA n) | Follow‐up period | Study population | Setting | Thromboprophylaxis |
|---|---|---|---|---|---|---|---|
| Hospital studies | |||||||
| Benito et al. (2020) (Spain) 13 | Prospective | March–April 2020 | 1275 (76) | Median (IQR): PE, 15.5 (9.8) days; no PE, 7 (11) days a , b | COVID‐19 patients admitted to hospital | Single hospital | Patients with PE: 28/32 LMWH, of which prophylactic, 93%; higher‐risk prophylactic, 7% Median (IQR), 6 (9) days of LMWH |
| Berger et al. (2020) (USA) 14 | Retrospective | March–April 2020 | 2377 (NR) | 35–73 daysc | COVID‐19‐positive patients admitted to hospital | Multi‐hospital health system | Before admission, 201/2377 (8.5%) |
| Bilaloglu et al. (2020) (USA) 15 | Retrospective | March–April 2020 | 3334 (NR) | 45–120 daysc | COVID‐19‐positive patients admitted to hospital, including 829 in ICU | Single hospital | Low‐dose prophylaxis was used in most patients after admission |
| Bunel et al. (2021) (France) 16 | Retrospective | March–April 2020 | 1097 (157) | NR | COVID‐19 patients admitted to hospital | Single hospital | LMWH, varying intensity Patients with CTPA (due to stagnation or worsening): 94.3%; 100% in ICU; 93% in ward. Patients with PE: 100% in ICU; 86.7% in ward |
| Doses: none, 5.7%: low prophylactic, 24.8%; high prophylactic, 57.3%; therapeutic, 12.1% | |||||||
| Choi et al. (2020) (USA) 18 | Retrospective | March–May 2020 | 1739 (571d) | 21–64 daysc | COVID‐19‐positive patients admitted to hospital | Community hospital and quaternary referral center | Enoxaparin or UFH, varying dose |
| COVID‐ICU group (2021) (France, Belgium, and Switzerland) 17 | Prospective cohort study | February–May 2020 | 4244 (NR) | 90 days | Critically ill adults with COVID‐19 treated in ICU (including 2233 on mechanical ventilation from Day 1 in ICU) | 138 hospitals | NR |
| Esenwa et al. (2021) (USA) 19 | Retrospective | March–May 2020 | 4299 (NR) | NR | COVID‐19‐positive patients admitted to hospital | Three New York hospitals | 47.0%–50.4% across ethnicity groups |
| Fang et al. (2020) (UK) 20 | Retrospective | March–April 2020 | 2157 (93) | 14–41 daysc | COVID‐19‐positive patients, including 1200 admitted to hospital | Single hospital | NR |
| Fauvel et al. (2020) (France) 21 | Retrospective | February–April 2020 | 2878 (1240) | Median (IQR), 6.8 (5–12) daysa | COVID‐19 patients admitted to hospitale | 24 centers | Before hospitalization, PE versus no PEf: Therapeutic dose anticoagulation, 4.9% versus 11.5% (p = 0.044) |
| During hospitalization, PE versus no PE (LMWH or UFH): Prophylactic dose, 18.4% versus 67.0% (p < 0.001) Intermediate dose, 5.1% versus 8.8% | |||||||
| Giannis et al. (2021) (USA) 22 | Retrospective | March–April 2020 | 10,871 (NR) | NR | COVID‐19 patients admitted to hospitalg | Multi‐hospital system | Of patients with VTE or death within 8 h of presentation: None, 99.3% Prophylactic, 0.7% |
| Gonzalez‐Fajardo et al. (2021) (Spain) 23 | Retrospective | March–April 2020 | 2943 (NR) | NR | COVID‐19‐positive patients admitted to hospital who required assessment for thrombotic events (261 in ICU) | Single hospital | Of 58 patients with PE: Prophylactic dose, 15 (26%) Therapeutic dose, 1 (2%) |
| Grillet et al. (2020) (France) 8 | Retrospective | To April 2020 | 2003 (100) | NR | COVID‐19‐positive patients, including 280 admitted to hospital | Single hospital | NR |
| Gupta et al. (2020) (USA) 24 | Retrospective | March–April 2020 | 2215 (NR) | 61–92 daysc | COVID‐19‐positive patients admitted to ICU | Multiple US hospital ICU departments | 8.4% using home anticoagulation before admission |
| Kartsios et al. (2021) (UK) 25 | Retrospective | February–April 2020 | 1583 (NR) | 8–97 daysc | COVID‐19‐positive patients admitted to hospital | Three hospitals | On thromboprophylaxis at time of VTE (not all PE), 5 of 11 patients with VTE post COVID‐19 diagnosis |
| Lee et al. (2021) (USA) 9 | Retrospective | March–May 2020 | 3727 (86) | NR | COVID‐19‐positive patients | Hartford healthcare system | Nearly all inpatients received at least systemic and mechanical thromboprophylaxis; regimens varied |
| Linschoten et al. (2020) (13 countries) 26 | Retrospective | March–July 2020 | 3011 (NR) | Median (IQR), 7 (4–14) daysa | COVID‐19 patients admitted to hospital, including 837 in ICUh | International patient registry | NR |
| Miró et al. (2020) (Spain, France) 11 | Retrospective | March–April 2020 | 8880 (320) | NA | COVID‐19 patients treated in EDi | 8 EDs | NR |
| Miró et al. (2021) (Spain) 12 | Retrospective | March–April 2020 | 63,822 (NR) | NA | COVID‐19 patients diagnosed in EDc | 50 EDs | NR |
| Monfardini et al. (2020) (Italy) 27 | Retrospective | March 2020 | 1207 (34) | ≥2 weeksj | COVID‐19‐positive patients admitted to hospital | Single hospital | Before PE: 8/25 received LMWH (32%) |
| Nordberg et al. (2021) (Sweden) 28 | Retrospective | March–May 2020 | 1162 (NR) | Median 162 days | COVID‐19‐positive patients admitted to hospital | Single hospital | Patients who developed PE while in hospital (n = 22), 14 (64%) were treated with LMWH (prophylactic, 8; elevated prophylactic, 5; treatment dose, 1). Patients with PE at admission, no prior anticoagulation was reported |
| Piazza et al. (2020) (USA) 10 | Retrospective | March–April 2020 | 1114 (NR) | 30 days | COVID‐19‐positive patients: outpatients, 715; on ward, 229; in ICU, 170 | Integrated health network | Therapeutic/prophylactic: ICU, 25.3%/89.4% Ward, 9.2%/84.7% Outpatient, 2.3%/0.1% |
| Planquette et al. (2021) (France) 29 | Retrospective | March–April 2020 | 1042 (269) | 30–81 daysc | |||
| COVID‐19 patients admitted to hospitale | Two Paris hospitals | Patients with PE: prophylactic anticoagulation, 55%; therapeutic anticoagulation, 6.9% | |||||
| Rashidi et al. (2021) (Iran) 7 | Prospective | February–April 2020 | 1529 (12) | ≥45 days | Patients discharged after hospitalization for COVID‐19, 45‐day follow‐up | Three hospitals | Prophylaxis (LMWH or UFH), 1490/1529 (97.4%) |
| Spiegel‐enberg et al. (2021) (Netherlands) 30 | Retrospective | March–May 2020 | 1154 (NR) | Median (IQR): TAC use, 7 (4–11) days; no TAC use, 7 (4–13) daysa | COVID‐19 patients admitted to hospitalk | Six hospitals | 16.5% were previously using therapeutic anticoagulants due to AF (76%), history of VTE (13%), or other reasons 88.8% of the remaining patients received LMWH during hospitalization After PS matching, 164 patients with therapeutic anticoagulation and 410 without were included |
| Tsakok et al. (2021) (UK) 31 | Retrospective |
March–April 2020 December 2020–January 2021 |
1560 (wave 1, 35; wave 2, 193) | NR | COVID‐19 patients admitted to hospital | Single hospital | NR |
| Whyte et al. (2020) (UK) 32 | Retrospective | March–May 2020 | 1477 (214) | NR | COVID‐19 patients admitted to hospital (including 222 in ICU) | Single hospital | Admitted patients: LMWH (UFH in ICU) Of patients scanned >24h after admission, 124/132 (93.9%) were on anticoagulation |
| Database studies | |||||||
| Aktaa et al. (2021) (UK) 33 | Retrospective, database study | March–July 2020 | 84,728 (NR) | NR | COVID‐19‐positive patients admitted to hospital with any TE | English hospital admissions | NR |
| Cates et al. (2020) (USA) 34 | Retrospective, database study | March–May 2020 | 3948 (NR) | Median (IQR), 8.6 (3.9–18.6) daysa | COVID‐19 patients admitted to hospital | Veterans Health Administration database | NR |
| Cho et al. (2021) (South Korea) 36 | Retrospective, database study | February–May 2020 | 7327 (NR) | Mean (SD), 20.9 (13.1) days | All adult COVID‐19‐positive patients in HIRA database, including 6911 hospitalized patients | Korea HIRA database | NR |
| Dalager‐Pedersen et al. (2021) (Denmark) 37 | Retrospective, database study | February–May 2020 | 9460 (NR) | 30 days | All COVID‐19 cases in Denmark, including 1540 hospitalized patients | National databases: Danish CRS database, Microbiological database, National Patient Registry and National Prescription database | NR |
| Piroth et al. (2021) (France) 35 | Retrospective | March–April 2020 | 89,530 (NR) | Until end of hospital stay | COVID‐19‐positive patients admitted to hospital | All hospital admissions in France—PMSI database | NR |
| Ray et al. (2021) (Canada) 38 | Retrospective, database study | January–June 2020 | 6991 (NR) | 31–198 daysc | All COVID‐19‐positive patients in healthcare database | Ontario healthcare database | NR |
Abbreviations: AF, atrial fibrillation; CTPA, computed tomography pulmonary angiogram; ED, emergency department; EHR, electronic health record; HCO, healthcare organization; HIRA, Health Insurance Review and Assessment; ICU, intensive care unit; IQR, interquartile range; LMWH, low‐molecular‐weight heparin; NA, not applicable; NOAC, non‐vitamin K antagonist oral anticoagulant; NR, not reported; PE, pulmonary embolism; PMSI, Programme de Médicalisation des Systèmes d'Information; PS, propensity score; SD, standard deviation; TAC, therapeutic anticoagulant; TE, thromboembolism; UFH, unfractionated heparin; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Duration of hospitalization.
IQR reported as width of range.
Maximum follow‐up period for patients in hospital at the end of the enrollment period.
Compression ultrasound or CTPA.
Diagnosis based on PCR or typical imaging characteristics.
Mostly VKA and NOAC in PE group.
Diagnosis method not reported.
Includes all adult patients admitted to hospital with highly suspected COVID‐19; PCR testing was not a requirement.
Clinical or microbiological diagnosis.
All patients were dead or discharged after 2 weeks.
Diagnosis by PCR (97%) or clinical features in combination with a CT scan with a very high level of suspicion.
Six studies described analyses of large health databases 33 , 34 , 35 , 36 , 37 , 38 ; of these, three studies described exclusively hospitalized cohorts, 33 , 34 , 35 and three described all COVID‐19‐positive patients in the databases. 36 , 37 , 38
Most of the included studies described populations with COVID‐19 during the first wave of infection, with data collected up to July 2020 (most commonly March–April 2020). A single study compared patients treated in hospital in March–April 2020 with those treated in December 2020–January 2021. 31
The only relevant outcome of interest for this study that was reported in the included studies was PE. No hospital studies suitable for inclusion were identified that described CTEPH, endothelial damage, microthrombi, or thrombosis in situ; in addition, no imaging‐based diagnostic standard for in situ thrombosis was identified.
Overall incidence of PE
Across the included studies, PE was reported by a median of 0.4% of outpatients, 7 , 8 , 9 , 10 , 11 , 12 , 36 , 37 , 38 2.3% of patients admitted to hospital, 8 , 10 , 13 , 14 , 15 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 37 and 7.3% of patients treated in ICU 15 , 17 , 24 , 26 , 32 (Figure 2a). In analyses of patients who all underwent CTPA, PE was reported by a median of 34.2% of outpatients treated in ED, 11 , 12 8.3% of previously discharged outpatients (in a single study), 7 18.5% of hospitalized patients, 9 , 13 , 18 , 21 , 27 , 29 , 31 22.1% of hospitalized non‐ICU patients, 8 , 16 , 20 , 32 and 44.9% of patients treated in ICU 8 , 16 , 20 , 32 (Figure 2b).
Figure 2.
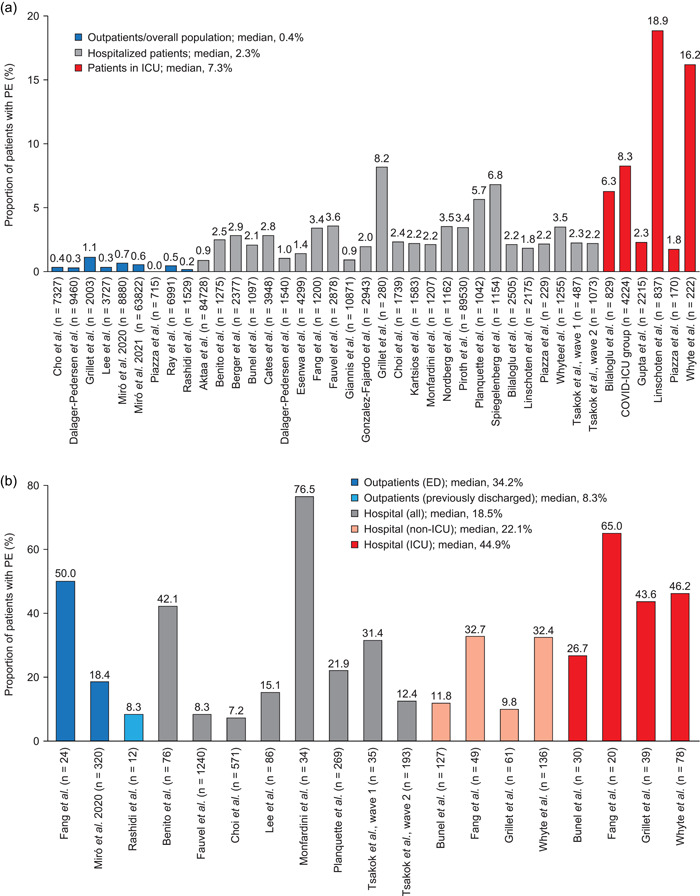
Incidence of PE among (a) all patients; (b) patients who underwent CTPA. The outpatient/overall population group in Fang et al. was excluded because a majority of patients were hospitalized. Where separate hospitalized and ICU cohorts are not shown, data for hospitalized patients may include those treated in ICU. ED, emergency department; ICU, intensive care unit; IQR, interquartile range; PE, pulmonary embolism.
PE in outpatients
A total of four studies assessed PE among all recorded COVID‐19 cases within a healthcare system. Cho et al. found 26 cases of PE among 7237 patients with a positive COVID‐19 test in South Korea (0.36%). 36 In Denmark, 28 patients with PE were identified among 9460 positive cases (0.30%; 16 cases occurred among 1540 hospitalized patients [1.0%]). 37 Lee et al. reported an analysis of a combined total of 3727 COVID‐19‐positive patients across inpatient and outpatient settings in a US regional healthcare system: in total, 86 patients required CTPA, and 13 had PE (0.35%). 9 Similarly, Ray et al. found a total of 33 cases of PE among the 6991 patients with a positive COVID‐19 test in Ontario, Canada (0.47%). 38
Two further studies of hospital outpatients found rates of PE of 0% (0 of 715) and 1.1% (23 of 2003) in the USA and France, respectively. 8 , 10
Miró et al. (2020 and 2021) reported two studies of PE among patients with COVID‐19 who attended hospital EDs but were not necessarily admitted. 11 , 12 Across eight EDs in France and Spain, 59 of 8880 patients were diagnosed with PE (0.66%); there was considerable variation, with incidence ranging from 0.07% to 2.9% across EDs. 11 In a larger study in 50 Spanish EDs, 353 of 63,822 patients had PE (0.55%). 12
PE in hospitalized patients
Esenwa et al. described a study of patients admitted to three US hospitals: 62 of 4299 patients were diagnosed with PE (1.4%); the incidence of PE ranged from 0.8% in Asian patients to 1.6% in Hispanic patients. 19 In a further US multi‐hospital system, 101 of 10,871 patients (0.93%) were diagnosed with PE within 8 h of admission. 22
Three database studies reviewed all hospitalized patients within a healthcare system. Piroth et al. found that in France, 3.4% of patients admitted to hospital with COVID‐19 in March and April 2020 (n = 89,530) had a diagnosis code for PE. 35 Similarly, Cates et al. found that 2.8% of the 3948 COVID‐19‐positive admissions in March–May 2020 in the US Veterans Health Administration database had PE. 34 Aktaa et al. investigated UK hospital admissions for TE: the incidence of PE among admitted patients with COVID‐19 was 886 per 100,000 admissions (0.89%) 33
Most other analyses of hospitalized patients (17 of 20) identified in our literature search reported the incidence of PE to range from 1.0% to 3.6%. 10 , 13 , 14 , 15 , 16 , 18 , 20 , 21 , 23 , 25 , 26 , 27 , 28 , 31 , 32 , 37 Three studies found higher rates, of 5.7%–8.2%; this may reflect the possible inclusion of ICU patients, who were not reported separately. 8 , 29 , 30
PE in patients treated in ICU
The COVID‐ICU group found that in 149 ICU departments in 138 centers in France, Belgium, and Switzerland, 350 of 4224 patients (8.3%) had PE, including 207 of 2233 (9.3%) of those requiring mechanical ventilation. 17 By contrast, Gupta et al. reported only 51 PE events among 2215 patients (2.3%) treated in US hospital ICU departments. 24 Smaller ICU studies reported varying rates of PE, ranging from 1.8% in another US study to 18.9% in a European registry study. 10 , 15 , 26 , 32
Comparison between pandemic waves
Tsakok et al. compared COVID‐19‐positive patients admitted to a UK hospital in December 2020–January 2021 with those hospitalized in March–April 2020. 31 Overall, the proportion of patients with PE was similar in the two waves (2.3% in 2020 and 2.2% in 2021). In the subset of patients who underwent CTPA, the proportion who had PE was lower in the second wave than in the first—this may suggest increased awareness of the risk of thromboembolism and a lower threshold for CTPA examination. The proportion of CTPA requests originating from ICU decreased from 10% in the first wave to 3% in the second wave, whereas the proportion originating from the respiratory high‐dependency unit increased from 7% in the first wave to 16% in the second wave. 31
Location of PE
The locations of PEs were described in eight studies (Figure 3). 8 , 13 , 16 , 20 , 27 , 28 , 29 , 32 In general, segmental arteries were the most common location for PE, although one study found that more than half of patients had lobar or proximal emboli. 28 Bilateral PE was frequent, with two studies reporting that the majority of patients with PE had bilateral emboli. 8 , 27 Three studies found PE in subsegmental arteries—which may represent in situ thrombosis—in around 30% of events. 13 , 20 , 32
Figure 3.
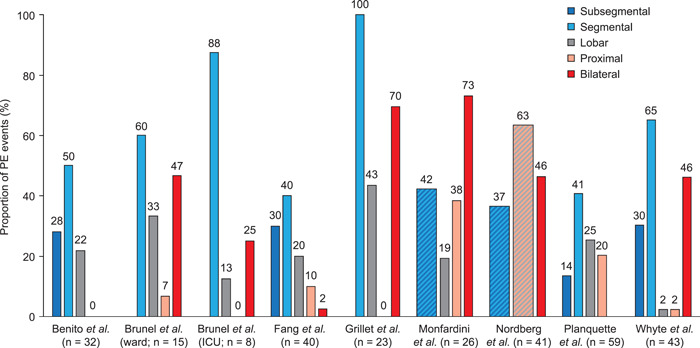
Location of PE. n = 43 for Whyte et al. refers to unilateral PE only; a further 37 events of 80 in total were bilateral with location not reported. n = 40 for Fang et al. refers to unilateral PE only; a further 1 event of 41 in total was bilateral with location not reported. Cross‐hatched columns represent reporting of combined categories (e.g., segmental or subsegmental). PE, pulmonary embolism.
Risk factors for development of PE
A total of eight studies reported risk factors for the development of PE, or significant differences between groups of patients with and without PE (Table 2). 8 , 13 , 16 , 20 , 21 , 29 , 30 , 32 Significant risk factors for PE included male sex, older age, and mechanical ventilation, as well as higher C‐reactive protein and d‐dimer levels.
Table 2.
Significant risk factors for PE
| Study | Risk factors for PE |
|---|---|
| Significant differences between groups with and without PE | |
| Benito et al. (2020) (Spain) 13 |
|
|
|
|
|
| Bunel et al. (2021) (France) 16 |
|
| Fang et al. (2020) (UK) 20 |
|
| Whyte et al. (2020) (UK) 32 |
|
|
|
|
|
| Risk analysis | |
| Fauvel et al. (2020) (France) 21 | Multivariable analysis OR (95% CI): |
|
|
|
|
|
|
|
|
|
|
| Grillet et al. (2020) (France) 8 | Multivariable analysis OR (95% CI): |
|
|
|
|
| Planquette et al. (2021) (France) 29 | OR (95% CI): |
|
|
|
|
| Spiegelenberg et al. (2021) (Netherlands) 30 | RR (95% CI): |
|
|
|
|
Abbreviations: BMI, body mass index; CI, confidence interval; CTPA, computed tomography pulmonary angiogram; IQR, interquartile range; OR, odds ratio; PE, pulmonary embolism; PS, propensity score; SD, standard deviation.
Outcomes of patients with PE
Clinical outcomes (hospital events and mortality) associated with PE were described in 10 studies (Table 3). 13 , 15 , 17 , 20 , 21 , 23 , 24 , 27 , 28 , 29 In studies that compared outcomes between patients with and without PE, those with PE were more likely to be admitted to ICU 13 , 21 and to require mechanical ventilation, 13 , 21 , 29 and had longer median hospital stays. 13 , 29 However, only one of six studies found patients with PE to have statistically significantly higher mortality than those without. 17
Table 3.
Hospital events and mortality among patients with PE
| Study | Hospital events | Mortality | |||
|---|---|---|---|---|---|
| Event | Patients with PE | Patients without PE | Patients with PE | Patients without PE | |
| Benito et al. (2020) (Spain) 13 | ICU admission | 15/32 (47%) | 10/44 (23%); p = 0.027 | 3/32 (9%) | 5/44 (11%) |
| MV | 14/32 (44%) | 8/44 (18%); p = 0.015 | ‐ | ‐ | |
| Hospital stay, median (IQR) | 15.5 (9.8) days | 7 (11) days; p = 0.010 | ‐ | ‐ | |
| Bilaloglu et al. (2020) (USA) 15 | ‐ | ‐ | ‐ | Ward, 7/54 (13%) | Ward, 282/2216 (12.7%) |
| ‐ | ‐ | ‐ | ICU, 33/52 (63%) | ICU, 305/585 (52.1%)a | |
| COVID‐ICU group (2021) (France, Belgium, and Switzerland) 17 | ‐ | ‐ | ‐ | 135/350 (38.6%)b | 1163/3874 (30.0%)b |
| Fang et al. (2020) (UK) 20 | ‐ | 6/41 (15%) | 5/52 (10%)c | ‐ | ‐ |
| Fauvel et al. (2020) (France) 21 | ICU admission | 32/103 (31.1%) | 153/1137 (13.5%); p < 0.001 | 9/103 (8.7%) | 142/1137 (12.5%) |
| MV | 25/103 (24.3%) | 83/1137 (7.3%); p < 0.001 | ‐ | ‐ | |
| Gonzalez‐Fajardo et al. (2021) (Spain) 23 | ‐ | ‐ | ‐ | 7/58 (12%) | ‐ |
| Gupta et al. (2020) (USA) 24 | ‐ | ‐ | ‐ | 19/51 (37%) | ‐ |
| Monfardini et al. (2020) (Italy) 27 | ‐ | ‐ | ‐ | 10/26 (38%) | ‐ |
| Nordberg et al. (2021) (Sweden) 28 | ‐ | ‐ | ‐ | 10/41 (24%) | ‐ |
| Planquette et al. (2021) (France) 29 | MV | 25/59 (42.4%) | 25/118 (21.2%) | 12/59 (20.3%) | 19/118 (16.1%)c |
| Hospital stay, median (IQR)d | 12 (3–18) days | 8 (2–15) daysc | ‐ | ‐ | |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; MV, mechanical ventilation; PE, pulmonary embolism; SD, standard deviation.
Patients with no thrombotic events.
At Day 90; calculated; study reports PE to be significantly more common in patients who had died after 90 days than in those who were alive (10.4% vs. 7.3%; p < 0.001).
Patients who underwent CTPA.
IQR reported as width of range. p values are shown where statistically significant differences are reported.
Events after initial hospital treatment
A single study reported PE events occurring after initial hospital treatment and discharge. 7 A cohort of patients (n = 1529) discharged from three hospitals in Iran after hospitalization for COVID‐19 were followed prospectively for 45 days. A total of 52 patients were evaluated for suspicion of thromboembolism, and one was diagnosed with PE. A further two patients died during the follow‐up period, with PE given as the likely cause of death; overall, the incidence of definite or likely PE among discharged patients was 0.2%. 7
TE in autopsy studies
In total, nine autopsy studies were included in the literature review (Table 4). 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 Of these, six reported data on PE, 39 , 40 , 41 , 43 , 45 , 47 seven described pulmonary thrombi or microthrombi, 40 , 41 , 42 , 44 , 45 , 46 , 47 and four reported endothelial damage. 40 , 41 , 42 , 46
Table 4.
Autopsy studies
| Study (country) | Study period | N | Study population | Setting | Study description | Thromboprophylaxis |
|---|---|---|---|---|---|---|
| Benzakoun et al. (2020) (France) 39 | March–April 2020 | 64 | Deceased patients with suspected COVID‐19 (clinical context and lung CT) | Institute of Forensic Medicine of Paris | Unenhanced post‐mortem CT analysis | NR |
| Borczuk et al. (2020) (USA/Italy) 40 | March–April 2020 | 68 | Consecutive deceased patients PCR‐positive for COVID‐19 | Two hospitals in New York and one in Padua | Analysis of lung tissue from autopsies | 48 of 68 patients received anticoagulation |
| Bryce et al. (2021) (USA) 41 | March–June 2020 | 100a | Consecutive deceased patients PCR‐positive for COVID‐19 | Mount Sinai Hospital (New York) | Autopsy, multiple organ systems | 82 of 100 patients received anticoagulation |
| Carsana et al. (2020) (Italy) 42 | February–March 2020 | 38 | Consecutive deceased patients PCR‐positive for COVID‐19 | Two hospitals in Milan and Bergamo | Autopsy, lungs only | NR |
| Edler et al. (2020) (Germany) 43 | March–April 2020 | 80 | Consecutive deceased patients PCR‐positive for COVID‐19 | University Medical Center Hamburg‐Eppendorf | Autopsy, multiple organ systems; focus on lungs | NR |
| Mauad et al. (2021) (Brazil) 44 | March–June 2020 | 41 | Deceased patients PCR‐positive for COVID‐19 | University of Sao Paulo Medical School | Ultrasound‐guided minimally invasive autopsy, lungs only | 33 of 37 patients received anticoagulation in ICU (NR for four patients) |
| Menter et al. (2020) (Switzerland) 45 | To April 2020 | 21 | Deceased patients PCR‐positive for COVID‐19 | Two hospitals in Basel canton | Autopsy, multiple organ systems | 11 of 21 patients received anticoagulation |
| Prieto‐Pérez et al. (2020) (Spain) 46 | March–April 2020 | 20b | Deceased patients PCR‐positive for COVID‐19 | University hospital Fundación Jiménez Díaz (Madrid) | Autopsy, lungs and bone marrow | NR |
| Skok et al. (2021) (Austria) 47 | March–June 2020 | 28 | Deceased patients PCR‐positive for COVID‐19 (35% of deceased COVID‐19 cases in study period) | Hospital Graz II (Graz) | Autopsy, multiple organ systems; focus on lungs | NR |
Abbreviations: CT, computed tomography; NR, not reported; PCR, polymerase chain reaction; PE, pulmonary embolism; TE, thromboembolism.
Autopsy results for 25 patients were previously reported in Borczuk et al. (2020).
20 lung samples were evaluated from 33 patients.
The results of the autopsy studies are summarized in Figure 4. PE was reported in 6%–23% of deceased patients. 39 , 40 , 41 , 43 , 45 , 47 Other TE were more common. Thrombosis in situ—described as large thrombi, pulmonary artery thrombosis, or thrombi in small intra‐acinar branches of the pulmonary artery—was found in 43%–100% of patients. 40 , 44 , 47 Microthrombi and alveolar capillary microthrombi—reported to be fibrinous and to affect small arteries, arterioles, and capillaries—were seen in 45%–91% of samples. 40 , 41 , 42 , 44 , 45 , 46 For example, Borczuk et al. described microthrombi containing fibrin and platelets and found in small arteries (< 1.0 mm) in lungs from 57 of 68 patients (84%). 40
Figure 4.
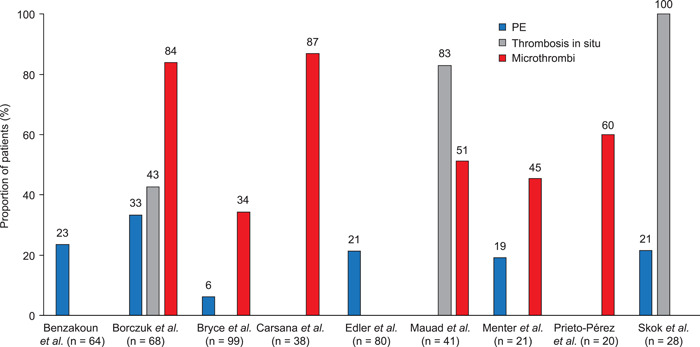
Thromboembolic events in autopsy studies. Thrombosis in situ: Borczuk et al., large thrombi (diffuse in 22 cases, focal in 7); Bryce et al., intravascular fibrin thrombi within small arteries or arterioles (in addition, 21 of 23 samples on which CD61 stains were performed had platelet aggregates and/or thrombi in small arteries, arterioles, and capillaries); Mauad et al., thrombi in small intra‐acinar branches of pulmonary artery; Skok et al., pulmonary artery thrombosis. Microthrombi: Borczuk et al., diffuse in 44 cases, focal in 13; Carsana et al., platelet‐fibrin thrombi in small arterial vessels (<1 mm); Mauad et al., thrombi in alveolar capillaries. PE, pulmonary embolism.
Bryce et al. reported intravascular fibrin thrombi within small arteries or arterioles, with platelet aggregates or thrombi in small arteries, arterioles, and capillaries in 91% of samples on which CD61 stains were performed. 41
Four of the included studies also described pulmonary endothelial damage. 40 , 41 , 42 , 46 Borczuk et al. reported that vascular injury without prominent diffuse alveolar damage was observed in 11 of 68 patients (16%). 40 Similarly, Bryce et al. described capillary proliferation with inflammation and injury resembling endotheliitis in the lungs of 19 of 99 deceased patients with COVID‐19 (19%). 41 Carsana et al. reported endothelial necrosis in the lungs of 29 of 38 deceased patients with COVID‐19 (76%). 42 In addition, Prieto‐Pérez et al. described endothelial damage visible with electron microscopy, but this was performed only for two samples. 46
DISCUSSION
The incidence of PE varied widely across the studies included in this SLR. This variation is likely to reflect the heterogeneity of the included studies and the conditions in which they were conducted, mainly during the early stages of the pandemic in which healthcare systems were strained and CTPA was not always routinely used. Among large studies of the incidence of TE, PE was the only event of interest reported, with no data identified on other events of interest for the current SLR (CTEPH, endothelial damage, microthrombi, and peripheral/small artery thrombosis) and limited reporting of long‐term events occurring after hospital treatment. In contrast to the results of the cohort and database studies, the majority of studies describing autopsies of patients with COVID‐19 reported thrombosis in situ and microthrombi, with several studies also reporting pulmonary endothelial damage. Together, these findings suggest that the broader picture of thrombosis in COVID‐19 is not yet clearly understood, with a focus on the acute phase of the disease but less clarity on the mechanisms underlying the initial dysfunction or on the potential downstream sequelae, both of PE and of other vascular dysfunctions.
As demonstrated by Tsakok et al., 31 PE has remained common in patients with COVID‐19 despite advances in therapy. The Dutch COVID and Thrombosis Coalition showed a similar pattern for overall thrombotic complications in patients with COVID‐19: the risk of thrombotic complications was comparably high in the second and first waves of the pandemic (with PE being the most frequent thrombotic complication in both waves), although overall mortality was reduced by 47% in the second wave. 48 Thus, thrombotic complications (particularly in the lungs) remain an ongoing challenge in COVID‐19. Tsakok et al. found a reduced rate of CTPA requests from ICU in the second wave, reflecting a change in practice (most patients with COVID‐19 were managed on respiratory high‐dependency units in the second wave). 31 The Dutch COVID and Thrombosis Coalition found that the risk of thrombotic complications decreased in ICU but increased in the ward in the second wave. This may reflect a lower threshold for diagnostic testing in the second wave, resulting in earlier diagnosis of thrombotic complications (before patients were transferred to ICU from the ward). 48
The reported location of PE in the studies included in our systematic review was often segmental or subsegmental. It has been suggested that PE limited to the subsegmental arteries would represent in situ thrombosis caused by thromboinflammatory disease; 3 , 49 this would be consistent with autopsy findings, 2 but there is otherwise little supporting evidence to date. A study of patients with severe COVID‐19 pneumonia requiring extracorporeal membrane oxygenation found concordant distribution of pulmonary arterial thrombosis and wedge parenchymal perfusion defects in only 30% of cases, suggesting the presence of separate pathophysiologic processes for micro‐ and macrovascular disease. 1 , 50 In‐depth studies are needed to confirm the mechanisms underlying pulmonary vascular dysfunction and thromboinflammatory disease, to understand the association of pulmonary thromboinflammatory disease with COVID‐19 and other pulmonary diseases/infections, and to ascertain the optimal prevention of and treatment for pulmonary thromboinflammatory disease. Of note, one of the potential explanations for the inconsistent findings of large thromboprophylaxis randomized trials in hospitalized patients with COVID‐19 is the impossibility of differentiating “conventional thromboembolism” from in situ immunothrombosis as an outcome measure. Therefore, another key issue to be addressed by future research is the differentiation of pulmonary thromboinflammatory disease from thromboembolic disease based on clinical and/or imaging data. From an imaging perspective, this is technically very challenging in small vessels where there is very little to differentiate the two phenomena. Biomarkers may therefore play a role alongside imaging data, in particular perfusion studies.
Although the studies included in this systematic review mostly focused on acute thromboembolic complications of COVID‐19, evidence is emerging of longer‐term pulmonary vascular sequelae. For example, the single study with long‐term follow‐up in our systematic review reported cases of PE occurring after initial hospital treatment and discharge. 7 Consistent with this, a self‐controlled study of the Scottish general population has found that while the likelihood of thromboembolism was highest in the 7 days following a positive test, the risk remained significantly elevated 8 weeks later. 51 Similarly, a recent study using the US Veterans Affairs health databases showed an increased risk of PE beyond the first 30 days after a positive COVID‐19 test compared with contemporary and historical controls. 52 Another USA‐based study showed an increased risk of PE and pulmonary hypertension beyond the acute phase of COVID‐19 infection, compared with contemporary and historical controls. 53 In a recent self‐controlled case series analysis using Swedish national registry data, the incidence rate ratio for PE remained elevated during Days 91–180 after COVID‐19. 4 The different designs of these studies illustrate the challenge of selecting an appropriate control group: the self‐controlled design may limit generalizability whereas the use of contemporary and historical controls may cause confounding.
It has been suggested that patients with COVID‐19 are at risk of developing various forms of pulmonary hypertension, 53 , 54 including CTEPH, which is a well‐known long‐term complication of acute PE. 55 Notably, reports of CTEPH following COVID‐19 were not found in the SLR, potentially due to insufficient follow‐up time. The absence of such reports to date is not conclusive: in patients without COVID‐19, the interval between acute PE and subsequent development of CTEPH can range from months to several years. 56 , 57 Incomplete thrombus resolution has been reported in a subset of patients with COVID‐19 and PE. 58 , 59 , 60 Therefore, cases of CTEPH following COVID‐19‐associated acute PE may yet emerge. 61 Consistent with this, a report from the USA found more diagnoses of pulmonary hypertension than expected among COVID‐19 survivors. 53 It, therefore, remains possible that an increased incidence of CTEPH may be an important long‐term consequence of widespread COVID‐19 infection. The incidence of CTEPH following COVID‐19‐associated pulmonary TE should be investigated in long‐term follow‐up studies. 62
In the United Kingdom, the rate of CTEPH referrals has actually fallen during the pandemic. However, a recent report has suggested that the decline in referral rate is due to the varied impacts of the pandemic on the healthcare system itself, including limited access to diagnostics and reductions in hospital follow‐up appointments, and that hundreds of CTEPH cases may have been undiagnosed. 63
CTEPH work‐up requires either access to nuclear medicine facilities for ventilation/perfusion scanning, a dedicated cardiothoracic radiologist who can confidently make diagnoses on the basis of CTPA alone, or cardiopulmonary magnetic resonance imaging (MRI), depending on local practice. 64 , 65 If the normal incidence of CTEPH following PE holds for PE caused by COVID‐19, it will be a huge challenge to optimally manage the number of CTEPH cases to be expected following the pandemic, within the existing capacity of healthcare systems, particularly in developing countries. Simple noninvasive diagnostic algorithms (e.g., “CTEPH rule‐out criteria” based on ECG characteristics and levels of N‐terminal pro‐brain natriuretic peptide 66 , 67 , 68 ) and artificial intelligence (AI)‐based algorithms may help to reduce the burden on healthcare systems. Studies are needed to develop AI‐based algorithms for the identification of patients with COVID‐19 who have additional acute/chronic pulmonary vascular conditions, and for the prediction of treatment outcomes. This will require the use of large, machine‐readable databases of imaging/clinical data such as that generated by the Radiological Cooperative Network (RACOON) project in Germany. 69
Studies included in the SLR showed little standardization of TE reporting (location, definition, etc.) and a low rate of detection of vasculopathy during the acute phase of COVID‐19 infection. Combined with the emerging data showing elevated long‐term risks of pulmonary vascular sequelae after COVID‐19, 4 , 52 , 53 these findings suggest that the current approach used to identify vascular abnormalities (visual assessment by a single radiologist) is not sufficient to reliably detect pulmonary vascular disease that might have an important chronic component. 70 Several studies have demonstrated the potential of CT scans for the prediction or early detection of CTEPH. 70 , 71 , 72 , 73 , 74 However, those studies did not focus on patients with COVID‐19, for whom microvascular disease may have a predictive role. As discussed by Weir‐McCall et al., microvascular disease may result in subtle hypoperfusion which could be missed by visual assessment of contrast‐enhanced CT. 50 Early identification of patients at risk of developing long‐term pulmonary sequelae will require the use of sensitive tools to detect subtle morphological abnormalities and to quantify changes over time. Such tools may include dual‐energy CT (DECT) 75 , 76 and hyperpolarized xenon 129 MRI. 77 Patel et al. used DECT to assess pulmonary perfusion in patients with severe COVID‐19 pneumonia, and detected perfusion abnormalities in all patients with assessable lobes. 76 In a study of nine patients who had been previously hospitalized for COVID‐19 and who were evaluated at least 3 months after discharge, hyperpolarized xenon 129 MRI showed alveolar‐capillary diffusion limitation in all cases, despite normal or nearly normal results at CT. 77
Automated quantitative imaging methods may also help to ease the burden of CTEPH work‐up. For example, Rahaghi et al. have quantified vascular changes associated with CTEPH by generating three‐dimensional reconstructions of the intraparenchymal vasculature from CT images. 78 In other studies, CT images from patients with COVID‐19 have been used to quantify the percentage of pulmonary blood volume in vessels with a cross‐sectional area of 1.25–5 mm2 (BV5%); BV5% was found to be lower in patients with COVID‐19 than in healthy controls and patients with ARDS, and was associated with death and intubation. 79 , 80 , 81
This SLR has some limitations. First, the heterogeneity of the included studies makes interpreting the data on the incidence of TE challenging. Second, most of the studies did not perform routine CTPA (reasons for this are likely to include hospital system pressure and infection control considerations), suggesting probable underdiagnosis—data for overall populations are therefore likely to be subject to significant confounding and should be treated with caution. Third, while the focus of this article has been on the pulmonary vasculature, the long‐term pulmonary sequelae of COVID‐19 may also have parenchymal and airway components. The relative contributions of these three components and their interactions are not yet known. Fourth, the majority of the included studies were conducted in the first half of 2020 when vaccines and antiviral therapies were not yet available. It is unknown how the major changes in the general management of COVID‐19 as well as updated thromboprophylaxis strategies have influenced the rate and outcome of COVID‐19 pulmonary vascular complications; the two studies described above that compared the first and second waves found that PE remained common in the second wave, but would not have captured the impact of widespread vaccination and use of antiviral treatments or differences in the incidence of TE among more recent SARS‐CoV‐2 variants. Other limitations of this SLR include its restriction to English‐language papers (which may mean some relevant studies published in other languages were missed) and its use of size thresholds: the large number of potentially relevant studies meant that only the largest studies were reviewed, which may mean that smaller studies focusing on TE other than PE were missed.
CONCLUSION
Pulmonary thromboembolic complications of COVID‐19 have posed a continuing challenge throughout the first and following waves of the pandemic. Although much of current literature has focused on the acute phase of COVID‐19, it has become clear that patients remain at increased risk of thromboembolic complications such as PE for a substantial period of time following the initial infection. Therefore, it may be considered to perform adequate imaging studies of the chest in patients with new or persistent respiratory symptoms following the acute phase of COVID‐19 to rule out the presence of acute or chronic PE or pulmonary thromboinflammatory disease. The authors propose several key objectives for future research (Box 1).
| Key objectives for future research—authors' proposals |
|
|
|
|
|
AUTHOR CONTRIBUTIONS
Pareen Vora, Sylvia Nikkho, Claire Mulligan, and Paul M. Overton were involved in the conception and design of the study. Claire Mulligan and Paul M. Overton conducted the searches and data extraction and wrote the first draft of the manuscript. All authors analyzed and interpreted the data, revised the manuscript critically for important intellectual content, approved the final manuscript, and agree to be accountable for its overall content.
CONFLICTS OF INTEREST
Paul M. Overton and Claire Mulligan are directors of Beacon Medical Communications Ltd, which received funding for this project from Bayer AG, and additional project funding from Bayer AG outside the submitted work. Mark Toshner received funding from Bayer and Actelion/Jansen; he disclosed he is a member of MorphogenIX scientific advisory board. Pareen Vora and Sylvia Nikkho are employees of Bayer AG. Jan de Backer and Ben R. Lavon are employees of FLUIDDA, which develops imaging‐derived metrics of pulmonary vascular health. Frederikus A. Klok received research support from Bayer, Actelion, the Dutch thrombosis association, Actelion, BSCI, The Netherlands Organisation for Health Research and Development, the Dutch Heart Foundation, and the Horizon Europe program.
ETHICS STATEMENT
Not applicable.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Bayer AG (Berlin, Germany). All authors agree to be accountable for the overall content of the manuscript.
Overton PM, Toshner M, Mulligan C, Vora P, Nikkho S, de Backer J, Lavon BR, Klok FA, the PVRI Innovative Drug Development Initiative . Pulmonary thromboembolic events in COVID‐19—a systematic literature review. Pulmonary Circulation. 2022;5:e12113. 10.1002/pul2.12113
REFERENCES
- 1. Poor HD. Pulmonary thrombosis and thromboembolism in COVID‐19. Chest. 2021;160(4):1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, Muscarella G, Orlandi M. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Dam LF, Kroft LJM, van der Wal LI, Cannegieter SC, Eikenboom J, de Jonge E, Huisman MV, Klok FA. Clinical and computed tomography characteristics of COVID‐19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res. 2020;193:86–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katsoularis I, Fonseca‐Rodríguez O, Farrington P, Jerndal H, Lundevaller EH, Sund M, Lindmark K, Fors Connolly AM. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid‐19: nationwide self‐controlled cases series and matched cohort study. BMJ. 2022;377:e069590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14–7. [PMC free article] [PubMed] [Google Scholar]
- 6. The Joanna Briggs Institute . Critical appraisal tools for use in JBI systematic reviews: checklist for prevalance studies. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf. (2017). Accessed 17 Feb 2022.
- 7. Rashidi F, Barco S, Kamangar F, Heresi GA, Emadi A, Kaymaz C, Jansa P, Reis A, Rashidi A, Taghizadieh A, Rezaeifar P, Moghimi M, Ghodrati S, Mozafari A, Foumani AA, Tahamtan O, Rafiee E, Abbaspour Z, Khodadadi K, Alamdari G, Boodaghi Y, Rezaei M, Muhammadi MJ, Abbasi M, Movaseghi F, Koohi A, Shakourzad L, Ebrahimi F, Radvar S, Amoozadeh M, Fereidooni F, Naseari H, Movalled K, Ghorbani O, Ansarin K. Incidence of symptomatic venous thromboembolism following hospitalization for coronavirus disease 2019: prospective results from a multi‐center study. Thromb Res. 2021;198:135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID‐19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee E, Krajewski A, Clarke C, O'Sullivan D, Herbst T, Lee S. Arterial and venous thromboembolic complications of COVID‐19 detected by CT angiogram and venous duplex ultrasound. Emerg Radiol. 2021;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, Almarzooq Z, Goldhaber SZ. Registry of arterial and venous thromboembolic complications in patients with COVID‐19. J Am Coll Cardiol. 2020;76(18):2060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miró Ò, Llorens P, Aguirre A, Lozano L, Beaune S, Roussel M, Le Borgne P, Chouihed T, Freund Y, Spanish‐French Emergency Department Investigative Team. Association between Covid‐19 and pulmonary embolism (AC‐19‐PE study). Thromb Res. 2020;196:322–4. [DOI] [PMC free article] [PubMed]
- 12.Miró Ò, Llorens P, Jiménez S, Piñera P, Burillo‐Putze G, Martín A, Martín‐Sánchez FJ, González Del Castillo J, Spanish Investigators on Emergency Situations TeAm (SIESTA) network. Frequency of five cardiovascular/hemostatic entities as primary manifestations of SARS‐CoV‐2 infection: results of the UMC‐19‐S(2). Int J Cardiol. 2021;330:268–72. [DOI] [PMC free article] [PubMed]
- 13. Benito N, Filella D, Mateo J, Fortuna AM, Gutierrez‐Alliende JE, Hernandez N, Gimenez AM, Pomar V, Castellvi I, Corominas H, Casademont J, Domingo P. Pulmonary thrombosis or embolism in a large cohort of hospitalized patients with Covid‐19. Front Med (Lausanne). 2020;7:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, Cao M, Goldenberg R, Hindenburg A, Horowitz J, Parnia S, Petrilli C, Reynolds H, Simon E, Slater J, Yaghi S, Yuriditsky E, Hochman J, Horwitz LI. Prevalence and outcomes of d‐Dimer elevation in hospitalized patients with COVID‐19. Arterioscler Thromb Vasc Biol. 2020;40(10):2539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID‐19 in a New York City health system. JAMA. 2020;324(8):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bunel V, Saker L, Ajzenberg N, Timsit JF, Najem S, Lortat‐Jacob B, Gay J, Weisenburger G, Mal H, Khalil A. Pulmonary embolism detected by CT pulmonary angiography in hospitalized COVID‐19 patients. Pulmonology. 2021;27:348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. COVID‐ICU Group . Clinical characteristics and day‐90 outcomes of 4244 critically ill adults with COVID‐19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi JJ, Wehmeyer GT, Li HA, Alshak MN, Nahid M, Rajan M, Liu B, Schatoff EM, Elahjji R, Abdelghany Y, D'Angelo D, Crossman D, Evans AT, Steel P, Pinheiro LC, Goyal P, Safford MM, Mints G, DeSancho MT. d‐dimer cut‐off points and risk of venous thromboembolism in adult hospitalized patients with COVID‐19. Thromb Res. 2020;196:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esenwa C, Unda SR, Altschul DJ, Patel NK, Malaviya A, Seiden J, Lendaris A, Moncrieffe K, Labovitz DL. The effect of race on composite thrombotic events in patients with COVID‐19. Thromb Res. 2021;199:10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang C, Garzillo G, Batohi B, Teo J, Berovic M, Sidhu PS, Robbie H. Extent of pulmonary thromboembolic disease in patients with COVID‐19 on CT: relationship with pulmonary parenchymal disease. Clin Radiol. 2020;75(10):780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, Benmansour O, Godeau G, Mecheri Y, Lebourdon R, Yvorel C, Massin M, Leblon T, Chabbi C, Cugney E, Benabou L, Aubry M, Chan C, Boufoula I, Barnaud C, Bothorel L, Duceau B, Sutter W, Waldmann V, Bonnet G, Cohen A, Pezel T, Critical Covid‐19 France Investigators. Pulmonary embolism in COVID‐19 patients: a French multicentre cohort study. Eur Heart J. 2020;41(32):3058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannis D, Barish MA, Goldin M, Cohen SL, Kohn N, Gianos E, Chatterjee S, Lesser M, Coppa K, Hirsch JS, McGinn T, Spyropoulos AC, COVID‐19 Consortium Group. Incidence of venous thromboembolism and mortality in patients with initial presentation of COVID‐19. J Thromb Thrombolysis. 2021;51(4):897–901. [DOI] [PMC free article] [PubMed]
- 23. Gonzalez‐Fajardo JA, Ansuategui M, Romero C, Comanges A, Gómez‐Arbeláez D, Ibarra G, Garcia‐Gutierrez A. Mortality of COVID‐19 patients with vascular thrombotic complications. Med Clin (Engl Ed). 2021;156(3):112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg‐Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Sutherland A, Green A, Shehata AM, Goyal N, Vijayan A, Velez J, Shaefi S, Parikh CR, Arunthamakun J, Athavale AM, Friedman AN, Short S, Kibbelaar ZA, Abu Omar S, Admon AJ, Donnelly JP, Gershengorn HB, Hernán MA, Semler MW, Leaf DE, STOP‐COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–47. [DOI] [PMC free article] [PubMed]
- 25. Kartsios C, Lokare A, Osman H, Perrin D, Razaq S, Ayub N, Daddar B, Fair S. Diagnosis, management, and outcomes of venous thromboembolism in COVID‐19 positive patients: a role for direct anticoagulants? J Thromb Thrombolysis. 2021;51(4):947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linschoten M, Peters S, van Smeden M, Jewbali LS, Schaap J, Siebelink HM, Smits PC, Tieleman RG, van der Harst P, van Gilst WH, Asselbergs FW, CAPACITY‐COVID collaborative consortium. Cardiac complications in patients hospitalised with COVID‐19. Eur Heart J Acute Cardiovasc Care. 2020;9(8):817–23. [DOI] [PMC free article] [PubMed]
- 27. Monfardini L, Morassi M, Botti P, Stellini R, Bettari L, Pezzotti S, Alì M, Monaco CG, Magni V, Cozzi A, Schiaffino S, Bnà C. Pulmonary thromboembolism in hospitalised COVID‐19 patients at moderate to high risk by Wells score: a report from Lombardy, Italy. Br J Radiol. 2020;93(1113):20200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nordberg M, Bankler S, Everhov ÅH, Saraste D. Central pulmonary embolism in patients with Covid‐19. Infect Dis (Lond). 2021;53:1–8. [DOI] [PubMed] [Google Scholar]
- 29. Planquette B, Le Berre A, Khider L, Yannoutsos A, Gendron N, de Torcy M, Mohamedi N, Jouveshomme S, Smadja DM, Lazareth I, Goudot G, Fournier L, Bruel C, Diehl JL, Mourad JJ, Meyer G, Priollet P, Messas E, Sanchez O, Beaussier H, Mirault T, Zins M, Chatelier G, Emmerich J. Prevalence and characteristics of pulmonary embolism in 1042 COVID‐19 patients with respiratory symptoms: a nested case‐control study. Thromb Res. 2021;197:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spiegelenberg JP, van Gelder M, Maas ML, Hovens M, Esselink A, Dofferhoff A, Janssen R, van de Maat J, Janssen N, Blaauw M, Hassing RJ, van Apeldoorn M, Kerckhoffs A, Veerman K, Hoogerwerf J, Kramers C, Leentjens J. Prior use of therapeutic anticoagulation does not protect against COVID‐19 related clinical outcomes in hospitalized patients: a propensity score‐matched cohort study. Br J Clin Pharmacol. 2021;87:4839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsakok MT, Qamhawi Z, Lumley SF, Xie C, Matthews P, Gleeson F, Benamore R. COVID‐19 CT pulmonary angiogram examinations and reported pulmonary embolism incidence: comparison between peak first wave and early second wave. Clin Radiol. 2021;76(4):310–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID‐19. Thromb Res. 2020;195:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aktaa S, Wu J, Nadarajah R, Rashid M, de Belder M, Deanfield J, Mamas MA, Gale CP. Incidence and mortality due to thromboembolic events during the COVID‐19 pandemic: multi‐sourced population‐based health records cohort study. Thromb Res. 2021;202:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cates J, Lucero‐Obusan C, Dahl RM, Schirmer P, Garg S, Oda G, Hall AJ, Langley G, Havers FP, Holodniy M, Cardemil CV. Risk for in‐hospital complications associated with COVID‐19 and influenza—veterans health administration, United States, October 1, 2018‐May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert‐Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID‐19 and seasonal influenza: a nationwide, population‐based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cho KH, Kim SW, Park JW, Do JY, Kang SH. Effect of sex on clinical outcomes in patients with coronavirus disease: a population‐based study. J Clin Med. 2020;10(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dalager‐Pedersen M, Lund LC, Mariager T, Winther R, Hellfritzsch M, Larsen TB, Thomsen RW, Johansen NB, Søgaard OS, Nielsen SL, Omland LH, Lundbo LF, Israelsen SB, Harboe ZB, Pottegård A, Nielsen H, Bodilsen J. Venous thromboembolism and major bleeding in patients with COVID‐19: a nationwide population‐based cohort study. Clin Infect Dis. 2021;73:2283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ray JG, Vermeulen MJ, Schull MJ, Park AL. ABO blood group, SARS‐CoV‐2 infection, and risk of venous thromboembolism: population‐based cohort study. Clin Appl Thromb Hemost. 2021;27:10760296211008986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benzakoun J, Hmeydia G, Delabarde T, Hamza L, Meder JF, Ludes B, Mebazaa A. Excess out‐of‐hospital deaths during COVID‐19 outbreak: evidence of pulmonary embolism as a main determinant. Eur J Heart Fail. 2020;22:1046–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, Elsoukkary S, He B, Del Vecchio C, Fortarezza F, Pezzuto F, Navalesi P, Crisanti A, Fowkes ME, Bryce CH, Calabrese F, Beasley MB. COVID‐19 pulmonary pathology: a multi‐institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, AlRasheed MR, Chen J, Li L, Wang D, Corben A, Haines GK, Westra WH, Umphlett M, Gordon RE, Reidy J, Petersen B, Salem F, Fiel MI, El Jamal SM, Tsankova NM, Houldsworth J, Mussa Z, Veremis B, Sordillo E, Gitman MR, Nowak M, Brody R, Harpaz N, Merad M, Gnjatic S, Liu WC, Schotsaert M, Miorin L, Aydillo Gomez TA, Ramos‐Lopez I, Garcia‐Sastre A, Donnelly R, Seigler P, Keys C, Cameron J, Moultrie I, Washington KL, Treatman J, Sebra R, Jhang J, Firpo A, Lednicky J, Paniz‐Mondolfi A, Cordon‐Cardo C, Fowkes ME. Pathophysiology of SARS‐CoV‐2: the Mount Sinai COVID‐19 autopsy experience. Mod Pathol. 2021;34:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schädler J, Steurer S, Mushumba H, Sperhake JP, Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schädler J, Steurer S, Mushumba H, Sperhake JP. Dying with SARS‐CoV‐2 infection‐an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mauad T, Duarte‐Neto AN, da Silva LFF, de Oliveira EP, de Brito JM, do Nascimento E, de Almeida Monteiro RA, Ferreira JC, de Carvalho C, do Nascimento Saldiva PH, Dolhnikoff M. Tracking the time course of pathological patterns of lung injury in severe COVID‐19. Respir Res. 2021;22(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Post‐mortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prieto‐Pérez L, Fortes J, Soto C, Vidal‐González Á, Alonso‐Riaño M, Lafarga M, Cortti MJ, Lazaro‐Garcia A, Pérez‐Tanoira R, Trascasa Á, Antonio A, Córdoba R, Rodríguez‐Pinilla SM, Cedeño O, Peces‐Barba G, Fernández‐Ormaechea I, Díez Medrano MJ, López de Las Heras M, Cabello A, Petkova E, Álvarez B, Carrillo I, Silva AM, Castellanos M, Calpena S, Valverde‐Monge M, Fresneda D, Rubio‐Martín R, Cornejo I, Astilleros Blanco de Cordova L, de la Fuente S, Recuero S, Górgolas M, Piris MA. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID‐19 infection. Mod Pathol. 2020;33(11):2139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skok K, Vander K, Setaffy L, Kessler HH, Aberle S, Bargfrieder U, Trauner M, Lax SF. COVID‐19 autopsies: procedure, technical aspects and cause of fatal course. Experiences from a single‐center. Pathol Res Pract. 2021;217:153305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutch COVID & Thrombosis Coalition, Kaptein F, Stals M, Grootenboers M, Braken S, Burggraaf J, van Bussel B, Cannegieter SC, Ten Cate H, Endeman H, Gommers D, van Guldener C, de Jonge E, Juffermans NP, Kant KM, Kevenaar ME, Koster S, Kroft L, Kruip M, Leentjens J, Marechal C, Soei YL, Tjepkema L, Visser C, Klok FA, Huisman MV. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID‐19 in the second and first wave. Thromb Res. 2021;199:143–8. [DOI] [PMC free article] [PubMed]
- 49. Desborough MJR, Doyle AJ, Griffiths A, Retter A, Breen KA, Hunt BJ. Image‐proven thromboembolism in patients with severe COVID‐19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weir‐McCall JR, Galea G, Mun Mak S, Joshi K, Agrawal B, Screaton N, Toshner M, Ruggiero A, Benedetti G, Brozik J, Machin R, Das I, Kotnik M, Sun J, Mackay, M , Jacob J, Rodrigues JCL, Camporota L, Vuylsteke A. Vascular thrombosis in severe coronavirus disease 2019 requiring extracorporeal membrane oxygenation: a multicenter study. Crit Care Med. 2021;50:624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ho FK, Man KKC, Toshner M, Church C, Celis‐Morales C, Wong I, Berry C, Sattar N, Pell JP. Thromboembolic risk in hospitalized and nonhospitalized COVID‐19 patients: a self‐controlled case series analysis of a nationwide cohort. Mayo Clin Proc. 2021;96(10):2587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xie Y, Xu E, Bowe B, Al‐Aly Z. Long‐term cardiovascular outcomes of COVID‐19. Nat Med. 2022;28(3):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, Lipsitch M, Cohen K. Risk of clinical sequelae after the acute phase of SARS‐CoV‐2 infection: retrospective cohort study. BMJ. 2021;373:n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cascino TM, Desai AA, Kanthi Y. At a crossroads: coronavirus disease 2019 recovery and the risk of pulmonary vascular disease. Curr Opin Pulm Med. 2021;27(5):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL, ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019;41(4):543–603. [DOI] [PubMed] [Google Scholar]
- 56. Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, Jenkins D, Kim NH, Humbert M, Jais X, Vonk Noordegraaf A, Pepke‐Zaba J, Brénot P, Dorfmuller P, Fadel E, Ghofrani HA, Hoeper MM, Jansa P, Madani M, Matsubara H, Ogo T, Grünig E, D'Armini A, Galie N, Meyer B, Corkery P, Meszaros G, Mayer E, Simonneau G. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;57(6):2002828. [DOI] [PubMed] [Google Scholar]
- 58. Ritchie CA, Johnson MM, Stowell JT, Idrees H, Toskich B, Paz‐Fumagalli R, Montazeri S, Fortich S, Franco‐Mesa C, Gloviczki P, Bjarnason H, Rivera C, Shaikh M, Moreno‐Franco P, Sanghavi D, Marquez CP, McBane RD, Park MS, O'Horo JC, Meschia JF, Erben Y. Resolution of acute pulmonary embolism using anticoagulation therapy alone in coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2022;10:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Whyte MB, Barker R, Kelly PA, Gonzalez E, Czuprynska J, Patel RK, Rea C, Perrin F, Waller M, Jolley C, Arya R, Roberts LN. Three‐month follow‐up of pulmonary embolism in patients with COVID‐19. Thromb Res. 2021;201:113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Dam LF, Kroft LJM, van der Wal LI, Cannegieter SC, Eikenboom J, de Jonge E, Huisman MV, Klok FA. More on clinical and computed tomography characteristics of COVID‐19 associated acute pulmonary embolism. Thromb Res. 2020;196:435–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cueto‐Robledo G, Roldan‐Valadez E, Graniel‐Palafox LE, Garcia‐Cesar M, Torres‐Rojas MB, Enriquez‐Garcia R, Cueto‐Romero HD, Rivera‐Sotelo N, Perez‐Calatayud AA. Chronic thromboembolic pulmonary hypertension (CTEPH): a review of another sequel of severe post‐Covid‐19 pneumonia. Curr Probl Cardiol . 2022:101187. 10.1016/j.cpcardiol.2022.101187 [DOI] [PMC free article] [PubMed]
- 62. Kruip M, Cannegieter SC, Ten Cate H, van Gorp E, Juffermans NP, Klok FA, Maas C, Vonk‐Noordegraaf A, Dutch COVID Thrombosis Coalition study group. Caging the dragon: research approach to COVID‐19‐related thrombosis. Res Pract Thromb Haemost. 2021;5(2):278–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Newman J, Boubriak I, Jenkins D, Ng C, Ruggiero A, Screaton N, Cannon J, Toshner M. Rising COVID‐19 related acute pulmonary emboli but falling national chronic thromboembolic pulmonary hypertension referrals from a large national dataset. ERJ Open Res. 2021;7(4):00431–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 65. Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Klok FA, Surie S, Kempf T, Eikenboom J, van Straalen JP, van Kralingen KW, van Dijk AP, Vliegen HW, Bresser P, Wollert KC, Huisman MV. A simple non‐invasive diagnostic algorithm for ruling out chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Thromb Res. 2011;128(1):21–6. [DOI] [PubMed] [Google Scholar]
- 67. Klok FA, Tesche C, Rappold L, Dellas C, Hasenfuß G, Huisman MV, Konstantinides S, Lankeit M. External validation of a simple non‐invasive algorithm to rule out chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Res. 2015;135(5):796–801. [DOI] [PubMed] [Google Scholar]
- 68.Boon G, Ende‐Verhaar YM, Bavalia R, El Bouazzaoui LH, Delcroix M, Dzikowska‐Diduch O, Huisman MV, Kurnicka K, Mairuhu A, Middeldorp S, Pruszczyk P, Ruigrok D, Verhamme P, Vliegen HW, Vonk Noordegraaf A, Vriend J, Klok FA, InShape II study group . Non‐invasive early exclusion of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: the InShape II study. Thorax. 2021;76(10):1002–9. [DOI] [PubMed] [Google Scholar]
- 69. Salg GA, Ganten MK, Bucher AM, Kenngott HG, Fink MA, Seibold C, Fischbach RE, Schlamp K, Velandia CA, Fervers P, Doellinger F, Luger A, Afat S, Merle U, Diener MK, Pereira PL, Penzkofer T, Persigehl T, Othman A, Heußel CP, Baumhauer M, Widmann G, Stathopoulos K, Hamm B, Vogl TJ, Nikolaou K, Kauczor HU, Kleesiek J. A reporting and analysis framework for structured evaluation of COVID‐19 clinical and imaging data. NPJ Digit Med. 2021;4(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lorenz G, Saeedan MB, Bullen J, Klok FA, Kroft L, Meijboom LJ, Heresi GA, Sripariwuth A, Renapurkar RD. CT‐Based biomarkers for prediction of chronic thromboembolic pulmonary hypertension after an acute pulmonary embolic event. AJR Am J Roentgenol. 2020;215(4):800–6. [DOI] [PubMed] [Google Scholar]
- 71. Ende‐Verhaar YM, Meijboom LJ, Kroft LJM, Beenen L, Boon G, Middeldorp S, Nossent EJ, Symersky P, Huisman MV, Bogaard HJ, Vonk Noordegraaf A, Klok FA. Usefulness of standard computed tomography pulmonary angiography performed for acute pulmonary embolism for identification of chronic thromboembolic pulmonary hypertension: results of the InShape III study. J Heart Lung Transplant. 2019;38(7):731–8. [DOI] [PubMed] [Google Scholar]
- 72. Braams NJ, Boon G, de Man FS, van Es J, den Exter PL, Kroft L, Beenen L, Huisman MV, Nossent EJ, Boonstra A, Vonk Noordegraaf A, Ruigrok D, Klok FA, Bogaard HJ, Meijboom LJ. Evolution of CT findings after anticoagulant treatment for acute pulmonary embolism in patients with and without an ultimate diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;58(6):2100699. [DOI] [PubMed] [Google Scholar]
- 73. Boon G, Ende‐Verhaar YM, Beenen LFM, Coolen J, Delcroix M, Golebiowski M, Huisman MV, Mairuhu A, Meijboom LJ, Middeldorp S, Pruszczyk P, van Rooden CJ, Vonk Noordegraaf A, Kroft L, Klok FA. Prediction of chronic thromboembolic pulmonary hypertension with standardised evaluation of initial computed tomography pulmonary angiography performed for suspected acute pulmonary embolism. Eur Radiol. 2022;32(4):2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boon G, Jairam PM, Groot GMC, van Rooden CJ, Ende‐Verhaar YM, Beenen L, Kroft L, Bogaard HJ, Huisman MV, Symersky P, Vonk Noordegraaf A, Meijboom LJ, Klok FA. Identification of chronic thromboembolic pulmonary hypertension on CTPAs performed for diagnosing acute pulmonary embolism depending on level of expertise. Eur J Intern Med. 2021;93:64–70. [DOI] [PubMed] [Google Scholar]
- 75. Zantonelli G, Cozzi D, Bindi A, Cavigli E, Moroni C, Luvarà S, Grazzini G, Danti G, Granata V, Miele V. Acute pulmonary embolism: prognostic role of computed tomography pulmonary angiography (CTPA). Tomography. 2022;8(1):529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, Ledot S, Morgan C, Passariello M, Price S, Singh S, Thakuria L, Trenfield S, Trimlett R, Weaver C, Wort SJ, Xu T, Padley S, Devaraj A, Desai SR. Pulmonary angiopathy in severe COVID‐19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202(5):690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grist JT, Chen M, Collier GJ, Raman B, Abueid G, McIntyre A, Matthews V, Fraser E, Ho LP, Wild JM, Gleeson F. Hyperpolarized (129)Xe MRI abnormalities in dyspneic patients 3 months after COVID‐19 pneumonia: preliminary results. Radiology. 2021;301(1):E353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rahaghi FN, Ross JC, Agarwal M, González G, Come CE, Diaz AA, Vegas‐Sánchez‐Ferrero G, Hunsaker A, San José Estépar R, Waxman AB, Washko GR. Pulmonary vascular morphology as an imaging biomarker in chronic thromboembolic pulmonary hypertension. Pulm Circ. 2016;6(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Morris MF, Pershad Y, Kang P, Ridenour L, Lavon B, Lanclus M, Godon R, De Backer J, Glassberg MK. Altered pulmonary blood volume distribution as a biomarker for predicting outcomes in COVID‐19 disease. Eur Respir J. 2021;58(3):2004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lins M, Vandevenne J, Thillai M, Lavon BR, Lanclus M, Bonte S, Godon R, Kendall I, De Backer J, De Backer W. Assessment of small pulmonary blood vessels in COVID‐19 patients using HRCT. Acad Radiol. 2020;27(10):1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Thillai M, Patvardhan C, Swietlik EM, McLellan T, De Backer J, Lanclus M, De Backer W, Ruggiero A. Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID‐19. Thorax. 2021;76(2):182–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


