Abstract
Objectives
In this study covering all of Turkey, we aimed to define cutaneous and systemic adverse reactions in our patient population after COVID‐19 vaccination with the Sinovac/CoronaVac (inactivated SARS‐CoV‐2) and Pfizer/BioNTech (BNT162b2) vaccines.
Methods
This prospective, cross‐sectional study included individuals presenting to the dermatology or emergency outpatient clinics of a total of 19 centers after having been vaccinated with the COVID‐19 vaccines. Systemic, local injection site, and non‐local cutaneous reactions after vaccination were identified, and their rates were determined.
Results
Of the 2290 individuals vaccinated between April 15 and July 15, 2021, 2097 (91.6%) received the CoronaVac vaccine and 183 (8%) BioNTech. Systemic reactions were observed at a rate of 31.0% after the first CoronaVac dose, 31.1% after the second CoronaVac dose, 46.4% after the first BioNTech dose, and 46.2% after the second BioNTech dose. Local injection site reactions were detected at a rate of 35.6% after the first CoronaVac dose, 35.7% after the second CoronaVac dose, 86.9% after the first BioNTech dose, and 94.1% after the second BioNTech dose. A total of 133 non‐local cutaneous reactions were identified after the CoronaVac vaccine (2.9% after the first dose and 3.5% after the second dose), with the most common being urticaria/angioedema, pityriasis rosea, herpes zoster, and maculopapular rash. After BioNTech, 39 non‐local cutaneous reactions were observed to have developed (24.8% after the first dose and 5% after the second dose), and the most common were herpes zoster, delayed large local reaction, pityriasis rosea, and urticaria/angioedema in order of frequency. Existing autoimmune diseases were triggered in 2.1% of the patients vaccinated with CoronaVac and 8.2% of those vaccinated with BioNTech.
Conclusions
There are no comprehensive data on cutaneous adverse reactions specific to the CoronaVac vaccine. We determined the frequency of adverse reactions from the dermatologist's point of view after CoronaVac and BioNTech vaccination and identified a wide spectrum of non‐local cutaneous reactions. Our data show that CoronaVac is associated with less harmful reactions while BioNTech may result in more serious reactions, such as herpes zoster, anaphylaxis, and triggering of autoimmunity. However, most of these reactions were self‐limiting or required little therapeutic intervention.
1. INTRODUCTION
The pandemic caused by the novel coronavirus disease 2019 (COVID‐19) has had an unprecedented impact on overall health and the global economy. Rapidly developed public health strategies helped control the spread of COVID‐19, but were not enough to reduce the impact of the disease. Vaccination is one of the most powerful tools available in the ongoing battle against the COVID‐19 disease. Following emergency use approval issued by the Turkish Medicines and Medical Devices Agency, Sinovac/CoronaVac (inactivated SARS‐CoV‐2) has been implemented since January 13, 2021, and mRNA‐based Pfizer/BioNTech (BNT162b2) since April 2, 2021, in Turkey. CoronaVac was the first choice of vaccine in Turkey for protection against the disease.
Fatigue and pain have been reported as the most common systemic and local adverse reactions in phase 3 clinical trials of both vaccines. 1 , 2 Population‐based, 3 hospital‐based, 4 and international registry‐based 5 studies have also been undertaken to investigate the cutaneous adverse reactions to the BioNTech vaccine. However, there are only small‐scale studies 6 and case series 7 , 8 concerning the cutaneous adverse reactions to the CoronaVac vaccine.
In this study covering all of Turkey, we aimed to define cutaneous and systemic adverse reactions in our patient population after COVID‐19 vaccination with the Sinovac/CoronaVac (inactivated SARS‐CoV‐2) and Pfizer/BioNTech (BNT162b2) vaccines.
2. METHODS
2.1. Study design and participants
We conducted a nationwide, multicenter, prospective, cross‐sectional study. The study group consisted of patients aged over 18 years, who presented to dermatology or emergency outpatient clinics after having been vaccinated with the CoronaVac or BioNTech vaccine between 15 April and 15 July 2021. The dermatologist himself/herself made the evaluation in patients admitted to the emergency department and in all patients. Patients from 19 centers selected according to population density and by covering all geographical regions of Turkey were included in the sample.
All individuals who developed or did not develop a reaction after both doses of either vaccine were included in the study. For both vaccines, the second dose was administered four‐six weeks after the first dose. To identify adverse reactions, the patients that received the first vaccine dose were followed up in terms of their second dose to ensure safety surveillance. In addition, individuals who developed a reaction within 20 days of the first dose were also included in the study without waiting for their second dose. The aim here was not to miss these individuals who had not yet received their second vaccine dose. Only reactions within the first 20 days after vaccination were considered to be associated with the vaccine. Patients using analgesics and antibiotics and those having cutaneous reactions were not included in the study. In addition, patients using immunosuppressant drugs, such as corticosteroids and cyclosporine, were excluded. There were no pregnant/breastfeeding participants; this group generally avoided the vaccine.
Age, sex, occupation, presence of comorbidities, personal and family history of allergic diseases (such as asthma, atopic dermatitis), COVID‐19 vaccine type and dose information, and post‐vaccination adverse reactions were recorded for all the participants. Post‐vaccination adverse reactions were defined as systemic reactions, local injection site reactions, and non‐local cutaneous reactions. Systemic reactions included early anaphylaxis, late anaphylaxis, fever, headache, nausea/diarrhea, myalgia, and fatigue. Cases accompanied by skin and mucosal signs and respiratory symptoms and/or hypotension were considered as early anaphylaxis if they occurred within the first 30 minutes and late anaphylaxis if observed after 30 minutes. Local injection site reactions included injection site pain, erythema, and edema. While detailing non‐local cutaneous reactions, a detailed history was taken from all the patients, and they all underwent physical and dermatological examinations. Routine blood tests and skin punch biopsy, if necessary, were performed in these cases. Biopsy was preferred only in cases where clinical findings were not sufficient for the diagnosis. These were cases in which biopsy was indispensable in the differential diagnosis, such as bullous dermatoses. Non‐local cutaneous reactions were defined based on the presence of the following four criteria: (i) typical clinical presentation, (ii) blood and tissue eosinophilia and/or clinical‐pathological correlation, (iii) no history of suspicious drug use, and (iv) reaction being observed within the first 20 days after vaccination. As an exception to the autoimmune or autoinflammatory group, the first two months after the second vaccination was considered as the limit for the development of a new dermatological disease. In addition, any post‐dose trigger of existing dermatological or autoimmune diseases was recorded.
2.2. Ethics approval statement
Ethical approval was obtained from the local ethics committee (date: 27/04/2021, number: 2021.106.04.01), and the study was also approved by the Turkish Ministry of Health (date: 14/04/2021, number: 14T_16_46_07). Informed consent was obtained from all the participants.
2.3. Outcomes
The primary outcome of our study was to identify systemic and cutaneous adverse reactions after vaccination and determine their rates. The secondary outcome was to determine the possible relationship of systemic and cutaneous adverse reactions with demographic characteristics, comorbidities, and allergic diseases.
2.4. Statistical analysis
SPSS v. 15.0 software package for Windows was used for statistical analyses. Descriptive statistics were presented as numbers and percentages for categorical variables, and mean, standard deviation, minimum, maximum, and median values for numerical variables. The chi‐square test was used to compare the rates between independent single, paired, and multiple groups. Since the normal distribution conditions were not met, the comparison of numerical variables was undertaken using the Mann–Whitney U‐test for two independent groups and the Kruskal–Wallis test for more than two groups. The statistical alpha significance level was accepted as p < 0.05.
3. RESULTS
3.1. Demographic characteristics
The study included a total of 2290 people vaccinated against the COVID‐19 disease. Table 1 presents the detailed demographic and basic characteristics of the patients according to the vaccine type and dose.
TABLE 1.
Demographic characteristics of the study group
| Total a (n = 2290) | First dose | Total (n = 2189) | Second dose | |||||
|---|---|---|---|---|---|---|---|---|
| CoronaVac (n = 2097) | BioNTech (n = 183) | p value | CoronaVac (n = 2060) | BioNTech (n = 119) | p value | |||
| Sex n (%) | ||||||||
| Female | 1291 (56.4%) | 1192 (56.8%) | 91 (49.7%) | 0.057 | 1236 (56.5%) | 1170 (56.8%) | 58 (48.7%) | 0.085 |
| Male | 999 (43.6%) | 905 (43.2%) | 92 (50.3%) | 953 (43.5%) | 890 (43.2%) | 61 (51.3%) | ||
| Age Mean ± SD (Min‐Max) | 50.4 ± 17.9 (20–96) | 50.3 ± 18.3 (20–96) | 50.6 ± 11.4 (24–90) | 0.909 | 50.4 ± 18.1 (20–96) | 50.2 ± 18.4 (20–96) | 52.5 ± 10.9 (24–81) | 0.233 |
| Occupation n (%) | ||||||||
| Healthcare professional | 1167 (51.0%) | 1.124 (53.6%) | 43 (23.5%) | <0.001 | 1145 (52.3%) | 1115 (54.1%) | 30 (25.2%) | <0.001 |
| Other | 265 (11.6%) | 186 (8.9%) | 79 (43.2%) | 229 (10.5%) | 178 (8.6%) | 51 (42.9%) | ||
| Unemployed | 858 (37.5%) | 787 (37.5%) | 61 (33.3%) | 815 (37.2%) | 767 (37.2%) | 38 (31.9%) | ||
| Comorbidities | 1050 (45.9%) | 975 (46.5%) | 66 (36.1%) | <0.001 | 1015 (46.4%) | 958 (46.5%) | 48 (40.3%) | 0.189 |
| Hypertension + CAD + CHF | 679 (29.7%) | 631 (30.1%) | 40 (21.9%) | <0.001 | 660 (30.2%) | 621 (30.1%) | 31 (26.1%) | 0.343 |
| Diabetes mellitus | 339 (14.8%) | 310 (14.8%) | 24 (13.1%) | 0.006 | 325 (14.8%) | 304 (14.8%) | 16 (13.4%) | 0.694 |
| CD, ED, AD | 211 (9.2%) | 196 (9.3%) | 14 (7.7%) | 0.746 | 203 (9.3%) | 192 (9.3%) | 10 (8.4%) | 0.737 |
| Oncological diseases | 45 (2.0%) | 40 (1.9%) | 4 (2.2%) | 0.212 | 41 (1.9%) | 37 (1.8%) | 3 (2.5%) | 0.478 |
| Other | 179 (7.8%) | 170 (8.1%) | 9 (4.9%) | 0.199 | 172 (7.9%) | 167 (8.1%) | 5 (4.2%) | 0.125 |
| Allergic disease history | 402 (17.6%) | 370 (17.6%) | 29 (15.8%) | 0.484 | 382 (17.5%) | 361 (17.5%) | 18 (15.1%) | 0.502 |
| Asthma | 120 (5.2%) | 113 (5.4%) | 5 (2.7%) | 0.033 | 119 (5.4%) | 112 (5.4%) | 5 (4.2%) | 0.561 |
| Urticaria/angioedema | 81 (3.5%) | 72 (3.4%) | 9 (4.9%) | 0.483 | 74 (3.4%) | 71 (3.4%) | 3 (2.5%) | 0.796 |
| Rhinoconjunctivitis | 212 (9.3%) | 200 (9.5%) | 11 (6.0%) | 0.287 | 204 (9.3%) | 196 (9.5%) | 7 (5.9%) | 0.185 |
| Drug/food allergy | 108 (4.7%) | 96 (4.6%) | 1 (6.0%) | 0.498 | 104 (4.8%) | 94 (4.6%) | 9 (7.6%) | 0.134 |
| Atopic dermatitis | 70 (3.1%) | 65 (3.1%) | 3 (1.6%) | 0.004 | 67 (3.1%) | 62 (3.0%) | 3 (2.5%) | 1.000 |
Abbreviations: AD, autoimmune diseases; CAD, coronary artery disease; CD, connective diseases; CHF, congestive heart failure; ED, endocrinological diseases.
Type of vaccine applied not known for 10 participants.
3.2. Adverse reaction prevalence
The most common adverse event observed after the first and second doses of either vaccine was local injection site reactions [n = 908 (39.7%) and n = 849 (38.8%), respectively]. At least one local injection site reaction was observed in 747 (35.6%) of the 2097 participants that had received the first CoronaVac dose and 159 (86.9%) of the 183 participants that had received the first BioNTech dose. At least one local injection site reaction was detected in 735 (35.7%) of the 2060 participants that had received the second CoronaVac dose and 112 (94.1%) of the 119 participants that had received the second BioNTech dose. Pain was determined to be the most common local reaction after both vaccines.
A total of 133 non‐local cutaneous reactions were observed to have developed after CoronaVac [60 (2.9%) after the first dose and 73 (3.5%)after the second dose], with the most common being urticaria/angioedema [n = 16 (0.8%)and n = 24 (1.2%), respectively], pityriasis rosea [n = 9 (0.4%)and n = 12 (0.6%), respectively],herpes zoster [n = 9 (0.4%)and n = 10 (0.5%), respectively], maculopapular rash [n = 7 (0.3%) and n = 8 (0.4%), respectively], and contact dermatitis [n = 7 (0.3%)and n = 7 (0.3%), respectively]. After BioNTech, there were a total of 39 non‐local cutaneous reactions [33 (4.8%) after the first dose and 6 (5%)after the second dose], with the most common being herpes zoster [n = 8 (4.4%)after the first dose], delayed large local reactions [n = 7 (3.8%) after the first dose], pityriasis rosea [n = 3 (1.6%)after the first dose and n = 3 (2.5%) after the second dose], and urticaria/angioedema [n = 5 (2.7%) and n = 1 (0.8%), respectively].
At least one systemic reaction developed in 650 (31.0%) of the 2097 participants that had received the first CoronaVac dose, 85 (46.4%) of the 183 participants that had received the first BioNTech dose, 641 (31.1%) of the 2060 participants that had received the second CoronaVac dose, and 55 (46.2%) of the 119 participants that had received the second BioNTech dose. After both vaccines, fatigue was the most common systemic reaction. Fatigue, headache, myalgia, fever after both doses, and nausea/diarrhea after only the first dose were statistically significantly higher in the BioNTech group compared to CoronaVac. Characteristics of adverse reactions after COVID‐19 vaccination are seen in Table 2. Of the anaphylaxis cases, three emerged after the first dose (n = 1 for CoronaVac and n = 2 for BioNTech) and one after the second dose of BioNTech. When the first and second dose groups were compared separately, there was no statistically significant difference in the development of anaphylaxis between BioNTech and CoronaVac. However, when both vaccines are compared regardless of the dose, and early‐late anaphylaxis is evaluated together; anaphylaxis was detected in 0.05% after CoronaVac and 1.6% after BioNTech, which was statistically significant (p = 0.002, p < 0.05). Regardless of dose, there was no significant difference between CoronaVac and BioNTech for the development of early anaphylaxis (0.05% vs. 0.5%; p = 0.153, p > 0.05), while there was a statistically significant difference for the development of late anaphylaxis (0.0% vs. 1.1%; p = 0.006, p < 0.05). For BioNTech, there was no statistically significant difference in the rate of systemic reactions between the first and second doses (p = 0.362). Table S1 presents the comparison of the development of adverse reactions after the first and second doses of the two vaccines. The rates of local injection site reactions were significantly higher after the first dose of both the CoronaVac and BioNTech vaccines compared to their second doses (p < 0.001 and p = 0.023, respectively). The rate of non‐local cutaneous reactions associated with CoronaVac was higher after the first dose compared to the second dose (p = 0.022). For BioNTech, the post‐vaccination development of non‐local cutaneous reactions was similar between the first and second doses (p = 1.000) (Table S1).
TABLE 2.
Characteristics of adverse reactions after COVID‐19 vaccination a
| Total (n = 2290) | First dose | Total (n = 2189) | Second dose | |||||
|---|---|---|---|---|---|---|---|---|
| CoronaVac (n = 2097) | BioNTech (n = 183) | p value | CoronaVac (n = 2060) | BioNTech (n = 119) | p value | |||
| Local injection site reactions | 908 (39.7%) | 747 (35.6%) | 159 (86.9%) | <0.001 | 849 (38.8%) | 735 (35.7%) | 112 (94.1%) | <0.001 |
| Pain | 678 (29.6%) | 547 (26.1%) | 130 (71.0%) | <0.001 | 515 (23.5%) | 445 (21.6%) | 68 (57.1%) | <0.001 |
| Erythema | 124 (5.4%) | 80 (3.8%) | 43 (23.5%) | <0.001 | 56 (2.6%) | 46 (2.2%) | 8 (6.7%) | 0.008 |
| Edema | 117 (5.1%) | 88 (4.2%) | 29 (15.8%) | <0.001 | 64 (2.9%) | 58 (2.8%) | 6 (5.0%) | 0.159 |
| Systemic reactions | 739 (32.3%) | 650 (31.0%) | 85 (46.4%) | <0.001 | 700 (32.0%) | 641 (31.1%) | 55 (46.2%) | 0.002 |
| Fatigue | 411 (17.9%) | 350 (16.7%) | 58 (31.7%) | <0.001 | 263 (12.0%) | 236 (11.5%) | 26 (21.8%) | 0.001 |
| Headache | 341 (14.9%) | 297 (14.2%) | 44 (24.0%) | 0.001 | 206 (9.4%) | 189 (9.2%) | 17 (14.3%) | 0.064 |
| Myalgia | 250 (10.9%) | 207 (9.9%) | 41 (22.4%) | <0.001 | 172 (7.9%) | 149 (7.2%) | 22 (18.5%) | <0.001 |
| Fever | 57 (2.5%) | 38 (1.8%) | 18 (9.8%) | <0.001 | 29 (1.3%) | 21 (1.0%) | 8 (6.7%) | <0.001 |
| Nausea/diarrhea | 32 (1.4%) | 22 (1.0%) | 10 (5.5%) | <0.001 | 9 (0.4%) | 8 (0.4%) | 1 (0.8%) | 0.397 |
| Early anaphylaxis | 2 (0.09%) | 1 (0.05%) | 1 (0.5%) | 0.157 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Late anaphylaxis | 1 (0.04%) | 0 (0.0%) | 1 (0.5%) | 0.083 | 1 (0.05%) | 0 (0.0%) | 1 (0.8%) | 0.055 |
| Non‐local cutaneous reactions | 94 (4.1%) | 60 (2.9%) | 33 (18.0%) | <0.001 | 81 (3.7%) | 73 (3.5%) | 6 (5.0%) | 0.442 |
| Diagnosed by | ||||||||
| Dermatologist | 85 (90.4%) | 53 (88.3%) | 31 (93.9%) | 0.540 | 79 (97.5%) | 71 (97.3%) | 6 (100%) | 1.000 |
| Emergency physician | 9 (9.6%) | 7 (11.7%) | 2 (6.1%) | 2 (2.5%) | 2 (2.7%) | 0 (0.0%) | ||
| Diagnosis | ||||||||
| Herpes zoster | 17 (0.7%) | 9 (0.4%) | 8 (4.4%) | <0.001 | 10 (0.5%) | 10 (0.5%) | 0 (0.0%) | 1.000 |
| Pityriasis rosea | 12 (0.5%) | 9 (0.4%) | 3 (1.6%) | 0.065 | 15 (0.7%) | 12 (0.6%) | 3 (2.5%) | 0.045 |
| Petechial rash | 1 (0.05%) | 1 (0.05%) | 0 (0.0%) | 1.000 | – | – | – | – |
| Dyshidrotic eczema | 4 (0.2%) | 2 (0.1%) | 2 (1.1%) | 0.035 | 1 (0.05%) | 1 (0.05%) | 0 (0.0%) | 1.000 |
| Urticaria/angioedema within 24 hours | 6 (0.3%) | 4 (0.2%) | 2 (1.1%) | 0.078 | 6 (0.3%) | 6 (0.3%) | 0 (0.0%) | 1.000 |
| Urticaria/angioedema after 24 hours | 15 (0.7%) | 12 (0.6%) | 3 (1.6%) | 0.113 | 19 (0.9%) | 18 (0.9%) | 1 (0.8%) | 1.000 |
| Lichenoid reaction | 2 (0.1%) | 2 (0.1%) | 0 (0.0%) | 1.000 | 2 (0.1%) | 2 (0.1%) | 0 (0.0%) | 1.000 |
| Erythromelalgia | 1 (0.05%) | 0 (0.0%) | 1 (0.5%) | 0.080 | – | – | – | |
| Contact dermatitis | 9 (0.4%) | 7 (0.3%) | 2 (1.1%) | 0.159 | 8 (0.4%) | 7 (0.3%) | 1 (0.8%) | 0.358 |
| Delayed large local reaction | 8 (0.4%) | 1 (0.05%) | 7 (3.8%) | <0.001 | 1 (0.05%) | 1 (0.05%) | 0 (0.0%) | 1.000 |
| SDRIFE | 1 (0.05%) | 1 (0.05%) | 0 (0.0%) | 1.000 | – | – | – | |
| Disseminated herpes zoster | 1 (0.05%) | 0 (0.0%) | 1 (0.5%) | 0.080 | – | – | – | |
| Disseminated herpes simplex | – | – | – | – | 1 (0.05%) | 1 (0.05%) | 0 (0.0%) | 1.000 |
| Acneiform eruption | 1 (0.05%) | 0 (0.0%) | 1 (0.5%) | 0.080 | – | – | – | – |
| Maculopapular rash | 9 (0.4%) | 7 (0.3%) | 2 (1.1%) | 0.159 | 9 (0.4%) | 8 (0.4%) | 1 (0.8%) | 0.393 |
| Erythroderma | 1 (0.05%) | 0 (0.0%) | 1 (0.5%) | 0.080 | – | – | – | – |
| Bullous pemphigoid | 2 (0.1%) | 2 (0.1%) | 0 (0.0%) | 1.000 | – | – | – | – |
| Chilblain | – | – | – | – | 2 (0.1%) | 2 (0.1%) | 0 (0.0%) | 1.000 |
| Cutaneous vasculitis | – | – | – | – | 2 (0.1%) | 2 (0.1%) | 0 (0.0%) | 1.000 |
| Psoriasis vulgaris | 2 (0.1%) | 1 (0.05%) | 1 (0.5%) | 0.154 | 3 (0.1%) | 2 (0.1%) | 1 (0.8%) | 0.153 |
| Cutaneous sarcoidosis | – | – | – | – | 1 (0.05%) | 0 (0.0%) | 1 (0.8%) | 0.054 |
| Linear IgA bullous dermatosis | – | – | – | – | 1 (0.05%) | 1 (0.05%) | 0 (0.0%) | 1.000 |
Abbreviations: SDRIFE, symmetrical drug‐related intertriginous and flexural exanthema.
Type of vaccine not known for four of the local injection reactions, eight of the systemic reactions, and three of the non‐local cutaneous reactions.
For 168 of the 175 non‐local cutaneous reactions, there was information on how many days after vaccination the reaction started. Non‐local cutaneous reactions occurred after a mean duration of 6.2 ± 4.3 (median: 6; range: 1–20) days after the first CoronaVac dose, 7.6 ± 6 (median: 7; range: 1–30) days after the second CoronaVac dose, 7.2 ± 5.8 (median: 5.5; range: 1–19) days after the first BioNTech dose, and 8.8 ± 5.5 (median: 8.5; range: 2–15) days after the second BioNTech dose. Table S1 shows the onset times of non‐local cutaneous reactions according to the type and dose of vaccines. Figure 1 presents the images of some of the cutaneous reactions that developed after CoronaVac and BioNTech vaccination and Table 3 gives the detailed characteristics of these reactions. Of all the non‐local cutaneous reactions, 84.6% developed within the first 10 days after the first dose and 72.7% within the first 10 days after the second dose (Figure 2). Non‐local cutaneous reactions that developed earliest within the first day after the first dose were petechial rash, dyshidrotic eczema, and urticaria/angioedema for CoronaVac and maculopapular rash, erythroderma, and urticaria/angioedema for BioNTech. Of the urticaria/angioedema cases that developed within 24 hours, six occurred after the first dose (n = 4 for CoronaVac and n = 2 for BioNTech), and six occurred after the second dose (all CoronaVac). The rate of these serious reactions, such as anaphylaxis and urticaria/angioedema developing within the first 24 hours, was 0.5% for CoronaVac compared to 2.72% for BioNTech.
FIGURE 1.
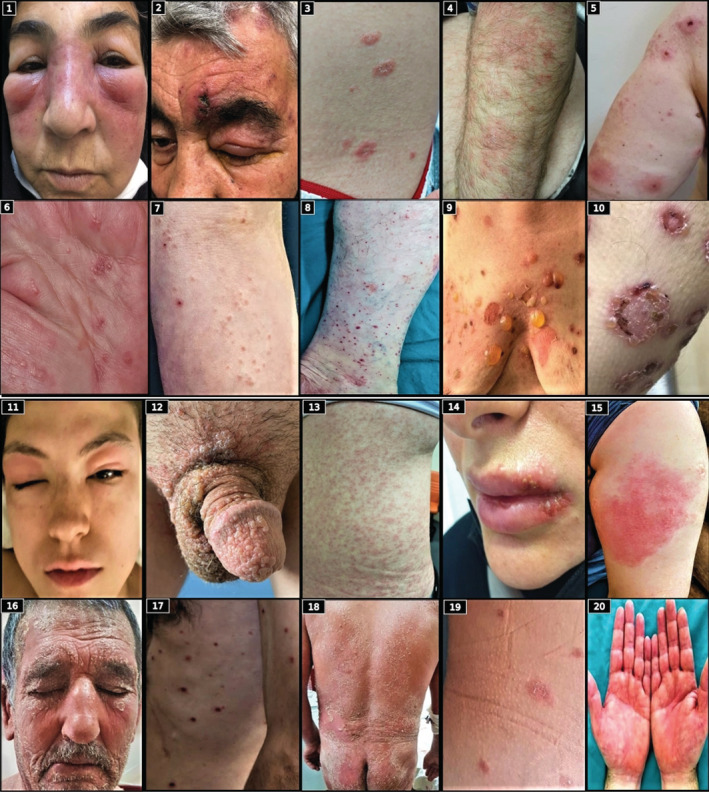
(1) Urticaria/angioedema. (2) Ophthalmic herpes zoster. (3) Pityriasis rosea. (4) Allergic eczematous contact dermatitis. (5) Erythema multiforme. (6) Dyshidrotic eczema. (7) Lichenoid reaction. (8) Leukocytoclastic vasculitis. (9) Bullous pemphigoid. (10) Linear IgA bullous dermatosis. (11) Urticaria/angioedema. (12) Herpes zoster. (13) Maculopapular rash. (14) Herpes labialis. (15) Delayed large local reaction. (16) Allergic eczematous contact dermatitis. (17) Disseminated Herpes zoster. (18) Erythroderma. (19) Pityriasis rosea. (20) Erythromelalgia.
TABLE 3.
Characteristics of some cases with cutaneous reactions to BioNTech and CoronaVac vaccines
| Case number | Age/sex | Type of vaccine | Dose after which adverse event was seen | Non‐local cutaneous reactions | Onset time of reaction | Localization | Other symptoms | Comorbidity |
|---|---|---|---|---|---|---|---|---|
| 1 | 50s/F | CoronoVac | Second dose | Urticaria/angioedema | Day 5 | Face, trunk, back Extremities, neck Groin, axilla |
Fever, tachycardia Pruritus, dyspnea |
Diabetes hypertension Asthma |
| 2 | 60s/M | CoronoVac | Single dose | Ophthalmic herpes zoster | Day 17 | Face, scalp |
Fever Facial pain |
Hypertension |
| 3 | 20s/M | CoronoVac | Second dose | Pityriasis rosea | Day 7 | Trunk | None | None |
| 4 | 70s/M | CoronoVac | Second dose | AECD | Day 1 | Trunk, back Extremities, palm‐sole |
Pruritus Eosinophilia |
Hypertension |
| 5 | 60s/M | CoronoVac | First dose | Erythema multiforme | Day 10 |
Trunk, back Extremities |
Headache, fever Leukocytosis |
None |
| 6 | 70s/F | CoronoVac | First dose | Dyshidrotic eczema | Day 1 | Hands, feet | Burning, pruritus | Hypertension |
| 7 | 60s/F | CoronoVac | Second dose | Lichenoid reaction | Day 3 | Bilateral forearms | Pruritus | Endocrinological Disease, CAD |
| 8 | 60s/M | CoronoVac | Second dose | Leukocytoclastic vasculitis | Day 20 | Lower extremities |
Burning Pain in the legs |
None |
| 9 | 60s/F | CoronoVac | Both doses | Bullous pemphigoid | Day 10 | Trunk, back, extremities |
Pruritus, burning Eosinophilia |
Hypertension Bullous pemphigoid |
| 10 | 70s/M | CoronoVac | Second dose | Linear IgA bullous dermatosis | Day 30 | Trunk, neck, groin Axilla | Burning | Hypertension |
| 11 | 20s/F | BioNTech | Both doses | Urticaria/angioedema | Day 1 |
Face, trunk Extremities |
Pruritus, dyspnea Tachycardia Hypotension |
None |
| 12 | 30s/M | BioNTech | First dose | Herpes zoster | Day 17 | Genital, gluteal | Pain, anemia | Immunodeficiency |
| 13 | 40s/F | BioNTech | First dose | Maculopapular rash | Day 1 |
Trunk, back Extremities |
Pruritus, Eosinophilia | None |
| 14 | 30s/F | BioNTech | First dose | Herpes labialis | Day 5 | Lips | Fever, pain | None |
| 15 | 20s/M | BioNTech | First dose | Delayed large local reaction | Day 4 | Arm | Fever, pain | None |
| 16 | 50s/M | BioNTech | First dose | AECD | Day 14 | Face, trunk, back Extremities | Pruritus Eosinophilia | Diabetes, hypertension |
| 17 | 40s/M | BioNTech | First dose | Disseminated Herpes zoster | Day 14 |
Face, trunk, back Extremities |
Fever, headache nausea, vomiting meningeal irritation lymphopenia |
Kidney transplant (patient with immunodeficiency) |
| 18 | 60s/M | BioNTech | First dose | Erythroderma | Within 10 hours |
Face, groin, axilla Trunk, extremities Oral‐ genitalmucosa |
Fever Leukocytosis |
Hypertension, diabetes |
| 19 | 30s/F | BioNTech | First dose | Pityriasis rosea | Day 4 | Trunk and back | Fever, pruritus | None |
| 20 | 40s/M | BioNTech | First dose | Erythromelalgia | Day 14 |
Palm‐sole Urticarial lesions |
Pruritus | Cranial tumor |
Abbreviations: AECD, allergic eczematous contact dermatitis; CAD, coronary artery disease; F, female; M, male.
FIGURE 2.
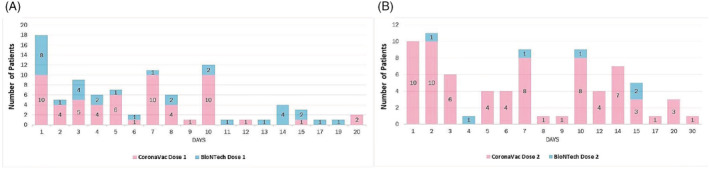
Of all the non‐local cutaneous reactions, 84.6% developed within the first 10 days after the first dose and 72.7% within the first 10 days after the second dose.
Pre‐existing autoimmune diseases were triggered in 44 (2.1%) of the patients vaccinated with CoronaVac and 15 (8.2%) of those vaccinated with BioNTech. The diseases listed in Table S2 were cases with new‐onset after vaccination. The most common diseases triggered after CoronaVac were chronic urticaria (n = 15, 0.7%) and psoriasis vulgaris (n = 13, 0.6%). Similarly, the most common diseases triggered after BioNTech were psoriasis vulgaris (n = 8, 4.4%) and chronic urticaria (n = 4, 2.2%). Triggering of herpes simplex from mucosal lesions was observed at similar rates after the two vaccines [n = 90 (4.3%) for CoronaVac and n = 9 (4.9%) for BioNTech] (Table S2).
3.3. Risk factors associated with adverse reactions
We also examined whether adverse reactions varied according to the characteristics of individuals, such as sex, age, presence of comorbidities, and history of allergic diseases. After CoronaVac, the rate of local injection site reactions was statistically significantly higher in women, patients aged <55 years, those without chronic diseases, and those with a history of allergic diseases (p < 0.001 for all).
After CoronaVac, non‐local cutaneous reactions were more common in patients aged >55 years, those with chronic diseases, and those with allergic diseases (p = 0.005, p = 0.001, and p = 0.023, respectively), but no significant difference was found according to sex (p = 0.462). For BioNTech, while the development of non‐local cutaneous reactions was more common in patients aged <55 years (p = 0.028), there was no significant difference in relation to sex, presence of comorbidities, and history of allergic diseases (p = 0.827, p = 0.689, and p = 0.281, respectively).
After CoronaVac, systemic reactions were statistically significantly higher in women, patients aged <55 years, those without comorbidities, and those with a history of allergic diseases (p < 0.001 for all). For BioNTech, no significant difference was found in relation to any of the possible risk factors (Figure 3).
FIGURE 3.
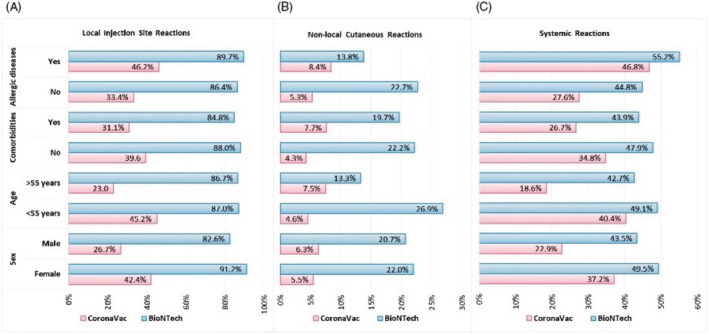
Distribution of adverse reactions after the CoronaVac and BioNTech vaccines by sex, age, presence of comorbidities, and history of allergic diseases. Local injection site reactions (a), non‐local cutaneous reactions (b), and systemic reactions (c).
4. DISCUSSION
In this study, we investigated the rates of adverse reactions that developed after the administration of two COVID‐19 vaccines and were mostly diagnosed by dermatologists. In addition to the commonly observed systemic and local injection site reactions, we defined non‐local cutaneous reactions together with their onset times.
In this study, the most common reactions after both doses of the CoronaVac vaccine were pain at the injection site, fatigue, and headache at the rates of 26.1%, 16.7%, and 14.2%, respectively, after the first dose and 21.6%, 11.5%, and 9.2%, respectively, after the second dose. The most frequently observed adverse reactions reported in the phase 3 clinical trials of the CoronaVac vaccine are similar to our study. The rate of pain at the injection site has been reported as 2.36%–60.3%, 1 , 9 , 10 , 11 fatigue 8.22%–26.8%, 1 , 9 , 10 , 11 and headache 5.91%–48.5%. 1 , 9 , 10 , 11
The non‐local cutaneous reactions we detected after the CoronaVac vaccine were heterogeneous, and the most common reactions after the first and second dose were similar and were urticaria/angioedema and pityriasis rosea. In a study by Durmaz et al., who used an e‐mail survey to evaluate 221 healthcare professionals vaccinated with CoronaVac, 33 cutaneous reactions were reported, with the most common being urticaria. 6 In two case series, one including seven patients 8 and the other including six patients, 7 urticaria/angioedema was the most observed cutaneous reactions. In the current study, after the CoronaVac vaccine, type 1 immediate type reactions were observed, such as urticaria/angioedema and anaphylaxis and type 4 delayed‐type reactions, including petechial rash, dyshidrotic eczema, lichenoid reaction, erythema multiforme, contact dermatitis, delayed large local reaction, symmetrical drug‐related intertriginous and flexural exanthema, and maculopapular rash. In addition, we detected diseases associated with the human herpes virus family, including herpes zoster, pityriasis rosea, and disseminated herpes simplex. Such herpetic reactivations have also been described after the SARS‐CoV‐2 infection 12 , 13 and mRNA COVID‐19 vaccines. 3 , 5 As a possible mechanism, it has been suggested that a strong specific immune response against SARS‐CoV‐2 or the inactivated virus used in vaccines may suppress cellular immune response to another latent virus. 3 In our study, we also reported the development of some immune‐mediated skin diseases. Among these, cutaneous vasculitis and chilblain have also been described after the SARS‐CoV‐2 infection 12 and mRNA COVID‐19 vaccines. 3 , 5 Immune‐mediated skin diseases such as bullous pemphigoid, psoriasis vulgaris, and linear IgA bullous dermatosis that we reported have also been observed after various other vaccines 14 , 15 and COVID‐19 mRNA vaccines. 3 , 16 , 17 This situation may possibly be related to the inactive viral antigen inducing an autoimmune disease in people with a genetic predisposition, but it has been emphasized that there is a need for further studies to clarify this finding. 18 , 19 Unlike previous case reports, 6 , 7 , 8 , 20 , 21 , 22 in our study, we identified contact dermatitis, bullous pemphigoid, psoriasis vulgaris, and linear IgA bullous dermatosis after the CoronaVac vaccine was administered.
In this study, the most common reactions observed after the first and second doses of the BioNTech vaccine were pain at the injection site (71.0% and 57.1%, respectively), fatigue (31.7% and 21.8%, respectively), and headache (24.0% and 14.3%, respectively). This is consistent with the results of previous studies. 2 , 23 We observed a wide spectrum of non‐local cutaneous reactions after BioNTech. We also detected the presence of type 1 reactions, such as urticaria/angioedema, type 4 reactions ranging from maculopapular rash to erythroderma, and rare conditions; for example, erythromelalgia, acneiform eruption, and sarcoidosis. The most common non‐local cutaneous reactions after the first dose were herpes zoster and urticaria/angioedema, and after the second dose, it was pityriasis rosea. Although we only commented on the rates of adverse reactions due to the significant difference in the number of participants vaccinated with CoronaVac and BioNTech, we also had some important observations in this study. First, the rates of local injection site reactions and systemic reactions were higher in patients vaccinated with BioNTech compared to those vaccinated with CoronaVac for both doses. Second, non‐local cutaneous reactions were generally more common after the first dose of BioNTech compared to CoronaVac. Third, after BioNTech vaccination, the rates of herpes zoster, dyshidrotic eczema, and delayed large local reactions were particularly higher following the first dose, while that of pityriasis rosea was higher following the second dose compared to CoronaVac. Lastly, pre‐existing autoimmune diseases were triggered in a higher number of patients vaccinated with BioNTech compared to CoronaVac.
Several recent reviews examined the pathophysiological mechanism of cutaneous adverse reactions associated with COVID‐19 vaccines in light of available data. 24 , 25 Immediate reactions to COVID‐19 vaccines may be IgE‐dependent or independent of IgE. In BioNTech, polyethylene glycol (PEG), also known as macrogol, is a possible adjuvant responsible for immediate reactions. 25 The CoronaVac vaccine contains SARS‐CoV‐2 (strain CZ02) inactivated with β‐propiolactone and aluminum hydroxide as an adjuvant; thus, immediate reactions may be due to the inactivated vaccine component or the adjuvant. 26 Lichen planus, maculopapular rash, vesicular rash, erythema multiforme, and pityriasis rosea are among the reactions that occur as a result of the typical antiviral response to the vaccine, and they are characterized as Th1‐polarized cellular immune responses. 24 , 27 Many components of vaccines can act like hapten and trigger a Th2‐polarized inflammatory reaction, resulting in urticarial reactions, and exacerbation of atopic dermatitis, contact dermatitis, and autoimmune bullous dermatoses. Contact dermatitis can also be related to the dressings and infectants used. Components such as aluminum in CoronaVac and lipid nanoparticles and PEG in BioNTech may play an important role in this inflammatory model. 24 , 28 , 29 With the vaccine stimulating the innate immune system, skin‐resident memory T cells may be activated and a Th17/Th22‐predominant environment may be formed. This may cause neutrophil flow and trigger psoriasiform and pustular reactions. 24 Vaccine components may trigger the inflammatory process resulting in granulomatous reactions, and vaccine‐associated trauma and disruption of the extracellular matrix may initiate a fibrogenic inflammatory response. This paves the wave for diseases such as granuloma annulare, cutaneous sarcoidosis, and morphea. 24 Cutaneous vasculitis may also occur with minor vessel wall damage as a result of the vaccine triggering the accumulation of immune complexes that activate the complement cascade. 30 In addition, maculopapular, urticarial, vesicular, and chilblain‐like lesions that develop after vaccination against COVID‐19 have also been observed after an active SARS‐CoV‐2 infection, 12 suggesting that the related vaccines elicit similar immunological mechanisms to the SARS‐CoV‐2 infection. 24
In this study, we identified several risk factors associated with the development of adverse events after post‐COVID‐19 vaccination. After vaccination with CoronaVac, women, young people, patients with a history of allergic diseases, and those without comorbidities were more prone to developing local injection site and systemic reactions. Our findings have some commonalities with the results of other studies. In a CoronaVac phase 3 study conducted in Chile, local and systemic adverse reactions were observed more frequently in the younger age group. 9 In a study evaluating healthcare workers in China, being female, having comorbidities, having a history of adverse reactions to other vaccines, and having a history of allergic reactions were associated with the adverse reactions of the CoronaVac vaccine. 11 In studies evaluating BioNTech, local and systemic reactions were reported to be more common in young people 2 , 23 and women. 23 , 31 In contrast, in our study, there was no relationship between risk factors and local injection site and systemic reactions after BioNTech. This may be due to the low number of participants vaccinated with BioNTech. The higher incidence of adverse reactions in women may be related to their higher tendency to report such reactions, greater reactogenicity of women to vaccines, 32 and some immunological differences between the two sexes. 33 In our study, after CoronaVac, non‐local cutaneous reactions were more common in older people, patients with chronic diseases, and those with a history of allergic diseases. Interestingly, after BioNTech, these reactions were more frequently seen in young people. Although there are limited data on the risk factors of non‐local cutaneous reactions, they have been reported to have a higher rate among women after BioNTech. 3 , 4
In our study, there were fewer local injection site reactions associated with both vaccines after the second doses. Systemic reactions were observed at a lower rate after the second CoronaVac dose, but there was no difference between the first and second doses of BioNTech. The few studies have reported conflicting results. Studies on BioNTech have reported that local reactions are either similar between the two doses 2 or more common after the first dose, 23 while systemic reactions are more common and severe after the second dose. 2 , 23 In one study, the authors observed more adverse reactions after the second dose of the BioNTech vaccine compared to the first dose. 34 In our study, non‐local cutaneous reactions were more common after the second dose of CoronaVac, but there was no difference between the two doses of BioNTech.
We determined the early non‐local cutaneous reactions as petechial rash and dyshidrotic eczema after CoronaVac and maculopapular eruption and erythroderma after BioNTech. The late reactions were herpes zoster, cutaneous vasculitis, and linear IgA dermatosis after CoronaVac and dyshidrotic eczema, erythromelalgia, and pityriasis rosea after BioNTech.
This study has certain limitations. In Turkey, vaccination against COVID‐19 began with the CoronaVac vaccine and was made available first for healthcare professionals and elderly individuals. Since the data collection period was short and BioNTech vaccination had just started to be used in Turkey at the time of the study, our findings on this vaccine are limited. Many case reports and studies of hair involvement reported after COVID‐19 vaccination have been published in the literature. 35 , 36 , 37 Nonetheless, since our study focused on cutaneous reactions, hair involvement after COVID‐19 vaccination was not examined separately. However, the current study also had several strengths, such as more than 95% of non‐local cutaneous reactions being diagnosed by a dermatologist and the participants being questioned through one‐to‐one interviews.
In conclusion, in this study, we determined the frequency of adverse effects of COVID‐19 vaccines and defined their characteristic features from the perspective of a dermatologist. We revealed the relationship between individual characteristics, such as age, sex and comorbidities and adverse reactions. This will help clinicians inform patients more clearly about the possibility of adverse reaction development following any vaccine, not specific at the COVID vaccinations. In addition, we caution clinicians to inquire about their patients' vaccination status when they face diseases such as sarcoidosis and linear IgA bullous dermatosis. The rate of serious reactions such as anaphylaxis and urticaria/angioedema developing within the first 24 hours is higher in BioNTech than in CoronaVac, which is a very important finding. Our data show that CoronaVac is associated with less harmful reactions while BioNTech may result in more serious reactions, such as herpes zoster, anaphylaxis, and triggering of autoimmunity. However, most of the reactions were self‐limiting and required little or no therapeutic intervention.
AUTHOR CONTRIBUTIONS
Filiz Cebeci Kahraman, Sevil Savaş Erdoğan, Nurhan Döner Aktaş, Hülya Albayrak, Dursun Türkmen, Murat Borlu, Deniz Aksu Arıca, Abdullah Demirbaş, Atiye Akbayrak, Algün Polat Ekinci, Gözde Emel Gökçek, Hilal Ayvaz Çelik, Mustafa Kaan Taşolar, İsa An, Selami Aykut Temiz, Emel Hazinedar, Erhan Ayhan, Pelin Hızlı, Eda Öksüm Solak, Arzu Kılıç, and Ertan Yılmaz involved in concept. Filiz Cebeci Kahraman, Sevil Savaş Erdoğan, Nurhan Döner Aktaş, and Hülya Albayrak involved in design. Filiz Cebeci Kahraman, Sevil Savaş Erdoğan, Nurhan Döner Aktaş, Hülya Albayrak, and Selami Aykut Temiz involved in supervision. None of the authors involved in funding. Filiz Cebeci Kahraman, Sevil Savaş Erdoğan, Nurhan Döner Aktaş, Hülya Albayrak, Dursun Türkmen, Murat Borlu, Deniz Aksu Arıca, Abdullah Demirbaş, Atiye Akbayrak, Algün Polat Ekinci, Gözde Emel Gökçek, Hilal Ayvaz Çelik, Mustafa Kaan Taşolar, İsa An, Selami Aykut Temiz, Emel Hazinedar, Erhan Ayhan, Pelin Hızlı, Eda Öksüm Solak, Arzu Kılıç, and Ertan Yılmaz involved in materials. Filiz Cebeci Kahraman, Sevil Savaş Erdoğan, Nurhan Döner Aktaş, Hülya Albayrak, Dursun Türkmen, Murat Borlu, Deniz Aksu Arıca, Abdullah Demirbaş, Atiye Akbayrak, Algün Polat Ekinci, Gözde Emel Gökçek, Hilal Ayvaz Çelik, Mustafa Kaan Taşolar, İsa An, Selami Aykut Temiz, Emel Hazinedar, Erhan Ayhan, Pelin Hızlı, Eda Öksüm Solak, Arzu Kılıç, and Ertan Yılmaz involved in data collection and/or processing. Filiz Cebeci Kahraman, Sevil Savaş Erdoğan, Nurhan Döner Aktaş, Hülya Albayrak, and Selami Aykut Temiz involved in analysis and/or interpretation, literature search, writing, and critical review.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
Supporting information
Supplemental Table 1. Comparison of adverse reactions after the first and second doses of the two vaccines in patients that had received both doses Onset times of non‐local cutaneous reactions after vaccination
Supplemental Table 2.Skin or autoimmune diseases triggered by COVID‐19 vaccination
Cebeci Kahraman F, Savaş Erdoğan S, Aktaş ND, et al. Cutaneous reactions after COVID‐19 vaccination in Turkey: A multicenter study. J Cosmet Dermatol. 2022;00:1‐12. doi: 10.1111/jocd.15209
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398:213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Català A, Muñoz‐Santos C, Galván‐Casas C, et al. Cutaneous reactions after SARS‐CoV‐2 vaccination: a cross‐sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2021;186:142‐152. doi: 10.1111/bjd.20639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson LB, Fu X, Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID‐19 vaccines. JAMA Dermatol. 2021;157:1000‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durmaz K, Temiz SA, Metin Z, et al. Allergic and cutaneous reactions following inactivated SARS‐CoV‐2 vaccine (CoronaVac[®] ) in healthcare workers. Clin Exp Dermatol. 2021;47:171‐173. doi: 10.1111/ced.14896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akdaş E, Öğüt B, Erdem Ö, Öztaş MO, İlter N. Cutaneous reactions following CoronaVac COVID‐19 vaccination: a case series of six healthcare workers from a single Centre. J Eur Acad Dermatol Venereol. 2021;35:e861‐e864. doi: 10.1111/jdv.17592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu JN, Angeles CB, Lim HG, Chavez C, Roxas‐Rosete C. Cutaneous reactions to inactivated SARS‐CoV‐2 vaccine and ChAdOx1‐S (recombinant) vaccine against SARS‐CoV‐2: a case series from The Philippines. J Eur Acad Dermatol Venereol. 2021;35:e841‐e845. doi: 10.1111/jdv.17575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bueno SM, Abarca K, González PA, et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS‐CoV‐2 in healthy chilean adults in a phase 3 clinical trial. medRxiv. 2003;2021(2021):2031.21254494. doi: 10.1101/2021.03.31.21254494 [DOI] [Google Scholar]
- 10. Palacios R, Patiño EG, de Oliveira PR, et al. Double‐blind, randomized, placebo‐controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID‐19 (inactivated) vaccine manufactured by Sinovac ‐ PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang MX, Zhang TT, Shi GF, et al. Safety of an inactivated SARS‐CoV‐2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021;20:891‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agnihothri R, Fox LP. Clinical patterns and morphology of COVID‐19 dermatology. Dermatol Clin. 2021;39:487‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz J, Yue S, Xue W. Herpes simplex and herpes zoster viruses in COVID‐19 patients. Ir J Med Sci. 2021;1‐5:1093‐1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2021;84:563‐568. doi: 10.1016/j.jaad.2021.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin MS, Kim SJ, Kim SH, Kwak YG, Park HJ. New onset guttate psoriasis following pandemic H1N1 influenza vaccination. Ann Dermatol. 2013;25:489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID‐19 vaccination. J Allergy Clin Immunol. 2021;148:750‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coto‐Segura P, Fernández‐Prada M, Mir‐Bonafé M, et al. Vesiculobullous skin reactions induced by COVID‐19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2021;47:141‐143. doi: 10.1111/ced.14835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferretti F, Cannatelli R, Benucci M, et al. How to manage COVID‐19 vaccination in immune‐mediated inflammatory diseases: an expert opinion by IMIDs study group. Front Immunol. 2021;12:656362. doi: 10.3389/fimmu.2021.656362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vadalà M, Poddighe D, Laurino C, Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8:295‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orenay OM, Balta I, Yigit D, Eksioglu M. Systemic drug‐related intertriginous and flexural exanthema like eruption after CoronaVac vaccine. J Eur Acad Dermatol Venereol. 2021;35:e634‐e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cebeci F, Kartal İ. Petechial skin rash associated with CoronaVac vaccination: first cutaneous side effect report before phase 3 results. Eur J Hosp Pharm. 2021;ejhpharm‐2021‐002794. doi: 10.1136/ejhpharm-2021-002794. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Temiz SA, Abdelmaksoud A, Dursun R, Vestita M. Acral chilblain‐like lesions following inactivated SARS‐CoV‐2 vaccination. Int J Dermatol. 2021;60:1152‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menni C, Klaser K, May A, et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niebel D, Novak N, Wilhelmi J, et al. Cutaneous adverse reactions to COVID‐19 vaccines: insights from an Immuno‐dermatological perspective. Vaccines. 2021;9:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available COVID‐19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stone CA Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune‐mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694‐2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eyerich K, Eyerich S. Immune response patterns in non‐communicable inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2018;32:692‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20:615‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rutkowski K, Mirakian R, Till S, Rutkowski R, Wagner A. Adverse reactions to COVID‐19 vaccines: a practical approach. Clin Exp Allergy. 2021;51:770‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen SR, Prussick L, Kahn JS, Gao DX, Radfar A, Rosmarin D. Leukocytoclastic vasculitis flare following the COVID‐19 vaccine. Int J Dermatol. 2021;60:1032‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med. 2021;384:1273‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626‐638. [DOI] [PubMed] [Google Scholar]
- 34. Andrzejczak‐Grządko S, Czudy Z, Donderska M. Side effects after COVID‐19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25:4418‐4421. [DOI] [PubMed] [Google Scholar]
- 35. Rossi A, Magri F, Michelini S, et al. Recurrence of alopecia areata after covid‐19 vaccination: a report of three cases in Italy. J Cosmet Dermatol. 2021;20(12):3753‐3757. [DOI] [PubMed] [Google Scholar]
- 36. Gallo G, Mastorino L, Tonella L, Ribero S, Quaglino P. Alopecia areata after COVID‐19 vaccination. Clin Exp Vaccine Res. 2022;11:129‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aryanian Z, Balighi K, Hatami P, Afshar ZM, Mohandesi NA. The role of SARS‐CoV‐2 infection and its vaccines in various types of hair loss. Dermatol Ther. 2022;35:e15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Comparison of adverse reactions after the first and second doses of the two vaccines in patients that had received both doses Onset times of non‐local cutaneous reactions after vaccination
Supplemental Table 2.Skin or autoimmune diseases triggered by COVID‐19 vaccination
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


