Abstract
The risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hospitalization and death, and the effects of SARS-CoV-2 vaccines in solid organ transplant recipients (SOTRs) is still debated. We performed a nationwide, population-based, matched cohort study, including all Danish SOTRs (n = 5184) and a matched cohort from the general population (n = 41 472). Cox regression analyses were used to calculate incidence rate ratios (IRRs). SOTRs had a slightly increased risk of SARS-CoV-2 infection and were vaccinated earlier than the general population. The overall risk of hospital contact with COVID-19, severe COVID-19, need for assisted respiration, and hospitalization followed by death was substantially higher in SOTRs (IRR: 32.8 95%CI [29.0–37.0], 9.2 [6.7–12.7], 12.5 [7.6–20.8], 12.4 [7.9–12.7]). The risk of hospitalization and death after SARS-CoV-2 infection decreased substantially in SOTRs after the emergence of the Omicron variant (IRR: 0.45 [0.37–0.56], 0.17 [0.09–0.30]). Three vaccinations reduced the risk of SARS-CoV-2 infection only marginally compared to two vaccinations, but SOTRs with three vaccinations had a lower risk of death (IRR: 022 [0.16–0.35]). We conclude that SOTRs have a risk of SARS-CoV-2 infection comparable to the general population, but substantially increased the risk of hospitalization and death following SARS-CoV-2 infection. A third vaccination only reduces the risk of SARS-CoV2 infection marginally, but SOTRs vaccinated 3 times have reduced mortality.
KEYWORDS: clinical research/practice, infectious disease, infection and infectious agents - viral, infection and infectious agents – viral: SARS-CoV-2/COVID-19, solid organ transplantation
Abbreviations: 95%CI, 95% confidence interval; COVID-19, coronavirus disease 2019; DNPR, Danish National Patient Register; ICD-10, international classification of diseases version 10; IRRs, incidence rate ratios; PCR, polymerase chain reaction; PYR, person years of observation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplantation; SOTR, solid organ transplant recipient; WHO, World Health Organization
1. INTRODUCTION
In late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported as the infectious agent leading to coronavirus disease 2019 (COVID-19).1 Globally, more than 517 million cases and 6.2 million deaths of COVID-19 have been confirmed by the WHO (World Health Organization) as of May 2022, and more than 11.6 billion vaccine doses have been given.2 Vaccines successfully reduce the risk of transmission of the virus and in particular the severity of the infection.3 , 4 The protection offered by vaccination and past infection remains to be fully elucidated, as the virus mutates, and reinfections and breakthrough infections become more frequent. Recently, the Omicron variant has become the main SARS-CoV-2 variant in most countries including Denmark.
Solid-organ transplantation (SOT) recipients (SOTRs) comprise a heterogeneous group of recipients of mainly kidney, heart, lung, and liver grafts. SOT candidates have a multitude of serious underlying medical conditions making them eligible for transplantation. To reduce the risk of organ rejection, SOTRs require lifelong immunosuppressive treatment5 making them more prone to infections.6 During the 2010 influenza A H1N1 pandemic, this infection caused increased morbidity in SOTRs compared to non-SOTRs.7
Studies of severity and mortality of SARS-CoV-2 infection in SOTRs have shown inconsistent results, with both increased, decreased, and unaltered risk compared to non-SOTRs.8, 9, 10 An Italian study from July 2020 found that 30-day mortality among hospitalized SOT COVID-19 patients was similar to 30-day mortality among non-SOT COVID-19 patients.11 In a US study from December 2020, the authors did not find worse outcomes for SOTRs than for non-SOTRs hospitalized with COVID-19.12 A US study from January 2021 found higher mortality in SOTRs vs non-SOTRs hospitalized with COVID-19.13 A meta-analysis from July 2021 comprising results from 15 studies (including the three mentioned above) concluded that SOTRs were more prone to serious courses of COVID-19 with higher mortality than non-SOTRs with COVID-19.14 Previous studies of outcomes of SARS-CoV-2 infection in SOTRs often recruited patients from specialized centers and did not include a comparison cohort representing a random sample from the general population.
We used Danish national health registers to perform a nationwide, population-based study of the impact of SARS-CoV-2 infection in SOTRs compared with a matched cohort from the general population. The specific objectives were to compare the risk of infection, hospitalization, and death in SOTRs with the general population and to clarify the association between vaccination status and risk of SARS-CoV-2 infection, including the Omicron variant, hospitalization, and death in SOTRs.
2. METHODS
2.1. Study design
The study was performed as a nationwide, population-based, matched cohort study comparing outcomes of SARS-CoV-2 infection in SOTRs and the general population.
2.2. Settings
In Denmark the first case of SARS-CoV-2 was detected on February 27, 2020 and the first vaccine was administered on December 27, 2020.15 Pfizer/BioNTech Comirnaty and Moderna mRNA-1273 were the first vaccines to be approved, and by May 15, 2022 82% of the 5.8 million people living in Denmark had been vaccinated at least once and 61.5% three times with the Pfizer, Moderna, Vaxzeria (previously AstraZeneca) or Janssen vaccines. The Danish tax-funded health care system provides vaccination against and testing and treatment for SARS-CoV-2 free-of-charge to all Danish Residents. The Danish vaccination program was rolled out by a personal invitation letter starting with risk groups defined by age or comorbidity. As of May 15, 2022, 2.98 million individuals (51% of the population) had tested positive for SARS-CoV-2 at least once and more than 121 million individually registered PCR and antigen tests had been performed in the country.
2.3. Data sources
We used data from the Danish Civil Registration System that has recorded information on all Danish residents since 1968, using the unique personal identification number (PIN) assigned to all residents at birth or upon immigration into Denmark.16 Among other data, this national register has information on vital status, date of birth, sex, date of immigration and emigration, date of death, and demographic data.17 The unique PIN number is also used for registering SARS-CoV-2 PCR tests and vaccination records.
A national COVID-19 surveillance system is maintained by the Statens Serum Institut. It captures the results of all SARS-CoV-2 tests performed in public (including hospitals) and private testing facilities. From this register, we extracted data on SARS-CoV-2 PCR and antigen tests.18
SARS-CoV-2 vaccination status was retrieved from the Danish Vaccination Register which contains individual-level information on the date and type of vaccines given in Denmark.19
Data on morbidity and comorbidity was extracted from the Danish National Patient Register (DNPR).20 The DNPR includes information on the date of admission, date of discharge, diagnoses, and procedures recorded using the International Classification of Diseases classification system (ICD-10)21 and the Danish Health Care Authorities’ Classification System (SKS).22
2.4. Study population
We included all Danish SOTRs alive on the date of study inclusion, which was the latest of March 1 2022, or the date of SOT. The date of SOT was the first date of a transplant diagnosis of Z940 (kidney), Z941 (heart), Z942 (lung), and Z943 (heart and lung), Z944 (liver) or a procedure code of KFQA (heart), KGDG (lung), KKAS (kidney), and KJJC (liver) in the DNPR. We included 50 individuals who received more than one organ, and these multiorgan-transplanted individuals were included in the heart/lung transplanted group if they were heart or lung transplanted irrespective of other solid organ transplantations, and the kidney transplanted group if they had a kidney and liver transplantation. Patients with a kidney transplant who were registered with consecutive procedures of dialysis (BJFD) for more than 90 days and were still on dialysis on the date of study inclusion were excluded. For each SOTR we extracted eight population controls at random from the general population, matched on sex and date of birth (born within 7 days of the SOTR), who were alive and residing in Denmark on the date of study inclusion. Individuals below 18 years of age on January 1, 2020, were excluded. Population controls had the same date of inclusion as the SOTRs to whom they were matched.
2.5. Outcomes
Outcomes were calculated as time to the following events:
Date of first positive SARS-CoV-2 test: First date the individual was registered in the national COVID-19 surveillance system with a positive test for SARS-CoV-2, either by a PCR or antigen test.
Date of second positive SARS-CoV-2 test: First date the individual was registered with a positive test for SARS-CoV-2 (either by a PCR or antigen) more than 90 days after the first positive SARS-CoV-2 test.
Date of full second and third vaccination: 14 days after the individual’s second or third vaccination as registered in the Danish Vaccination Register.
Date of first hospital contact with a diagnosis of COVID-19: First date an individual was hospitalized (inpatient or outpatient) with a diagnosis in the DNPR of either ICD10 codes B342 (coronavirus infection, unspecified sites) and/or DB972 (severe coronavirus infection) and/or procedures involving mechanical ventilation using the SKS code BGDA (ventilator treatment and other assisted ventilation/breathing), combined with a positive test for SARS-CoV-2 within 14 days of diagnosis.
Date of the first hospitalization with severe COVID-19: First date of hospitalization with a diagnosis in the DNPR of either DB972 and/or procedures involving mechanical ventilation (SKS code: BGDA) combined with a positive SARS-CoV-2 test within 14 days of diagnosis.
Date of the first hospitalization with COVID-19 with procedures involving mechanical ventilation: First date of hospitalization with a procedure involving mechanical ventilation (SKS code: BGDA) combined with a positive SARS-CoV-2 test within 14 days of diagnosis.
Date of hospital contact with COVID-19 leading to death: Date of death in case an individual died within 30 days of hospitalization with a diagnosis of Covid-19 (as described above).
2.6. Statistical analyses
We calculated incidence rate ratios (IRRs) in SOTRs vs individuals in the matched control cohort for the outcomes described above. Time was calculated from the date of study inclusion until the date of emigration, death, kidney graft failure, May 1, 2022, or the outcome of interest. In analyses of time to vaccination, time was calculated from January 1, 2021 and in analyses of the impact of vaccination from November 1, 2021. In analyses of time to hospitalization and death after a first positive SARS-CoV-2 test, time was calculated from the date of the first positive test.
We computed Kaplan–Meier life tables using calendar time as a time scale. In analyses of time to hospitalization and death after a first positive SARS-CoV-2 test, we used days after a positive test as a time scale. We used Cox regression to calculate IRR as estimates of relative risks. In analyses of time to hospitalization, time to death after a SARS-CoV-2 test, and vaccine effects, estimates were adjusted for age and sex. To elucidate the impact of the Omicron variant we stratified analyses of time to hospitalization and death on whether the individual tested SARS-CoV-2 positive before or after January 1, 2022.
In sub-analyses, we stratified the results into three groups: individuals with (1) kidney, (2) heart/lung, and (3) liver transplantation. We also made estimates for the group of multiorgan-transplantation.
Data were analyzed using STATA 14 statistical software.23
2.7. Ethical considerations
This study was performed as a national surveillance study under the authority task of the Danish national infectious disease control institute the Statens Serum Institut. The study was approved by The Danish Data Protection Agency (permission no 21/04383). According to Danish regulations, national surveillance activities as well as studies solely relying on registered information, do not require individual consent nor approval from an ethics committee.
3. RESULTS
We included 5184 SOTRs including 3640 kidney, 839 heart/lung, (492 heart, 331 lung, and 16 lung and heart) and 705 liver transplant individuals, and 41 472 matched population controls. In the SOTRs population, 50 individuals were transplanted with more than one organ (16 lung/heart, 4 heart/liver, 6 heart/kidney, 4 lung/liver or kidney, and 20 kidney/liver). More than 60% were males and the median age was 55.9 years (IQR 45.4–65.4 years). The study included 9327 years of follow-up in the SOTR cohort and 76 853 years in the control cohort ( Table 1). In the study period, 2238 SOTRs individuals tested positive for SARS-CoV-2, of whom 2203 (98.4%) were PCR SARS-CoV-2 positive ( Table 2). The SOTRs suffered from considerably more comorbidity than did population controls (Table 1).
TABLE 1.
Study population characteristics, solid organ transplant recipients (SOTR), and population controls in Denmark March 1, 2020—May 1, 2022
| SOTR patients (total) | SOTR (kidney) | SOTR (heart/lung) | SOTR (liver) | Population controls | |
|---|---|---|---|---|---|
| Total number (n) | 5184 | 3640 | 839a | 705 | 41 472 |
| Males (n) | 3144 (61%) | 2235 (61%) | 529 (63%) | 380 (54%) | 25 152 (61%) |
| Age [years] Median, IQR | 55.9 (45.4–65.4) | 55.4 (45.4–65.4) | 58.9 (48.1–66.2) | 53.9 (42.6–63.9) | 55.9 (45.4–65.4) |
| Time from SOT diagnosis to study inclusion [years] Median, IQRb | 6.8 (2.1–13.7) | 5.7 (1.0–12.6) | 6.0 (1.0–12.5) | 5.1 (0.7–11.7) | NA |
| Number transplanted after January 1, 2019 | 1073 (21%) | 742 (20%) | 174 (21%) | 157 (22%) | NA |
| Total observation period (PYRc) | 10 113 | 7136 | 1589 | 1388 | 83 202 |
| Charlson indexd | |||||
| 0 (%) | 443 (8.6) | 135 (3.7) | 41 (4.9) | 267 (37.9) | 31 338 (75.6) |
| 1 (%) | 336 (6.5) | 30 (0.8) | 207 (24.7) | 99 (14.0) | 4727 (11.4) |
| 2 (%) | 1548 (29.9) | 1274 (35.0) | 135 (16.1) | 139 (19.7) | 3039 (7.3) |
| 3 (%) | 870 (16.8) | 624 (17.1) | 161 (19.2) | 85 (12.1) | 1140 (2.8) |
| 4 (%) | 696 (13.4) | 520 (14.3) | 126 (15.0) | 50 (7.1) | 485 (1.2) |
| 5 (%) | 540 (10.4) | 440 (12.1) | 78 (9.3) | 22 (3.1) | 239 (0.6) |
| ≥6 (%) | 751 (14.5) | 617 (17.0) | 91 (10.9) | 43 (6.1) | 504 (1.2) |
Abbreviations: NA, not applicable; SOTR, solid organ transplant recipient.
Heart: 492, lung: 331, heart & lung: 16.
Interquartile range.
Person years at risk.
At the date of study entry.
TABLE 2.
Risk of first positive SARS-CoV-2 test, vaccination, hospitalization, and death in solid organ transplant recipients (SOTR) vs. controls in Denmark, March 1, 2020—May 1, 2022
| Relative risk: SOTR vs population controls |
||||
|---|---|---|---|---|
| SOTR population | Total | Kidney | Heart/lung | Liver |
| Outcome | IRR (95%CI) | |||
| Time to first positive SARS-CoV-2 testa | 1.07 (1.03–1.12) | 1.11 (1.05–1.17) | 0.98 (0.87–1.10) | 0.98 (0.86–1.10) |
| Time to second positive SARS-CoV-2 testa,b | 1.08 (0.87–1.35) | 1.29 (1.02–1.64) | 0.50 (0.20–1.23) | 0.57 (0.28–1.17) |
| Time to first hospital contact with COVID-19a | 32.8 (29.0–37.0) | 34.7 (30.1–40.1) | 40.3 (29.2–55.7) | 19.0 (13.9–26.0) |
| Time to hospitalization with severe COVID-19a | 9.2 (6.7–12∙7) | 10.0 (6.9–14.5) | 18.6 (7.6–45.5) | 1.26 (0.28–5.58) |
| Time to hospitalization with assisted ventilationa | 12.5 (7.6–20.8) | 14.9 (8.3–27.0) | 29.8 (6.2–143.6) | NA |
| Time to hospitalization with COVID-19 followed by deatha | 12.4 (7.2–21.2) | 13.6 (7.2–25.8) | 20.3 (5.3–78.6) | 2.0 (0.2–18.3) |
| Time to hospitalizationc after first positive SARS-CoV-2 testd Pre-Omicron periode |
10.0 (7.9–12.7)f | 10.7 (8.0–14.0)f | 13.0 (7.5–22.5)f | 6.0 (3.1–11.5)f |
| Time to hospitalizationc after first positive SARS-CoV-2 testd Omicron periode |
9.6 (7.9–11.7)f | 9.7 (7.6–12.3)f | 8.1 (5.1–12.9)f | 115 (7.1–18.5)f |
| Time to death after first positive SARS-CoV-2 testd Pre-Omicron periode |
8.5 (5.1–14.0)f | 10.7 (6.0–19.1)f | 10.6 (2.9–38.2)f | NA |
| Time to death after hospitalization for SARS-CoV-2d Omicron periode |
7.4 (3.7–14.8)f | 8.1 (2.9–22.5)f | 9.6 (3.2–28.6)f | 2.3 (0.3–27.7)f |
Note: Outcomes are the time to the event.
Abbreviations: IRR, Incidence rate ratio; NA, not applicable; PYR, person years of observation; SOT, solid organ transplantation; SOTR, solid organ transplant recipient; 95%CI, 95% confidence interval.
Time was calculated from the date of study inclusion (latest of March 1, 2020 or date of SOT) to date of outcome with calendar time as a scale.
A positive SARS-CoV-2 test >90 days after the first positive SARS-CoV-2 test was categorized as the second positive SARS-CoV-2 test.
Includes hospital admissions for ≥24 h.
Time was calculated from the date of the first positive SARS-CoV-2 test to the date of outcome with time from the first positive SARS-CoV-2 test as a time scale and censoring at 90 days.
Pre-Omicron period is time until January 1, 2022; Omicron period is the time from January 1, 2022.
Adjusted for age and sex.
3.1. Risk of a first positive SARS-CoV-2 test
The risk of a first positive SARS-CoV-2 test in SOTRs was slightly higher than in the general population (IRR = 1.07, 95%CI 1.03–1.12) and the increased risk was only observed in the kidney transplanted patients (Table 2, Figure 2; Figure S1). In all SOTR groups, ≥97% of the positive tests were confirmed by PCR (data not shown). SOTRs were tested slightly more frequently at the beginning of the epidemic and less frequently than the population controls in later periods (Figure S9).
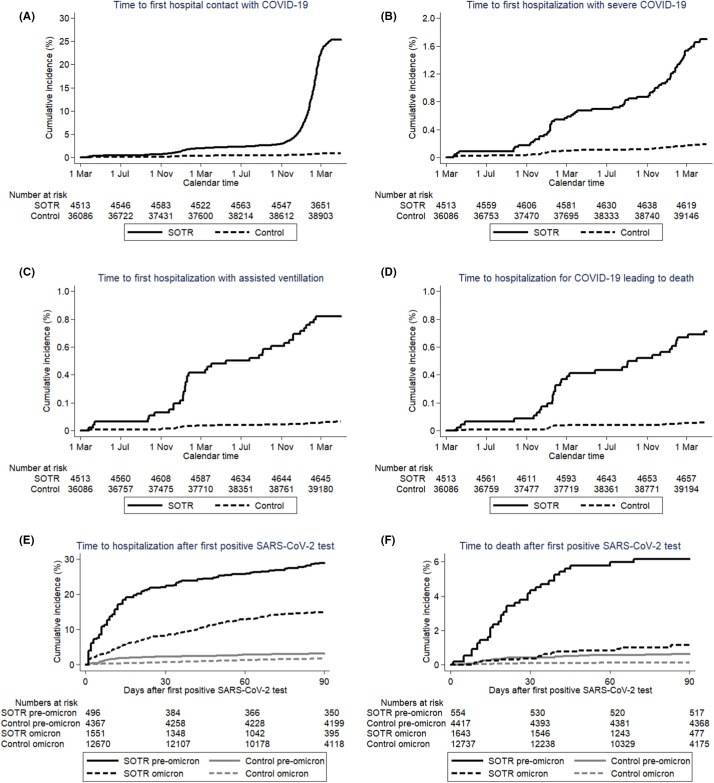
FIGURE 2.
Time to (A) the first hospitalization with a COVID-19 diagnosis, (B) hospitalization with severe COVID-19, (C) hospitalization with assisted ventilation, (D) hospitalization with COVID-19 leading to death within 90 days (outcomes described in detail in methods), (E) time to hospitalization ≥24 h after first positive SARS-CoV-2 test, (F) time to death after first positive SARS-CoV-2 test. Solid line: SOTR; dotted line: population controls. Time starts on March 1, 2020 or the date of a positive test for SARS-CoV-2, respectively. [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Vaccination for SARS-CoV-2
The SOTRs received their second and third vaccine doses earlier than the controls and on May 1, 2022, 96.3% (95%CI 95.7–96.8%) and 94.5% (95%CI 93.8–95.1%) of the SOTRs were vaccinated 2 and 3 times compared with 92.4% (95%CI 92.1–92.7%) and 89.8% (95%CI 89.5–90.0%) in the general population ( Figure 1B). Time to vaccination differed only marginally between SOTR groups (Figure S2).
FIGURE 1.
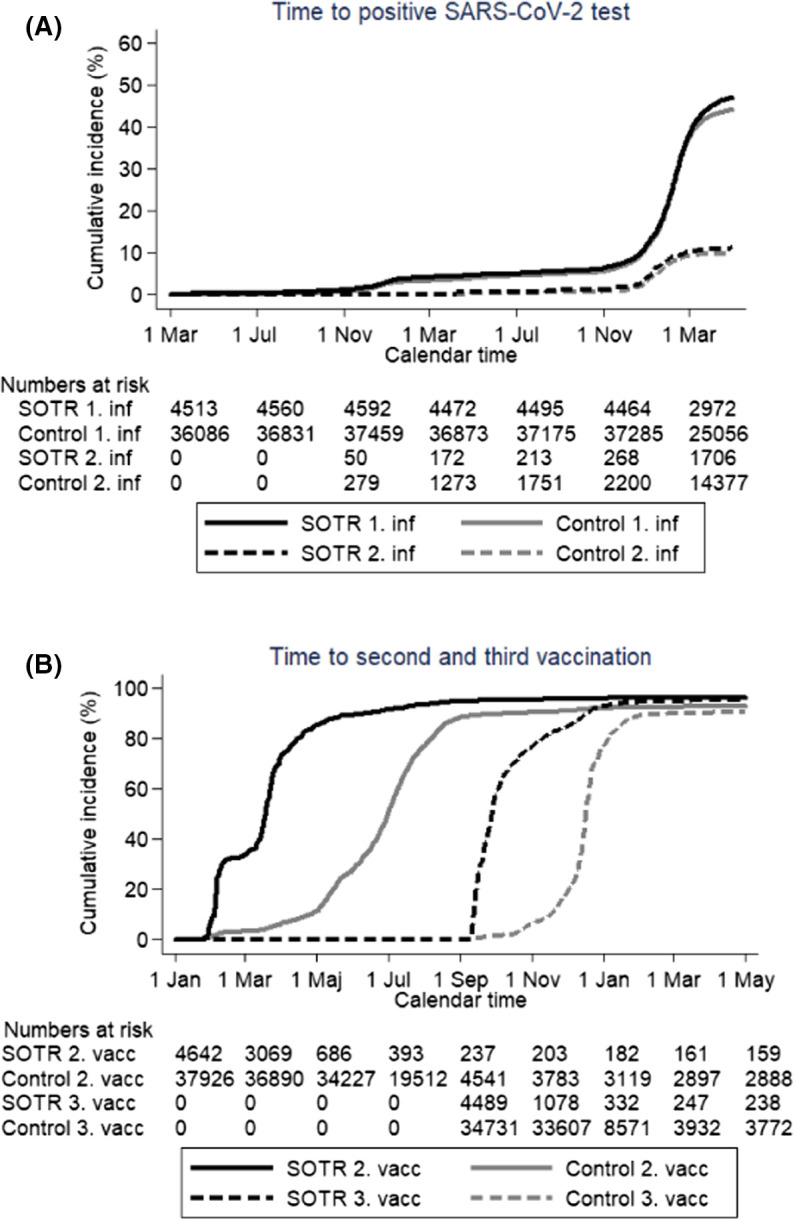
(A) Time to first and second positive test for SARS-CoV-2; black line: SOTRs, gray line: population controls, solid line: first positive test, dotted line: second positive test, time starts March 1, 2020 and ends May 1, 2022. (B) Time to second and third vaccination for SARS-CoV-2: black line: SOTR, gray line: population controls, solid line: second vaccination, dotted line: third vaccination, time starts January 1, 2021 and ends May 1, 2022. [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Risk of hospitalization and death from COVID-19
The risk of hospital contact with COVID-19 was highly increased for SOTRs compared to the general population, IRR = 32.8, 95%CI 29.0–37.0 for first hospital contact with a diagnosis of COVID-19, IRR = 9.2, 95%CI 6.7–12.7 for the first hospitalization with severe COVID-19, IRR = 12.5, 95%CI 7.6–20.8 for the first hospitalization involving mechanical ventilation; and IRR = 12.4, 95%CI 7.2–21.2 for the first hospitalization with COVID-19 leading to death (Table 2, Figure 2A–D). There was no major difference in the risk between kidney and heart/lung transplanted individuals, but the risk appeared substantially lower in liver transplanted individuals (Table 2; Figures S3–S6).
Both in the pre-Omicron and the Omicron period overall risk of hospitalization and death after a first positive SARS-CoV-2 test was substantially higher in SOTRs compared to population controls (Table 2, Figure 2E; Figure S7). The risk of death after a first positive SARS-CoV-2 test appeared considerably lower in liver transplanted individuals compared with kidney and heart/lung transplanted individuals (Table 2; Figure S8). SOTRs infected with SARS-CoV-2 in the Omicron period had a substantially decreased risk of hospitalization (IRR = 0.45, 95%CI 0.37–0.56) and death (IRR = 0.17, 95%CI 0.09–0.30) compared to SOTRs infected in the pre-Omicron period. The risk of death after hospitalization with a SARS-CoV-2 infection was increased in SOTRs compared to the general population (IRR = 1.89, 95%CI 1.19–3.00). SOTRs over 60 years of age had an increased risk of hospitalization (IRR = 1.5, 95%CI 1.2–1.9) and considerably increased risk of death (IRR = 13.1, 95%CI 6.1–27.8) after a first positive SARS-CoV-2 test compared with SOTRs 60 years or younger. Individuals transplanted after January 1, 2019 had an increased risk of hospitalization (IRR = 1.4, 95%CI 1.1–1.8) and decreased risk of death (IRR = 0.30, 95%CI 0.09–0.96) compared with individuals transplanted before January 1, 2019. In the multiorgan-transplanted group risk of SAR-CoV-2 infection, coverage of vaccination, hospital contact for COVID-19, and hospitalization after a positive SARS-CoV-2 test did not differ substantially from the other SOTR groups (Figures S1D, S2D, S3D, and S7D). In this group, ≤3 individuals had severe outcomes of COVID-19 or died from the disease.
3.4. Vaccine effects
When comparing SOTRs who had received two COVID-19 vaccine doses with those who had received three doses, those who had received three doses had a slightly decreased risk of a first positive SARS-CoV-2 test (IRR = 0.83, 95%CI 0.70–0.99), identical risk of hospital contact with a diagnosis of COVID-19 (IRR = 1.00, 95%CI 0.79–1.27), but a substantially reduced risk of death (IRR = 0.22, 95%CI 0.16–0.36) ( Figure 3A–C). Our study did not have the statistical power to calculate reliable estimates of vaccine effects in the SOTR subgroups.
FIGURE 3.
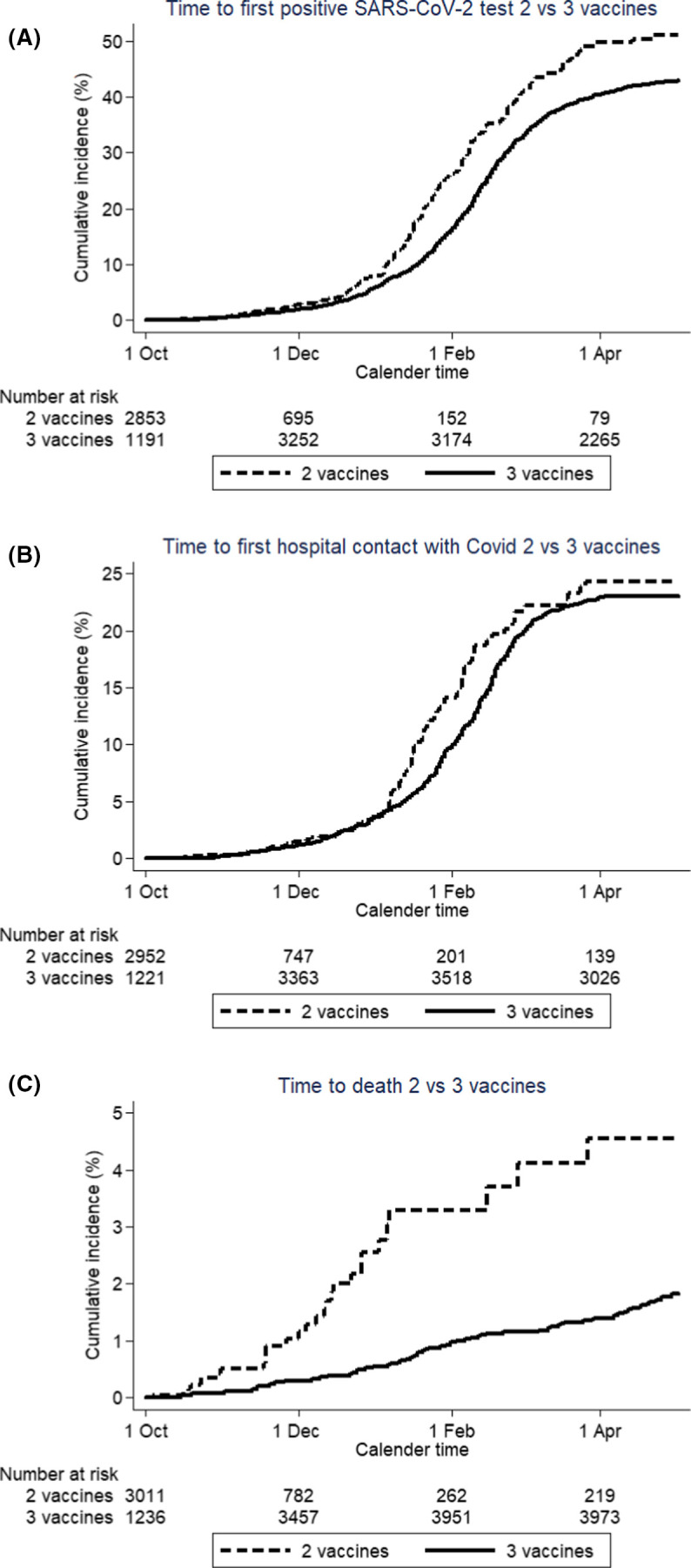
Time to (A) first positive SARS-CoV-2 test, (B) the first hospitalization with COVID-19, and (C) death for SOTRs before and after getting fully vaccinated (14 days after second vaccination or third vaccination). Dotted line: time with two vaccinations, full line: time with three vaccinations. Time starts on October 1, 2021 and ends May 1, 2022. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this study, we demonstrated that the risk of testing positive for SARS-CoV-2 was almost identical in SOTRs compared with the general population in Denmark. SOTRs were generally vaccinated before the general population, and at the end of 2021, almost all SOTRs had received the third dose of vaccination. The risk of hospitalization and death from a SARS-CoV-2 infection was, however, substantially higher in the SOTR population. The third vaccination for SARS-CoV-2 did not substantially reduce risk of SARS-CoV-2 infection or hospital contact in the SOTR population but was associated with a reduced risk of death.
The test strategy for SARS-CoV-2 changed during the study period, which potentially could infer bias. In the beginning of the epidemic, when test capacity was limited, mainly symptomatic and high-risk individuals were tested. In later periods test activity for non-symptomatic individuals increased, as a negative SARS-CoV-2 test for not-fully vaccinated citizens was mandatory for participating in social and cultural activities in specific periods during the study period (Corona certificate). Probably as a result of these test strategies, we observed a higher frequency of SARS-CoV-2 tests in SOTRs in the beginning of the epidemic and a higher test frequency in the general population in the summer of 2021, when the Corona certificate was introduced in Denmark. Most of the patients included in our study either tested positive with PCR or had a positive antigen test confirmed with PCR. In Denmark, the rate of home testing for SARS-CoV-2 has increased so the differences in frequency of home testing in SOTRs and non-SOTRs cannot be excluded and may also have influenced the estimates. This may especially have been the case for kidney transplant patients who had a slightly increased risk of testing positive for SARS-CoV-2. To our knowledge, no previous study has, on a national level, compared the risk of SARS-CoV-2 infection in SOTRs with the risk in the general population. It could be hypothesized that immune suppression may motivate SOTRs to self-isolate during a pandemic and thereby decrease their risk of SARS-CoV-2 infection. On the contrary, the vulnerable condition of SOTRs may increase test frequency, which would lead to an increased risk of testing positive for SARS-CoV-2. Our data do not support any of these scenarios and indicate that the risk of infection in SOTRs was almost equivalent to that observed in the general Danish population.
Danish health authorities initially prioritized immunosuppressed individuals when access to vaccines was limited. Consequently, SOTRs were among the first to be invited to vaccination for SARS-CoV-2. Our data demonstrate that this strategy led to a high vaccine coverage of Danish SOTRs, with almost all SOTRs fully vaccinated with the third vaccine by May 1, 2022. Yet, our analyses demonstrate a substantially higher risk of hospitalization, ventilator-assisted treatment, and death following SARS-CoV-2 infection in SOTRs compared with the general population. We did not observe a substantially increased risk of SARS-CoV-2 infection or decreased probability of being vaccinated in the SOTR group. We, therefore, conclude that the increased risk of serious progression of SARS-CoV-2 infection in the SOTR population is not due to an increased risk of infection or a lower vaccination coverage, but originates from the increased risk of organ manifestations from SARS-CoV-2 when infected, which again most likely is related to the immune suppression, graft dysfunction and comorbidities present in the SOTRs. Our data substantiate a considerable role of comorbidity in the risk of hospitalization and death in the SOTRs (Table 1).
Our study was conducted as a population-based, nationwide study. Most previous studies of SARS-CoV-2 infection in SOTRs include individuals recruited from centers specialized in treating SOTRs.6 , 7 These cohorts may therefore include SOTRs with more serious SARS-CoV-2 infection. As we had access to data from all Danish SOTRs, our analyses are not prone to over- or underestimation of risk in SOTRs. We observed a substantially increased risk of hospitalization in the SARS-CoV-2 positive SOTRs compared to the general population. In contrast, the risk of death in the hospitalized SOTRs was less than two times higher than in the non-SOTR cohort. Presumably, the threshold for hospitalization of SOTRs is lower than population controls. The differences in overall and hospital-admission-related risk of death may partly explain why some previous studies have found minor differences in mortality between SOTR and non-SOTR hospitalized patients.11 This finding also underlines the weaknesses of studies that include only hospitalized individuals. In a study of 126 liver transplanted individuals, Mansoor et al observed that 40% of the patients required hospitalization, which is in line with our findings.24
We observed a smaller risk of hospitalization and death in liver transplanted patients compared to kidney transplanted and heart/lung transplanted patients. We presume that this difference is related to less immunosuppression in liver transplanted patients.
The risk of death was substantially lower in SOTRs after the introduction of the Omicron variant in late 2021. This observation is in accordance with what has been reported for the background population.25
In a meta-analysis of 15 studies with a total of 450 SOTRs, the authors found a slightly increased risk of death in the SOTR population. The risk of death differed substantially between the included studies, as did the mortality rate in the comparison groups.14 Trapani et al. found a two-fold relative risk of death (IRR = 2.0, 95%CI 1.7–2.4) for COVID-19 infected SOTR compared with non-SOTR with COVID-19, which is comparable to the relative risk we observed.26 Similar estimates were seen in a US study comparing death in SOT and non-SOT hospitalized patients (IRR = 1.9, 95%CI 1.2–3.2).13 In a propensity-matched cohort study, Pereira et al. observed similar overall outcomes (including death) in SOTR hospitalized with COVID-19 compared to non-SOT recipients. In this study, the authors observed a mortality of 23% which is considerably higher than what we observed and may be related to the high mortality that was observed in the initial phase of the epidemic.27 In a multicenter study including 496 kidney transplanted patients with COVID-19, the authors observed a 16.9% risk of death on day 28.28 A comparable risk of death was found in a meta-analysis of 1522 liver-transplanted patients.29 This risk is higher than we observed, but these studies included more hospitalized patients. Other studies did not find an increased risk of death in SOT patients compared to non-SOT patients.11 Of importance, these studies included small and selected study populations. As demonstrated in our study, the characteristics of the SOTRs, such as age and type of transplanted organ, are strongly associated with the risk of death, why the differences in risk estimates between the studies may also be influenced by differences in characteristics of the SOT populations and non-SOT comparison cohorts.
The immune response induced by a SARS-CoV-2 vaccination is lower in SOTRs than in non-SOTR, but discordance between vaccine-induced cell-mediated and neutralizing humoral immunities has been observed.30 Although SOTRs are known to be immunocompromised, our data demonstrate that SOTRs vaccinated three times had a substantially lower risk of death than SOTRs who were only vaccinated two times. This justifies prioritizing the SOTR group in vaccine programs. The Danish health authorities recommended the third vaccination for COVID-19 in immunocompromised patients from August 2021 and in the general population a few months later, why we restricted our analyses to comparing 2 vs. 3 vaccines in SOTRs in the period after November 1, 2021. In a US study, Aslam et al. demonstrated a considerable reduction in risk of SARS-CoV-2 infection in vaccinated SOTRs compared to unvaccinated SOTRs (IRR = 0.2, 95%CI 0.1–0.5),31 but the study was underpowered to demonstrate the impact of vaccination on clinical outcomes. Differences in vaccine effectiveness between variants of SARS-CoV-2 and confounding from differences in SARS-CoV-2 positive rates over calendar periods may partly explain differences in observed effects of vaccines. We found a substantially reduced risk of death in SOTRs vaccinated three times compared with two times, but we cannot exclude that the more resourceful SOTRs are more likely to be vaccinated three times which may have confounded our estimates (Table 2, Figure 3).
A noteworthy strength of this study is the long follow-up, which included the complete period of the Danish SARS-CoV-2 epidemic in a country with a high PCR testing capacity. Our access to nationwide registers of SARS-CoV-2 tests, vaccination status, hospital diagnosis, and vital status allowed us to construct population-based nationwide cohorts of SOTRs and an age- and sex-matched cohort of population controls. This design also allowed us to follow more than 5000 SOTRs for outcomes from the first phase of the SARS-CoV-2 epidemic to May 1, 2022 and thereby gave the study high statistical power. As the study included the complete Danish population of SOTR, we believe that our results are generalizable to other western world settings.
Possible study limitations include reliance on register-based discharge diagnoses, which may be inaccurate and incomplete. We assume that diagnoses of organ transplantation and procedure codes for assisted ventilation have high predictive values, and a validation study of COVID-19 discharge diagnoses found high sensitivity and positive predictive value.32 Also, we presume that a possible misclassification of outcomes is non-differential, for which reason the impact on the relative risk estimates would be minor. We included all individuals who were categorized with a condition of kidney, heart, lung, or liver transplantation. For the latter three categories, the condition as SOTR is life-long, but a fraction of the kidney transplant individuals will have a failure of the kidney graft and will return to kidney replacement dialyzes. Consequently, their immunosuppression will likely be reduced and in some individuals stopped. We, therefore, excluded kidney transplanted individuals who were on continuous dialyzes at study inclusion. To obtain statistical power we combined heart/lung transplanted individuals in one group, which deprived us of the opportunity to look at these two groups separately. For comparison, we used a random sample from the general population matched on age and sex why the comparison cohort may have included patients with primary immunodeficiencies or on immunosuppressive treatment.
In conclusion, the Danish SOTRs had almost the same probability of testing positive for SARS-CoV-2 as the general population but had a substantially higher risk of hospitalization and death following COVID-19 infection. In Denmark, SOTRs received SARS-CoV-2 vaccine coverage earlier than the general population and SOTRs vaccinated three times had a substantially reduced risk of death.
ACKNOWLEDGMENTS
We thank the staff in test sites and the hospital Departments of Clinical Microbiology. We further wish to thank the staff at the Department of Virus & Microbiological Special Diagnostics, TestCenter Denmark, and the staff of the Data Integration and Analysis Secretariat (DIAS), all at Statens Serum Institut.
FUNDING INFORMATION
This research received no specific funding.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
De-identified participant-level data are available for access to members of the scientific and medical community for non-commercial use only. Applications should be submitted to Forskerservice at The Danish Health Data Authority, where they will be reviewed on the basis of relevance and scientific merit. Data are available now, with no defined end date.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Appendix S1
REFERENCES
- 1.Gorbalenya AE, Baker SC, Baric R, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2022;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (SARS-COV-2) dashboard 2021. Published 2021. Accessed March 12, 2022. https://covid19.who.int/.
- 3.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karam S, Wali RK. Current state of immunosuppression: past, present, and future. Crit Rev Eukaryot Gene Expr. 2015;25(2):113–134. doi: 10.1615/critreveukaryotgeneexpr.2015011421. [DOI] [PubMed] [Google Scholar]
- 6.Timsit J-F, Sonneville R, Kalil AC, et al. Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients. Intensive Care Med. 2019;45(5):573–591. doi: 10.1007/s00134-019-05597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar D, Michaels MG, Morris MI, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10(8):521–526. doi: 10.1016/S1473-3099(10)70133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll E, Fernández-Ruiz M, Padilla M, et al. COVID-19 in solid organ transplant recipients in spain throughout 2020: catching the wave? Transplantation. 2021;105(10):2146–2155. doi: 10.1097/TP.0000000000003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumortier J, Duvoux C, Roux O, et al. Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol. 2021;45(4):101639. doi: 10.1016/j.clinre.2021.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinaldi M, Bartoletti M, Bussini L, et al. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population. Transplant Infect Dis. 2021;23(1):e13421. doi: 10.1111/tid.13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery RK, Chiang TPY, Marr KA, et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: a retrospective cohort. Am J Transplant. 2021;21(7):2498–2508. doi: 10.1111/ajt.16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher AM, Schlauch D, Mulloy M, et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States. Clin Transplant. 2021;35(4):e14216. doi: 10.1111/ctr.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ao G, Wang Y, Qi X, et al. The association between severe or death COVID-19 and solid organ transplantation: a systematic review and meta-analysis. Transplant Rev. 2021;35(3):100628. doi: 10.1016/j.trre.2021.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danish Health Authority. Så er vi i gang - i dag bliver de første vaccineret mod COVID-19. Accessed March 12, 2022. https://www.sst.dk/da/nyheder/2020/saa-er-vi-i-gang---i-dag-bliver-de-foerste-vaccineret-mod-covid-19#:~:text=S%C3%A5%20er%20vi%20i%20gang,vaccineret%20mod%20COVID%2D19%20%2D%20Sundhedsstyrelsen
- 16.Pedersen CB, Gøtzsche H, Møller JØ, Mortensen PB. The Danish civil registration system. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 17.Frank L. When an entire country is a cohort. Science. 2000;287(5462):2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 18.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gram MA, Nielsen J, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death when combining a first dose ChAdOx1 vaccine with a subsequent mRNA vaccine in Denmark: A nationwide population-based cohort study. PLoS Med. 2021;18(12):e1003874. doi: 10.1371/journal.pmed.1003874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268. [PubMed] [Google Scholar]
- 21.World Health Organization. International statistical classification of diseases and related health problems. - 10th revision, edition 2010.
- 22.Authority TDHD. The health care authorities’ classification system SKS (Sundhedsvæsenets Klassifikations System) 2021.
- 23.StataCorp L. StataCorp stata statistical software: Release 14. College Station, TX, USA; 2015.
- 24.Mansoor E, Perez A, Abou-Saleh M, et al. Clinical characteristics, hospitalization, and mortality rates of coronavirus disease 2019 among liver transplant patients in the United States: a multicenter research network study. Gastroenterology. 2021;160(1):459–462. doi: 10.1053/j.gastro.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bager P, Wohlfahrt J, Bhatt S, Stegger M, Legarth R, Møller C, Skov RL, Valentiner-Branth P, Voldstedlund M, Fischer TK, Simonsen L, Kirkby NS, Thomsen MK, Spiess K, Marving E, Larsen NB, Lillebaek T, Ullum H, Mølbak K, Krause TG, Omicron-Delta study group Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study. Lancet Infect Dis. 2022;22(7):967–976. doi: 10.1016/S1473-3099(22)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study. Am J Transplant. 2021;21(7):2509–2521. doi: 10.1111/ajt.16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira MR, Arcasoy S, Farr MA, et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis. 2021;23(4):e13637. doi: 10.1111/tid.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goffin E, Candellier A, Vart P, et al. COVID-19 related mortality in kidney transplant and hemodialysis patients: a comparative, prospective registry based study. Nephrol Dial Transplant. 2021;36(11):2094–2105. doi: 10.1093/ndt/gfab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni AV, Tevethia HV, Premkumar M, et al. Impact of COVID-19 on liver transplant recipients–A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Ruiz M, Almendro-Vázquez P, Carretero O, et al. Discordance Between SARS-CoV-2–specific Cell-mediated and Antibody Responses Elicited by mRNA-1273 Vaccine in Kidney and Liver Transplant Recipients. Transplant Direct. 2021;7(12):e794. doi: 10.1097/TXD.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aslam S, Adler E, Mekeel K, Little SJ. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transplant Infect Dis. 2021;23(5):e13705. doi: 10.1111/tid.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kluberg SA, Hou L, Dutcher SK, et al. Validation of diagnosis codes to identify hospitalized COVID-19 patients in health care claims data. Pharmacoepidemiol Drug Saf. 2022;31(4):476–480. doi: 10.1002/pds.5401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
De-identified participant-level data are available for access to members of the scientific and medical community for non-commercial use only. Applications should be submitted to Forskerservice at The Danish Health Data Authority, where they will be reviewed on the basis of relevance and scientific merit. Data are available now, with no defined end date.