Abstract
Background and purpose
Long‐term outcome after COVID‐19 in patients with multiple sclerosis (pwMS) has scarcely been studied, and controlled data are lacking. The objective of this study was to compare long‐term outcome after COVID‐19 in pwMS to a matched control group of pwMS without COVID‐19.
Methods
We included pwMS with polymerase chain reaction‐confirmed diagnosis of COVID‐19 and ≥6 months of follow‐up and, as a control group, pwMS matched 1:1 for age, sex, disability level, and disease‐modifying treatment type.
Results
Of 211 pwMS with COVID‐19 (mean age = 42.6 years [SD = 12.2], 69% female, median Expanded Disability Status Scale = 1.5 [range = 0–7.5], 16% anti‐CD20), 90.5% initially had a mild COVID‐19 course. At follow‐up, 70% had recovered completely 3 months (M3) after COVID‐19, 83% after 6 months (M6), and 94% after 12 months (M12). Mild initial COVID‐19 course was the only significant predictor of complete recovery (odds ratio [OR] = 10.5, p < 0.001). The most frequent residual symptoms were fatigue (M3: 18.5%, M6: 13.7%, M12: 7.3%), hyposmia (M3: 13.7%, M6: 5.2%, M12: 1.7%), and dyspnea (M3: 7.1%, M6: 6.6%, M12: 2.8%). Compared to matched controls, fatigue, hyposmia, and dyspnea were significantly more frequent at M3 and still slightly more frequent at M6, whereas there was no difference at M12. pwMS with COVID‐19 had neither a significantly increased risk for relapses (OR = 1.1, p = 0.70) nor disability worsening (OR = 0.96, p = 0.60).
Conclusions
Long‐term outcome of COVID‐19 is favorable in a large majority of pwMS, with only a small proportion of patients suffering from persistent symptoms usually resolving after 3–6 months. COVID‐19 is not associated with increased risk of relapse or disability.
Keywords: COVID‐19, long term, multiple sclerosis, outcome, SARS‐CoV‐2
In a nation‐wide multicenter cohort of 211 patients with multiple sclerosis (pwMS) surviving COVID‐19, 70% had recovered completely 3 months (M3) after COVID‐19, 83% after 6 months (M6), and 94% after 12 months (M12). Compared to a matched control group of pwMS without COVID‐19, residual symptoms (fatigue, hyposmia, and dyspnea) were significantly more frequent at M3 and still slightly more frequent at M6, whereas there was no difference at M12. pwMS with COVID‐19 had neither a significantly increased risk for relapses nor disability worsening.

INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐associated disease (coronavirus disease 2019 [COVID‐19]) has caused >5.9 million deaths worldwide (as of 23 February 2022), with mortality and clinical severity of COVID‐19 strongly depending on age and comorbidities [1].
A broad body of evidence indicates that in patients with multiple sclerosis (pwMS), COVID‐19 severity is also primarily determined by age and concomitant comorbidities, but also the degree of physical disability, whereas multiple sclerosis (MS) itself is not associated with increased risk of severe COVID‐19 [2, 3, 4, 5]. Reassuringly, most immunotherapies used for disease‐modification (disease‐modifying therapy [DMT]) in MS are not associated with COVID‐19 severity, although B‐cell‐depleting anti‐CD20 monoclonal antibodies seem to be associated with a moderately increased risk [2, 6].
Whereas it is well established that the large majority of pwMS have a mild initial COVID‐19 course evading hospitalization or worse, long‐term outcome of pwMS surviving COVID‐19 is scarcely studied and controlled data are lacking [5, 7]. Thus, it is not clear whether pwMS display differences in the degree of long‐term sequelae or speed of recovery, nor whether COVID‐19 influences parameters of MS outcome such as frequency of relapses or physical disability. Some reports have hinted at an increased risk of MS relapse in relation to SARS‐CoV2 infection, but these studies are hampered by low sample size and lack of a control group [8, 9].
The objective of this study was therefore to describe long‐term outcome after COVID‐19 in pwMS in comparison to a matched control group of pwMS without COVID‐19.
METHODS
Patients and definitions
The Austrian MS‐COVID‐19 registry (AUT‐MuSC) comprises patients with a confirmed diagnosis of MS aged ≥18 years and with a diagnosis of COVID‐19 (defined by a positive SARS‐CoV‐2 polymerase chain reaction [PCR]) recruited in an ongoing nationwide multicenter observational study. Details of the study design and the data collected are described elsewhere [4, 10].
For the present study, we included all patients from AUT‐MuSC with (i) symptomatic and survived PCR‐confirmed diagnosis of COVID‐19 established between 1 January 2020 and 30 June 2021 and (ii) clinical follow‐up of ≥6 months.
As a control cohort, we included patients from the Vienna MS database with (i) confirmed MS diagnosis according to McDonald criteria and (ii) no history of a positive SARS‐CoV‐2 PCR or clinical suspicion of COVID‐19, who were matched with the AUT‐MuSC cohort for age (±5 years), sex, disability (Expanded Disability Status Scale [EDSS] ± 0.5 if AUT‐MuSC EDSS was <4 and EDSS ± 0 if AUT‐MuSC EDSS was ≥4), and DMT group.
The primary endpoint for long‐term COVID‐19 outcome was defined as complete recovery of all COVID‐19‐associated symptoms at 3 (M3), 6 (M6), and 12 (M12) months of follow‐up after SARS‐CoV2 infection. As secondary endpoints, specific COVID‐19‐associated symptoms (new or increased hyposmia, new or increased dyspnea, new or increased fatigue) were assessed at M3, M6, and M12 and classified as either completely remitted or persistent.
Endpoints for long‐term MS outcome were occurrence of relapse (defined as patient‐reported symptoms with objectively confirmed signs typical of an acute central nervous system inflammatory demyelinating event with a duration of at least 24 h in the absence of fever or infection, separated from the last relapse by at least 30 days) and disability worsening (defined as a confirmed EDSS increase of ≥1.5 points in patients with a baseline score of 0, ≥1.0 points in patients with a baseline score of 1.0–5.5, or ≥0.5 points in patients with a baseline score of >5.5 sustained for at least 3 months) [11, 12].
DMT groups were defined according to DMT status at the time of SARS‐CoV‐2 infection as receiving either no DMT, moderately effective DMT (comprising dimethyl fumarate, glatiramer acetate, interferon‐beta preparations, and teriflunomide); highly effective DMT (comprising alemtuzumab, cladribine, fingolimod, natalizumab, ozanimod, and siponimod), or anti‐CD20 monoclonal antibodies (CD20; comprising ocrelizumab, ofatumumab and rituximab) to account for differences in the risk for severe COVID‐19 course as well as differences in efficacy in preventing MS disease activity.
Severity of initial COVID‐19 was defined as the clinical status at the most severe point of COVID‐19 course as either a mild course (no pneumonia or mild pneumonia without hospitalization) or a severe course requiring hospitalization and fulfilling at least one of five criteria (breathing rate > 30/min; SpO2 ≤ 93%; PaO2/FiO2 ratio < 300; pulmonary infiltrate > 50% within 24–48 h; requirement of noninvasive ventilation, high‐flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation).
A priori risk of COVID‐19 severity was quantified according to an established risk score (MS‐COV‐risk; range from −6 to 15, with higher scores predicting an increased COVID‐19 severity) taking into account age, EDSS, smoking status, obesity (body mass index ≥ 30), and presence of cardiovascular disease (coronary heart disease and/or ischemic heart failure and/or cardiac valve disease), arterial hypertension, chronic kidney disease, chronic pulmonary disease (asthma, chronic obstructive pulmonary disease, or pulmonary fibrosis), and diabetes mellitus [13].
Statistical analysis
Statistical analysis was performed using SPSS 26.0 (IBM). Categorical variables were expressed in frequencies and percentages. Continuous variables were tested for normal distribution by Lilliefors test and expressed as mean and SD or median and range as appropriate. Univariate group comparisons were conducted by independent t‐test, Mann–Whitney U‐test, or chi‐squared test as appropriate. Univariate correlation analyses were calculated by Pearson or Spearman test as appropriate.
To investigate predictors of long‐term COVID‐19 outcome, we performed a multivariate binary logistic regression model in the MS + COVID group with complete recovery as the dependent variable and a priori risk of COVID‐19 severity (MS‐COV‐risk), sex (age and EDSS are already included in the MS‐COV‐risk score), severity of initial COVID‐19, MS disease course (relapsing–remitting vs. progressive MS), MS disease duration, and DMT group as independent variables.
To assess predictors of long‐term MS outcome, we calculated multivariate binary logistic regression models with relapse and disability worsening as dependent variables and group (MS + COVID vs. controls) as the independent variable adjusted for sex, MS‐COV‐risk score (age and EDSS are already included in the MS‐COV‐risk score), severity of initial COVID‐19, MS disease course (relapsing–remitting vs. progressive MS), MS disease duration, and DMT group.
Robustness of the statistically significant differences to unidentified confounders was quantified with Rosenbaum sensitivity test for Hodges–Lehmann Γ [14]. Missing values were handled by multiple (20 times) imputation using the missing not at random approach with pooling of estimates according to Rubin's rules [15]. A two‐sided p‐value < 0.05 was considered statistically significant.
Ethics
The study was designed and conducted in accordance with the Declaration of Helsinki, the General Data Protection Regulations, and the STROBE (Strengthening Reporting of Observational Studies in Epidemiology) guidelines (Table A1 in Appendix A) and was approved by the ethics committee of the Medical University Vienna (ethical approval number: EK 1338–2020). Patients included were informed about the objective of the study, and written informed consent was obtained.
RESULTS
Of 320 eligible pwMS, we included 211 pwMS with COVID‐19 (MS + COVID) from the AUT‐MuSC registry, and recruited 211 matched controls. Overall characteristics of the study groups are given in Table 1.
TABLE 1.
Characteristics of MS patients with COVID‐19 and MS controls
| Characteristic | MS + COVID, n = 211 | MS controls, n = 211 | p |
|---|---|---|---|
| Female a | 146 (69.2) | 146 (69.2) | 0.999 d |
| Age, years b | 42.6 (12.2) | 43.4 (12.6) | 0.508 e |
| BMI b | 24.7 (4.4) | 25.1 (4.9) | 0.378 e |
| Smokers a | 33 (15.8) | 36 (17.1) | 0.793 d |
| Ethnicity a | |||
| Caucasian a | 208 (98.6) | 205 (97.2) | 0.503 d |
| Other a | 3 (1.4) | 3 (2.7) | |
| Disease duration, years b | 12.1 (9.3) | 11.2 (10.4) | 0.349 e |
| Disease course a | |||
| RRMS a | 170 (80.6) | 172 (81.5) | 0.963 d |
| SPMS a | 30 (14.2) | 29 (13.7) | |
| PPMS a | 11 (5.2) | 10 (4.7) | |
| EDSS c | 1.5 (0–7.5) | 2.0 (0–7.5) | 0.498 f |
| On DMT a | 158 (74.9) | 158 (74.9) | 0.999 d |
| Moderately effective | 76 (36.0) | 76 (36.0) | 0.999 d |
| Interferon‐beta a | 16 (7.6) | 8 (3.8) | 0.359 d |
| Glatiramer acetate a | 16 (7.6) | 18 (8.5) | |
| Dimethyl fumarate a | 36 (17.1) | 40 (19.0) | |
| Teriflunomide a | 8 (3.8) | 10 (4.7) | |
| Highly effective | 48 (22.8) | 48 (22.8) | 0.999 d |
| Natalizumab a | 19 (9.0) | 21 (10.0) | 0.804 d |
| Fingolimod a | 20 (9.5) | 18 (8.5) | |
| Siponimod a | 1 (0.5) | 2 (1.0) | |
| Cladribin a | 5 (2.4) | 6 (2.8) | |
| Alemtuzumab a | 3 (1.4) | 1 (0.5) | |
| CD20 a | 34 (16.1) | 34 (16.1) | 0.999 d |
| Ocrelizumab a | 16 (7.6) | 13 (6.2) | 0.758 d |
| Ofatumumab a | 1 (0.5) | 1 (0.5) | |
| Rituximab a | 17 (8.1) | 20 (8.5) | |
| Lymphopenia at last lab before SARS‐CoV‐2 infection a | 28 (13.3) | 26 (12.3) | 0.884 d |
| Grade 3 or lower a | 16 (7.6) | 15 (7.1) | 0.999 d |
| Comorbidities | |||
| Any a | 71 (33.6) | 68 (32.2) | 0.836 d |
| Cardiovascular disease a | 6 (2.8) | 8 (3.8) | 0.787 d |
| Arterial hypertension a | 27 (12.8) | 31 (14.7) | 0.573 d |
| Diabetes mellitus a | 7 (3.3) | 6 (2.8) | 0.999 d |
| Chronic kidney disease a | 5 (2.4) | 6 (2.8) | 0.999 d |
| Obesity [BMI > 30] a | 35 (16.6) | 38 (18.0) | 0.797 d |
| Chronic pulmonary disease a | 5 (2.4) | 8 (3.8) | 0.575 d |
| A priori risk score of COVID‐19 [MS‐COV‐risk] e | 0 (−6 to 11) | 0 (−6 to 12) | 0.763 f |
| Initial COVID‐19 course | |||
| Mild course a | 191 (90.5) | NA | NA |
| Severe course a | 20 (9.5) | NA | |
Abbreviations: BMI, body mass index; CD20, anti‐cluster of differentiation 20 monoclonal antibodies (ofatumumab, ocrelizumab, rituximab); DMT, disease‐modifying treatment; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; PPMS, primary progressive MS; RRMS, relapsing–remitting MS; SPMS, secondary progressive MS.
Absolute number and percentage.
Fisher exact test.
Mean and standard deviation.
Independent t‐test.
Median and minimum–maximum range.
Mann–Whitney U‐test.
COVID‐19 outcome
At follow‐up, the MS + COVID group had achieved complete recovery in 70.1% (148/211) at M3 after COVID‐19, 83.4% (176/211) at M6, and 93.8% (167/178) at M12 (Figure 1a).
FIGURE 1.
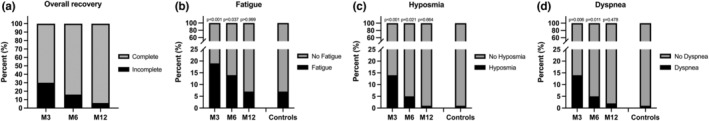
Overall recovery and residual symptoms in multiple sclerosis (MS) patients after COVID‐19 compared to MS controls. M3, 3 months after COVID‐19 diagnosis; M6, 6 months after COVID‐19 diagnosis; M12, 12 months after COVID‐19 diagnosis. Probability values were calculated by Fisher exact test comparing frequency at M3/M6/M12 in the MS + COVID cohort to MS controls
The most frequently reported residual symptom in the MS + COVID group was new or increased fatigue, in 18.5% (39/211) at M3, in 13.7% (29/211) at M6, and in 7.3% (13/178) at M12 (Figure 1b). In MS controls, new or increased fatigue occurred in 7.1% (15/211), which differed significantly from frequency of fatigue in the MS + COVID group at M3 (p < 0.001) and at M6 (p = 0.037), but not at M12 (p > 0.99).
New or increased hyposmia was reported by MS + COVID patients in 13.7% (29/211) at M3, in 5.2% (11/211) at M6, and in 1.7% (3/178) at M12 (Figure 1c). Compared to MS controls (0.9% [2/211]), there was a significant difference at M3 (p < 0.001) and M6 (p = 0.021), but not at M12 (p = 0.67).
New or increased dyspnea was reported by MS + COVID patients in 7.1% (15/211) at M3, in 6.6% (14/211) at M6, and in 2.8% (5/178) at M12 (Figure 1d). In comparison to MS controls (1.4%, 3/211), this was significantly more frequent at M3 (p < 0.001) and at M6 (p = 0.037), but not at M12 (p > 0.99).
In univariate analyses, complete recovery at M6 was significantly associated with severity of initial COVID‐19 course (89.9% after mild course vs. 50% after severe course, p < 0.001) and with a priori risk of COVID‐19 severity (ρ = −0.180, p = 0.049), but not with sex (15.8% in males vs. 16.9% in females, p > 0.999), disease course (15.3% in relapsing–remitting MS vs. 22.0% in progressive MS, p = 0.59), disease duration (ρ = −0.067, p = 0.54), or DMT group (p = 0.34).
The multivariate regression model identified severity of initial COVID‐19 course as the only significant predictor of complete recovery, with a mild course indicating an approximately 10‐fold increased probability of complete recovery (odds ratio [OR] = 10.5, 95% confidence interval [CI] = 2.7–55.6, p < 0.001) after adjusting for sex, a priori risk of COVID‐19 severity (which includes age and EDSS), MS course, disease duration, and DMT group (Table 2).
TABLE 2.
Predictors of recovery from COVID‐19 in patients with MS
| Predictor | Probability of complete recovery | ||
|---|---|---|---|
| OR a | 95% CI | p | |
| Sex | |||
| Male | Reference | ||
| Female | 0.98 | 0.90–1.10 | 0.903 |
| MS‐COV‐risk score (per point) b | 0.89 | 0.75–1.16 | 0.367 |
| MS disease course | |||
| RRMS | Reference | ||
| PMS | 0.74 | 0.55–1.22 | 0.403 |
| MS disease duration (per year) | 0.98 | 0.88–1.07 | 0.837 |
| DMT | |||
| No DMT | Reference | ||
| M‐DMT c | 1.04 | 0.82–1.31 | 0.782 |
| H‐DMT d | 1.01 | 0.80–1.28 | 0.971 |
| Anti‐CD20 e | 0.92 | 0.56–1.53 | 0.231 |
| Initial COVID‐19 course | |||
| Severe course | Reference | ||
| Mild course | 10.5 | 2.7–55.6 | <0.001 |
| Overall | R 2 overall = 0.671, p < 0.001 | ||
Note: Calculated by a multivariate binary logistic regression model with complete recovery as the dependent variable and a priori risk of COVID‐19 severity (MS‐COV‐risk), sex (age and Expanded Disability Status Scale are already included in the MS‐COV‐risk score), severity of initial COVID‐19, MS disease course (RRMS vs. PMS), MS disease duration, and DMT group as independent variables.
Abbreviations: CI, confidence interval; DMT, disease‐modifying treatment; MS, multiple sclerosis; OR, odds ratio; PMS, progressive MS; RRMS, relapsing–remitting MS.
Values >/< 1 indicate higher/lower probability of complete recovery from COVID‐19 symptoms.
MS‐COV‐risk score: MS COVID‐19 severity risk score (range from −6 to 15), with higher scores predicting an increased COVID‐19 severity (see Rosenbaum and Rubin [14]).
Defined as moderately effective DMT comprising dimethyl fumarate, glatiramer acetate, interferon‐beta preparations, and teriflunomide.
Defined as highly effective DMT comprising alemtuzumab, cladribine, fingolimod, natalizumab, ozanimod, ponesimod, and siponimod.
Defined as anti‐CD20 monoclonal antibodies comprising ocrelizumab, ofatumumab, and rituximab.
Sensitivity analyses evaluating the impact of vaccination by removal of vaccinated patients (n = 21) did not change results significantly.
MS outcome
Occurrence of relapse (11.8% [25/211] vs. 10.4% [22/211], p = 0.76) and disability worsening (6.2% [13/211] vs. 6.6% [14/211], p > 0.99) were not significantly increased in the MS + COVID group compared to the matched MS control group during the observation period (Figure 2).
FIGURE 2.
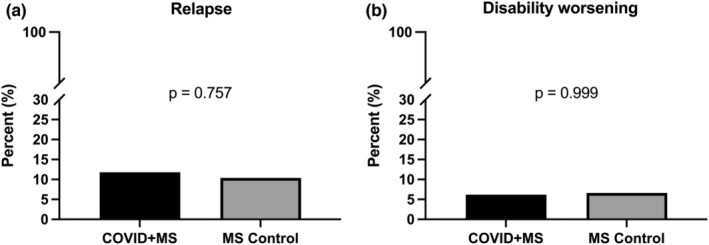
Frequency of relapse (a) and disability worsening (b) in multiple sclerosis (MS) patients with COVID‐19 compared to MS controls. Probability values were calculated by Fisher exact test
DMT was changed in 7.6% (16/211) of MS + COVID patients and in 9.0% (19/211) of controls (p = 0.725), whereas employment status remained unchanged in 96.7% (204/211) after COVID‐19 and 97.2% (205/211) in controls (p > 0.99).
Multivariate regression models revealed that the MS + COVID group was prone to neither a higher likelihood for relapse (OR = 1.1, 95% CI = 0.67–2.4, p = 0.70) nor disability worsening (OR = 0.96, 95% CI = 0.54–2.1, p = 0.60) with reference to MS controls and after adjusting for sex, MS‐COV‐risk score, severity of initial COVID‐19, MS course, disease duration, and DMT group (Table 3).
TABLE 3.
Predictors of relapse and disability worsening in MS patients with COVID‐19 and matched MS controls
| Predictor | Probability of relapse | Probability of disability worsening | ||||
|---|---|---|---|---|---|---|
| OR a | 95% CI | p | OR a | 95% CI | p | |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 1.03 | 0.94–1.15 | 0.818 | 0.94 | 0.79–1.07 | 0.635 |
| MS‐COV‐risk score (per point) b | 0.89 | 0.75–1.16 | 0.367 | 1.11 | 0.87–1.33 | 0.353 |
| Initial COVID‐19 course | ||||||
| Severe course | Reference | Reference | ||||
| Mild course | 1.10 | 0.62–2.11 | 0.721 | 0.97 | 0.54–1.81 | 0.762 |
| MS disease course | ||||||
| RRMS | Reference | Reference | ||||
| PMS | 0.53 | 0.26–0.81 | <0.001 | 2.56 | 1.78–4.62 | <0.001 |
| MS disease duration (per year) | 0.98 | 0.88–1.07 | 0.837 | 1.14 | 0.90–1.40 | 0.252 |
| DMT | ||||||
| No DMT | Reference | Reference | ||||
| M‐DMT c | 0.93 | 0.84–1.03 | 0.128 | 0.97 | 0.80–1.13 | 0.415 |
| H‐DMT d | 0.74 | 0.60–0.87 | <0.001 | 0.86 | 0.74–0.97 | 0.021 |
| Anti‐CD20 e | 0.73 | 0.58–0.91 | <0.001 | 0.83 | 0.67–0.99 | 0.049 |
| Initial COVID‐19 course | ||||||
| MS controls | Reference | Reference | ||||
| MS + COVID | 1.10 | 0.67–2.4 | 0.698 | 0.96 | 0.54–2.1 | 0.597 |
| R 2 overall = 0.724, p < 0.001 | R 2 overall = 0.645, p < 0.001 | |||||
Note: Calculated by a multivariate binary logistic regression model with relapse/disability worsening as the dependent variable and group (MS + COVID vs. controls) as the independent variable adjusted for sex, MS‐COV‐risk score (age and Expanded Disability Status Scale are already included in the MS‐COV‐risk score), severity of initial COVID‐19, MS disease course (RRMS vs. PMS), MS disease duration, and DMT group.
Abbreviations: CI, confidence interval; DMT, disease‐modifying treatment; MS, multiple sclerosis; OR, odds ratio; PMS, progressive MS; RRMS, relapsing–remitting MS.
Values >/< 1 indicate higher/lower probability of severe COVID‐19.
MS‐COV‐risk score: MS COVID‐19 severity risk score (range from −6 to 15), with higher scores predicting an increased COVID‐19 severity (see Rosenbaum and Rubin [14]).
cDefined as moderately effective DMT comprising dimethyl fumarate, glatiramer acetate, interferon‐beta preparations, and teriflunomide.
dDefined as highly effective DMT comprising alemtuzumab, cladribine, fingolimod, natalizumab, ozanimod, ponesimod, and siponimod.
eDefined as anti‐CD20 monoclonal antibodies comprising ocrelizumab, ofatumumab, and rituximab.
DISCUSSION
In this nationwide multicenter study, we investigated long‐term outcome after COVID‐19 in pwMS in comparison to a closely matched group of pwMS without COVID‐19.
Complete recovery from COVID‐19‐associated symptoms was found in 70% after 3 months, 83% after 6 months, and 94% after 12 months.
A recent study in the UK using online questionnaires of self‐reported symptoms showed complete recovery of COVID‐19 symptoms in 87% of pwMS after ≥12 weeks, with female sex, higher pre‐COVID‐19 disability, and probable anxiety/depression as predictors of incomplete recovery [7]. In our study, mild initial COVID‐19 course was the only significant predictor of long‐term outcome, indicating a 10‐fold increased likelihood of complete recovery, with no significant effect of sex, MS course, level of disability, or disease‐modifying treatment. This discrepancy is most likely explained by the UK study excluding patients who were hospitalized due to COVID‐19, therefore excluding patients with severe course, and using reported (questionnaire) instead of confirmed data [7]. The rates of recovery are well within the margin reported in the general population, although these vary significantly between 2% and 30% after 3 months, mostly depending on the method of assessment and definition of complete recovery [16, 17, 18]. Whereas larger studies providing thorough and especially objective measures are needed to assess frequency and degree of COVID‐19 sequelae, neither MS itself nor immunotherapy seem to be associated with increased risk of incomplete recovery from COVID‐19.
In the present cohort, the most frequently reported residual symptoms of COVID‐19 were new or increased fatigue, hyposmia, and dyspnea. Against the background of frequencies in the MS control cohort, which was matched for age, sex, disability, and DMT, all three symptoms were significantly more frequent after 3 months and still slightly more frequent after 6 months, but not more frequent after 12 months. Thus, COVID‐19 sequelae seem to resolve after 3–6 months in most patients. However, further studies with larger sample size and control groups of previously healthy COVID‐19 patients and pwMS without COVID‐19 are needed to establish the risk of COVID‐19 sequelae in MS.
In addition to the question of the risk for direct COVID‐19 sequelae in pwMS, concerns have been raised regarding whether SARS‐CoV2 infection could cause an increase of MS disease activity, that is, relapses or disability progression. Experimental studies suggested that SARS‐CoV2 may misdirect host immune responses and may therefore exacerbate preexisting autoimmunity [19, 20]. One study reported that 20% of pwMS develop new MS symptoms, and another showed a twofold increased risk of relapse within 2 weeks before and 4 weeks after SARS‐CoV2 infection [8, 9]. However, these studies are hampered by a low sample size, lack of a control cohort, and lack of objective assessment of symptoms, probably explaining the increased relapse rate. In our study, which includes a closely matched control cohort of pwMS without COVID‐19, we found neither a significant increased risk of relapses (OR = 1.1) nor disability worsening (OR = 0.96).
Previous studies have suggested an increased risk of MS exacerbations associated with other infections. However, these studies were uncontrolled and lacked methodologically sound distinction of relapses from pseudorelapses, that is, Uhthoff's phenomena, typically occurring in association with infection‐induced fever [21, 22, 23, 24, 25]. The consistently reported absence of an association between infections and occurrence of new magnetic resonance imaging lesions—an objective measure of MS disease activity—portends that the apparent increase of MS exacerbations in timely association with infections is probably not caused by aggravated autoimmunity [21, 22].
Strengths and limitations
The main strengths of this study are its population‐based approach and the detailed characterization of the study cohort provided by the high‐quality data from certified specialized MS centers. We have to acknowledge some potential limitations inherent to the study design.
The endpoints of incomplete/complete recovery were primarily based on patient reports rather than objective testing. This is especially important for symptoms such as dyspnea or hyposmia, where subjective perception and objective degree of impairment may vary. Also, we were unable to investigate or adjust for the effect of SARS‐CoV2 vaccines, as there were only 21 patients with complete vaccination included in the MS + COVID cohort, which did not allow inclusion into the regression models as this would have resulted in overfitting. However, we conducted sensitivity analyses evaluating the robustness of results to the impact of vaccination by removal of vaccinated patients, which did not indicate a significant change in results. Also, we could not consider the effect of reinfection, as there were no reinfections included in the cohort. Similarly, we did not have PCR sequencing results available and therefore could not investigate the potential effect of different subtypes of SARS‐CoV‐2. Due to the study period, our cohort likely comprises the wild‐type as well as the Delta variant.
There may also be confounders influencing long‐term outcome of COVID‐19 in pwMS unaccounted for in this study. However, Rosenbaum bounds indicated only a small potential impact of hidden bias not accounted for in the multivariate models [14].
CONCLUSIONS
We showed in a population‐based cohort of pwMS suffering from COVID‐19 that long‐term outcome is generally favorable in a large majority of pwMS, with only a small proportion of patients suffering from prolonged symptoms usually resolving after 3–6 months. Against the background of a closely matched MS control group, COVID‐19 is not associated with increased risk of relapse or disability.
AUTHOR CONTRIBUTIONS
Gabriel Bsteh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Gabriel Bsteh: Study concept and design, patient recruitment, acquisition of data, statistical analysis and interpretation of data, drafting of manuscript, conceptualization, data curation, writing–original draft. Hamid Assar, Christiane Gradl, Bettina Heschl, Maria‐Sophie Hiller, Nik Krajnc, Franziska Di Pauli, Gudrun Zulehner, Michael Guger, Christian Enzinger, Harald Hegen: Patient recruitment, acquisition of data, critical revision of manuscript for intellectual content. Gerhard Traxler, Fritz Leutmezer, Peter Wipfler: Acquisition of data, critical revision of manuscript for intellectual content. Harald Hegen: Drafting of manuscript. Thomas Berger: Study concept and design, patient recruitment, interpretation of data, critical revision of manuscript for intellectual content. Christiane Gradl, Bettina Heschl, Maria‐Sophie Hiller, Nik Krajnc, Franziska Di Pauli, Harald Hegen, Gerhard Traxler, Fritz Leutmezer, Peter Wipfler, Gudrun Zulehner, Michael Guger: Methodology, writing–review and editing. Hamid Assar: Investigation, writing–review and editing. Christian Enzinger: Methodology, supervision, writing–review and editing. Thomas Berger: Conceptualization, supervision, writing–review and editing.
CONFLICT OF INTEREST
G.B. has participated in meetings sponsored by and/or received speaker honoraria and/or travel funding from Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva, and has received honoraria for consulting from Biogen, Celgene/BMS, Novartis, Roche, Sanofi‐Genzyme, and Teva. He has received research grants from Celgene/BMS and Novartis. H.A. has participated in meetings sponsored by and/or received honoraria (advisory boards, consultations) and/or travel funding from Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme, Celgene/BMS, Janssen‐Cilag, and Teva. C.G. has participated in meetings sponsored by and/or received honoraria (lectures, consultations) and/or travel funding from Biogen, D‐Pharma, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. B.H. has received funding for travel or speaker honoraria from Bayer, Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. M.‐S.H. has nothing to disclose. N.K. has participated in meetings sponsored by and/or received speaker honoraria and/or travel funding from Roche, Novartis, and Merck, and held a grant for a Multiple Sclerosis Clinical Training Fellowship Program from ECTRIMS. F.D.P. has participated in meetings sponsored by and/or received honoraria (lectures, advisory boards, consultations) and/or travel funding from Almirall, Bayer, Biogen, Celgene/BMS, Janssen, Merck, Novartis, Sanofi‐Genzyme, Roche, and Teva. Her institution has received research grants from Roche. H.H. has participated in meetings sponsored by and/or received speaker honoraria and/or travel funding from Bayer, Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme, Siemens, and Teva, and has received honoraria for consulting from Biogen, Celgene/BMS, Novartis, and Teva. G.T. has participated in meetings sponsored by and/or received honoraria (lectures, advisory boards, consultations) and/or travel funding from Biogen, Celgene/BMS, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. F.L. has participated in meetings sponsored by and/or received honoraria for acting as an advisor/speaker for Bayer, Biogen, Celgene/BMS, MedDay, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. P.W. has received funding for travel and honoraria (lectures, advisory boards) from Bayer, Biogen, Celgene/BMS, Janssen‐Cilag, Merck, Novartis, Roche, Sandoz, Sanofi‐Genzyme, and Teva. G.Z. has participated in meetings sponsored by and/or received travel funding or speaker honoraria from Biogen, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva. M.G. has received support and honoraria for research, consultation, lectures, and education from Almirall, Biogen, Celgene/BMS, Genzyme, Janssen, Merck, Novartis, Roche, Sanofi Aventis, and TEVA Ratiopharm. C.E. has received funding for travel and speaker honoraria from Biogen, Bayer, Merck, Novartis, Roche, Shire, Genzyme, and Teva; has received research support from Biogen, Merck, and Teva; and is serving on scientific advisory boards for Bayer, Biogen, Celgene/BMS, Merck, Novartis, Roche, and Teva. T.B. has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Celgene/BMS, GSK, Janssen‐Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi‐Genzyme, and Teva. His institution has received financial support in the past 12 months through unrestricted research grants (Bayer, Biogen, Celgene/BMS, Merck, Novartis, Sanofi Aventis, Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Celgene/BMS, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, and Teva.
ACKNOWLEDGMENTS
We thank all the AUT‐MuSC investigators, clinical research staff, and especially the patients for helping to collect these data. The named individuals were not compensated for their help.
APPENDIX A.
TABLE A1.
STROBE statement: Checklist of items that should be included in reports of observational studies
| Section | Item | Recommendation | Page No. | Relevant text from manuscript |
|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract | 3 | |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 3 | |||
| Introduction | ||||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 4 | |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 4 | |
| Methods | ||||
| Study design | 4 | Present key elements of study design early in the paper | 5–7 | |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow‐up, and data collection | 5 | |
| Participants | 6 | (a) Cohort study: Give the eligibility criteria, and the sources and methods of selection of participants; describe methods of follow‐up | 5 | |
| Case–control study: Give the eligibility criteria, and the sources and methods of case ascertainment and control selection; give the rationale for the choice of cases and controls | ||||
| Cross‐sectional study: Give the eligibility criteria, and the sources and methods of selection of participants | ||||
| (b) Cohort study: For matched studies, give matching criteria and number of exposed and unexposed | ||||
| Case–control study: For matched studies, give matching criteria and the number of controls per case | ||||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers; give diagnostic criteria, if applicable | 5–6 | |
| Data sources/measurement | 8 a | For each variable of interest, give sources of data and details of methods of assessment (measurement); describe comparability of assessment methods if there is more than one group | 5 | |
| Bias | 9 | Describe any efforts to address potential sources of bias | 6–7, 12–13 | |
| Study size | 10 | Explain how the study size was arrived at | 6–7, 9 | |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses; if applicable, describe which groupings were chosen and why | 6–7 | |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 6–7 | |
| (b) Describe any methods used to examine subgroups and interactions | 6–7 | |||
| (c) Explain how missing data were addressed | 7 | |||
| (d) Cohort study: If applicable, explain how loss to follow‐up was addressed | ||||
| Case–control study: If applicable, explain how matching of cases and controls was addressed | ||||
| Cross‐sectional study: If applicable, describe analytical methods taking account of sampling strategy | ||||
| (e) Describe any sensitivity analyses | 7 | |||
| Results | ||||
| Participants | 13 a | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow‐up, and analyzed | 9–10 | |
| (b) Give reasons for nonparticipation at each stage | ||||
| (c) Consider use of a flow diagram | ||||
| Descriptive data | 14 a | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 9, Table 1 | |
| (b) Indicate number of participants with missing data for each variable of interest | 9–10 | |||
| (c) Cohort study: Summarize follow‐up time (eg, average and total amount) | 9 | |||
| Outcome data | 15 a | Cohort study: Report numbers of outcome events or summary measures over time | 9–10 | |
| Case–control study: Report numbers in each exposure category, or summary measures of exposure | ||||
| Cross‐sectional study: Report numbers of outcome events or summary measures | ||||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder‐adjusted estimates and their precision (e.g., 95% confidence interval); make clear which confounders were adjusted for and why they were included | 9–10, Figures 1 and 2 | |
| (b) Report category boundaries when continuous variables were categorized | 9–10, Figures 1 and 2 | |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | NA | |||
| Other analyses | 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses | 10 | |
| Discussion | ||||
| Key results | 18 | Summarize key results with reference to study objectives | 11–13 | |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision; discuss both direction and magnitude of any potential bias | 12–13 | |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 11–13 | |
| Generalizability | 21 | Discuss the generalizability (external validity) of the study results | 11–13 | |
| Other information | ||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 14 | |
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the websites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe‐statement.org.
Abbreviation: STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
Give information separately for cases and controls in case–control studies and, if applicable, for exposed and unexposed groups in cohort and cross‐sectional studies.
APPENDIX B.
AUT‐MuSC‐19 investigators
Aigner Doris (LKH Bruck, Bruck, Austria)
Assar Hamid (Kepler University Hospital, Linz, Austria)
Berger Thomas (Medical University of Vienna, Vienna, Austria)
Böck Klaus (Kepler University Hospital, Linz, Austria)
Bsteh Christian (Private practice neurologist, Salzburg, Austria)
Bsteh Gabriel (Medical University of Vienna, Vienna, Austria)
Di Pauli Franziska (Medical University of Innsbruck, Innsbruck, Austria)
Enzinger Christian (Medical University of Graz, Graz, Austria)
Gradl Christiane (Medical University of St. Pölten, St. Pölten, Austria)
Gruber Elisabeth (Hospital Diakonissen Schladming, Schladming, Austria)
Guger Michael (Med Campus III, Kepler University Hospital GmbH, Linz, Austria)
Hegen Harald (Medical University of Innsbruck, Innsbruck, Austria)
Heschl Bettina (Medical University of Graz, Graz, Austria)
Hiller Marie‐Sophie (Barmherzige Brüder Hospital Eisenstadt, Eisenstadt, Austria)
Kornek Barbara (Medical University of Vienna, Vienna, Austria)
Lemmerer Heidi (Barmherzige Brüder Hospital Graz, Graz, Austria)
Leutmezer Fritz (Medical University of Vienna, Vienna, Austria)
Lex Camillo (Hietzing Hospital, Vienna, Austria)
Mayr Markus (District Hospital Kufstein, Kufstein, Austria)
Morgenstern Gabriele (Private practice neurologist, Lienz, Austria)
Oel Dirk (Klinikum Wels‐Grieskirchen, Wels‐Grieskirchen, Austria)
Rommer Paulus (Medical University of Vienna, Vienna, Austria)
Schnabl Peter (Private practice neurologist, Velden, Austria)
Schneider‐Koch Gabriela (Ottakring Hospital, Vienna, Austria)
Schrotter Gabriele (LKH West Graz, Graz, Austria)
Traxler Gerhard (Clinic for Neurology 2, Med Campus III, Kepler University Hospital GmbH, Linz, Austria)
Wipfler Peter (Paracelsus Medical University of Salzburg, Salzburg, Austria)
Zulehner Gudrun (Medical University of Vienna, Vienna, Austria)
Zrzavy Tobias (Medical University of Vienna, Vienna, Austria).
Bsteh G, Assar H, Gradl C, et al. Long‐term outcome after COVID‐19 infection in multiple sclerosis: A nation‐wide multicenter matched‐control study. Eur J Neurol. 2022;00:1‐11. doi: 10.1111/ene.15477
AUT‐MuSC‐19 investigators listed in Appendix B
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and can be accessed from and upon approval by the ethics committee of the Medical University Vienna.
REFERENCES
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sormani MP, Rossi ND, Schiavetti I, et al. Disease‐modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89:780‐789. doi: 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079‐1088. doi: 10.1001/jamaneurol.2020.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bsteh G, Assar H, Hegen H, et al. COVID‐19 severity and mortality in multiple sclerosis are not associated with immunotherapy: insights from a nation‐wide Austrian registry. PLoS ONE. 2021;16:e0255316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barzegar M, Mirmosayyeb O, Gajarzadeh M, et al. COVID‐19 among patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sormani MP, Salvetti M, Labauge P, et al. DMTs and Covid‐19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neur. 2021;8:1738‐1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garjani A, Middleton RM, Nicholas R, Evangelou N. Recovery from COVID‐19 in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;9:e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barzegar M, Vaheb S, Mirmosayyeb O, Afshari‐Safavi A, Nehzat N, Shaygannejad V. Can coronavirus disease 2019 (COVID‐19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Dis. 2021;52:102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garjani A, Middleton RM, Hunter R, et al. COVID‐19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult Scler Relat Dis. 2021;52:102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bsteh G, Dürauer S, Assar H, et al. Humoral immune response after COVID‐19 in multiple sclerosis: a nation‐wide Austrian study. Mult Scler J. 2021;27:2209‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hegen H, Bsteh G, Berger T. ‘No evidence of disease activity’ – is it an appropriate surrogate in multiple sclerosis? Eur J Neurol. 2018;359:1221‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162‐173. [DOI] [PubMed] [Google Scholar]
- 13. Bsteh G, Bitschnau C, Hegen H, et al. Multiple sclerosis and COVID‐19: how many are at risk? Eur J Neurol. 2020;28:3369‐3374. doi: 10.1111/ene.14555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516‐524. [Google Scholar]
- 15. National Research Council (US) Panel on Handling Missing Data in Clinical Trials . The Prevention and Treatment of Missing Data in Clinical Trials. National Academies Press (US); 2010. doi: 10.17226/12955 [DOI] [PubMed] [Google Scholar]
- 16. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Premraj L, Kannapadi NV, Briggs J, et al. Mid and long‐term neurological and neuropsychiatric manifestations of post‐COVID‐19 syndrome: a meta‐analysis. J Neurol Sci. 2022;434:120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitaker M, Elliott J, Chadeau‐Hyam M, et al. Persistent symptoms following SARS‐CoV‐2 infection in a random community sample of 508,707 people. Medrxiv. 2022. doi: 10.1101/2021.06.28.21259452 [DOI] [Google Scholar]
- 19. Sormani MP, Schiavetti I, Carmisciano L, et al. COVID‐19 severity in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;9:e1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID‐19. Nature. 2021;595:283‐288. [DOI] [PubMed] [Google Scholar]
- 21. Edwards S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64:736‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buljevac D, Flach HZ, Hop WCJ, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952‐960. [DOI] [PubMed] [Google Scholar]
- 23. Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652‐659. [DOI] [PubMed] [Google Scholar]
- 24. Marrodan M, Alessandro L, Farez MF, Correale J. The role of infections in multiple sclerosis. Mult Scler J. 2018;25:891‐901. [DOI] [PubMed] [Google Scholar]
- 25. Frohman TC, Davis SL, Beh S, Greenberg BM, Remington G, Frohman EM. Uhthoff's phenomena in MS—clinical features and pathophysiology. Nat Rev Neurol. 2013;9:535‐540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and can be accessed from and upon approval by the ethics committee of the Medical University Vienna.


