Abstract
Background
Chronic disease causes skeletal muscle loss that contributes to morbidity and mortality. There are limited data on the impact of dynamic muscle loss on clinical outcomes in COVID‐19. We hypothesized that acute COVID‐19‐related muscle loss (acute sarcopenia) is associated with adverse outcomes.
Methods
A retrospective analysis of a prospective clinical registry of COVID‐19 patients was performed in consecutive hospitalized patients with acute COVID‐19 (n = 95) and compared with non‐COVID‐19 controls (n = 19) with two temporally unique CT scans. Pectoralis muscle (PM), erector spinae muscle (ESM) and 30 day standardized per cent change in cross sectional muscle area were quantified. Primary outcomes included mortality and need for intensive care unit (ICU) admission. Multivariate linear and logistic regression were performed. Cox proportional hazard ratios were generated for ICU admission or mortality for the per cent muscle loss standardized to 30 days.
Results
The COVID‐19 CT scan cohort (n = 95) had an average age of 63.3 ± 14.3 years, comorbidities including COPD (28.4%) and diabetes mellitus (42.1%), and was predominantly Caucasian (64.9%). The proportion of those admitted to the ICU was 54.7%, with 10.5% requiring tracheostomy and overall mortality 16.8%. Median duration between CT scans was 32 days (IQR: 16–63 days). Significant reductions in median per cent loss was noted for PM (−2.64% loss [IQR: −0.28, −5.47] in COVID‐19 vs. −0.06 loss [IQR: −0.01, −0.28] in non‐COVID‐19 CT controls, P < 0.001) and ESM (−1.86% loss [IQR: −0.28, −5.47] in COVID‐19 vs. −0.06 loss [IQR: −0.02, −0.11]) in non‐COVID‐19 CT controls, P < 0.001). Multivariate linear regression analysis of per cent loss in PM was significantly associated with mortality (−10.8% loss [95% CI: −21.5 to −0.19]) and ICU admission (−11.1% loss [95% CI: −19.4 to −2.67]), and not significant for ESM. Cox proportional hazard ratios demonstrated greater association with ICU admission (adj HR 2.01 [95% CI: 1.14–3.55]) and mortality (adj HR 5.30 [95% CI: 1.19–23.6]) for those with significant per cent loss in PM, and greater association with ICU admission (adj HR 8.22 [95% CI: 1.11–61.04]) but not mortality (adj HR 2.20 [95% CI: 0.70–6.97]) for those with significant per cent loss in ESM.
Conclusions
In a well‐characterized cohort of 95 hospitalized patients with acute COVID‐19 and two temporally distinct CT scans, acute sarcopenia, determined by standardized reductions in PM and ESM, was associated with worse clinical outcomes. These data lay the foundation for evaluating dynamic muscle loss as a predictor of clinical outcomes and targeting acute sarcopenia to improve clinical outcomes for COVID‐19.
Keywords: Acute sarcopenia, COVID‐19, Standardized reduction; pectoralis muscle area, Erector spinae muscle area
Introduction
The COVID‐19 pandemic has affected nearly 494.6 million patients globally and is responsible for over 6.2 million deaths. 1 , 2 Even though COVID‐19 causes multisystem injury and a systemic inflammatory response, the effects of COVID‐19 on skeletal muscle loss, a frequent and debilitating disease in patients with acute illness, have not been systematically studied. 3 , 4 Previous studies of critically ill patients have demonstrated that rapid muscle loss (or acute sarcopenia) increases the risk for morbidity and mortality. 5 , 6 Clinical contributors to muscle loss in COVID‐19 include inflammatory responses, immobilization during acute illness, lack of enteral nutrition, and medications (i.e. steroids). 3 , 7 , 8 , 9 To date, there are limited data on the impact of acute COVID‐19 on temporal changes in skeletal muscle mass.
Loss of muscle mass contributes to symptoms including fatigue and delayed return to premorbid functional capacity after recovery, known as post‐acute COVID‐19 syndrome or ‘long COVID’. 10 The few published studies on the clinical consequences of sarcopenia due to COVID‐19 report the consequences of low muscle mass based on a single measure of muscle area. 11 , 12 , 13 , 14 , 15 , 16 These data show that patients with lower muscle mass have higher risk of severe COVID‐19 which impacts post recovery outcomes. These studies on muscle mass or malnutrition in COVID‐19, however, do not report the temporal changes in muscle mass and their impact on clinical outcomes.
A number of instruments have been used to quantify muscle mass, but image analysis of computed tomogram (CT) scans of the abdomen or thorax has become the current standard approach to quantify muscle mass due to high reproducibility and precision in measuring muscle area. 17 , 18 The diagnosis of acute sarcopenia, or muscle loss during hospitalization for acute illness, is often based on a single measure of muscle area on imaging, and can be difficult to establish because the premorbid muscle mass is often not known. Since muscle loss is a dynamic process, serial measurements allow changes in muscle mass to be quantified. Protocol CTs with standardized time intervals are not routinely performed in patients with acute illness. Retrospective analyses of clinical scans with standardized interscan intervals have been used to measure the rate of abdominal/pelvic muscle loss and shown to be independent risk factors for adverse outcomes in cirrhosis and ovarian cancer. 19 , 20 Foundational studies show that low pectoralis and erector spinae muscle (PM, ESM) area are associated with adverse clinical outcomes in chronic obstructive pulmonary disease (COPD), 18 , 21 , 22 but the rate of muscle loss in thoracic muscles has not been reported. Also, unlike chronic diseases where the rate of muscle loss is gradual, acute sarcopenia due to infectious or inflammatory insults occurs more rapidly. 4 , 5 , 6 The rate of muscle loss may be related to disease severity and serve as a better predictor of adverse clinical outcomes in acute illnesses. We tested the hypothesis that COVID‐19 is associated with acute sarcopenia as compared with controls (hospitalized patients without COVID‐19), and that dynamic muscle loss in COVID‐19 predicts mortality and adverse clinical outcomes. We utilized complementary statistical approaches (i.e. regression analyses and Cox proportional hazard ratios) to identify the most consistent associations with outcomes. We studied consecutive hospitalized patients enrolled in a prospective acute COVID‐19 registry who had temporally distinct chest CTs to determine the rate of muscle loss and its association with clinical outcomes.
Patients and methods
From March 2020 to December 2020, we performed a retrospective analysis of a prospective observational clinical registry known as the Cleveland Clinic COVID‐19 Research Registry (CCCRR), 23 during which time 75 808 patients tested positive for COVID‐19 at the Cleveland Clinic (main campus and regional facilities) and 12 524 were hospitalized (Figure 1). Testing was confirmed by a reverse transcription–polymerase chain reaction SARS‐CoV‐2 assay that was validated in the Cleveland Clinic Robert J. Tomsich Pathology and Laboratory Medicine Institute. This assay used an extraction kit (MagNA Pure; Roche) and 7500 DxReal‐Time PCR System instruments (Applied Biosystems). Testing for specific variants of SARS‐CoV‐2 was not routinely performed during the time period included in this study. Other laboratory assays included a comprehensive metabolic panel, C‐Reactive protein, ferritin, procalcitonin, d‐dimer and complete blood counts. Demographic data included sex, age, and race and were recorded using standard protocols by trained clinical personnel. Co‐morbidities including COPD, diabetes mellitus, hypertension and congestive heart failure were based on a diagnosis established before the diagnosis of COVID‐19. Hospitalized patients with acute COVID‐19 and clinically indicated chest CT scan (i.e. two scans, 3 or more days apart, of sufficient quality to allow for accurate measurements) were included (n = 95) and referred to as the CT COVID cohort. This cohort was compared with the rest of the COVID‐19 registry (n = 12 429) (Supporting Information, Tables S1 and S2) to determine if the CT COVID cohort had disease severity comparable with that of the other patients in the COVID registry. Follow‐up CT scans were performed as clinically indicated either during the index hospitalization (i.e. the first time the patient was hospitalized), as an outpatient follow‐up scan, or during a hospital readmission. Prehospitalization labs (2 weeks‐3 days prior to hospital stay) and hospital labs (within 7 days of admission) were included (Table 1). Additional clinical characteristics including alcohol use were quantified as part of an exploratory analysis of risk factors associated with muscle loss because of the recognized increase in alcohol sales and consumption during the COVID‐19 pandemic and that alcohol causes sarcopenia (Table S3). We defined a standard drink as a beverage containing 0.6 fluid ounces or 14 g of pure alcohol. In addition to clinical, demographic and laboratory findings, PM and ESM area on chest CT scans were quantified by an image analysis programme. 18
Figure 1.
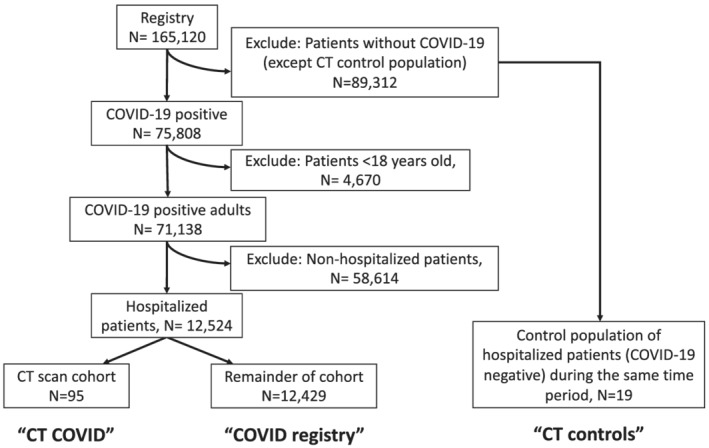
Flow chart of patients included from the COVID‐19 registry. Of the 75 782 patients who tested positive for COVID‐19 in the CCCRR between March 2020 and December 2020, 12 524 were hospitalized, 2343 had at least one CT of the chest during their hospital stay, and 95 met our inclusion criteria of having two CT scans of the chest at least 3 days apart during or after their hospital stay.
Table 1.
Laboratory test results in CT COVID cohort (n = 95)
| Pre hospitalization 1 | Admission 2 | P‐value 3 | |
|---|---|---|---|
| Lab value | |||
| WBC 4 (103 cells/μL) 6 | 7.08 (3.82) | 7.24 (3.47) | 0.595* |
| Absolute neutrophil count (cells/μL) | 5.37 (2.49) | 6.24 (4.55) | 0.017 |
| Absolute lymphocyte count (cells/μL) | 0.95 (0.71) | 1.04 (1.17) | 0.188 |
| Absolute eosinophil count (cells/μL) | 0.05 (0.06) | 0.08 (0.01) | 0.487* |
| Platelets (×1000) | 195 (81.6) | 251 (131) | <0.001 |
| Albumin (g/dL) | 3.57 (0.65) | 3.50 (0.57) | 0.422 |
| Alkaline phosphatase (U/L) | 102 (78.8) | 109 (104) | 0.992* |
| ALT 4 (U/L) 5 | 38.8 (50.0) | 40.7 a (42.9) | 0.897* |
| AST 4 (U/L) 5 | 54.5 a (70.1) | 101 a (51.1) | 0.002* |
| Bicarbonate (mmol/L) | 25.0 (3.68) | 24.1 (4.07) | 0.154 |
| BUN (mmol/L) | 25.0 (3.73) | 24.1 (15.8) | 0.631* |
| Creatinine (mg/dL) | 1.81 (2.63) | 1.17 (0.97) | 0.968* |
| Haematocrit (%) | 37.2 (5.98) | 34.9 (6.71) | <0.001 |
| Haemoglobin (g/dL) | 12.2 (2.15) | 11.3 (2.38) | <0.001 |
| Potassium (mmol/L) | 4.12 (0.73) | 4.17 (0.56) | 0.195 |
| Sodium (mmol/L) | 136 (4.59) | 137 (4.90) | 0.106 |
| Total bilirubin (mg/dL) | 0.69 (1.67) | 0.75 (2.09) | 0.960* |
Pre testing labs were missing in 30% of subjects. Average time between pre‐hospitalization and admission labs was 5(±1.5) days.
Admission labs were missing in 5% of subjects.
P‐values represent pairwise Student's two‐tailed t‐tests for normally distributed continuous variables and Wilcoxon–Mann–Whitney rank sum test for non‐normally distributed variables (denoted by *). Difference from control, P < 0.05.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; WBC = white blood cell.
Values represent plasma concentrations.
Values represent whole blood concentrations.
Represents liver enzyme values which are above the upper limit of normal.
The control group (CT controls) consisted of a hospitalized non‐COVID‐19 group (n = 19) admitted during the same period who tested negative for COVID‐19 and had at least two CT scans, one of which was performed during the index hospital stay (i.e. the first admission in case a patient was re‐admitted). The proportion of CT control patients who were admitted to the regular medical unit versus the ICU was similar to the CT COVID cohort (Table S4). The proportion of comorbidities was also similar in the CT control and CT COVID groups, and we did not exclude conditions such as congestive heart failure or cancer known to cause sarcopenia in our control population because these comorbidities increase the risk for poor outcomes in COVID‐19.
The primary outcomes were mortality and need for ICU admission. Secondary outcomes included need for proning, high flow nasal cannula, NIPPV (non‐invasive positive pressure ventilation), IMV (invasive mechanical ventilation), length of ICU stay, or need for dialysis. Survival was determined from the date of the first CT scan to evaluate the rate of muscle loss as previously described. 19 Median duration between CT scans was 32 days (IQR: 16–63 days). For survival analysis, subjects were followed 90 days after their initial CT scan and then censored as reported in previous studies. 24 Clinical characteristics were retrieved from the electronic medical record using standard algorithms and manually verified. Study data were collected and managed using REDCap®. The study was approved by Cleveland Clinic's central IRB (21‐1277) with waiver of written informed consent due to minimal risk to research subjects.
CT scan analysis
Quantitative assessments of PM and ESM cross‐sectional area (CSA) on chest CT scans were performed (Figure 2) using Aquarius iNtuition (TeraRecon Inc, Durham, NC) as previously reported by us. 18 Muscles were selected based on an attenuation range of −50 to +90 Hounsfield units, consistent with the values for skeletal muscle as previously described. 18 In brief, single axial slice of the CT scan was used for PM just above the aortic arch and for ESM at the level of the lower margin of the 12th thoracic vertebra. The total area (in cm2) of PM and ESM on both sides were used for these analyses.
Figure 2.
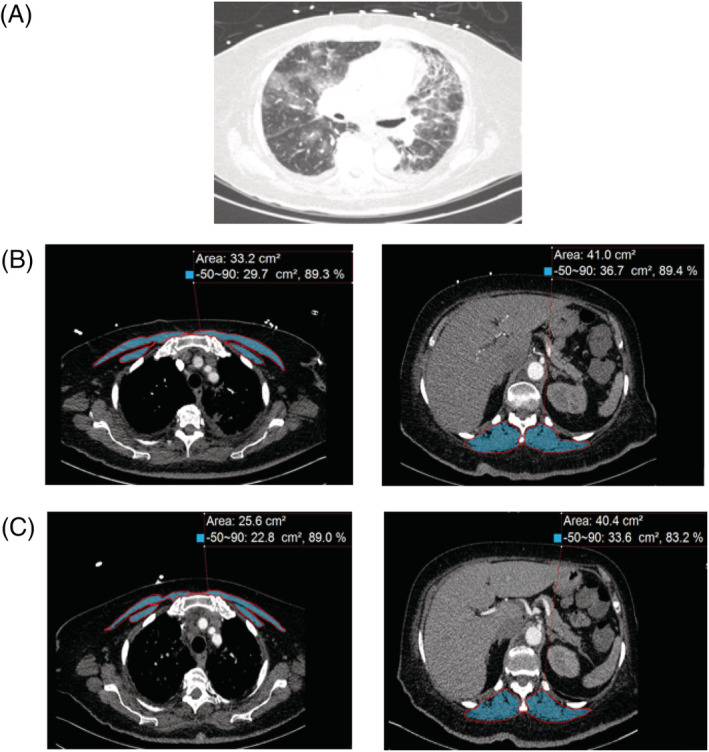
Representative computed tomography (CT) scans at thoracic level used to determine muscle area in patients with COVID‐19. (A) Representative CT image utilizing lung windows demonstrates evidence of COVID‐19 related bilateral pneumonia. (B) Representative CT image for pectoralis muscle and erector spinae muscle imaging from the initial CT scan are shaded. Skeletal muscle CSAs are measured in cm2. (C) Representative CT image for pectoralis muscle and erector spinae muscles from the subsequent CT scan are shaded. Skeletal muscle mass CSAs are measured in cm2.
Quantification of the muscle area was done by an experienced investigator (AA) who was masked to the diagnosis, outcomes or time interval between the scans. Change in PM and ESM CSA on temporally distinct CT scans were expressed as absolute ((Musclefinal − Muscleinitial)/(days between CT scan)) × (30 days) and per cent ((((Musclefinal − Muscleinitial)/(Muscleinitial)*100)/(days between CT scan)) × (30 days) change standardized to 30 days. Standardization across a predefined time interval allows for comparisons across patients with variable interscan intervals, an approach reported earlier. 19 , 20 We also performed an analysis of outcomes related to static analysis: (Muscleinitial and outcomes, Musclefinal and outcomes).
Statistical analyses
Continuous variables are presented as mean ± SD. Qualitative variables were compared using a chi square test. For quantitative variables, the Student's t‐test or analysis of variance were used for normally distributed variables and Wilcoxon–Mann–Whitney rank sum for non‐normally distributed variables. Non‐normally distributed variables are presented as median with interquartile range. For differences between medians, the ‘median of difference’, that is, the median change between pre‐CT and post‐CT scan muscle area was determined; 2 × 2 χ 2 was used to compare alcohol use and elevated liver function enzymes. Our previous analysis of a COPD population demonstrated that a reduction of 4.92 cm2 CSA of the PM was associated with poor clinical outcomes. 18 Therefore, using a two‐tailed analysis, we predicted the need for 37 subjects to have 90% power to detect a change in PM of at least 4.92 cm2 which we then doubled given our analysis also included ESM and took into account the rapidity of muscle loss in addition to static measures. We then performed a power analysis subsequent to the initiation of the study comparing mean per cent reduction in PM and ESM (CT COVID cohort vs. CT controls) using a two‐sample unbalanced design and determined that n = 19 for CT controls had greater than 90% power (alpha = 0.05) to determine significant differences in muscle loss. Multivariate linear and logistic regression models were generated and adjusted for age, sex, BMI, and race. For our logistic regression analysis, significant muscle loss for PM and ESM was determined using Youden's optimal cutpoint criteria 25 to calculate the optimum cutoff based on sensitivity and specificity for absolute muscle loss standardized to 30 days. Multivariate Cox proportional hazard analysis was performed to determine the time to event for ICU admission or mortality based on the rate of muscle loss (adjusted for age, sex, BMI, and race). A receiver operating characteristic curve was generated and Youden's optimal cutpoint criteria 25 was determined with admission to the ICU or death as the outcome variable. To preserve degrees of freedom, we compared African‐American race versus all other races. A P‐value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R (version 4.0.2).
Results
Of the 75 782 patients who tested positive for COVID‐19 in the CCCRR (March 2020 to December 2020), 12 524 were hospitalized, and 95 met our inclusion criteria of having two CT scans of the chest at least 3 days apart during or after their hospital stay (median 32.0; IQR 16.0 to 63.0 days (Figure 1). This cohort, known as the CT COVID cohort, (n = 95) was then compared with the remainder of the COVID registry (n = 12 429) to determine any differences in disease severity between these populations. The baseline clinical and demographic data comparing the CT COVID cohort to the COVID registry are shown in Table S1. While baseline demographics were similar, the CT COVID cohort had more comorbidities than the COVID registry. A high mean BMI (COVID registry: 30.7 ± 8.4 kg/m2|CT COVID: 31.9 ± 8.9 kg/m2|P = 0.874) was consistent with previous reports of increased risk of severe COVID‐19 in obese subjects. Prior documented exposure to COVID‐19 was noted in over a third of patients in both populations. Shortness of breath (70.2% vs. 45.9%, P < 0.001), cough (65.6% vs. 45.3%), and fever (43.2% vs. 25.6%, P < 0.001) were more common in the CT COVID cohort than the COVID registry. Comorbidities including COPD (28.4% vs. 20.0%, P = 0.042), asthma (40.0% vs. 15.6%, P < 0.001), and congestive heart failure (27.4% vs. 18.4%, P = 0.024) were also more common in the CT COVID cohort than the COVID registry.
The clinical course for the hospitalized COVID registry patients was then compared with that of the CT COVID cohort (Table S2). The CT COVID cohort was more likely to require ICU admission than the COVID registry (54.7% vs. 23.3%, P < 0.001), and require advanced procedures like mechanical ventilation (38.9% vs. 9.3%, P < 0.001). However, mortality in the two cohorts was similar (CT COVID 16.8% vs. COVID registry 16.7%, P = 0.979). CT COVID patients were more likely to be discharged to an acute care hospital (9.5% vs. 2.7%, P < 0.001), and be readmitted (30 day readmission 35.8% vs. 12.3%, P < 0.001; 90 day readmission 61.5% vs. 18.0%, P < 0.001). Overall, these findings show that the CT COVID cohort had higher disease severity than the remainder of the COVID registry. Additional findings that were present in the CT COVID cohort but not in the overall COVID registry are shown in Table S3. In the CT COVID cohort, the majority of deaths were due to respiratory failure (68.8%) followed by liver failure (18.8%). We also noted that nearly a quarter of the CT COVID cohort drank more than five alcoholic drinks per week, which is a known risk factor for muscle loss.
The laboratory findings for the CT COVID cohort (prehospitalization and admission) are shown in Table 1. Significant differences were noted between prehospitalization and admission laboratory values for absolute neutrophil count, platelets, haematocrit, and haemoglobin. Aspartate amino transferase (AST) was elevated pre‐admission (54.5 ± 70.1 U/L) and both alanine amino transferase (ALT) (40.7 ± 42.9 U/L) and AST (101 ± 51.1 U/L) were elevated at admission. There was a negative correlation between CT scan interval and survival (R 2 = 0.30, P = 0.003).
We then quantified the degree of muscle loss in the CT COVID cohort and determined whether muscle loss was present in concurrent non‐COVID‐19 patients (‘CT controls’; n = 19) admitted to the hospital during the same period (Table S4). Quantitative assessments of PM and ESM CSA on chest CT scans were performed (Figure 2). Changes in muscle area are shown in Table S5 with significant reductions noted in PM and ESM in the CT COVID cohort as compared with the CT controls. When the per cent change in muscle mass was standardized to 30 days as a measure of the rate of muscle loss (Table S6), reductions in PM and ESM were minimal in the CT controls and pronounced in the CT COVID cohort (PM median change per 30 days −0.06 cm2 [IQR −0.01, −0.28] in CT controls vs. −2.64 cm2 [IQR −0.56, −6.60] in CT COVID, P < 0.001|ESM median change per 30 days −0.06 cm2 [IQR −0.02, −0.11] in CT controls vs. −1.86 cm2 [IQR −0.28, −5.47] in CT COVID, P < 0.001). These data show that the rate of muscle loss was significantly greater in the CT COVID cohort compared with CT controls.
The clinical outcomes of the CT COVID cohort were then compared among survivors and non‐survivors (Table 2). There was no difference in age, sex, or BMI between survivors and non‐survivors. Admission to the ICU was greater for those who died (81.2% vs. 49.4%, P = 0.039) and associated with lower initial ESM area (27.9 cm2 [IQR 24.5, 37.3] vs. 37.0 cm2 [IQR 30.8, 46.8], P = 0.025), and final ESM area (27.1 cm2 [IQR 23.7, 31.4] vs. 35.1 [IQR 27.2, 43.3], P = 0.009). The per cent loss of PM standardized to 30 days was greater (P = 0.037) in those who died (−2.69 cm2 [IQR −8.81, −1.54]) versus those who survived (−1.14 cm2 [IQR −5.17, −0.24]).
Table 2.
Demographics and clinical outcomes based on survival in CT COVID cohort (n = 95)
| Alive (n = 79) | Dead (n = 16) | P‐value 1 | |
|---|---|---|---|
| Clinical outcomes | |||
| Age (in years) (mean (SD)) | 63.1 (13.7) | 64.3 (17.4) | 0.773 |
| Sex (female) (%) | 37 (46.8) | 8 (50.0) | 1 |
| BMI 2 (mean (SD)) (kg/m2) | 32.5 (9.26) | 29.2 (6.39) | 0.177 |
| Race (African‐American) (%) | 21 (26.6) | 4 (25.0) | 1 |
| Hospital length of stay (in days) (median (IQR)) | 13.0 (6.0, 28.0) | 21.0 (13.8, 37.3) | 0.028* |
| ICU admission (%) | 39 (49.4) | 13 (81.2) | 0.039 |
| Mechanical ventilation (%) | 25 (69.4) | 13 (92.9) | 0.17 |
| PM CSA (initial) (cm2) (median (IQR)) | 33.1 (26.0, 47.0) | 31.0 (27.2, 34.9) | 0.383* |
| PM CSA (final) (cm2) (median (IQR)) | 32.1 (24.1, 42.2) | 27.6 (24.8, 29.1) | 0.076* |
| Per cent change in PM CSA (cm2) (standardized to 30 days) (median (IQR)) | −1.14 (−5.17,‐0.24) | −2.69 (−8.81,‐1.54) | 0.037 |
| ESM CSA (initial) (cm2) (median (IQR)) | 37.0 (30.8, 46.8) | 27.8 (24.5, 37.3) | 0.025* |
| ESM CSA (final) (cm2) (median (IQR)) | 35.1 (27.2, 43.3) | 27.10 (23.7, 31.4) | 0.009* |
| Per cent change in ESM CSA (cm2) (standardized to 30 days) (mean (SD)) | −0.81 (−4.62,‐0.10) | −2.18 (−10.9,‐0.70) | 0.089 |
| Lab results (on admission) (mean (SD)) | |||
| CRP 2 (mg/L) | 4.60 (1.75, 12.1) | 14.5 (2.80, 22.4) | 0.052* |
| Procalcitonin (ng/mL) | 0.17 (0.09, 0.33) | 0.24 (0.13, 0.82) | 0.154* |
| Lactate (mmol/L) | 1.63 (0.77) | 2.14 (3.01) | 0.359 |
| d‐dimer (ng/mL) | 1050 (600, 2760) | 3175 (1065, 5440) | 0.044* |
| WBC 2 (103 cells/μL) | 6.80 (5.12, 8.61) | 7.19 (5.02, 10.2) | 0.865* |
| Absolute lymphocyte count (cells/μL) | 1.03 (1.18) | 1.14 (1.16) | 0.740 |
P‐values represent pairwise Student's two‐tailed t‐tests for normally distributed continuous variables and Wilcoxon–Mann–Whitney rank sum test for non‐normally distributed variables (denoted by *). Non‐normally distributed variables are presented as median with interquartile range. Difference from control, P < 0.05.
BMI = body mass index; WBC = white blood cells; CRP = C‐reactive protein.
Linear regression analysis of clinical outcomes in CT COVID patients and per cent loss of PM and ESM (Table 3) showed that the per cent loss in PM was significantly associated with mortality (−10.8% loss [95% CI: −21.5 to −0.19]) and ICU admission (−11.1% loss [95% CI: −19.4 to −2.67]) in multivariate models, but were not significant for ESM. Other clinical outcomes including proning, high flow nasal cannula, NIPPV, IMV, ICU length of stay, or need for dialysis were not significantly associated with the rate of loss of PM or ESM.
Table 3.
Regression models analysing outcomes in CT COVID cohort (n = 95)
| Linear regression analysis comparing % PM reduction per 30 days | Linear regression analysis comparing % ESM reduction per 30 days | |||
|---|---|---|---|---|
| Unadjusted model | Adjusted1 model | Unadjusted model | Adjusted1 model | |
| Mortality | −4.74 (−24.8 to 16.3) | −10.8* (−21.5 to −0.19) | −5.02 (−12.01 to 1.97) | −5.88 (−13.1 to 1.32) |
| ICU2 admission | −2.44 (−17.7 to 12.8) | −11.1* (−19.4 to −2.76) | −0.75 (−6.30 to 4.81) | −1.75 (−7.72 to 4.22) |
| Proning | −1.07 (−20.0 to 17.9) | −2.72 (13.2 to 7.74) | −1.56 (−8.33 to 5.22) | −0.58 (−7.73 to 6.57) |
| High flow nasal cannula | 2.73 (−12.7 to 18.1) | −1.76 (−10.2 to 6.67) | −2.45 (−8.04 to 3.14) | −2.30 (−8.11 to 3.50) |
| NIPPV2 | 0.42 (−15.3 to 16.2) | −3.18 (−11.8 to 5.48) | −2.89 (−8.55 to 2.78) | −2.56 (−8.52 to 3.40) |
| IMV2 | −5.38 (−79.5 to 68.8) | −3.90 (−15.2 to 2.33) | −3.63 (−9.28 to 2.03) | −3.82 (−9.90 to 2.25) |
| ICU length of stay (per day) | −0.40 (−0.91 to 0.10) | −0.32 (−0.84 to 0.19) | 0.04 (−0.21 to 0.28) | 0.11 (−0.16 to 0.37) |
| Need for dialysis | 5.90 (−22.8 to 34.6) | −6.38 (−9.17 to 21.9) | −3.92 (−15.0 to 7.15) | −2.67 (−14.1 to 8.73) |
Multivariate model adjusted for age, sex, race (African American vs. other) and BMI. Of these covariates, sex was significant after adjustment for PM linear regression (P = 0.006) and trended toward significant for ESM linear regression (P = 0.056). The other covariates were not significant.
ICU = intensive care unit; NIPPV = non‐invasive positive pressure ventilation; IMV = invasive mechanical ventilation.
Values with * are statistically significant.
Logistic regression analysis showed a significant association of reduction in PM with clinical outcomes in unadjusted models (mortality: unadj OR 4.00 [95% CI: 1.27–13.3], ICU admission: unadj OR 2.44 [95% CI: 1.00–6.21], need for proning: unadj OR 2.91 [95% CI: 1.06–8.25], and NIPPV: unadj OR 2.47 [95% CI: 1.02–6.11]). Mortality remained associated with PM reduction in adjusted models as well (adj OR 4.60 [95% CI: 1.37–16.8]) (Table 4). For ESM, only need for proning (unadj OR 2.78 [95% CI: 1.01–7.84]) was significant in unadjusted models (Table 4). To incorporate the time‐to‐event for outcomes, we generated Cox proportional hazard models (Figure 3) and demonstrated that per cent loss in PM was associated with ICU admission (adj HR 2.01 [95% CI: 1.14–3.55]) and mortality (adj HR 5.30 [95% CI: 1.19–23.6]), and that per cent loss in ESM was associated with ICU admission (adj HR 8.22 [95% CI: 1.11–61.04]) but not mortality (adj HR 2.20 [95% CI: 0.70–6.97]).
Table 4.
Multivariate logistic regression model analysing outcomes in CT COVID patients (n = 95)
| Increased PM reduction per 30 days 1 | Increased ESM reduction per 30 days 1 | |||
|---|---|---|---|---|
| Unadjusted OR 4 | Adjusted OR 4 | Unadjusted OR 4 | Adjusted OR 4 | |
| Mortality | 4.00 (1.27–13.3)* | 4.60 (1.37–16.8)* | 1.51 (0.47–4.49) | 1.48 (0.45–4.61) |
| ICU 3 admission | 2.44 (1.00–6.21)* | 2.51 (0.95–7.01) | 1.93 (0.81–4.81) | 1.94 (0.76–5.21) |
| Proning | 2.91 (1.06–8.25)* | 2.11 (0.72–6.27) | 2.78 (1.01–7.84)* | 2.26 (0.79–6.65) |
| High flow nasal cannula | 1.81 (0.76–4.38) | 1.53 (0.61–3.87) | 2.34 (0.98–5.68) | 2.05 (0.83–5.14) |
| NIPPV | 2.47 (1.02–6.11)* | 2.03 (0.79–5.24) | 1.44 (0.59–3.46) | 1.15 (0.45–2.88) |
| IMV 3 | 1.95 (0.81–4.74) | 1.49 (0.58–3.82) | 1.63 (0.68–3.91) | 1.45 (0.57–3.67) |
| ICU 3 length of stay (per day) | 1.00 (0.97–1.05) | 0.99 (0.94–1.05) | 1.00 (0.96–1.04) | 0.99 (0.94–1.03) |
| Need for dialysis | 0.92 (0.12–4.98) | 0.67 (0.08–3.90) | 2.85 (0.45–22.5) | 2.27 (0.34–18.5) |
Significant absolute PM reduction per 30 days defined as >4.988 cm2 cross‐sectional area. Significant absolute ESM reduction per 30 days defined as >4.152 cm2 cross‐sectional area. Determined by Youden's optimal cutpoint criteria.
Multivariate model adjusted for age, sex, race (African American vs. other) and BMI.
ESM = Erector spinae muscle; ICU = Intensive Care Unit; IMV = Invasive mechanical ventilation; NIPPV = Non‐invasive positive pressure ventilation; OR = Odds ratio; PM = Pectoralis muscle.
Values denoted by * are statistically significant.
Figure 3.
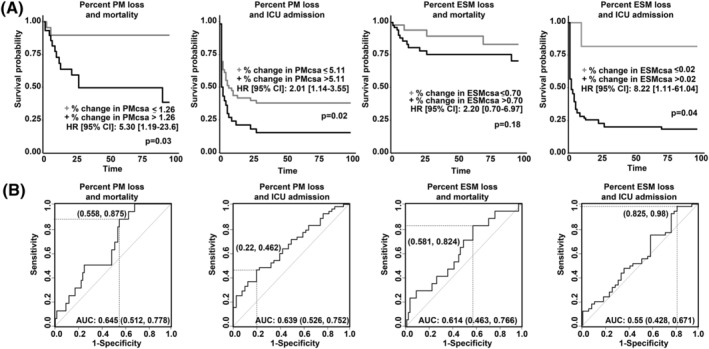
Mortality and ICU admission outcomes for patients with COVID‐19 who had decreased muscle area. (A) Cox proportional hazards ratio for mortality and ICU admission patients with COVID‐19 as determined by percentage change in pectoralis major (PM) and erector spinae (ES) muscle area. The cutoff value for PM and ESM areas corresponds to the optimum cutoff determined by a receiver operating characteristic curve based on sensitivity and specificity in this cohort for the standardized rate of muscle loss. The Cox proportional hazard ratio and P‐values for the optimum cutoff are presented. Optimum cutoffs are defined in the corresponding panel using Youden's optimal cutpoint criteria. (B) Receiver operating characteristic curves for per cent change in PM or ESM area.
As a part of our exploratory analysis of alcohol intake in CT COVID patients, we noted that alcohol consumption ≥5 drinks per week was associated with absolute reductions in PM in unadjusted (−7.38 cm2 [95% CI: −13.3 to −1.71], P = 0.007) and adjusted linear regression models (−5.93 cm2 [95% CI: −0.41 to −11.5]). Ingestion of ≥10 drinks per week was associated with absolute reductions in PM in our univariate model (−7.48 cm2 [95% CI: −13.3 to −1.71], P = 0.012) and showed a trend toward significance in our multivariate model (−5.97 cm2 [95% CI: −12.0 to 0.02], P = 0.051). Standardized change in ESM was not significantly associated with alcohol intake (Table 5), and per cent reductions in PM and ESM were also not significantly associated (data not shown).
Table 5.
Multivariate linear regression model analysing change in muscle mass and alcohol use in CT COVID patients (n = 95)
| Change in PM 3 per 30 days | ||||
|---|---|---|---|---|
| Unadjusted model 1 (95% CI) | P‐value | Adjusted model 1 (95% CI) | P‐value | |
| Alcohol abuse diagnosis | −2.62 (−7.82 to 13.1) | P = 0.619 | −0.77 (−11.3 to 9.74) | P = 0.884 |
| Drinks per week 1 | 0.01 (−0.19 to 0.18) | P = 0.949 | 0.06 (−0.13 to 0.24) | P = 0.529 |
| ≥5 drinks per week | −7.38 (−12.7 to −2.04) | P = 0.007 | −5.93 (−0.41 to −11.5) | P = 0.036 |
| ≥10 drinks per week | −7.48 (−13.3 to −1.71) | P = 0.012 | −5.97 (−12.0 to 0.02) | P = 0.051 |
| Change in ESM 3 per 30 days | ||||
|---|---|---|---|---|
| Unadjusted model 1 (95% CI) | P‐value | Adjusted model 1 (95% CI) | P‐value | |
| Alcohol abuse diagnosis | 4.88 (−8.92 to 18.7) | P = 0.484 | 5.12 (−9.24 to 19.5) | P = 0.480 |
| Drinks per week | −0.10 (−0.14 to 0.33) | P = 0.407 | 0.10 (−0.14 to 0.34) | P = 0.407 |
| ≥5 drinks per week | 2.25 (−4.36 to 8.85) | P = 0.50 | 4.07 (−2.68 to 10.8) | P = 0.234 |
| ≥10 drinks per week | −0.15 (−7.27 to 6.97) | P = 0.97 | −0.61 (−5.44 to 9.26) | P = 0.607 |
Multivariate model adjusted for age, sex, race (African American vs. other) and BMI. Numbers represent the beta coefficient and 95% confidence intervals. P < 0.05 considered significant. Bolded values are statistically significant. Italicized values approached statistical significance.
A standard drink was defined as a beverage containing 0.6 fluid ounces or 14 g of pure alcohol.
CI = Confidence interval; ESM = Erector spinae muscle; PM = Pectoralis muscle.
Outcomes related to static measures of PM and ESM CSA (based on initial and final CT scans) were then analysed in the CT COVID cohort (Table S7). As previously noted, reductions in ESM (initial and final CSA) were associated with mortality. Mechanical ventilation was more common in patients with a reduction in final ESM CSA (median 29.1 cm2 [IQR: 22.7, 39.4]] vs. 33.6 cm2 [26.5, 45.5], P = 0.028). Other outcomes (including ICU admission, high flow nasal cannula, NIPPV, IMV, and dialysis) were not significantly associated with static initial or final PM or ESM CSA.
Discussion
Acute sarcopenia, defined as skeletal muscle loss that occurs during hospitalization from an acute illness, 6 occurs in a number of inflammatory and infectious disorders and contributes to increased mortality and morbidity. 5 , 6 , 26 Our critical review of published literature of acute sarcopenia in COVID‐19 in retrospective and prospective cohorts (Table S8) showed that sarcopenia is frequent and variably associated with adverse clinical outcomes. Even though sarcopenia is a dynamic process, published literature to date has only focused on the relation between static measures of muscle mass and predefined outcomes in patients with COVID‐19. In the present study, we show that acute sarcopenia is a dynamic process, for PM in particular, and that the rate of muscle loss is related to multiple adverse clinical outcomes for PM, while static measures showed associations of ESM with mortality and need for mechanical ventilation demonstrating that not all muscles respond similarly during acute inflammatory states.
Despite effective vaccines against COVID‐19, a high proportion of the population globally remains unvaccinated and vaccine‐escape variants continue to appear, resulting in an increase in number of patients with COVID‐19. Clinical management has focused on supportive care for acute hypoxia and multiple organ dysfunction in patients with COVID‐19, 2 but nutritional consequences, including acute malnutrition and skeletal muscle loss are recognized in hospitalized patients using static measures. 3 , 4 In the present study, using a validated quantification of thoracic skeletal muscle area, 18 we show that standardized per cent reduction in PM was associated with the primary outcomes (mortality, ICU admissions) while change in ESM was associated with ICU admission in hospitalized patients with acute COVID‐19.
The clinical outcomes in our cohort of hospitalized acute COVID‐19 including in‐hospital mortality is similar to that reported by others. 2 Published data have focused on static measures of muscle loss, while our approach to measure the rate of muscle loss lays the foundation for future studies to directly compare static and dynamic measures of muscle loss due to COVID‐19 and other disorders. In the present study, we focused on acute sarcopenia, but prolonged symptoms of COVID‐19 (also known as ‘long COVID’) may be due to muscle loss, given that fatigue, dyspnoea, and joint pain are frequent sequalae of COVID‐19, 27 and significant impairment in exercise tolerance has been noted to be associated with long COVID. 28 Future studies are required to determine the impact of the rate of muscle loss on long term consequences of acute illnesses including COVID‐19.
Our exploratory analysis demonstrated that frequent alcohol intake was common, defined as ≥5 and 10 drinks per week, which was consistent with emerging data on alcohol sales/consumption during COVID‐19 lockdowns. 29 Frequent alcohol intake was also associated with significant absolute reductions in standardized change of PM per 30 days. Since excessive alcohol use causes sarcopenia or skeletal muscle loss directly and via liver injury, 19 these findings show that additional approaches are required to reverse muscle loss and improve clinical outcomes in these patients. Interestingly, elevated liver enzymes can occur in nearly half the patients with COVID‐19 and may represent liver injury due to a combination of excessive alcohol use, direct binding of SARS‐CoV‐2 to ACE2 positive cholangiocytes, and cytokine dysregulation leading to immune‐mediated hepatic injury 30 that may also contribute to the sarcopenia. We also noted significant increases in D‐dimer and ferritin levels in the CT COVID cohort consistent with previous reports that elevated levels of ferritin 31 and D‐dimer 32 occur with hypoxia, which causes sarcopenia by multiple mechanisms. 33
Our study demonstrated significant muscle loss in patients with COVID‐19; however, the mechanism(s) for muscle loss in these patients is likely multifactorial. Interruption of nutritional intake during hospitalization and COVID‐19 related gastrointestinal symptoms including reduced appetite, nausea, and vomiting could have potentially contributed to reduced nutrient intake. All subjects in our study required supplementary oxygen, and it is known that hypoxia causes anorexia, 33 suggesting another potential reason for poor oral intake in this population. There can also be delays in initiating nutrition for patients who required mechanical ventilation that may not be identified in the medical records. It is, therefore, possible that a component of muscle loss occurred due to insufficient nutritional intake/supplementation in the hospital which would require careful analyses of dietary data that were not part of the registry. Data abstraction from electronic health records (EHR) also has the potential to contain errors, and diagnoses based on billing codes are not always consistent with the medical record. However, given the nature of our COVID‐19 registry and that data was input and extracted by trained clinical personnel, this is less likely. We also note that certain parameters including the type of steroid medication taken (inhaled vs. oral vs. topical), reason for ICU transfer and cause of death were not a part of the registry and required manual abstraction from the EHR.
We recognize that the overall severity of illness in our cohort may not translate to a general COVID‐19 population. Patients with two chest CT scans either during the same hospital stay or follow‐up indicates either critical illness or significant pulmonary inflammation due to COVID‐19 that required follow‐up imaging. This is reflected by the high proportion of our subjects who were admitted to the ICU or required tracheostomy, which was greater than that in the general hospitalized cohort. 34 This indication bias suggests a higher severity of illness in our cohort than that in the overall COVID‐19 population. To mitigate this bias, we included a control population of non‐COVID‐19 patients (‘CT controls’) admitted to the hospital during the same period who also required two CT scans with a similar proportion of ICU and patients in the regular nursing units. In our COVID‐19 CT cohort, the rate of muscle loss was significantly greater in COVID‐19 patients as compared with CT controls. Since our control population included a range of medical conditions and was not specific to respiratory infections, our interpretation of the adverse impact of COVID‐19 on muscle loss was likely conservative and the true impact of COVID‐19 on acute sarcopenia may therefore be greater. Our observations in patients with COVID‐19 are similar to previous reports of respiratory infections like influenza that are associated with significant skeletal muscle loss, 35 , 36 suggesting that longitudinal studies of muscle mass are likely to help identify underlying mechanisms as well as define dynamic outcomes in these patients. Future studies are needed to determine whether COVID‐19 increases the risk for sarcopenia as compared with other severe respiratory infections.
There was no significant difference in age, sex, or BMI between survivors and non‐survivors in our analysis. Studies to date have noted that increased age, male sex, and increased BMI are associated with worse outcomes related to COVID‐19, 37 and therefore lack of statistical associations of these previously reported risk factors in our study may be due to smaller sample size (n = 95). Since studies in large COPD cohorts have demonstrated that sex, height, and weight may influence the interpretation of CT‐derived muscle mass, 21 we utilized models that adjusted for these variables.
We standardized muscle loss over 30 days to allow for variable scan intervals in different patients. Loss of muscle mass correlates with inflammatory markers, 38 which are more pronounced in the early phase of COVID‐19 and less pronounced in later stages. The variability in timing of the CT scans (median 32 days [IQR: 16–63 days]) and additional factors including inflammation are limitations to standardizing muscle loss. Our approach assumes a constant change of muscle mass between scans and during the standardization period, determining the rate of muscle mass can be challenging without protocol CT measurements, which pose logistical challenges. Despite these limitations, standardization over time intervals allows for evaluation of changes in muscle mass across patients. 6
We utilized complementary statistical approaches (i.e. regression analyses and Cox proportional hazard ratios) to analyse the data and identify the most consistent associations with outcomes. Using such methods, we observed that standardized PM as compared with ESM loss had a stronger association with adverse clinical outcomes. Even though the impact of dynamic changes in ESM on adverse outcomes was less than that of PM, we found that static reductions in ESM (both initial and final) were associated with mortality and static reduction in ESM (final) was associated with need for mechanical ventilation. These muscle specific differences may be related to ESM being an antigravity muscle that maintains normal posture, and ESM atrophy occurs in debilitated populations. 18 Therefore, initial reductions of ESM may be due to underlying chronic illness and portend an increased risk for poor outcomes in COVID‐19. Recently, quadriceps muscle and diaphragm thickness measured by ultrasound are being used to identify changes in muscle mass. 39 Despite ease of use and lower cost, ultrasound measurements can vary based on the sonographer and CT imaging remains the current standard for measuring muscle mass in the abdomen and thorax. 6 Our data show that PM muscle loss during COVID‐19 is a dynamic process and can provide insights into the patterns and/or contributors of muscle loss due to acute sarcopenia.
We show that standardized reductions in PM predict worse clinical outcomes including mortality in hospitalized patients with COVID‐19. We also observed differences in outcomes based on the specific muscle group, which lay the foundation for future mechanistic studies on context specificity of responses. Targeted interventions to improve muscle mass including pulmonary rehabilitation are an important area of future research in COVID‐19 and other acute respiratory illnesses.
Funding
Supported in part by NIH R01 GM119174; R01 DK113196; P50 AA024333; R01 AA021890; 3U01AA026976; U01 AA 026976; R56HL141744; U01 DK061732; 5U01 DK062470‐17S2; R21 AR 071046. A.H.A. and N.W. were supported by K12 HL141952; Nicole Welch is partially supported by NIH K08 AA028794 and the American College of Gastroenterology clinical research award.
Conflict of interest
The authors have no other conflicts of interest other than the grants listed above. The funders did not have any role in the design, analysis or interpretation of the data.
Supporting information
Table S1. Baseline clinical characteristics of hospitalized patients with COVID‐19
Table S2. In‐hospital outcomes for COVID‐19 patients
Table S3. Clinical characteristics of CT‐scan cohort (n = 95) with COVID‐19
Table S4. Baseline clinical characteristics of non‐COVID‐19 hospitalized CT cohort (n = 19) compared to CT COVID‐19 patients (95).
Table S5. Initial and final muscle area comparing non COVID‐19 hospitalized controls (n = 19) to COVID‐19 (n = 95)
Table S6. Quantitative CT related changes comparing hospitalized CT controls (n = 19) to CT COVID cohort patients (n = 95)
Table S7A. Comparing initial and final PM muscle mass with outcomes in CT COVID cohort (n = 95)
Table S7B. Comparing initial and final ESM muscle mass with outcomes in CT COVID cohort (n = 95)
Table S8. Review of published literature of acute sarcopenia in COVID‐19 to date
Acknowledgement
The authors of this manuscript comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Attaway A., Welch N., Dasarathy D., Amaya‐Hughley J., Bellar A., Biehl M., Dugar S., Engelen M. P. K. J., Zein J., and Dasarathy S. (2022) Acute skeletal muscle loss in SARS‐CoV‐2 infection contributes to poor clinical outcomes in COVID‐19 patients, Journal of Cachexia, Sarcopenia and Muscle, 13, 2436–2446, 10.1002/jcsm.13052
References
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID‐19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol 2020;49:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morley JE, Kalantar‐Zadeh K, Anker SD. COVID‐19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle 2020;11:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anker MS, Landmesser U, von Haehling S, Butler J, Coats AJS, Anker SD. Weight loss, malnutrition, and cachexia in COVID‐19: facts and numbers. J Cachexia Sarcopenia Muscle 2021;12:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–1600. [DOI] [PubMed] [Google Scholar]
- 6. Welch C, Majid Z, Greig C, Gladman J, Masud T, Jackson T. Interventions to ameliorate reductions in muscle quantity and function in hospitalised older adults: a systematic review towards acute sarcopenia treatment. Age Ageing 2021;50:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welch C, Greig C, Masud T, Wilson D, Jackson TA. COVID‐19 and Acute Sarcopenia. Aging Dis 2020;11:1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang PY, Li Y, Wang Q. Sarcopenia: An underlying treatment target during the COVID‐19 pandemic. Nutrition 2021;84:111104. 10.1016/j.nut.2020.111104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirwan R, McCullough D, Butler T, Perez de Heredia F, Davies IG, Stewart C. Sarcopenia during COVID‐19 lockdown restrictions: long‐term health effects of short‐term muscle loss. Geroscience 2020;42:1547–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halpin S, O'Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol 2021;93:1242–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGovern J, Dolan R, Richards C, Laird BJ, McMillan DC, Maguire D. Relation Between Body Composition, Systemic Inflammatory Response, and Clinical Outcomes in Patients Admitted to an Urban Teaching Hospital with COVID‐19. J Nutr 2021;151:2236–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bedock D, Bel Lassen P, Mathian A, Moreau P, Couffignal J, Ciangura C, et al. Prevalence and severity of malnutrition in hospitalized COVID‐19 patients. Clin Nutr ESPEN 2020;40:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giraudo C, Librizzi G, Fichera G, Motta R, Balestro E, Calabrese F, et al. Reduced muscle mass as predictor of intensive care unit hospitalization in COVID‐19 patients. PLoS ONE 2021;16:e0253433. 10.1371/journal.pone.0253433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wierdsma NJ, Kruizenga HM, Konings LA, Krebbers D, Jorissen JR, Joosten MI, et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID‐19 patients during and after hospital admission. Clin Nutr ESPEN 2021;43:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molfino A, Imbimbo G, Rizzo V, Muscaritoli M, Alampi D. The link between nutritional status and outcomes in COVID‐19 patients in ICU: Is obesity or sarcopenia the real problem? Eur J Intern Med 2021;91:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng Z, Zhao H, Kang W, Liu Q, Wu J, Bragazzi NL, et al. Association of Paraspinal Muscle Measurements on Chest Computed Tomography With Clinical Outcomes in Patients With Severe Coronavirus Disease 2019. J Gerontol A Biol Sci Med Sci 2021;76:e78–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Attaway AH, Welch N, Yadav R, Bellar A, Hatipoglu U, Meli Y, et al. Quantitative Computed Tomography Assessment of Pectoralis and Erector Spinae Muscle Area and Disease Severity in Chronic Obstructive Pulmonary Disease Referred for Lung Volume Reduction. COPD 2021;18:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welch N, Dasarathy J, Runkana A, Penumatsa R, Bellar A, Reen J, et al. Continued muscle loss increases mortality in cirrhosis: Impact of aetiology of liver disease. Liver Int 2020;40:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets‐Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle 2016;7:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross‐sectional study. Ann Am Thorac Soc 2014;11:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDonald MN, Diaz AA, Rutten E, Lutz SM, Harmouche R, San Jose Estepar R, et al. Chest computed tomography‐derived low fat‐free mass index and mortality in COPD. Eur Respir J 2017;50:1701134. 10.1183/13993003.01134-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jehi L, Ji X, Milinovich A, Erzurum S, Rubin BP, Gordon S, et al. Individualizing Risk Prediction for Positive Coronavirus Disease 2019 Testing: Results From 11,672 Patients. Chest 2020;158:1364–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salisbury R, Iotchkova V, Jaafar S, Morton J, Sangha G, Shah A, et al. Incidence of symptomatic, image‐confirmed venous thromboembolism following hospitalization for COVID‐19 with 90‐day follow‐up. Blood Adv 2020;4:6230–6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut‐point estimated from observations affected by a lower limit of detection. Biom J 2008;50:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Welch C, Greig CA, Masud T, Pinkney T, Jackson TA. Protocol for understanding acute sarcopenia: a cohort study to characterise changes in muscle quantity and physical function in older adults following hospitalisation. BMC Geriatr 2020;20:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carfì A, Bernabei R, Landi F. Persistent Symptoms in Patients After Acute COVID‐19. JAMA 2020;324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, et al. Persistent Exertional Intolerance After COVID‐19: Insights From Invasive Cardiopulmonary Exercise Testing. Chest 161:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stockwell T, Andreasson S, Cherpitel C, Chikritzhs T, Dangardt F, Holder H, et al. The burden of alcohol on health care during COVID‐19. Drug Alcohol Rev 2021;40:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alqahtani SA, Schattenberg JM. Liver injury in COVID‐19: The current evidence. United Eur Gastroenterol J 2020;8:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qi Y, Jamindar TM, Dawson G. Hypoxia alters iron homeostasis and induces ferritin synthesis in oligodendrocytes. J Neurochem 1995;64:2458–2464. [DOI] [PubMed] [Google Scholar]
- 32. Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res 2019;181:77–83. [DOI] [PubMed] [Google Scholar]
- 33. Langen RC, Gosker HR, Remels AH, Schols AM. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int J Biochem Cell Biol 2013;45:2245–2256. [DOI] [PubMed] [Google Scholar]
- 34. Zein JG, Whelan G, Erzurum SC. Safety of influenza vaccine during COVID‐19. J Clin Transl Sci 2021;5:e49. 10.1017/cts.2020.543 [DOI] [Google Scholar]
- 35. Radigan KA, Nicholson TT, Welch LC, Chi M, Amarelle L, Angulo M, et al. Influenza A Virus Infection Induces Muscle Wasting via IL‐6 Regulation of the E3 Ubiquitin Ligase Atrogin‐1. J Immunol 2019;202:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bartley JM, Pan SJ, Keilich SR, Hopkins JW, Al‐Naggar IM, Kuchel GA, et al. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle‐localized inflammation, and muscle atrophy. Aging (Albany NY) 2016;8:620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bloom CI, Drake TM, Docherty AB, Lipworth BJ, Johnston SL, Nguyen‐van‐Tam JS, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID‐19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med 2021;9:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bartali B, Frongillo EA, Stipanuk MH, Bandinelli S, Salvini S, Palli D, et al. Protein intake and muscle strength in older persons: does inflammation matter? J Am Geriatr Soc 2012;60:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pardo E, El Behi H, Boizeau P, Verdonk F, Alberti C, Lescot T. Reliability of ultrasound measurements of quadriceps muscle thickness in critically ill patients. BMC Anesthesiol 2018;18:205. 10.1186/s12871-018-0647-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline clinical characteristics of hospitalized patients with COVID‐19
Table S2. In‐hospital outcomes for COVID‐19 patients
Table S3. Clinical characteristics of CT‐scan cohort (n = 95) with COVID‐19
Table S4. Baseline clinical characteristics of non‐COVID‐19 hospitalized CT cohort (n = 19) compared to CT COVID‐19 patients (95).
Table S5. Initial and final muscle area comparing non COVID‐19 hospitalized controls (n = 19) to COVID‐19 (n = 95)
Table S6. Quantitative CT related changes comparing hospitalized CT controls (n = 19) to CT COVID cohort patients (n = 95)
Table S7A. Comparing initial and final PM muscle mass with outcomes in CT COVID cohort (n = 95)
Table S7B. Comparing initial and final ESM muscle mass with outcomes in CT COVID cohort (n = 95)
Table S8. Review of published literature of acute sarcopenia in COVID‐19 to date


