Abstract
Recently, the inhibiting effects of a clinically approved drug Cepharanthine on severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) have attracted widespread attention and discussion. However, the public does not understand the relevant research progress very well. This paper aims to introduce a brief history of studies on the effects of cepharanthine against SARS‐CoV‐2, including “discovery of anti‐SARS‐CoV‐2 activity of cepharanthine in vitro”, “potential mechanisms of cepharanthine against SARS‐CoV‐2”, “confirmation of cepharanthine's anti‐SARS‐CoV‐2 activity in vivo”, “potential approaches for improving the druggability of cepharanthine” and “clinical trials of cepharanthine treating SARS‐CoV‐2 infection”. Taken together, cepharanthine is believed to be a promising old drug for coronavirus disease‐19 (COVID‐19) therapy.
Keywords: cepharanthine, COVID‐19, SARS‐CoV‐2
Cepharanthine is a clinically approved drug mainly purified from the root tuber of plants including Stephania epigaea Lo, Stephania cepharantha Hayata, Stephania yunnanensis H.S.Lo, and so on. Moreover, it has recently been reported to possess a broad‐spectrum antiviral ability including, SARS‐CoV‐2 and related variants. Cepharanthine is believed to be a promising drug candidate for treating COVID‐19.
1. Introduction
On May 10, 2022, the invention patent “Use of Cepharanthine for the Preparation of Drugs for the Treatment of severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) Infectious Diseases” (ZL 2021 1 0172158.7) was officially authorized in China. This news ignited heated discussions on Chinese internet platforms, and has even become the top list of Weibo, Baidu, Tik Tok, and other major platforms, with the number of views on Weibo once reaching 530 million on May 14, 2022. To some extent, this reflects the strong desire of the Chinese public for the arrival of powerful oral anti‐SARS‐CoV‐2 drugs in China as soon as possible.[ 1 ] Cepharanthine is an alkaloid from stephania japonica, a raw material of the traditional Chinese medicine, and is a member of the bis‐benzylisoquinoline family. It has been mainly used in clinical diseases such as leukopenia. In early 2020, our team used the SARS‐CoV‐2 like pangolin coronavirus GX_P2V[ 2 ] to identify SARS‐CoV‐2 inhibitors from clinically approved drugs, and identified the antiviral potential of cepharanthine against SARS‐CoV‐2 for the first time.[ 3 , 4 ] Subsequently, different groups around the world have found and verified the anti‐SARS‐CoV‐2 ability of cepharanthine in vitro and in vivo (Figure 1 ).[ 5 , 6 , 7 ]
Figure 1.
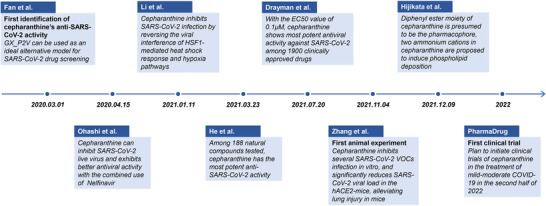
A timeline of critical events in research progress on inhibition of SARS‐CoV‐2 by cepharanthine. Since March 2020, when cepharanthine was first discovered as a potential anti‐SARS‐CoV‐2 drug, a large number of studies on the inhibition of cepharanthine against SARS‐CoV‐2 infection and its mechanism of action are underway. These events were summarized and displayed in the form of a timeline.
2. Discovery of Anti‐SARS‐CoV‐2 Activity of Cepharanthine In Vitro
Before the coronavirus disease‐19 (COVID‐19) epidemic outbreak, a coronavirus named GX_P2V sharing 86.0% genome nucleotide sequence and 92.2% amino acid identity of spike protein with SARS‐CoV‐2 isolate Wuhan‐hu‐1 was isolated and kept in our lab.[ 2 ] Moreover, both GX_P2V and SARS‐CoV‐2 utilize angiotensin converting enzyme 2 (ACE2) as the entry receptor, and GX_P2V is non‐pathogenic to human, we proposed GX_P2V can be used as an ideal alternative model for SARS‐CoV‐2 drug screening.[ 3 ] After the outbreak of COVID‐19 epidemic, we immediately decided to use GX_P2V for drug screening against SARS‐CoV‐2. The cytopathic effects (CPE) of each of 2406 clinically approved drugs on GX_P2V were observed by microscope, and cepharanthine was found to be the most powerful compound to inhibit CPE of this SARS‐CoV‐2 related coronavirus infection. And then more experiments were conducted and repeated to confirm the virustatic effect of cepharanthine, the anti‐SARS‐CoV‐2 effect of cepharanthine on authentic SARS‐CoV‐2 was confirmed by our collaborators in Bio‐level 3 laboratory very soon (data not published). Our research further suggested that cepharanthine inhibited GX_P2V infection in Vero E6 cells with the EC50 of 0.98 × 10−6 m and the CC50 of 39.30 × 10−6 m. Relevant achievements were declared priority as a Chinese patent for the “Use of Cepharanthine for the Preparation of Drugs for the Treatment of SARS‐CoV‐2 Infectious Diseases” on February 16, 2020, and the related data was published online in the Chinese Medical Journal on March 1, 2020.[ 3 ]
Later on April 15, 2020, Ohashi et Al. from Japan posted a preprint article in bioRxiv and cited the above result from our team as follow “A recent study reported that CEP exhibited anti‐SARS‐CoV‐2 activity (Fan et al., 2020), these authors speculated that CEP targeted both the entry and viral replication phase.” Their study reported that cepharanthine possessed a more potent antiviral ability against SARS‐CoV‐2 authenic virus than remdesivir, and eventually published on iScience in April 2021. In the SARS‐CoV‐2 infected VeroE6/TMPRSS2 model, the EC50 of cepharanthine was 0.35 × 10−6 m, which was lower than the 1.58 × 10−6 m of remdesivir. Moreover, the time‐of‐addition assay identified cepharanthine acted at the entry stage of viral infection, and speculated that cepharanthine interacted with viral spike protein and blocked the combination with ACE2. The single treatment with cepharanthine (3.20 × 10−6 m) or nelfinavir (2.24 × 10−6 m) reduced viral RNA level to 6.3% or 5.8% of the untreated control, respectively, while the combination with cepharanthine and nelfinavir reduced the viral RNA level to 0.068% of the untreated control, highlighting the synergetic effect of cepharanthine and nelfinavir in anti‐SARS‐CoV‐2 infection.[ 5 ]
On July 20, 2021, one study published on Science by a group from the University of Chicago showed that cepharanthine exhibited the most potent inhibition against SARS‐CoV‐2 in vitro among the 1900 clinically approved drugs. The EC50 of cepharanthine in A549‐ACE2 cells was only 0.1 × 10−6 m, followed by desloratidine, remdesivir, flupenthixol, trimipramine, lapatinib, and benztropine with the EC50 of 0.7 × 10−6, 0.72 × 10−6, 0.76 × 10−6, 1.5 × 10−6, 1.6 × 10−6, and 1.8 × 10−6 m, respectively. In brief, this study first conducted the in vitro screening of 1900 clinically approved drugs using human coronavirus OC43. After two rounds of screening, 108 drugs were identified as qualified, among which only five possessed the EC50 lower than 1 × 10−6 m. The drugs that effectively inhibited OC43 were tested for the inhibitory ability in SARS‐CoV‐2. Interestingly, the most effective drugs against HCoV‐OC43 infection (elbavir and amphotericin B) were not effective on SARS‐CoV‐2 infection, whereas cepharanthine was the most effective drugs that inhibited SARS‐CoV‐2. These data suggested that cepharanthine exhibited good inhibitory effect on both SARS‐CoV‐2 and HCoV‐OC43 infection in vitro and was the most potent SARS‐CoV‐2 inhibitor among 1900 clinically approved drugs. Moreover, they confirmed that cepharanthine had no inhibitory effect on SARS‐CoV‐2 3CL protease. Overall, this research confirmed cepharanthine exhibited the most potent anti‐SARS‐CoV‐2 ability among all clinically approved drugs, far more effective than remdesivir which had been approved to treat SARS‐CoV‐2 infection, and masitinib (EC50 = 3.2 × 10−6 m) which had been focused in their research.[ 8 ]
3. Potential Mechanisms of Cepharanthine against SARS‐CoV‐2
On January 11, 2021, our team proposed the possible mechanisms of cepharanthine inhibiting the GX_P2V infection in Vero cells by RNA‐Seq analysis. Cepharanthine was speculated to exert the antiviral effect through reversing the viral interference of HSF1‐mediated heat shock response, ER stress/response to unfolded protein, and hypoxia pathways. In addition, the single‐cell transcriptome analysis of COVID‐19 patients showed the upregulation of genes related to heat shock response and stress response. These results from COVID‐19 patients were in accordance with the potential mechanism of cepharanthine against GX_P2V at the cell culture level, providing an important basis for the therapeutic targets of COVID‐19.[ 9 ]
On March 23, 2021, a study from Huang's team in Chongqing Medical University (China) conducted an antiviral screening of 188 natural compounds using pseudotyped SARS‐CoV‐2, among which cepharanthine was the most effective. Cepharanthine showed significant inhibitory effect on S‐G614 pseudovirus in three different cell lines, with the EC50 of 0.351 × 10−6, 0.759 × 10−6, and 0.911 × 10−6 m in 293T‐ACE2, Calu‐3, and A549‐ACE2 cells, respectively, and the EC50 of cepharanthine on SARS‐CoV‐2 S‐D614, mutant N501Y.V1 (B.1.1.7), and N501Y.V2 (B.1.351) pseudoviruses in 293T‐ACE2 cells were 0.054 × 10−6, 0.047 × 10−6, and 0.296 × 10−6 m, respectively. Cepharanthine was also effective against pseudotyped SARS‐CoV and MERS‐CoV S‐protein infection, whose authentic viruses could cause serious diseases, with the EC50 of 0.042 × 10−6 and 0.140 × 10−6 m, respectively. The above data indicated that cepharanthine could effectively inhibit the infection of SARS‐CoV‐2 mutants and different coronaviruses in vitro. Further studies showed that the blockade of SARS‐CoV‐2 S pseudovirus entry by cepharanthine was largely depended on the calcium homeostasis. Cepharanthine eliminated S‐ACE2‐mediated membrane fusion by targeting the host calcium channel. At the same time, cepharanthine could upregulate the intracellular cholesterol level, which might also help to inhibit the viral infection.[ 6 ]
As mentioned above, cepharanthine effectively inhibits the infection of SARS‐CoV‐2 wide type and mutant strains including B.1.1.7 and B.1.351.[ 6 ] Our latest research progress shows that cepharanthine also has a good inhibitory effect on pseudotyped Omicron, a novel variant that is prevailing. In addition, it has been reported to have a good inhibitory effect on SARS‐CoV,[ 6 ] MERS‐CoV,[ 6 ] HCoV‐OC43,[ 8 ] and important human pathogens such as HIV‐1,[ 10 ] EboV,[ 7 ] Zika,[ 7 ] HSV‐1,[ 11 ] HTLV‐1,[ 12 ] etc. And we hypothesize that cepharanthine might inhibit viral infection by regulating the expression of key host factors in viral infection, or by regulating antiviral immunity as interferons. Moreover, cepharanthine was found to inhibit the activity of SARS‐CoV‐2 helicase. Andrew et al. suggested cepharanthine as a potential inhibitor of SARS‐CoV‐2 nsp13 (helicase) by virtual screening and estimated its IC50 value by nsp13 ATPase activity assay to be 0.4 × 10−3 m.[ 13 ] The amino acid sequences of the wild type with those of five SARS‐CoV‐2 VOCs including Omicron were compared, and the sequence similarity of nsp13 was greater than 99%. Therefore, the mutants of SARS‐CoV‐2 may not have a great impact on the clinical application of cepharanthine for the treatment of COVID‐19.
In addition to cepharanthine, chloroquine was also used to treat SARS‐CoV‐2 infected patients in the early stage of COVID‐19 epidemic, which is a manifestation of the “repurposing of clinically approved drugs” strategy. Among them, chloroquine was used as a therapy of malaria, it can play anti‐inflammatory and antiviral role through inhibiting extracellular metalloproteinases and regulating intracellular pH, respectively. Hoffmann and colleagues found that chloroquine could inhibit cathepsin L‐depended SARS‐CoV‐2 entry route through the regulation of pH,[ 14 ] however, the entry can also be induced by transmembrane protease serine 2, limiting the effectiveness of chloroquine treatment. Compared with chloroquine, cepharanthine can play multiple antiviral roles and show satisfactory inhibitory effects in different cell lines and hACE2 mouse, thus is promising in the further clinical application.
4. Confirmation of Cepharanthine's Anti‐SARS‐CoV‐2 Activity In Vivo
On November 4, 2021, the researchers from Tsinghua University and Peking Union Medical College published their study on Cell Research. They first identified the host factors interacting with SARS‐CoV‐2, Zika and Ebola virus by ChIRP‐MS, and then expounded drugs targeting vRNA‐host factor interactions (from open‐source chemical databases). Among the screened drugs, cepharanthine showed a high antiviral activity against clinical isolate SARS‐CoV‐2 (IPBCAMS‐YL01/2020) in A549‐ACE2 cell lines, with the IC50 value of 1.67 × 10−6 m, the CC50 value of 30.92 × 10−6 m. Furthermore, the antiviral activity of cepharanthine on SARS‐CoV‐2 authenic virus in hACE2‐transgenic mice was also evaluated. Compared with the control group, cepharanthine‐treated group (intranasally administered 24 h prior to SARS‐CoV‐2 challenge) showed a significant reduction in viral load at 5 d post infection and the expression levels of inflammatory factors including TNF and IL‐6 in the mice, which explained the less lung injury in cepharanthine‐treated group compared with the control group. Interestingly, cepharanthine exerted strong antiviral effect against Beta (B.1.351) variant in A549‐ACE2 cells, with the IC50 of 0.24 × 10−6 m, and with the IC50 of 0.06 × 10−6 m in Huh7.5.1 cells. In addition, the combination therapy of cepharanthine and trifluoperazine (5 × 10−6 m each) reduced vRNA levels to less than 0.01% compared with control group. The above results showed that cepharanthine could play antiviral roles at a low concentration, suggesting its potential effectiveness and safety.[ 7 ] It is worth noting that cepharanthine showed strong inhibitory effect to SARS‐CoV‐2 variants and therapeutic effect on SARS‐CoV‐2 infected hACE2‐transgenic mice, suggesting that cepharanthine has the value of further development in clinical application of treating COVID‐19.[ 15 ] Although the antiviral efficacy data of cepharanthine obtained by different teams differed by more than ten times, the fluctuation of the EC50 was within a reasonable range. Similar with some other anti‐SARS‐CoV‐2 drugs (e.g., remdesivir, molnupiravir, paxlovid), the EC50 of cepharanthine fluctuates in different cell lines, different SARS‐CoV‐2 strains and different research groups.
5. Potential Approaches for Improving the Druggability of Cepharanthine
On December 9, 2021, Hijikata et al. from Japan also reported the remarkable anti‐SARS‐CoV‐2 ability of cepharanthine in cell culture. The EC50 of cepharanthine was 1.90 × 10−6 m in VeroE6/TMPRSS2 cells.[ 15 ] According to the computer simulation, the diphenyl ester moiety of cepharanthine was presumed to be the pharmacophore, but the key viral target protein of cepharanthine was awaiting to be found. Moreover, the chemical structure of cepharanthine was analyzed and was speculated that two ammonium cations in cepharanthine could induce phospholipid deposition, which is common in drug development. The authors proposed an idea of substituting ammonium cations without affecting the antiviral effect of cepharanthine.[ 15 ]
To effectively improve the solubility and bioavailability of cepharanthine, a variety of approaches could be performed to perfect the druggability of cepharanthine. Preparation of dissolvable salt forms from the medicinal alkaloids cepharanthine by using either inorganic or organic type acids can effectively enhance water solubility and provide for much more efficient dosage regimes for treatment of viral infections via oral, intravenous and pulmonary administrations. In addition, preparation of spray or transdermal patch by changing the dosage form of cepharanthine can also improve the bioavailability of cepharanthine. Moreover, construction of biomimetic cepharanthine nanoparticles for targeted delivery to improve the bioavailability of cepharanthine is a promising clinical treatment strategy.[ 4 ]
6. Clinical Trials of Cepharanthine Treating SARS‐CoV‐2 Infection
In terms of clinical trials of cepharanthine treating SARS‐CoV‐2 infection, PharmaDrug (a Canadian pharmaceutical company) obtained a U.S. patent about the enteric‐coated formulation of cepharanthine (PD‐001) (US 10,576,007 B2)[ 16 ] on March 3, 2020, and subsequently submitted a clinical trial application for PD‐001 in treating SARS‐CoV‐2 infection to the U.S. Food and Drug Administration (FDA) in June 2021. In September 2021, FDA granted a pre‐investigational new drug (pre‐IND) meeting with PharmaDrug for the clinical development of PD‐001, and then the pre‐IND meeting was successfully held, PharmaDrug had established the agreement with FDA on the path forward for the development of PD‐001 toward clinical studies for mild‐to‐moderate COVID‐19. PharmaDrug planned to initiate clinical studies in the second half of 2022, and this is the only clinical study of cepharanthine for the purpose to treat COVID‐19. Although cepharanthine showed effectiveness in the treatment of SARS‐CoV‐2 infection, the low oral bioavailability suggested the demand for intravenous administration, limiting the further clinical application. Therefore, PharmaDrug prepared cepharanthine into an enteric‐coated form (PD‐001) for oral administration. Compared with generic cepharanthine, PD‐001 showed well‐absorbance in rodent and nonrodent models, remarkably improving its bioavailability.[ 16 ]
7. Conclusion
In 1934, cepharanthine was purified by Japanese pharmacists Heisaburo Kondo for the first time, and it was named after one of its important sources, Stephania cepharantha. It has been widely used in Japanese medicine since 1951, mainly for the treatment of managing radiation‐induced leukopenia, alopecia pityrodes, alopecia areata, venomous snakes, and idiopathic thrombocytopenic purpura. At the same time, cepharanthine has been used clinically for the experimental treatment of pneumoconiosis in China for many years.[ 17 ] As an approved drug being used for more than 70 years, cepharanthine is mainly extracted, separated, and purified from the rhizomes of Stephania, and possesses mature production technique with low price. The raw material Stephania rotunda is a creeper that is native to China and now commonly found in the mountains of Cambodia and throughout Southeast Asia. In addition to the antiviral ability, cepharanthine is also reported to possess antiparasite, antitumor, anti‐inflammatory, and other activities. Given the remarkable in vitro and in vivo efficiency against SARS‐CoV‐2 infection and the mature production technique, cepharanthine is a promising drug candidate for treating COVID‐19. We look forward to the promotion of the clinical trial of cepharanthine for the treatment of COVID‐19, and hope cepharanthine‐based COVID‐19 therapy can contribute for ending the COVID‐19 epidemic (Table 1 ).
Table 1.
Features of research articles and patent on the effect of cepharanthine against SARS‐CoV‐2
| In vitro | In vivo | ||||||
|---|---|---|---|---|---|---|---|
| Team | Cell line | Virus | EC50/IC50 | CC50 | Subjects | Results of experiments | Underlying mechanisms |
| Fan et al.[ 3 ] | Vero E6 | GX_P2V | 0.98 × 10−6 m | 39.30 × 10−6 m | Inhibited both the viral entry and post‐entry processes. | ||
| Ohashi et al.[ 5 ] | Vero E6/TMPRSS2 | SARS‐CoV‐2 | 0.35 × 10−6 m | Acted at the entry stage of viral infection; cepharanthine interacts with viral spike protein and blocks the combination with ACE2. | |||
| Li et al.[ 9 ] | Vero E6 | GX_P2V | Exhibited antiviral activities through reversing the viral interference of HSF1‐mediated heat shock response, ER stress/response to unfolded protein, and hypoxia pathways. | ||||
| He et al.[ 6 ] |
293T‐ACE2,Caclu 3, A549‐ACE2; 293T‐ACE2; 293T‐ACE2; 293‐DPP4 |
SARS‐CoV‐2 S‐G614 pseudovirus; SARS‐CoV‐2 S‐D614, mutant N501Y.V1 (B.1.1.7), N501Y.V2 (B.1.351) pseudoviruses; SARS‐CoV S‐protein pseudovirus; MERS‐CoV S‐protein pseudovirus |
0.351 × 10−6 m, 0.759 × 10−6 m, 0.911 × 10−6 m; 0.0537 × 10−6 m, 0.047 × 10−6 m, 0.296 × 10−6 m; 0.0417 × 10−6 m; 0.140 × 10−6 m |
Blocked SARS‐CoV‐2 S pseudovirus entry by calcium homeostasis. Upregulation of intracellular cholesterol levels, which may also help to inhibit viral infection. |
|||
| Drayman et al.[ 8 ] |
A549; A549‐ACE2 |
OC43; SARS‐CoV‐2 |
0.1 × 10−6 m; 0.77 × 10−6 m |
Blocked SARS‐CoV‐2 infection without targeting 3CL protein . | |||
| Zhang et al.[ 7 ] |
A549‐ACE2; A549‐ACE2; Huh7.5.1 |
SARS‐CoV‐2; SARS‐CoV‐2 Beta variant; SARS‐CoV‐2 Beta variant |
1.67 × 10−6 m; 0.24 × 10−6 m; 0.06 × 10−6 m |
30.92 µM | hACE2‐ transgenic mice | The drug‐treated group showed a significant reduction in viral load on the fifth day after infection and significantly lower levels of inflammatory factors such as TNF and IL‐6 in the mice. | |
| Hijikata et al.[ 15 ] | VeroE6/TMPRSS2 | SARS‐CoV‐2 | 1.90 × 10−6 m |
According to computer simulation, the diphenyl ester moiety of cepharanthine was presumed to be the pharmacophore, but the key target protein of cepharanthine was await to be found. Two ammonium cations in cepharanthine could induce phospholipid deposition. |
|||
| CEP PharmaDrug Inc. US_10576077(1)[ 16 ] | human | Declare the clinical studie on the mild and moderate COVID‐19 patients in the second half of 2022. | |||||
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
H.F., S.‐t.H., P.H., B.H., K.L., M.L., and S.W. contributed equally to this work. H.F. and Y.T. conceived this study, H.F., S.H., P.H., B.H., K.L., M.L., and S.W. conducted this analysis and drafted the manuscript.
Acknowledgements
This research was supported by National Key Research and Development Program of China (Grant Nos. 2022YFC0867500, 2019YFC1200502, 2020YFA0712102, BWS21J025, and 20SWAQK22), National Natural Science Foundation of China (Grant No. 82151224), Fundamental Research Funds for Central Universities (Grant No. BUCTZY2022), and H&H Global Research and Technology Center (Grant No. H2021028).
Biographies
Huahao Fan is currently an associate professor in the College of Life Science and Technology, Beijing University of Chemical Technology. He received his Ph.D. degree from Peking University. He used to be a joint Ph.D. student at the University of Alabama at Birmingham and a postdoctoral fellow at the Tsinghua University. He is mainly engaged in bacteriophage, coronavirus and enterovirus drug screening, and virus‐host interaction mechanisms research of emerging infectious diseases.

Shi‐ting He received her bachelor's degree (B.E.) at the South China Agricultural University in 2020. She is currently pursuing her master's degree under the supervision of Prof. Yigang Tong and Prof. Huahao Fan at the Beijing University of Chemical Technology. Currently, her research focuses on coronavirus drug screening and drug mechanisms research.

Pengjun Han is studying for his doctor degree under the supervision of Prof. Yigang Tong and Prof. Huahao Fan at Beijing University of Chemical Technology. Currently, his research focuses on the mutual evolution between phage and host, the function of phage DNA polymerase, and the drug screening and mechanism of resistant bacteria.

Bixia Hong received her bachelor's degree (B.E.) at the Beijing Technology And Business University in 2019. She is currently pursuing her doctor degree under the supervision of Prof. Yigang Tong and Prof. Huahao Fan at the Beijing University of Chemical Technology.Currently, her research focuses on antiviral drug screening and mechanism research.

Ke Liu received her bachelor's degree (B.E.) at the Beijing University of Chemical Technology in 2021. She is currently pursuing her master's degree under the supervision of Prof. Yigang Tong and Prof. Huahao Fan at the Beijing University of Chemical Technology. Currently, her research focuses on drug screening and virus‐host interaction mechanisms of emerging infectious diseases (coronavirus).

Maochen Li received his bachelor's degree (B.E.) at the Beijing University of Chemical Technology in 2022. He is currently pursuing her master's degree under the supervision of Prof. Huahao Fan at the Beijing University of Chemical Technology. Currently, his research focuses on coronavirus immune escape.

Shuqi Wang received her bachelor's degree (B.E.) at the Beijing University of Chemical Technology in 2021. She is currently pursuing her master's degree under the supervision of Prof. Yigang Tong and Prof. Huahao Fan at the Beijing University of Chemical Technology. Currently, her research focuses on enterovirus drug screening and virus‐host interaction mechanisms.

Yigang Tong is currently a professor and the Dean of the College of Life Science and Technology, Beijing University of Chemical Technology. He received his bachelor's degree from Fudan University. He used to be a postdoctoral fellow at the University of British Columbia. He is mainly engaged in virology, genomics, bioinformatics, and emerging infectious diseases research. He acted as the chief expert of the Chinese aid team working in Sierra Leone for the fight against Ebola. He participated in the WHO–China joint study in Wuhan on the origin of SARS‐CoV‐2.

Fan H., He S.‐t., Han P., Hong B., Liu K., Li M., Wang S., Tong Y., Cepharanthine: A Promising Old Drug against SARS‐CoV‐2. Adv. Biology 2022, 2200148. 10.1002/adbi.202200148
Contributor Information
Huahao Fan, Email: fanhuahao@mail.buct.edu.cn.
Yigang Tong, Email: tongyigang@mail.buct.edu.cn.
References
- 1. Fan H., Lou F., Fan J., Li M., Tong Y., Lancet Microbe 2022, 3, e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam T. T.‐Y., Jia N.a, Zhang Y.‐W., Shum M. H.‐H., Jiang J.‐F., Zhu H.‐C., Tong Y.‐G., Shi Y.‐X., Ni X.‐B., Liao Y.‐S., Li W.‐J., Jiang B.‐G., Wei W., Yuan T.‐T., Zheng K., Cui X.‐M., Li J., Pei G.‐Q., Qiang X., Cheung W. Y.‐M., Li L.‐F., Sun F.‐F., Qin S., Huang J.‐C., Leung G. M., Holmes E. C., Hu Y.‐L., Guan Y., Cao W.‐C., Nature 2020, 583, 282. [DOI] [PubMed] [Google Scholar]
- 3. Fan H.‐H., Wang L.‐Q., Liu W.‐L., An X.‐P., Liu Z.‐D., He X.‐Q., Song L.‐H., Tong Y.‐G., Chin. Med. J. 2020, 133, 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu C., Zheng J., Ding Y., Meng Y., Tan F., Gong W., Chu X., Kong X., Gao C., Drug Delivery 2021, 28, 2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., Shirai T., Kanaya S., Ito Y., Kim K. S., Nomura T., Suzuki T., Nishioka K., Ando S., Ejima K., Koizumi Y., Tanaka T., Aoki S., Kuramochi K., Suzuki T., Hashiguchi T., Maenaka K., Matano T., Muramatsu M., Saijo M., Aihara K., Iwami S., Takeda M., Mckeating J. A., Wakita T., iScience 2021, 24, 102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He C.‐L., Huang L.‐Y., Wang K., Gu C.‐J., Hu J., Zhang G.‐J., Xu W., Xie Y.‐H., Tang N., Huang A.‐L., Signal Transduction Targeted Ther. 2021, 6, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang S., Huang W., Ren L., Ju X., Gong M., Rao J., Sun L., Li P., Ding Q., Wang J., Zhang Q. C., Cell Res. 2022, 32, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drayman N., Demarco J. K., Jones K. A., Azizi S.‐A., Froggatt H. M., Tan K., Maltseva N. I., Chen S., Nicolaescu V., Dvorkin S., Furlong K., Kathayat R. S., Firpo M. R., Mastrodomenico V., Bruce E. A., Schmidt M. M., Jedrzejczak R., Muñoz‐Alía M. Á., Schuster B., Nair V., Han K.‐Y., O'brien A., Tomatsidou A., Meyer B., Vignuzzi M., Missiakas D., Botten J. W., Brooke C. B., Lee H., Baker S. C., et al., Science 2021, 373, 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li S., Liu W., Chen Y., Wang L., An W., An X., Song L., Tong Y., Fan H., Lu C., Briefings Bioinf. 2021, 22, 1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okamoto M., Ono M., Baba M., Biochem. Pharmacol. 2001, 62, 747. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y., Chen L.i, Liu W., Li D., Zeng J., Tang Q., Zhang Y., Luan F., Zeng N., Front. Microbiol. 2021, 12, 795756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toyama M., Hamasaki T., Uto T., Uto T., Aoyama H., Okamoto M., Hashmoto Y., Baba M., Anticancer Res. 2012, 32, 2639. [PubMed] [Google Scholar]
- 13. White M. A., Lin W., Cheng X., J Phys Chem Lett 2020, 11, 9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann M., Mösbauer K., Hofmann‐Winkler H., Kaul A., Kleine‐Weber H., Krüger N., Gassen N. C., Müller M. A., Drosten C., Pöhlmann S., Nature 2020, 585, 588. [DOI] [PubMed] [Google Scholar]
- 15. Hijikata A., Shionyu‐Mitsuyama C., Nakae S., Shionyu M., Ota M., Kanaya S., Hirokawa T., Nakajima S., Watashi K., Shirai T., FEBS Open Bio 2022, 12, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., Naninck T., Pizzorno A., Lemaitre J., Gonçalves A., Kahlaoui N., Terrier O., Fang R. H. T., Enouf V., Dereuddre‐Bosquet N., Brisebarre A., Touret F., Chapon C., Hoen B., Lina B., Calatrava M. R., Van Der Werf S., De Lamballerie X., Le Grand R., Nature 2020, 585, 584. [DOI] [PubMed] [Google Scholar]
- 17. Rogosnitzky M., Okediji P., Koman I., Pharmacol. Rep. 2020, 72, 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]


