Abstract
Up‐to‐date information on coronavirus disease 2019 (COVID‐19) outcomes and risk factors in haematopoietic cell transplantation (HCT) recipients is required to inform on decisions about cancer treatment and COVID‐19 mitigation strategies. We performed a meta‐analysis to address this knowledge gap. All studies with at least five patients who reported COVID‐19‐related deaths in HCT recipients were included. The primary outcome was COVID‐19‐related death. Secondary outcomes were COVID‐19‐related mechanical ventilation (MV) and intensive care unit (ITU) admission. The cumulative COVID‐19‐related death rate among HCT recipients was 21% (95% confidence interval [CI] 18%–24%), while MV and ITU admission rates were 14% (95% CI 11%–17%) and 18% (95% CI 14%–22%), respectively. Subgroup analysis showed higher death rates in patients who developed COVID‐19 within 12 months of HCT (risk ratio [RR] 1.82, 95% CI 1.09–3.03), within 6 months of receiving immunosuppressant drugs (RR 2.11, 95% CI 1.38–3.20) or in the context of active graft‐versus‐host disease (RR 2.38, 95% CI 1.10–5.16). Our findings support the idea that HCT should remain an integral part of cancer treatment during the COVID‐19 pandemic but also highlight the need to prioritise preventative measures in those patients who are at increased risk of adverse COVID‐19 outcomes.
Keywords: COVID‐19, SCT, stem cell transplantation
1. INTRODUCTION
It has been demonstrated that patients with cancer appear particularly vulnerable to coronavirus disease 2019 (COVID‐19) caused by the SARS‐CoV‐2 virus owing in part to the immunosuppressive effects of anticancer therapies [1, 2, 3]. Within this group, haematopoietic cell transplant (HCT) recipients remain one of the most immunosuppressed cohorts and are considered to be at a high risk of COVID‐19‐related complications and death. Despite this, COVID‐19 outcomes for HCT recipients in single‐centre [4, 5, 6] or multicentre studies [7, 8] vary, with different studies reporting different mortality rates and risk factors. Consequently, the true risk of COVID‐19 in HCT recipients is unclear. Addressing this knowledge gap is essential in order to weigh the risks and benefits of HCT during the COVID‐19 pandemic, particularly as recent studies have shown that HCT recipients produce a less effective immune response to COVID‐19 vaccines compared to the general population [9, 10, 11]. In light of these considerations, we performed a systematic review and meta‐analysis to quantify the clinical outcomes of HCT recipients with COVID‐19, as well as a comprehensive subgroup analysis to determine clinical factors associated with adverse outcomes.
2. METHODS
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) [12] and the Joanna Briggs Institute (JBI) guidelines.
2.1. Inclusion criteria
All studies reporting COVID‐19 outcomes in HCT recipients were screened, and all those reporting COVID‐19‐related deaths in cohorts of at least five patients were selected irrespective of whether they involved paediatric (age <18 years old) or adult (age ≥18 years old) patients.
2.2. Search strategy
We identified relevant studies using the PubMed and Embase databases. We also sought preprint articles from medRxiv and bioRxiv, as well as conference proceedings from relevant international scientific meetings organised by the American Society of Clinical Oncology, American Society of Hematology, European Society for Blood and Marrow Transplantation (EBMT) and European Haematology Association. We used the search terms ‘([coronavirus] OR [COVID‐19] OR [SARS‐CoV‐2] OR [COVID‐2019])’ AND ‘([stem cell transplantation] OR [bone marrow transplantation] OR [hematopoietic cell transplantation] OR [HCT] OR [SCT] OR [HCT] OR [BMT])’. This was last updated on 20 December 2021. In addition, reference lists of studies, systematic reviews, narrative reviews and case reports were also scrutinised for eligible studies.
2.3. Study selection
Two reviewers (Yeong Jer Lim and Umair Khan) independently evaluated all titles and abstracts identified through the initial search and excluded studies that clearly did not meet the inclusion criteria. The full texts of the remaining studies were evaluated for eligibility, and any differences of opinion were resolved by discussion.
2.4. Data extraction
Using a preformulated template, two reviewers (Yeong Jer Lim and Umair Khan) independently extracted the following data: study characteristics (author, centre(s), region, inclusion period), sample size, patient characteristics (age, gender, major comorbidities/haematopoietic cell transplantation‐specific comorbidity index [HCT‐CI] score, indication for HCT), description of HCT received (allogeneic or autologous HCT, conditioning regimen, donor and graft type, graft‐versus‐host disease [GvHD] prophylaxis received, time since transplant, active GvHD or immunosuppressant use at time of COVID‐19), diagnostic criteria for COVID‐19 and duration of follow‐up.
The primary outcome was the cumulative rate of COVID‐19‐related deaths among HCT recipients, defined as death from any cause following a diagnosis of COVID‐19. The secondary outcomes were COVID‐19‐related mechanical ventilation (MV) and intensive care unit (ITU) admission rates, defined as any episode of MV or ITU admission following COVID‐19. Predetermined subgroup analyses were also conducted that related the primary outcome to age, gender, types of HCT, time since HCT, recent immunosuppressant use and active GvHD at the time of COVID‐19 onset.
2.5. Risk of bias assessment
All included studies were independently assessed for risk of bias by two reviewers (Yeong Jer Lim and Umair Khan) using the JBI critical appraisal checklist for studies reporting prevalence data [13] (Table S1). Briefly, this checklist comprised nine yes/no answers and assessed the following areas in each study: the method of study participant selection and the suitability of included participants to represent the target population; the adequacy of the sample size reported on; the description of study subjects and setting; the coverage of subgroups of interest; the validity of the identification and assessment of the condition; the suitability of the statistical analysis performed; and the adequacy of the study response rate. The adequacy of the sample size in each study was compared to sample size calculations [14] using an estimated risk of COVID‐19‐related death of 25% with a precision of 0.1 (95% confidence interval [CI] width of 20%). The estimated risk of COVID‐19‐related death was the average cumulative death rate among the two largest available datasets [7, 8] on COVID‐19‐related deaths in HCT patients at the time of writing.
The risk of bias was assessed based on the number of ‘yes’ answers to each question in the checklist. Any disagreements between reviewers were resolved by discussion. Risk of bias was classified as high in studies with one to four out of nine ‘yes’ answers, moderate in studies with five to six ‘yes’ answers and low in studies with seven or more ‘yes’ answers. Furthermore, publication bias was assessed by Begg's funnel plot analysis with Egger's test, and a p‐value <0.05 was deemed significant.
2.6. Statistical analysis
We used RevMan version 5.3 and the R package ‘meta’ to perform the meta‐analysis and Microsoft Excel 2010 for data compilation. For the primary and secondary outcomes, all included studies were pooled into a single arm forest plot where the combined incidence rate and its 95% CIs were calculated. For subgroup analysis, forest plots were constructed for each predefined subgroup, and a pooled relative risk with 95% CIs was calculated as well as the overall effect of each variable on COVID‐19‐related death rates. The threshold for statistical significance of the overall effect was p ≤0.05. Heterogeneity was judged to be significant if p ≤0.10 on the chi‐square test, while the assigned I 2 values of 25%–49.9%, 50%–74.9% and 75%–100% were deemed low, moderate and high degrees of heterogeneity, respectively. A random effects model was used for all statistical analyses. Finally, we have also performed a sensitivity analysis of the primary outcome by excluding studies deemed to have a moderate to high risk of bias, lower sample size (n < 10) or those only reporting on paediatric HCT recipients (age <18 years old).
3. RESULTS
3.1. Study selection
The results of the literature search are summarised in Figure 1. Through a systematic search strategy, we identified 1634 records, of which 94 records were assessed in full text for eligibility. In total, we found 36 records reporting 2141 patients, all in English and published between January 2020 and December 2021, which met the predetermined inclusion criteria. Conversely, 58 records were excluded because they did not meet the inclusion criteria.
FIGURE 1.
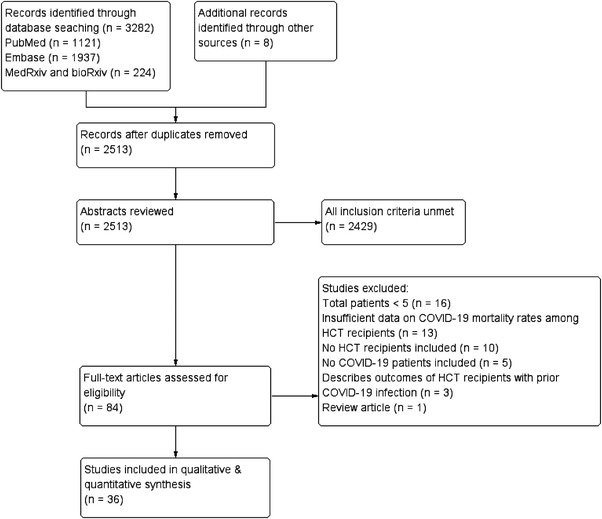
Preferred Reporting Items for Systematic Reviews and Meta‐Analyes (PRISMA) flow diagram detailing how studies were identified and selected
3.2. Characteristics of the selected studies
Patient demographics and study characteristics are summarised in Table S1. Selected studies reported retrospective observational data from single‐centre experiences [4–6, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26], multicentre experiences [27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41] or registry data [7, 8, 42, 43, 44, 45]. Seventeen studies [8, 15, 18, 19, 23, 24, 29, 31, 33, 34, 35, 37, 38, 39, 41, 44, 45] were from Europe (1153 patients), seven studies [4, 6, 17, 20, 22, 25, 36] were from North America (238 patients), seven studies [5, 16, 21, 26, 30, 32, 42] were from the Middle East (185 patients), two studies [28, 40] were from South America (41 patients), one study [27] was from Asia (28 patients) and two studies [7, 43] included patients from multiple regions (496 patients). Seventeen studies [7, 8, 16, 21, 24, 25, 26, 27, 28, 29, 30, 35, 38, 39, 40, 41, 45] included both adult and paediatric transplant recipients, 13 studies [4, 5, 6, 15, 18, 19, 20, 22, 23, 34, 36, 42, 43] only included adult patients ≥18 years old and five studies [17, 31, 32, 33, 37, 44] only included patients <18 years old. In most studies [4, 5, 8, 16, 17, 18, 19, 20, 21, 23, 24, 25, 27, 28, 30, 31, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44], the diagnosis of COVID‐19 was based on SARS‐CoV‐2 polymerase chain reaction or SARS‐CoV‐2 IgG antibody (IgG) positivity, while six studies [6, 7, 15, 22, 29, 32] additionally included patients with a strong clinical or radiological suspicion of COVID‐19. Eight studies included outcomes of HCT recipients within larger cohorts of patients with haematological malignancies (HM) [15, 17, 22, 31, 32, 41, 43] or solid organ transplant recipients [29], with limited available information on patient characteristics and treatment.
3.3. Characteristics of HCT recipients in the selected studies
Among the 2141 patients included in this meta‐analysis, 944 (44.1%) received an autologous HCT and 1191 (55.6%) received an allogeneic HCT, while details of the transplant were not available for six patients (0.3%). COVID‐19 was acquired within 12 months of HCT in 282 of 863 patients where this information was available (32.7%) and more than 1 year after HCT in 581/863 (67.3%). Within the allogeneic HCT cohort, 247/554 patients (44.6%) received immunosuppression therapy within 6 months of COVID‐19 diagnosis, 185/600 patients (30.8%) had acute GvHD at the onset of COVID‐19, and 194/580 patients (33.4%) had chronic GvHD at the onset of COVID‐19. Further details of allogeneic HCT recipients are summarised in Table S2.
3.4. Risk of bias assessment
Using the JBI checklist for prevalence studies, we identified 25 (69%) studies with a low risk of bias and 11 (31%) studies with moderate to high risk. The results for the risk of bias assessment and its justification are summarised in Table 1. When assessing for publication bias, asymmetry was seen in the funnel plot (Figure S1), with Egger's test (p = 0.001) showing the presence of significant publication bias in the included studies.
TABLE 1.
Risk of bias assessments for all selected studies using the Joanna Briggs Institute (JBI) critical appraisal checklist for studies reporting prevalence data
| Study | Q1: Was the sample frame appropriate to address the target population? | Q2: Were study participants sampled in an appropriate way? | Q3: Was the sample size adequate? | Q4: Were the study subjects and the setting described in detail? | Q5: Was the data analysis conducted with sufficient coverage of the identified sample? | Q6: Were valid methods used for the identification of the condition? | Q7: Was the condition measured in a standard, reliable way for all participants? | Q8: Was there appropriate statistical analysis? | Q9: Was the response rate adequate, and if not, was the low response rate managed appropriately? | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Agrawal et al. 2021 | Y | Y | N * | Y | Y | Y | Y | Y | Y | Low |
| Altuntas et al. 2020 | Y | Y | N * | Y | Y | Y | Y | Y | Y | Low |
| Averbuch et al. 2021 | Y | Y | N * | Y | N ∆ | Y | Y | Y | Y | Low |
| Bailén et al. 2020 | Y | Y | Y | Y | Y | U ¥ | U ¥ | Y | Y | Low |
| Basquiera et al. 2021 | Y | Y | N * | Y | N Ω | Y | Y | Y | Y | Low |
| Camargo et al. 2021 | Y | Y | N * | Y | Y | Y | Y | Y | Y | Low |
| Chari et al. 2020 | Y | Y | Y | N ◊ | N Ψ , γ | Y | Y | Y | Y | Low |
| Coll et al. 2020 | Y | Y | Y | N ◊ | U □ | Y | Y | Y | Y | Low |
| De Ramón et al. 2020 | Y | Y | Y | N ◊ | U □ | Y | Y | Y | Y | Low |
| Dwabe et al. 2021 | Y | Y | N * | Y | N † | Y | Y | Y | Y | Low |
| Fakih et al. 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| Faura et al. 2020 | Y | Y | N * | N ◊ | N ∆ , Ω | Y | Y | N/A ○ | Y | Mod |
| Fox et al. 2020 | Y | Y | N * | N ◊ | N γ | Y | Y | Y | Y | Mod |
| Haroon et al. 2020 | Y | Y | N * | Y | Y | Y | Y | N/A ○ | Y | Low |
| Jimenez‐Kurlander et al. 2020 | Y | Y | N * | N ◊ | N ∆ , Ω | U θ | Y | N/A ○ | Y | High |
| Kanellopoulos et al. 2020 | Y | Y | N * | Y | Y | Y | Y | N/A ○ | Y | Low |
| Karatas et al. 2021 | Y | Y | N * | Y | Y | Y | Y | Y | Y | Low |
| Kebudi et al. 2020 | Y | Y | N * | N ◊ | N ∆ | Y | Y | N/A ○ | Y | Mod |
| Ljungman et al. 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| Lucchini et al. 2021a | Y | Y | N * | Y | N ∆ , Ω | Y | Y | N/A ○ | Y | Mod |
| Lucchini et al. 2021b | Y | Y | Y | Y | N Ω | Y | Y | Y | Y | Low |
| Lupo‐Stanghellini et al. 2021 | Y | Y | N * | Y | N Ω | Y | Y | N/A ○ | Y | Mod |
| Machado et al. 2020 | Y | Y | N * | N ◊ | Y | Y | Y | Y | Y | Low |
| Malard et al. 2020 | Y | Y | N * | Y | Y | Y | Y | N/A ○ | Y | Low |
| Mico et al. 2020 | Y | Y | N * | Y | N Ω | Y | Y | Y | Y | Low |
| Mushtaq et al. 2021 | Y | Y | N * | Y | Y | Y | Y | Y | Y | Low |
| Passamonti et al. 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| Pinana et al. 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| Shah et al. 2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| Sharma et al. 2021 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Low |
| Sultan et al. 2020 | Y | Y | N * | Y | N ∆ , Ω | Y | Y | N/A ○ | Y | Mod |
| Tailor et al. 2020 | Y | Y | N * | Y | N γ | U ¥ | U ¥ | N/A ○ | Y | Mod |
| Varma et al. 2020 | Y | Y | N * | Y | Y | Y | Y | Y | Y | Low |
| Vicent et al. 2020 | Y | Y | N * | Y | N ∆ , Ω | Y | Y | N/A ○ | Y | Mod |
| Wang et al. 2020 | Y | Y | N * | N ◊ | N Ψ , γ | Y | Y | Y | Y | Mod |
| Xhaard et al. 2021 | Y | Y | N * | Y | N Ω | Y | Y | Y | Y | Low |
Abbreviations: HCT, haematopoietic cell transplantation; N, no; N/A, not applicable; SCT, stem cell transplantation; U, unclear; Y, yes.
Study sample size lower than estimated sample size required using estimated mortality risk of 25%, precision 0.1.
Insufficient description of patient/HCT treatment information.
Insufficient patient/treatment information provided to determine coverage.
Only HCT patients <50 years old included.
Descriptive report no meaningful statistical analysis performed.
Only allogeneic SCT recipients included.
Only multiple myeloma patients included.
Only autologous SCT recipients included.
Use of SARS‐CoV‐2 IgG positivity only as the inclusion criteria may introduce selection bias towards survivors of COVID‐19 infection.
Only one auto‐HCT recipient included.
Diagnostic criteria for COVID‐19 infection not provided.
3.5. Clinical outcomes
Data on COVID‐19 deaths were provided for all patients apart from 33 patients in two studies [8,29]. The median follow‐up period ranged from 21 to 282 days. Within the cohort of 2108 informative patients, the overall COVID‐19‐related death rate was 21% (95% CI 18%–24%) (Figure 2). Significant heterogeneity (I 2 = 49%; χ2 = 68.98; df = 35, p < 0.01) was detected between different studies.
FIGURE 2.
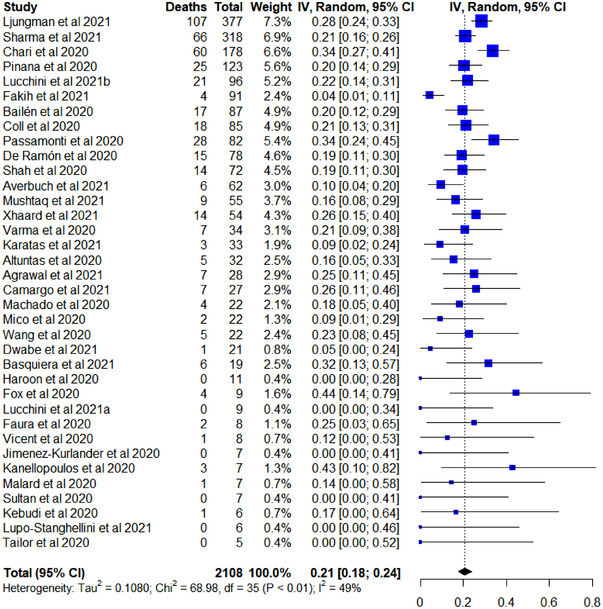
Forest plot of the COVID‐19‐related death rate in all selected studies
The overall COVID‐19‐related MV rate was 14% (95% CI 11%–17%) from a total of 868 informative patients, while the ITU admission rate was 18% (95% CI 14%–22%) from a total of 1033 informative patients (Figures S2 and S3). Heterogeneity was found to be statistically significant between studies reporting ITU admission rates (I 2 = 42%; χ2 = 32.90; df = 19, p = 0.02) but not between studies reporting COVID‐19‐associated MV rates (I 2 = 11%; χ2 = 21.32; df = 19, p = 0.32).
3.6. Subgroup analysis
Six predetermined variables were evaluated for their association with the primary outcome. Information on COVID‐19‐related deaths in relation to the time interval between transplant date and COVID‐19 onset was available in 894 patients from 12 studies. The death rate was significantly higher among patients who developed COVID‐19 within 12 months of HCT than among those who had their transplant more than 1 year previously (risk ratio [RR] 1.82, 95% CI 1.09–3.03; p = 0.02) (Figure 3). Significant heterogeneity was found between the 11 studies (I 2 = 52%; χ2 = 18.63; df = 9, p = 0.03).
FIGURE 3.
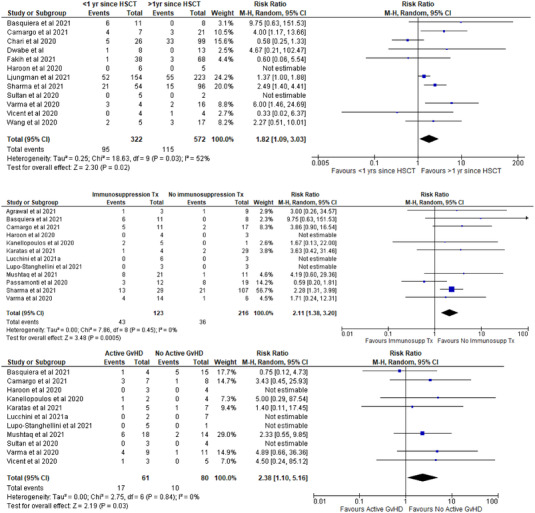
Subgroup analysis of COVID‐19‐related death rate by time from haematopoietic cell transplantation (HCT) to COVID‐19 onset (>1 year vs. <1 year; top), immunosuppressant therapy at the onset of COVID‐19 (middle), and active graft‐versus‐host disease (GvHD) at the onset of COVID‐19 (bottom)
Information on COVID‐19‐related deaths in relation to the use of immunosuppressant drug treatment to prevent GvHD was available in 339 patients from 12 studies. The death rate was significantly higher among patients who developed COVID‐19 within 6 months of receiving pharmacological immunosuppression than among those patients whose immunosuppression had been discontinued more than 6 months before the onset of COVID‐19 (RR 2.11, 95% CI 1.38–3.18; p = 0.0005) (Figure 3). No significant heterogeneity was found among the 12 studies (I 2 = 0%; χ2 = 7.86; df = 8, p = 0.45).
Information on COVID‐19‐related deaths in relation to active GvHD was available in 141 patients from 11 studies. The death rate was significantly higher among patients with active GvHD at the time of COVID‐19 onset than among those with no active GvHD (RR 2.38, 95% CI 1.10–5.16; p = 0.03) (Figure 3). No significant heterogeneity was found among the 11 studies (I 2 = 0%; χ2 = 2.75; df = 6, p = 0.84).
Notably, we did not observe any significant differences in the COVID‐19‐related death rate when comparing HCT recipients by age (<50 years vs. ≥50 years old; 208 patients from six studies), sex (697 patients from 12 studies) or type of transplant (autologous vs. allogeneic HCT; 1769 patients from 19 studies) (Figure S4).
3.7. Sensitivity analysis
Sensitivity analysis showed that the cumulative COVID‐19‐related death rate was not significantly affected by excluding studies with moderate or high risk of bias or with a low sample size (n < 10) or by excluding paediatric (age ≤18 years old) HCT recipients or those without laboratory confirmation of SARS‐CoV‐2 infection (Figure S5).
4. DISCUSSION
To our knowledge, this meta‐analysis, which utilised published data involving 2141 patients from 36 studies in four different continents, is the first to examine COVID‐19 outcomes in HCT recipients specifically. The cumulative COVID‐19‐related death rate was 21% (95% CI 18%–24%), with MV and ITU admission rates of 14% (95% CI 11%–17%) and 18% (95% CI 14%–22%), respectively. A significantly higher rate of COVID‐19‐related deaths was observed among HCT recipients who developed COVID‐19 within 1 year of HCT (RR 1.82, 95% CI 1.09–3.03; p = 0.02), within 6 months of receiving immunosuppressant drugs (RR 2.11, 95% CI 1.38–3.18, p = 0.0005), or in the setting of active GvHD (RR 2.38, 95% CI 1.10–5.16; p = 0.03). Crucially, some of these associations were not observed in individual studies and became apparent only when the data were pooled. The validity of our findings is supported by the sensitivity analysis, which demonstrated that the COVID‐19 death rate remained unchanged despite excluding studies with a moderate or high risk of selection bias or studies with a lower sample size or by excluding paediatric (age ≤18 years old) HCT recipients or those without laboratory confirmation of SARS‐CoV‐2 infection.
Interestingly, we found no statistically significant difference in the rate of COVID‐19‐related death between males versus females, allogeneic versus autologous HCT recipients, or HCT recipients aged ≥50 years versus <50 years. We believe the latter observation should be interpreted with caution for two reasons. First, due to inconsistency in reporting patient age groups, we were only able to obtain this information in 208 patients from six studies, which represents only 9.7% of the HCT recipients included in this review. Second, since age is a continuous variable, the risk of COVID‐19‐related death may vary significantly depending on which age cutoff is used. We chose a value of 50 years, as it was close to the median age of HCT recipients in most of our included studies. However, it may not have been optimal in separating patients into two groups based on COVID‐19 outcomes. The comparable COVID‐19‐related death rate between allogeneic and autologous HCT recipients is also surprising given the increased risk of posttransplant‐related complications and the degree of immunosuppression associated with allogeneic HCT. This observation was also supported by the two largest multicentre studies [7,8] of COVID‐19 outcomes in HCT recipients to date, which showed no significant difference in the overall survival between allogeneic and autologous HCT recipients at 4–6 weeks following COVID‐19. We suspect that these adverse risk factors associated with allogeneic HCT could be offset by the increased age generally found in autologous HCT recipients within our cohort of patients (where available, the median age among allogeneic HCT recipients ranged between 10 and 64 years old, while in autologous HCT, it was 40–65 years old). Furthermore, the variations in comorbidities and HCT indications between both HCT types may also have contributed to this observation, although we did not have sufficient pooled data to make any meaningful analysis in these two areas.
We did observe significant heterogeneity in the COVID‐19‐related death rate and ITU admission between different studies (χ2 = 68.98; p < 0.01 and chi2 = 32.90; p = 0.02, respectively). This likely reflects variation in the proportion of patients with risk factors for adverse COVID‐19 outcomes, including but not confined to the three identified in our subgroup analysis.
We recognise several limitations to our study. First, although our pooled subgroup analysis showed a significantly higher risk of COVID‐19‐related deaths in the first year following HCT, it is difficult to ascertain where these deaths were caused by the SARS‐CoV‐2 virus itself or by pre‐existing or subsequently acquired posttransplant‐related complications unrelated to the diagnosis of COVID‐19, which are known to be higher in the first year posttransplant [46]. Additionally, in most centres HCT recipients are followed up more closely within the first year. This may introduce reporting bias within this cohort of patients compared to those transplanted more than a year ago receiving less intensive follow‐up. We suspect that these two factors may have contributed to the significant heterogeneity seen within this subgroup analysis (χ2 = 18.63; p < 0.03).
Second, although a higher rate of COVID‐19‐related deaths was observed among HCT recipients in the setting of active GvHD, the information available for this subgroup of HCT recipients was insufficiently detailed to make any meaningful observation on whether this reflects those with acute versus chronic GvHD.
Third, we found evidence of duplicate publication bias. For example, we identified one study [7] involving Center for International Blood and Marrow Transplant Research data where some patient data may have been published separately as single‐centre experiences [6, 35], while another study [8] utilising EBMT registry data may have included data that were reported separately in other studies. Such ‘double reporting’ could potentially amplify the contribution of certain cases in determining the characteristics of the overall study population and therefore distort its true profile. In addition, limitations in resources and COVID‐19 testing capability during the initial stages of the pandemic are likely to have introduced selection bias towards symptomatic or hospitalised patients.
Fourth, due to variation in reporting across the selected studies, insufficient pooled data were available to meaningfully assess potential risk factors for adverse COVID‐19 outcomes such as comorbidity and performance status. However, we did identify five studies [7, 8, 28, 30, 38] that reported comorbidities and/or baseline fitness levels of HCT recipients and their correlation with COVID‐19‐related death rates. Despite consistencies in methods used to assess these risk factors, the presence of comorbidities [28, 30, 38] or variation in baseline fitness (assessed by ECOG performance status [8] or HCT‐CI score [7]) did not appear to correlate with a higher COVID‐19‐related death rate in any of these studies. Variation in reporting also precluded the meaningful analysis of other potential risk factors, including HCT conditioning regimen, indication for HCT, donor type or GvHD prophylaxis regimen used, as well as the effectiveness of different antiviral or anti‐inflammatory therapeutic interventions.
Finally, since all contributing studies were performed prior to COVID‐19 vaccine roll‐out, our meta‐analysis cannot provide direct information on COVID‐19 outcomes and risk factors in vaccinated HCT recipients. However, recent studies [9–11] have reported reduced immunogenicity of COVID‐19 vaccines among HCT recipients compared to the general population. This suggests that the COVID‐19 death rate and risk factors identified in our meta‐analysis may not be substantially different in the postvaccination era.
The COVID‐19 outcomes observed in our meta‐analysis of HCT recipients are notably better than those reported in a recent meta‐analysis [3] of unselected patients with HM who acquired COVID‐19 over the same time period. The latter study showed a pooled COVID‐19‐related death rate of 34% (95% CI 28%–39%) with MV rates and ITU admission rates of 17% and 21%, respectively. The differences in COVID‐19 outcomes between this study and ours may be explained by the stringent patient selection process for HCT, which is likely to result in enrichment of features such as minimal comorbidity, good performance status and control of the underlying disease. In keeping with this idea, large studies reporting on COVID‐19 outcomes in solid organ transplant recipients—another cohort of patients who undergo stringent selection—reported COVID‐19‐related death rates ranging from 19% to 27% [29, 47, 48, 49, 50]. In fact, the COVID‐19‐related death rate observed in our study of HCT recipients is similar to that of unselected patients hospitalised with COVID‐19 during the same time period (February–April 2020) [51, 52].
In summary, this meta‐analysis describes COVID‐19 outcomes in 36 studies involving 2141 HCT recipients across the globe. We found the COVID‐19‐related death rate to be 21%—lower than that of unselected patients with HM and similar to that of patients hospitalised with COVID‐19 in the general population during the same time period. COVID‐19‐related deaths were increased among HCT recipients who developed COVID‐19 within 1 year of HCT, within 6 months of receiving treatment with immunosuppressants, or in the context of active GvHD. These novel observations support the idea that HCT should remain an integral part of HM treatment protocols during the COVID‐19 pandemic. However, HCT recipients who are at increased risk of COVID‐19‐related death should be prioritised for surveillance and preventative measures.
FUNDING INFORMATION
No funding was received for this meta‐analysis.
AUTHOR CONTRIBUTIONS
Yeong Jer Lim, Yngvar Floisand and Muhammad Saif conceptualised the study. Yeong Jer Lim and Umair Khan performed the literature search, study selection and data analysis. Yeong Jer Lim wrote the first draft of the manuscript. Andrew R. Pettitt, Nagesh Kalakonda, Mats Remberger, Andrew Ross, Indrani Karpha, Muhammad Saif and Yngvar Floisand contributed to the subsequent edits of the manuscript.
CONFLICTS OF INTEREST
Andrew Ross, Indrani Karpha, Muhammad Saif and Yngvar Floisand are employed by Clatterbridge Cancer Centre NHS Foundation Trust. Yeong Jer Lim, Andrew R. Pettitt and Nagesh Kalakonda are employed by the University of Liverpool. Yeong Jer Lim is supported by the NW MRC fellowship scheme (award number MR/N025989/1). Mats Remberger is employed by Uppsala University Hospital, Sweden and supported by grants from the Swedish Research Council (VR 2017‐00355).
PATIENT CONSENT STATEMENT
Not applicable.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
None.
Lim YJ, Khan U, Karpha I, Ross A, Saif M, Remberger M, et al. COVID‐19 outcomes in haematopoietic cell transplant recipients: A systematic review and meta‐analysis. eJHaem. 2022;3:862–872. 10.1002/jha2.465
DATA AVAILABILITY STATEMENT
The extracted data are available upon request from the corresponding author.
REFERENCES
- 1. Russell B, Moss CL, Shah V, Ko TK, Palmer K, Sylva R, et al. Risk of COVID‐19 death in cancer patients: an analysis from Guy's Cancer Centre and King's College Hospital in London. Br J Cancer. 2021;125(7):939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID‐19: a meta‐analysis of patient data. JCO Global Oncol. 2020(6):799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136(25):2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camargo JF, Mendoza MA, Lin R, Moroz IV, Anderson AD, Morris MI, et al. Clinical presentation and outcomes of COVID‐19 following hematopoietic cell transplantation and cellular therapy. Transpl Infect Dis. 2021;23(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karataş A, Malkan ÜY, Velet M, Demiroğlu H, Büyükaşik Y, Telli Dizman G, et al. The clinical course of covid‐19 in hematopoietic stem cell transplantation (Hsct) recipients. Turk J Med Sci. 2021;51(4):1647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah GL, DeWolf S, Lee YJ, Tamari R, Dahi PB, Lavery JA, et al. Favorable outcomes of COVID‐19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020;130(12):6656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID‐19 in haematopoietic stem‐cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, et al. COVID‐19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35(10):2885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehmsen S, Asmussen A, Jeppesen SS, Nilsson AC, Østerlev S, Vestergaard H, et al. Antibody and T‐cell immune responses following mRNA COVID‐19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shem‐Tov N, Yerushalmi R, Danylesko I, Litachevsky V, Levy I, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID‐19 vaccine in haematopoietic stem cell transplantation recipients. Br J Haematol. 2022;196(4):884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhakal B, Abedin S, Fenske T, Chhabra S, Ledeboer N, Hari P, et al. Response to SARS‐CoV‐2 vaccination in patients after hematopoietic cell transplantation and CAR T‐cell therapy. Blood. 2021;138(14):1278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147‐53. [DOI] [PubMed] [Google Scholar]
- 14. Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1:9‐14. [Google Scholar]
- 15. Fox TA, Troy‐Barnes E, Kirkwood AA, Chan WY, Day JW, Chavda SJ, et al. Clinical outcomes and risk factors for severe COVID‐19 in patients with haematological disorders receiving chemo‐ or immunotherapy. Br J Haematol. 2020;191(2):194‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haroon A, Alnassani M, Aljurf M, Ahmed SO, Shaheen M, Hanbli A, et al. COVID‐19 post hematopoietic cell transplant, a report of 11 cases from a single center. Mediterr J Hematol Infect Dis. 2020;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jimenez‐Kurlander L, Antal Z, DeRosa A, Diotallevi D, Pottenger E, Wilson N, et al. COVID‐19 in pediatric survivors of childhood cancer and hematopoietic cell transplantation from a single center in New York City. Pediatr Blood Cancer. 2021;68(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lupo‐Stanghellini MT, Xue E, Mastaglio S, Oltolini C, Angelillo P, Messina C, et al. COVID‐19 in recipients of allogeneic stem cell transplantation: favorable outcome. Bone Marrow Transplant. 2021;56(9):2312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malard F, Genthon A, Brissot E, van de Wyngaert Z, Marjanovic Z, Ikhlef S, et al. COVID‐19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55(11):2180‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mushtaq MU, Shahzad M, Chaudhary SG, Luder M, Ahmed N, Abdelhakim H, et al. Impact of SARS‐CoV‐2 in hematopoietic stem cell transplantation and chimeric antigen receptor T‐cell therapy recipients. Transplant Cell Ther. 2021;27(9):796.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sultan AM, Mahmoud HK, Fathy GM, Abdelfattah NM. The outcome of hematopoietic stem cell transplantation patients with COVID‐19 infection. Bone Marrow Transplant. 2021;56(4):971‐3. [DOI] [PubMed] [Google Scholar]
- 22. Wang B, Van Oekelen O, Mouhieddine TH, Del Valle DM, Richter J, Cho HJ, et al. A tertiary center experience of multiple myeloma patients with COVID‐19: lessons learned and the path forward. J Hematol Oncol. 2020;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanellopoulos A, Ahmed MZ, Kishore B, Lovell R, Horgan C, Paneesha S, et al. COVID‐19 in bone marrow transplant recipients: reflecting on a single centre experience. Br J Haematol. 2020;190(2):e67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mico M, Pavoni C, Taurino D, Stefanoni P, Algarotti A, Finazzi M, et al. Outcome of covid 19 in allogeneic stem cells transplant patients: the single center experience from a severely affected area (Bergamo). Bone Marrow Transplant. 2021;56(1):112‐3. [Google Scholar]
- 25. Dwabe S, Savitala‐Damerla L, Rodman J, Ladha A, Tam E, Alachkar H, et al. Outcomes of COVID‐19 in hematopoietic stem‐cell transplant recipients: a single institution observational study. Blood. 2021;138(1):4896. [Google Scholar]
- 26. Tailor IK, Altaf SY, Alshehry NF, Alser AM, Marei M, Alfayez M, et al. Excellent outcomes of patients of middle eastern ethnic origin with autologous haemopoietic stem cell transplantation and COVID‐19: does a dampened immune system protect against severe COVID‐19? Blood. 2020;136(1):3.32614960 [Google Scholar]
- 27. Agrawal N, Singh R, Sharma SK, Naithani R, Bhargava R, Choudhary D, et al. Outcomes of COVID‐19 in hematopoietic stem cell transplant recipients: multicenter retrospective analysis. Indian J Hematol Blood Transfus. 2021:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basquiera AL, García MJ, Martinez Rolón J, Olmedo J, Laviano J, Burgos R, et al. Clinical characteristics and evolution of hematological patients and COVID‐19 in Argentina: a report from the Argentine Society of Hematology. Medicina (Mex). 2021;81(4):536‐45. [PubMed] [Google Scholar]
- 29. Coll E, Fernández‐Ruiz M, Sánchez‐Álvarez JE, Martínez‐Fernández JR, Crespo M, Gayoso J, et al. COVID‐19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21(5):1825‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El Fakih R, Haroon A, Alfraih F, Al‐Khabori MK, Alzahrani M, Alhuraiji A, et al. Clinical course and outcomes of COVID‐19 in hematopoietic cell transplant patients, a regional report from the Middle East. Bone Marrow Transplant. 2021;56(9):2144‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faura A, Rives S, Lassaletta Á, Sebastián E, Madero L, Huerta J, et al. Initial report on Spanish pediatric oncologic, hematologic, and post stem cell transplantation patients during SARS‐CoV‐2 pandemic. Pediatr Blood Cancer. 2020;67(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kebudi R, Kurucu N, Tuğcu D, Hacısalihoğlu Ş, Fışgın T, Ocak S, et al. COVID‐19 infection in children with cancer and stem cell transplant recipients in Turkey: a nationwide study. Pediatr Blood Cancer. 2021;68(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lucchini G, Furness C, Lawson S, Gibson B, Wynn R, Slatter M, et al. COVID‐19 infection in paediatric recipients of allogeneic stem cell transplantation: the UK experience. Br J Haematol. 2021;194(4):e74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piñana JL, Martino R, García‐García I, Parody R, Morales MD, Benzo G, et al. Risk factors and outcome of COVID‐19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varma A, Kosuri S, Ustun C, Ibrahim U, Moreira J, Bishop MR, et al. COVID‐19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34(10):2809‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vicent MG, Martinez AP, Trabazo Del Castillo M, Molina B, Sisini L, Morón‐Cazalilla G, et al. COVID‐19 in pediatric hematopoietic stem cell transplantation: the experience of Spanish Group of Transplant (GETMON/GETH). Pediatr Blood Cancer. 2020;67(9):e28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xhaard A, Xhaard C, D'Aveni M, Salvator H, Chabi ML, Berceanu A, et al. Risk factors for a severe form of COVID‐19 after allogeneic haematopoietic stem cell transplantation: a Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM‐TC) multicentre cohort study. Br J Haematol. 2021;192(5):e121‐e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lucchini G, Cozma E, Jackson A, Gilmour K, Protheroe RE, Tholouli E, et al. COVID‐19 infection of HSCT recipients is associated with high mortality but no detectable cytokine storm at presentation. Blood. 2021;138(1):1788. [Google Scholar]
- 40. Machado CM, Kerbauy MN, Colturato I, Arcuri LJ, dos Santos ACF, Silva FR, et al. Clinical characteristics and outcomes of COVID‐19 in HSCT recipients. Blood. 2020;136(1):19. [Google Scholar]
- 41. De Ramón C, Hernandez‐Rivas JA, Rodríguez García JA, Ocio EM, Gómez‐Casares MT, López Jiménez J, et al. Impact of Sars‐CoV2 infection on 491 hematological patients: the ecovidehe multicenter study. Blood. 2020;136(1):5‐6. [Google Scholar]
- 42. Altuntas F, Ata N, Yigenoglu TN, Bascı S, Dal MS, Korkmaz S, et al. COVID‐19 in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2021;56(4):952‐5. [DOI] [PubMed] [Google Scholar]
- 43. Chari A, Samur MK, Martinez‐Lopez J, Cook G, Biran N, Yong K, et al. Clinical features associated with COVID‐19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136(26):3033‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Averbuch D, De La Camara R, Corbacioglu S, Mikulska M, Piñana Sanchez JL, Tridello G, et al. COVID‐19 in children following hematopoietic cell transplantation: a multinational study of the European bone marrow transplantation society (EBMT) and the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH). Blood. 2021;138(1):2866. [Google Scholar]
- 45. Bailén R, Kwon M, Aguado B, Duarte RF, Calbacho M, Chinea A, et al. Impact of sars‐Cov‐2 infection in hematopoietic transplant patients: experience from the Madrid group. Blood. 2020;136(1):12‐3. [Google Scholar]
- 46. Penack O, Peczynski C, Mohty M, Yakoub‐Agha I, Styczynski J, Montoto S, et al. How much has allogeneic stem cell transplant–related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4(24):6283‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, et al. Outcomes following SARS‐CoV‐2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 2021;73(11):e4090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caillard S, Chavarot N, Francois H, Matignon M, Greze C, Kamar N, et al. Is COVID‐19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sánchez‐Álvarez JE, Pérez Fontán M, Jiménez Martín C, Blasco Pelícano M, Cabezas Reina CJ, Sevillano Prieto ÁM, et al. Situación de la infección por SARS‐CoV‐2 en pacientes en tratamiento renal sustitutivo. Informe del Registro COVID‐19 de la Sociedad Española de Nefrología (SEN). Nefrología. 2020;40(3):272‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID‐19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city area. JAMA. 2020;323(20):2052‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The extracted data are available upon request from the corresponding author.


