Abstract
Objectives
Thyroid nodules (TN) are common. Genetic and environmental factors as well as chronic inflammation play a role in occurrence of these nodules. The key point in diagnostic assessment is to rule out malignancy. Biomarkers that can show the possibility of malignancy continue to be investigated. We evaluated the relationship between sedimentation rate, leukocyte, fibrinogen, C-reactive protein (CRP), and pentraxin 3 (PTX3) inflammatory markers and characteristics and cytology of TN.
Methods
This study included a nodular goiter group with 55 persons and control group with 58 persons. Participants’ gender, age, family history, thyroid function tests, sedimentation, leukocyte, fibrinogen, CRP, and PTX3 serum levels were recorded. The number of nodules, the largest nodule diameter, nodular echogenicity, and nodule structures were examined on ultrasonography (US) and thyroid biopsy was performed.
Results
The number of TN in patients was between 1 and 4. The number of patients with two TN was higher (47.3%, n=26). Nodule diameters differed between 3 and 62 (mean 21) mm. In thyroid biopsy, papillary thyroid cancer was detected in 25.5% (n=14) of the patients. The number of nodules on US increased as CRP values increased (p=0.013). In addition, the number of nodules on US decreased as fibrinogen values increased (p=0.003). No significant difference was found between the groups in terms of sedimentation, leukocyte, and PTX3 values.
Conclusion
The number of TN was positively correlated with CRP and negatively correlated with fibrinogen levels. However, there was no difference between benign and malignant differentiation and biomarkers. CRP values that correlate with the increase in the number of nodules can be used in prognosis and clinical follow-up.
Keywords: C-reactive protein, erythrocyte sedimentation rate, fibrinogen, leukocyte, pentraxin 3, thyroid nodule
American thyroid association defines thyroid nodules (TNs) as “lesions radiologically distinct from thyroid parenchyma within the thyroid gland” and these modules are found in 50–60% of healthy persons.[1] TNs are thought to develop following the replication and hyperplasia of thyroid follicular cells with the chronic inflammation environment that develops with the predisposing effect of genetic and environmental factors.[2]
The prevalence of TNs has been reported in various studies as 2–6% by palpation, 19–35% with ultrasonography (US), and 8–65% in autopsy series.[3] The incidence of TNs increases by age in women, persons with iodine deficiency and those who are exposed to radiation.[2,3] While 90% of TNs are benign, thyroid carcinoma is found in 4–15%.[3,4]
At present, various biochemical tests, imaging methods, and cytopathological examinations are used to evaluate TN.[4,5] Many serum biomarkers such as serum thyrotropin thyroid-stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), calcitonin, thyroid peroxidase antibodies (TPOAb), and anti-thyroglobulin antibodies (TGAb) are used in determining the development and prognosis of TNs, although the search for other serum biomarkers may be inexpensive and effective continues.[5,6]
In this prospective study, we aimed to investigate utility of erythrocyte sedimentation rate (ESR), leukocyte, fibrinogen, C-reactive protein (CRP), and pentraxin 3 (PTX3) test that are today used as biomarkers in the diagnosis and prognosis of other autoimmune and inflammatory disease, in determining TN characteristics. In addition, we aimed to determine the relationship between these biomarkers and thyroid functions, auto-antibodies, and TN characteristics.
Methods
This prospective study was approved by the Local Ethics Committee with a 2019 dated and 18/350 numbered decision. The study included 55 patients and randomly selected 58 persons as the control group. All participants gave informed written consent. Participants’ age, gender, family history of nodular goiter, body mass index (BMI), and Charlson comorbidity score (CCS) were determined. Then, laboratory experiments, ultrasonographic examination and thyroid biopsy stages were initiated. Exclusion criteria: Pregnancy, smoking, alcohol consumption, use of steroids, and immunomodulators, patients with diabetes mellitus, renal failure, liver failure, malignancy, infectious diseases, and other known autoimmune diseases were excluded from the study.
Antecubital venous blood samples were collected from all participants in the morning following 12-h fasting. FT3 (reference range [RR]: 2.62–5.7 pmol/L), FT4 (RR: 9–19 pmol/L), and TSH (RR: 0.35–4.94 uIU/ml) levels were measured using chemiluminescent microparticle immunoassay method. TPOAb (RR: 0–5.6 IU/ml) and TGAb (RR: 0–4.1 IU/ml) levels were measured with electrochemiluminescent method. ESR (RR: 0–20 mm/h) was measured with the Westergren method. Leukocyte count (RR: 4.5–11 .103/uL) was measured using an automatic laser/impedance cell counter. CRP level (RR: <0.5 mg/dl) was measured with CRP kit and automatic analyzer. Fibrinogen level (RR: 170–400 mg/dl) was measured using Clauss method. Serum PTX3 level was measured with human PTX3 immunoassay kit and microplate reader. In our study, as in the reference similar studies, PTX3 median in our control group was 1.10 ng/mL; range, 0.39–8.08 ng/mL.[8]
Thyroid US was performed by a radiologist using a high-resolution US device equipped with a 12–15 mHz linear probe. Nodule sides as the right, left, and bilateral, the number of nodules, and nodule echogenicity, including hypoechoic, isoechoic, and hyperechoic, largest nodule diameter (mm), nodule structures, including solid, cystic, and semisolid were studied with US.
Following US, fine-needle biopsy was performed in all patients with TN by the same radiologists using a 20 mL syringe and 23 gauge needle.[9] The cytology smears were prepared when needle contents were expelled onto a glass slide and smeared using a second slide. Two types of slides were done for each lesion: One fixed in 95% ethanol and Papanicolaou stained, and other air dried and May–Grunwald–Giemsa stained. The examination was carried out by an experienced cytopathologist, benign malignant differentiation of nodules was made. In our study, the diagnosis of all malignant nodules was papillary thyroid cancer (PTC).
Statistical Analysis
SPSS 23.0 software package was used for statistical analyses of the data. Normality of quantitative variables was analyzed with Shapiro–Wilk test. Descriptive statistics are expressed as mean ± standard deviation for numerical variables, while categorical variables are given as frequency and percentage. Chi-square test was used to evaluate the difference between the groups for categorical variables. Quantitative variables were compared between two groups with Mann–Whitney U-test and between three groups with Kruskal–Wallis variance analysis. The correlation between two numerical variables was examined using Spearman’s correlation analysis. The results were evaluated at 95% confidence interval and p<0.05 values were considered statistically significant.
Results
Of all patients, 78.2% were female with an age range of 21–78 (mean 49.5) years. BMI value was in the range of 17–47.2 (mean 28.3) kg/m2. CCS was in the range of 0–3, and the score was 0 in 50.9% (n=28) of the patients. Of the patients, 41.8% (n=23) had a family history of nodular goiter. In the control group, 62% (n=36) of the participants were female with the age range of (mean 46.6) years. BMI was in the range of 17.9–40.6 (mean 27.4) kg/m2. CCS was in the range of 0–2, and the score was 0 in 58.6% (n=34) of the controls. Of these participants, 25.9% (n=15) had a history of nodular goiter.
The number of nodules differed between 1 and 4 (mean 1.8) in the patient group and the total number of nodules was. The number of nodules was mostly (47.3%, n=26) two. The largest nodule diameter of the patients was in the range of 3–62 (mean 21) mm. Nodule location was bilateral (47.3%, n=26), right (29.1%, n=16), and left (23.6%, n=13), while nodule echogenicity was mostly hypoechoic (36.4%, n=20), hyperechoic (36.4%, n=20), and isoechoic (27.3%, n=15). Nodule characteristics were solid (58.2%, n=32), semisolid (21.8%, n=12), and cystic (20%, n=11). Nodule biopsy was predominantly benign (74.5%, n=41).
When biochemical markers were compared between the patient and control groups; FT4 value was significantly higher in the patient group (p=0.041). ESR was markedly higher in the patient group, although the difference was not statistically significant (p=0.055). No significant difference was found between the patient and control groups in terms of the other biochemical tests (Table 1).
Table 1.
Comparison of the patient and control groups in terms of biochemical markers
| Biochemical Markers | Patient Group (n=55) Mean±SD | Control Group (n=58) Mean± SD | P* |
|---|---|---|---|
| TSH (uIU/ml) | 2.11±3.92 | 2.58±9.05 | 0.518 |
| FT3 (pmol/L) | 4.21±1.41 | 4.06±0.73 | 0.589 |
| FT4 (pmol/L) | 14.03±3.57 | 12.84±1.63 | 0.041 |
| TGAb (IU/ml) | 47.84±164.01 | 121.88±835.53 | 0.288 |
| TPOAb (IU/ml) | 293.36±848.56 | 113.37±327.85 | 0.411 |
| ESR (mm/h) | 12.42±7.24 | 10.91±9.22 | 0.055 |
| Leukocyte (103/uL) | 7.24±1.74 | 7.64±1.89 | 0.264 |
| CRP (mg/L) | 6.27±23.81 | 5.66±11.28 | 0.841 |
| Fibrinogen (mg/dl) | 303.5±88.4 | 316.8±73.4 | 0.367 |
| PTX3 (ng/ml) | 1.31±1.28 | 1.10±1.25 | 0.148 |
P*: Mann–whitney U-test; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; FT3: Free triiodothyronine; FT4: Free thyroxine; PTX3: Pentraxin 3; SD: Standard deviation; TGAb: Anti-thyroglobulin antibodies; TPOAb: Thyroid peroxidase antibodies; TSH: Thyrotropin.
As FT4 values increased, the largest nodule diameter values significantly decreased (p=0.029). As CRP values increased, the number of nodules on US also increased (p=0.013). As fibrinogen values increased, the number of nodules on US significantly decreased (p=0.003). Other biomarkers other than CRP and fibrinogen did not correlate with the number of nodules (Table 2 and Fig. 1).
Table 2.
Correlations between biochemical markers and the number of nodules, largest nodule diameter
| Biochemical markers | Number of nodules (n=55) | Largest nodule diameter (mm) (n=55) | P* |
|---|---|---|---|
| TSH (uIU/ml) | −0.173 | 0.185 | r |
| 0.206 | 0.177 | P | |
| FT3 (pmol/L) | 0.030 | −0.200 | r |
| 0.827 | 0.143 | P | |
| FT4 (pmol/L) | 0.177 | -0.295* | r |
| 0.196 | 0.029 | P | |
| TGAb (IU/ml) | −0.199 | 0.197 | r |
| 0.144 | 0.149 | P | |
| TPOAb (IU/ml) | −0.139 | −0.003 | r |
| 0.310 | 0.981 | P | |
| ESR (mm/h) | −0.175 | −0.041 | r |
| 0.203 | 0.766 | P | |
| Leukocyte (103/uL) | 0.131 | 0.031 | r |
| 0.339 | 0.825 | P | |
| CRP (mg/L) | 0.334 | −0.047 | r |
| 0.013 | 0.733 | P | |
| Fibrinogen (mg/dl) | −0.389 | −0.028 | r |
| 0.003 | 0.841 | P | |
| PTX3 (ng/ml) | −0.060 | −0.124 | r |
| 0.666 | 0.369 | P |
P*: Pearson correlation analysis; r: Correlation value; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; FT3: Free triiodothyronine; FT4: Free thyroxine; PTX3: Pentraxin 3; SD: Standard deviation; TGAb: Anti-thyroglobulin antibodies; TPOAb: Thyroid peroxidase antibodies; TSH: Thyrotropin.
Figure 1.
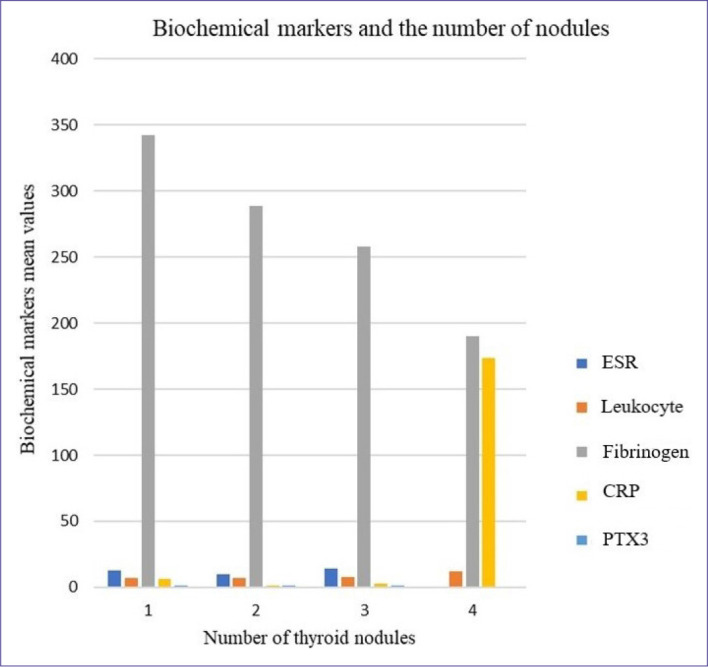
Relationship between serum biomarkers and number of nodules.
No statistically significant correlation was found between nodule echogenicity, nodule characteristic, nodule side, nodule cytology, and biochemical markers studied (p>0.05). On the other hand, in the comparison of thyroid cytology results, no statistically significant difference was found between 41 benign and 14 malignant patient subgroups (p>0.05) (Table 3).
Table 3.
Relationship between biochemical markers and thyroid nodule cytology
| Biochemical Markers | Benign nodule (n=41) Mean±SD | Malignant nodule (n=14) Mean±SD | P* |
|---|---|---|---|
| TSH (uIU/ml) | 1.63±2.04 | 3.51±6.96 | 0.164 |
| FT3 (pmol/L) | 4.39±1.54 | 3.70±0.77 | 0.071 |
| FT4 (pmol/L) | 14.39±3.84 | 13±2.47 | 0.156 |
| TGAb (IU/ml) | 37.4±140.95 | 78.4±221.93 | 0.288 |
| TPOAb (IU/ml) | 342.31±961.71 | 150.02±339.88 | 0.743 |
| ESR (mm/h) | 12.13±7.44 | 13.29±6.82 | 0.438 |
| Leukocyte (103/uL) | 7.26±1.78 | 7.16±1.68 | 0.794 |
| CRP (mg/L) | 6.95±27.13 | 4.29±9.12 | 0.534 |
| Fibrinogen (mg/dl) | 301.6±86.8 | 4.29± 9.12 | 0.629 |
| PTX3 (ng/ml) | 1.27±1.22 | 1.43± 1.48 | 0.977 |
P*: Mann-whitney u test; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate; FT3: Free triiodothyronine; FT4: Free thyroxine; PTX3: Pentraxin 3; SD: Standard deviation; TGAb: Anti-thyroglobulin antibodies; TPOAb: Thyroid peroxidase antibodies; TSH: Thyrotropin.
Discussion
TN is increasingly common in the population and the prevalence of palpable TN has been found to be approximately 5% in women and 1% in men.[1] Clinical significance of TN is the possibility of thyroid cancer that is found in 7–15% of the cases depending on age, gender, history of radiation exposure, family history, and other factors.[1,4] The annual incidence of thyroid cancer was 4.9/100000 in 1975, while this ratio raised to 14.3/100,000 in 2009.[10,11] Absolute increase in thyroid cancer is almost 4 folds higher in women than in men (6.5–21.4 = 14.9) per 100,000 women.[11] However, the rate of mortality from thyroid cancer remained constant between 1975 and 2009 (approximately 0.5 death/100,000).[11]
The formation and characteristic of nodules vary according to the sodium/iodine symporter activity elevation and mitotic activity of the thyrosides.[11] TSH is the main mitotic factor in nodule formation.[4,11] Radioactivity and increasing radiological examinations, habits such as smoking, some drugs, iodine, selenium, and iron deficiency, harmful chemicals such as nitrate, benzene, formaldehyde, pesticides, and bisphenol A that present in agricultural and industrial foods, obesity, metabolic syndrome, insulin resistance, and hyperinsulinemia, and genetic mutations such as SPOP (4/38), ZNF148 (6/38), RET, BRAF, and RAS contribute to TN and cancer formation.[4,12,13]
These mentioned factors may cause chronic inflammation and subsequently development of thyroid cancer by disrupt secretion regulation of TSH hormones on the one hand, and by activating Janus kinase/signal transducer and activator of transcription, phosphoinositide 3-kinase/protein kinase B/Akt, and/or mitogen-activated protein kinase pathways in thyroid tissue on the other hand, and with an increase in cytokines such as T and B lymphoids and interleukin (IL) 6, IL-7, IL-10, IL-13, tumor necrotizing factor (TNF), and vascular endothelial growth factor (VEGF).[14,15]
Studies have been conducted with various biomarkers in nodule etiology and to determine inflammation level to determine the prognosis. These studies have used phase proteins such as haptoglobin, galectin-3, IL6, VEGF, soluble intercellular adhesion molecule-1, neutrophil-lymphocyte ratio, albumin, prealbumin, and transferrin.[15-17]
Studies have reported that ESR, leukocyte, fibrinogen, and CRP were significantly increased in subacute thyroiditis and could be helpful in showing prognosis of thyroiditis.[18-21] In a comparative study with PTC and TN patients, ESR was found to be significantly higher in TN patients. However, it was reported that ESR has no potential value in PTC staging and predicting the prognosis.[22]
In our study, ESR was markedly higher in the patient group, although no statistically significantly difference was found between the groups. Again, as in our study, no correlation was observed between TN and thyroid cancer and leukocyte. In the present study, fibrinogen was higher in malignant nodules compared to the controls, but no statistically significant difference was found between the controls and differentiation of benign and malignant nodules. The number of nodules on US statistically significantly decreased as fibrinogen values increased.
Several thyroid studies, including nodular goiter, have reported that CRP levels have a limited value in the diagnosis of thyroid disease except for subacute thyroiditis.[23] CRP has been reported to have no potential value in PTC staging and predicting the prognosis.[17,22] In our study, CRP was higher in benign nodules compared to the patient group, although no statistically significant difference was found between patient and control groups and the differentiation of benign-malignant nodules. However, it was found that the number of nodules on US significantly increased as CRP values increased.
An acute phase protein, PTX3 is a member of pentraxin together with CRP (PTX1) and serum amiloid P (PTX2) that have both pro-inflammatory and inflammatory features.[7,24] However, it is longer compared to the others and is expressed by macrophages, neutrophils, dendritic cells, and vascular smooth muscle cells.[24] PTX3 induces active TGF-β and anti-inflammatory response and increases Akt phosphorylation in macrophages. It reduces NFκ-B activation caused by TNFα and decreased PTX3 of apoptotic cells stimulates phagocytic activity of macrophages.[24] This shows that PTX3 reduces and regulates excessive inflammatory activity in macrophages and supports the healing process.[25] İn our study, PTX3 was higher in malignant nodules compared to the patient group, although there was no statistically significant result in thyroid hormones, nodule characteristics, and the differentiation of malignant-benign nodules.
Study Limitations
We could have more patients, but the increasing cost of the study reduced the number of participants. On the other hand, if we could include other serum biomarkers of inflammation such as insulin-like growth factor-I, VEGF-C, TNF, and IL-6, it would be a more comprehensive study. Again, research costs limited the number of biomarkers that could be studied.
Conclusion
In our study, a significant correlation was found between CRP and fibrinogen levels and the number of nodules on US. CRP values can give us an idea in the follow-up of the increase in the number of nodules. In patients with TN, the increase in ESR was high enough to approach the limit of statistical significance. These situations show the importance of inflammation as one of the mechanisms underlying TN development. However, the number of patients with malignant and benign nodules was small in our study. This limited our study. Further studies with larger patients and larger inflammatory cytokines are needed for more effective evaluation.
Footnotes
Please cite this article as ”Destek S, Benturk B, Yapalak Y, Ozer OF. Clinical Significance of Erythrocyte Sedimentation Rate, Leukocyte, Fibrinogen, C-Reactive Protein, and Pentraxin 3 Values in Thyroid Nodules. Med Bull Sisli Etfal Hosp 2022;56(2):270–275”.
Disclosures
Ethics Committee Approval:
This prospective study was approved by the Bezmialem Vakıf University Ethics Committee with a 2019 dated and 18/350 numbered decision.
Peer-review:
Externally peer-reviewed.
Conflict of Interest:
None declared.
Authorship Contributions
Concept – S.D., B.B.; Design – S.D., Y.P.; Supervision – O.F.O.; Materials – B.B., Y.P.; Data collection &/or processing – Y.P., O.F.O.; Analysis and/or interpretation – S.D., O.F.O.; Literature search – S.D.; Writing – S.D.; Critical review – S.D., O.F.O.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kameyama K, Ito K, Takami H. Pathology of benign thyroid tumor. [Article in Japanese] Nihon Rinsho. 2007;65:1973–8. [PubMed] [Google Scholar]
- 3.Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901–11. doi: 10.1016/j.beem.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96:329–49. doi: 10.1016/j.mcna.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pemayun TG. Current diagnosis and management of thyroid nodules. Acta Med Indones. 2016;48:247–57. [PubMed] [Google Scholar]
- 6.Xu W, Huo L, Chen Z, Huang Y, Jin X, Deng J, et al. The relationship of TPOAb and TGAb with risk of thyroid nodules: a large epidemiological study. Int J Environ Res Public Health. 2017;14:723. doi: 10.3390/ijerph14070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoflach M, Kiechl S, Mantovani A, Cuccovillo I, Bottazzi B, Xu Q, et al. Pentraxin-3 as a marker of advanced atherosclerosis results from the Bruneck, ARMY and ARFY Studies. PLoS One. 2012;7:e31474. doi: 10.1371/journal.pone.0031474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, et al. Lipid Assessment Trial Italian Network (LATIN) Investigators Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–54. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 9.Sidoti M, Marino G, Resmini E, Augeri C, Cappi C, Cavallero D, et al. The rational use of fine needle aspiration biopsy (FNAB) in diagnosing thyroid nodules. Minerva Endocrinol. 2006;31:159–72. [PubMed] [Google Scholar]
- 10.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 11.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 12.Yildirim Simsir I, Cetinkalp S, Kabalak T. Review of factors contributing to nodular goiter and thyroid carcinoma. Med Princ Pract. 2020;29:1–5. doi: 10.1159/000503575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobel M. Etiopathology, clinical features, and treatment of diffuse and multinodular nontoxic goiters. J Endocrinol Invest. 2016;39:357–73. doi: 10.1007/s40618-015-0391-7. [DOI] [PubMed] [Google Scholar]
- 14.Fagin JA, Wells SA., Jr Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054–67. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provatopoulou X, Georgiadou D, Sergentanis TN, Kalogera E, Spyridakis J, Gounaris A, et al. Interleukins as markers of inflammation in malignant and benign thyroid disease. Inflamm Res. 2014;63:667–74. doi: 10.1007/s00011-014-0739-z. [DOI] [PubMed] [Google Scholar]
- 16.Daş T, Karadayı N, Yavuzer D, Topal CS. Role of galectin-3 and CD44v6 expression in the differentiation of the follicular carcinoma and adenoma of the thyroid. Sisli Etfal Hastan Tip Bul. 2016;50:131–6. [Google Scholar]
- 17.Ghoshal A, Garmo H, Arthur R, Carroll P, Holmberg L, Hammar N, et al. Thyroid cancer risk in the Swedish AMORIS study: the role of inflammatory biomarkers in serum. Oncotarget. 2017;9:774–82. doi: 10.18632/oncotarget.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erden S, Buyukozturk S, Vural P, Değirmencioğlu S. Acute-phase reactans in Hashimoto thyroiditis. Int Immunopharmacol. 2008;8:1863–5. doi: 10.1016/j.intimp.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Calapkulu M, Sencar ME, Sakiz D, Duger H, Ozturk Unsal I, Ozbek M, Cakal E. The prognostic and diagnostic use of hematological parameters in subacute thyroiditis patients. Endocrine. 2020;68:138–43. doi: 10.1007/s12020-019-02163-w. [DOI] [PubMed] [Google Scholar]
- 20.Cengiz H, Varim C, Demirci T, Cetin S. Hemogram parameters in the patients with subacute thyroiditis. Pak J Med Sci. 2020;36:240–5. doi: 10.12669/pjms.36.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czarnywojtek A, Owecki M, Zgorzalewicz-Stachowiak M, Woliński K, Szczepanek-Parulska E, Budny B, et al. The role of serum C-reactive protein measured by high-sensitive method in thyroid disease. Arch Immunol Ther Exp (Warsz) 2014;62:501–9. doi: 10.1007/s00005-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou X, Jiang L, Chen C, Zhu X, Ge M. Different expression of erythrocyte sedimentation rate and C-reactive protein in papillary thyroid carcinoma and nodular goiter. Clin Lab. 2015;61:793–9. doi: 10.7754/clin.lab.2015.150127. [DOI] [PubMed] [Google Scholar]
- 23.Pearce EN, Bogazzi F, Martino E, Brogioni S, Pardini E, Pellegrini G, et al. The prevalence of elevated serum C-reactive protein levels in inflammatory and noninflammatory thyroid disease. Thyroid. 2003;13:643–8. doi: 10.1089/105072503322239989. [DOI] [PubMed] [Google Scholar]
- 24.Magrini E, Mantovani A, Garlanda C. The dual complexity of PTX3 in health and disease: a balancing act? Trends Mol Med. 2016;22:497–510. doi: 10.1016/j.molmed.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiraki A, Kotooka N, Komoda H, Hirase T, Oyama JI, Node K. Pentraxin-3 regulates the inflammatory activity of macrophages. Biochem Biophys Rep. 2016;5:290–5. doi: 10.1016/j.bbrep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


