Abstract
The enlargement of multinodular goiter into the mediastinum through the thoracic inlet or ectopic thyroid tissues directly in the mediastinum is defined as Substernal Goiter (SG). However, there is no clear consensus in the literature on this definition. There are many definitions for SG in the literature. Most definitions are similar or overlapping. Since the thyroid is located in the neck above the thoracic inlet in its normal anatomical position, the simplest clinical definition should be preferred among the definitions regarding its descent below the thoracic inlet and adjacent to the mediastinal organs. In the American Thyroid Association guideline, SG is defined as clinical or radiological protrusion of the thyroid gland over the sternal notch or clavicle in a patient with a slightly extended neck in the supine position. SGs can be classified as primary or secondary according to their origins. In addition, there are combined SGs resulting from the enlargement of the primary SG, which is the growth of the cervical thyroid gland toward the mediastinum, and the secondary SG, which is defined as an ectopic mediastinal mass, together. We find it appropriate to define such SGs as mixed SGs. In this disease, which has the same etiology and etiopathogenesis as cervical goiter, the descent of the thyroid gland into the mediastinum due to some anatomical factors explains the physiopathology. Compression symptoms of mediastinal major vascular structures, trachea, and esophagus cause the symptoms and findings of SGs due to its localization. In addition, the relationship of SGs with possible malignancy risk and hyperthyroidism affecting the indications and methods of treatment has been discussed for a long time. In this study, we aimed to evaluate the definitions, classification, physiopathology, laboratory and imaging methods used for diagnosis, the relationship of SG with hyperthyroidism and malignancy, and briefly the treatment methods, according to the current studies from literature.
Keywords: Definitions, diagnostic methods, physiopathology, substernal goiter, treatment
Multinodular goiter (MNG) is the most common thyroid pathology that undergoes surgical procedure.[1] MNG may enlarge from the cervical region to the mediastinum through the thoracic inlet, or rarely, ectopic thyroid tissue may grow directly in the mediastinum. This pathology is defined as substernal goiter. The incidence of substernal goiter in thyroidectomy procedures varies between 2% and 19% due to the lack of a clear consensus on the definition of this pathology.[2]
Substernal goiter is biologically a type of nodular goiter and a variant of nodular goiter.[3]
Although the term substernal goiter is frequently used in the literature, it is noteworthy that different terms are used for it and its subtypes. These are different terms such as retrosternal goiter, retroclavicular goiter, subclavicular goiter, intrathoracic goiter, cervicomediastinal goiter, mediastinal goiter, aberrant goiter, mediastinal aberrant goiter, mobile goiter, plunging goiter, wandering goiter, and spring goiter.[2-6] In the present study, substernal goiter term is used.
Definitons
There is not any uniform definition of substernal goiter with consensus in literature. In 1749, Haller was the first to anatomically describe the substernal goiter as an extension of the thyroid to the thoracic inlet.[7]
Removal of the goiter beneath the sternum was first written by Klein in 1820.[8]
It is noteworthy that more than ten substernal definitions have been made in the literature. In both the sitting and supine positions, the inferior thyroid pole is normally located cranially to the thoracic inlet. According to the clinical criteria, if there is a retrosternal part of thyroid gland in the cervical examination without hyperextension, it is defined as substernal goiter.[9] Another definition is the thyroid gland being inferior to the manubrium sterni radiologically or clinically.[10]
Kocher defined the substernal goiter as the part of the thyroid gland that remained retrosternal permanently.[11]
Substernal goiter was defined by Torre et al. as the thyroid gland with lower position permanently below the sternal notch during the hyperextension.[12]
Lahey defined a substernal goiter as a goiter requiring surgical intervention in the upper mediastinum for performing surgery.[13]
Crile described that when the thyroid grows up to the aortic posterior level, it should be considered as a true intrathoracic goiter.[14]
On the other hand, Lindskog and Goldenberg defined substernal or mediastinal goiter as the enlargement of the goiter up to or below the transverse process of the fourth thoracic vertebra on X-ray examination.[15]
Katlic et al. defined it as more than 50% of the thyroid gland mass located retrosternally.[6]
Furthermore, Netterville et al. defined it as the presence of the major part of the goiter in the mediastinum.[16]
In another definition, substernal goiter is defined as a goiter localized partially or totally in the mediastinum and descending at least 3 cm below the manubrium sterni or two fingers below the thoracic inlet in the operating position.[17]
In supine position with a little neck extension, the presence of thyroid gland in below sternal notch or below clavicula on physical examination or radiological examination is defined as substernal goiter by the American Thyroid Association Guidelines.[18]
Most of definitions are similar to each other. Thyroid gland is located in superior of thoracic inlet normally. Of the definitions related to its descent below the thoracic inlet and adjacent to the mediastinal organs, the simplest clinical use should be preferred.
Classification
The definitions about substernal goiter (SG) generally are not related about mediastinal shift, adjacence to other organs, difficulty of thyroidectomy, surgical procedure type, or risk of complication. SG could be classified as primary or secondary according to its origin (Fig. 1, Fig. 2). The secondary SG develops from cervical thyroid gland’s shift to the mediastinum, and in generally, SG develops with this way from cervical thyroid. Both SGs in anterior mediastinum or posterior mediastinum are received their blood supply from inferior and superior thyroid artery. The secondary SG could be presented in anterior mediastinum commonly, and rarely in posterior mediastinum (10–15%) (Fig. 1, Fig. 2). The primary SG is obtained from ectopic thyroid cells derived from foregut endoderm that descends into mediastinum by embryological aortic arch and not related to cervical thyroid gland. It constitutes <1% of SG and may be localized in the anterior or posterior mediastinum. The primary SG is supplied by intrathoracic arteries (Fig. 3).[19]
Figure 1.
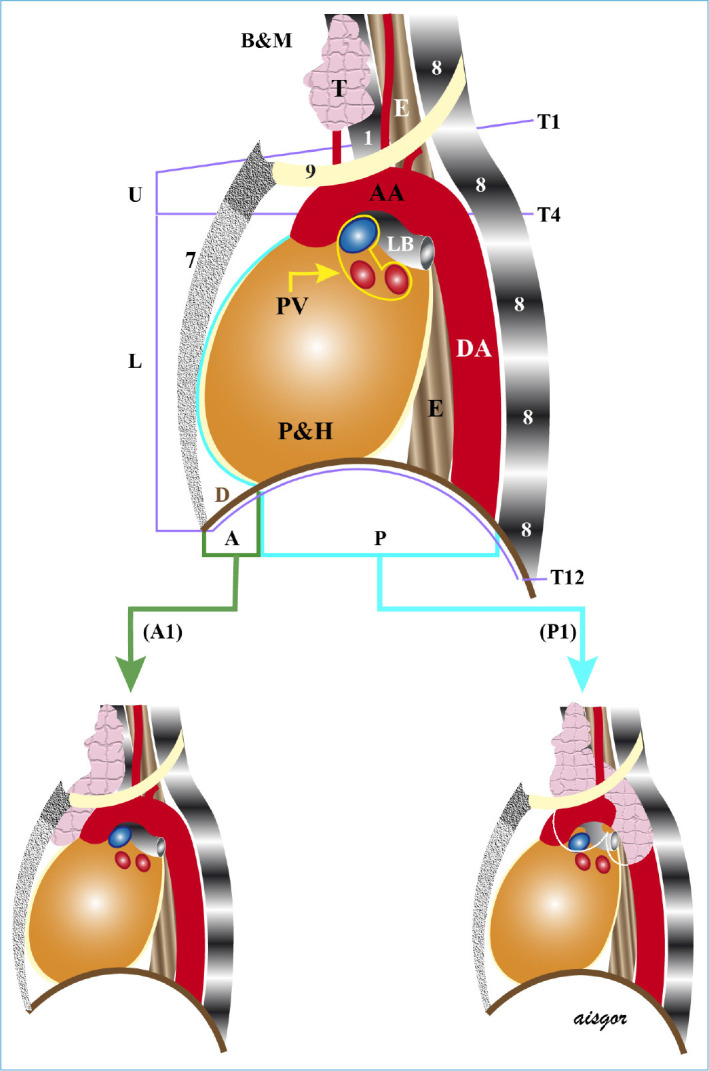
Lateral view of mediastinal parts and borders (B&M). A: Anterior mediastinum, P: posterior mediastinum, A1: anterior mediastinal subternal goiter, and P1: posterior mediastinal subternal goiter.
Figure 2.
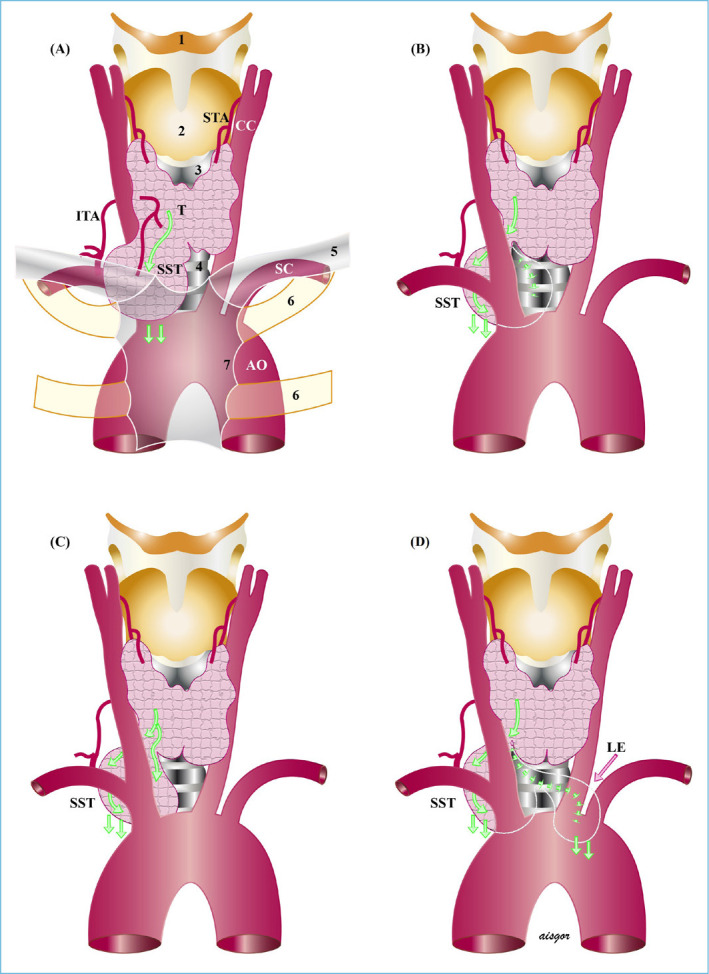
Secondary substernal goiter. (a) anterior mediastinal, (b) posterior to major vessels and trachea or esophagus, (c) posterior to major vessels, anterior to trachea, and (d) Extension to the left behind the major vessels and trachea or esophagus.
Figure 3.
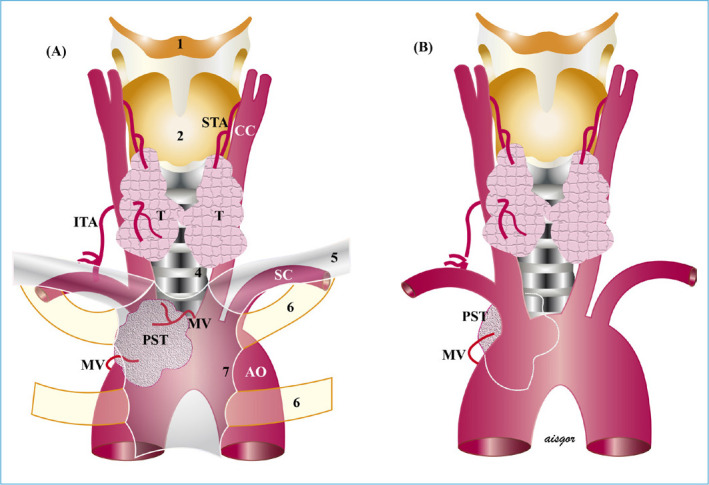
Primary substernal goiter. (a) Anterior mediastinal, (b) posterior mediastinal. It may be posterior to the major vessels, anterior to the trachea, or posterior to the major vessels and trachea.
In addition, thyroid remnants could be detected in the thyrotymic region in approximately 50% of the patients who underwent thyroidectomy, and 90% of these were smaller than 1 cm. Approximately 20% of these rests are isolated rests that are either connected to the thyroid by a thin fibrous band or are not directly connected, and nodular structures that will develop from these rests that may cause mediastinal goiter.[20]
Mediastinal masses developed from these remnants could be associated with parasitic mediastinal vessels and may be presented like primary SG. The primary SGs are generally associated with the heart and great vessels as a result of embryological development, and masses developing from such rests have no direct relationship with these structures. Combine SGs are rarely occured, through both substernal extension of cervical thyroid and enlargement of mediastinal ectopic thyroid synchronously. Our suggestion is that it would be appropriate to call this situation mixed SG (Fig. 4).
Figure 4.
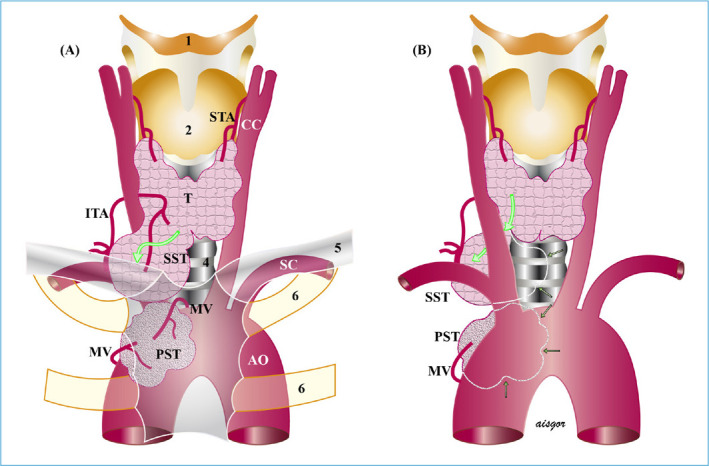
Mixed substernal goiter. (a) Anterior mediastinal, and (b) posterior mediastinal. Location is shown in Figure 2.
However, rarely, both the cervical thyroid can grow substernally and the thyrotymic rests that are not connected with the thyroid can grow synchronously. It should be taken into account that the cervical thyroid can push the thyrotymic ligament rest nodule toward the mediastinum and it may cause an appearence like mixed SGs.[21]
Huins et al. suggested classification system with three grades according to the relationship between SG and aortic arch and right atrium to predict patients may need intrathoracic interventions, preoperatively. Grade 1 SG is located above the aortic arch and can be operated by cervical incision. Grade 2 SG is located in between aortic arch and pericardium and can be operated with partially sternotomy. In addition, it is suggested that grade 3 SGs descend below the right atrium and can be operated with total sternotomy.[22]
Cohen and Cho divided SG into 4 categories (grades) according to the percentage of thyroid gland tissue in the mediastinum. If <25% of the thyroid gland tissue is in the mediastinum, it is grade 1, if 26–50% is grade 2, 51–75% is grade 3, and >75% is grade 4.[23,24]
Recently, Liddy et al. proposed a classification including anatomic features and localization in the mediastinum for safe surgery of SG. In this study, this classification will be evaluated in detail.
In this classification, types I and II are secondary SGs deriving from the cervical thyroid. Type I is anterior mediastinal growth and in this type, SG is located anterior to the recurrent laryngeal nerve (RLN), anterolateral to the trachea.[4,19]
The enlarging mass to the mediastinum is anterior to the subclavian and innominate vessels. The relationship between RLN and anterior mediastinal SG is similar like cervical goiter and the nerve is in the deep.[4]
Anterior mediastinal SGs were found to be more common on the left side. This situation could be explained by the location of anatomical structures. The ascending aorta is more anterior on the right. The ascending aorta and brachiocephalic artery show greater resistance to the growing in anterior mediastene enlargement of the thyroid on the right side. The aorta is curved posteriorly on the left and, therefore, is likely to allow the anteriorly located thyroid gland to more easily enter the anterior median on the left[25] (Table 1).
Table 1.
SG Classification
| Type | Localization | Anatomy |
|---|---|---|
| I | Anterior mediastinum | Located on anterior of the trachea, major vessels and RLN |
| II | Posterior mediastinum | Located on posterior of the trachea, major vessels and RLN |
| IIA | Ipsilateral enlargement | |
| IIB | Contralateral enlargement | |
| IIB1 | Enlargement to both posterior trachea and posterior esophagus | |
| IIB2 | Enlargement to between trachea and esophagus | |
| III | Mediastinal Goiter Only | Unrelated to the cervical thyroid |
Type 2 includes growths to the posterior mediastinum (Table 1). The SG enters posterior to the trachea, descends into the posterior mediastinum, and pushes the trachea anteriorly (Table 1). The mass first pushes the great vessels to the anterior, which, then, settles in the space of posterior of the innominate vein, carotid sheath contents, innominate and subclavian arteries, RLN, and inferior thyroid artery. It is important in posterior SGs to know the relationship between RLN and mass. RLN is located in the anterior of SGs inferior part. RLN can be stretched or even cut if not detected early. Even it could be trapped between the components of the posterior mediastinal goiter.[26]
In contrast to type I SG, type II SG is more likely to occur on the right side.[27]
Type 2 SG is classified as type 2A if the SG is derived from ipsilateral lobe and type 2B if it grows into the contralateral thorax with retrotracheal growth (Table 1). It is more common that growing into the right contralateral thorax due to the aortic arch and its branches obstructing the way to the left posterior mediastinum. It is defined as Type IIB1 if growth into the contralateral thoracic cavity in the posterior mediastinum occurs behind the trachea and esophagus, and type IIB2 if it occurs between the trachea and esophagus. In general, the caudal extension of the right thorax is limited at the level of the azygos arch.[4]
Physiopathology
The etiology and etiopathogenesis of SG are the same as cervical MNG. The etiopathogenesis of cervical MNG was presented in detail in our previous study and will not be detailed in this study.[1]
In the cervical region, there is limited anatomical space for the growth of nodular goiter. Thyroid grows toward the region, where the resistance is least. Posterior growth is limited by the trachea and vertebrae. Anterior growth is related to the resistance of the strep muscles, and the thyroid grows anteriorly according to the degree of stretching of the strep muscles and causing cervical goiter. At the level where the strep muscles can no longer stretch, the growth occurs inferiorly to the thoracic inlet and from there to the mediastinum. Because there is no anatomical structure between the inferior part of the thyroid and the thoracic inlet, there is only a thin areolar tissue.[3,23]
Traction force during swallowing, negative mediastinal pressure on inspiration, and gravity may contribute to the growth of the thyroid from the cervical region through the fascial planes into the inferior mediastinum.[3,21,23]
The short neck and strong neck muscles, along with the short cervical trachea, are additional anatomical factors for the faster growth of goiter down in patients.[28] The SG incidence is higher in individuals with short neck.[23]
Symptoms and Findings
SGs are more common in endemic areas, too. SGs grow slowly in years and, generally, were diagnosed at 5–7th decades of life.[2,29] In addition that, it can be occured in the ages between 15 and 95.[30] Female/male ratio is approximately 3/1–4/1.
Compression of adjacent structures due to long-term and progressive expansion of the SG causes symptoms and signs. It can be asymptomatic for long time. Most patients diagnosed and operated on are symptomatic. In a comparative multicentric study involving approximately 20,000 patients, patients with SG were more frequently symptomatic, with a rate of 73% in patients with SG and 29% in patients with cervical goiter.[5]
Patients with SG present with a mass in neck or with symptoms of compression of adjacent structures due to anatomic localization. About 69–97% of the patients present with cervical mass, 42–96% respiratory symptoms, 26–60% dysphagia, and 1–5% acute airway obstruction.[4]
Other less frequently reported symptoms are hoarseness, symptoms of thyrotoxicosis, and symptoms of superior vena cava syndrome.[27]
In detailed examination, one of three asymptomatic patients can be diagnosed according to their symptoms.[4]
Respiratory symptoms are the most common complaint in patients with SG. It occurs due to progressive enlargement of the goiter and airway compression and deviation.[23]
Mild symptoms are dyspnea, cough, choking sensation, exercise-induced dyspnea, and orthopnea.[27] Most of patients have chronic wheezing due to progressive tracheal compression, which is misdiagnosed as asthma.
The exercise induced dyspnea occurs when the trachea diameter is <8 mm; stridor and wheezing occur when it is <5 mm.[31] Acute airway obstruction symptoms may not occur until tracheal lümen is obstructed up to 75%.[32]
Sometimes, life-threatening acute airway obstruction may occur due to the sudden enlarging goiter mass. Acute airway obstruction is usually due to spontaneous or traumatic bleeding into the nodule, cystic degeneration. In addition, malignant degeneration, upper respiratory tract infections, and pregnancy can cause acute airway symptoms.
Pulmonary hypoperfusion, pleural effusion, decompensated right heart failure, secondary to compression of pulmonary artery, are the other rare causes which can make respiratory distress.[4]
Emergency intubation or tracheostomy may be required to secure the airway before definitive treatments for acute airway obstruction.[33] Surgery should be performed, while these patients are intubated and stable. After thyroid surgery, laryngeal examination should be performed to exclude laryngeal edema and evaluate vocal cord function before extubation.[4]
Although displacement of the esophagus is frequent, dysphagia is generally associated with retrooesophageal enlargement of the goiter. It is associated with compression of the esophagus between the trachea anteriorly and the thyroid mass posteriorly.[23]
It has been reported that dysphagia complaints in many patients improved significantly after surgery.[34]
The laryngopharyngeal reflux incidence is usually higher in SG patient and in these patients reflux should be investigated and treated specifically.[35]
Less than 5% of SG patients apply with Pemberton’s sign.[36] Pemperton’s sign is associated with moving down of SG below the thoracic inlet and an increase in transient venous obstruction. Complaints and findings may occur when the patient raises his arms, lies on his back, extends the neck, and looks to the right or left.[23] With these maneuvers in the patient; dilatation of the external jugular veins, facial flushing, sometimes cyanosis, and increased distress may occur. Temporary respiratory distress may also develops in these patients.
When the SG obstructs venous flow in thoracic inlet, it may cause superior vena cava syndrome. Clinical features are facial plethora, congestion, cyanosis, swelling of the arms, and prominent superficial chest veins for collateral drainage.[27] Superior vena cava syndrome is usually associated with malignancy. Malignancy should be excluded in these SG patients.[3,6]
Superior vena cava syndrome may cause the downhill esophageal varices which are occurred without portal hypertension. These varices are collaterals either bypass superior vena cava obstruction by azygos vein or to drain blood from head and neck to portal vein if the superior vena cava and azygos are obstructed. Bleeding from varices and hematemesis as a result of this bleeding may be occurred in these patients.[3,6]
Tracheal varices secondary to tracheal venous hypertension may rarely be occurred as a result of superior vena cava syndrome.[37]
Pain and tenderness are rare symptoms of SG and could be developed secondary to abscess or malignancy.[6] Furthermore, transient ischemik attack, cerebral edema, vocal cord paralysis due to compression of nervus recurrens, Horner syndrome, Chylothorax due to compression of ductus thoracicus, and pericardial effusion may be developed.[3,4,6]
Rarely, tracheomalacia may develop due to destruction of the tracheal rings due to SG compression. SG pressing on the trachea for more than 5 years is a possible risk factor for tracheomalacia.[2] These patients may develop progressive airway collapse after surgery.[3] It should be kept in mind that intubation trauma may develop in the posterior tracheal membranous wall during intubation in patients with SG.[4]
Substernal Goiter and Hyperthyroidism
Hyperthyroidism can be seen in substernal goiter and this entity can be a part of a spectrum which starts with subclinical hyperthyroidism. Patients may be presented without symptoms at the beginning of the follow-up and after increased autonomy of the nodules hyperthyroidism can turn into overt thyrotoxicosis.[3]
In the literature, hyperthyroidism in substernal goiter was reported between 13.1% and 28.8%.[5,38,39]
In addition, in one study, 19,662 patients were evaluated and in this study cervical goiter patients and substernal goiter patients were assessed, thyrotoxicosis was higher in the substernal goiter group compared to the cervical goiter group (28.8% vs. 16.7%, 95% CI: 1.76–2.33, p<0.001).[5]
There are several reasons that may cause increased percentage of hyperthyroidism in substernal goiter. In Graves disease, continuous stimulations may cause enlargement of thyroid gland. Autonomous growth may also be combined with autonomous function and this can cause an increased nodule size and decreased serum TSH levels.[3,40]
Hyperthyroidism can be corrected with surgery in suitable patients; however, radioiodine therapy may be helpful in elder patients has comorbidities.[41]
Substernal Goiter and Malignancy
Malignancy or suspicion of malignancy is an important indication for surgery in substernal goiter patients. Several studies have mentioned on malignancy in substernal goiter and rate of malignancy was reported as 3.7% and 23.3%.[2,5]
In a study which evaluated 19,662 goiter patients, malignancy rate was higher in substernal goiter patients compated to cervical goiter patients (10.4% vs. 23.3%, 95% CI: 2.25–3.04; p<0.001). Histologic subtypes of lesions in substernal goiter were papillary carcinoma (72.4%), follicular carcinoma (26.8%), and medullary carcinoma (0.8%). In the same study, increased sternotomy rate was observed in substernal goiter with malignancy.[5]
In substernal goiters with malignancy, findings of invasion to the trachea are important and preoperative preparation should be made such as flexible laryngoscopy and small intubation tubes. Self expandable metallic stents for trachea may be used in some cases.[3,38]
Laboratory Assessment
Thyroid function tests should be seen as the first step in SGs, as in cervical goiters. Especially, TSH and T4 levels are the most important laboratory findings. Although there are studies recommending thyroid peroxidase and anti-thyroglobulin antibody tests in the literature, there is no definite judgment for the necessity of these tests.[42] However, although there is a thyroid nodule with autonomous function, TSH value can be seen within the normal range, especially in iodine-deficient regions.
Although thyroglobulin measurement is not considered necessary in the preoperative period, calcitonin measurement may be requested in cases with suspected medullary carcinoma.[18] However, conditions such as Hashimoto’s thyroiditis, chronic kidney failure, and hypercalcemia that will cause a false positive calcitonin value should be kept in mind when this test is requested.
Imaging Methods
Chest Radiograph
SGs are mostly diagnosed by deviation or narrowing of the trachea on posteroanterior chest radiographs. Due to its frequent use and cost-effectiveness among imaging methods, incidental SG detection after PA chest radiograph is common (Fig. 5).
Figure 5.
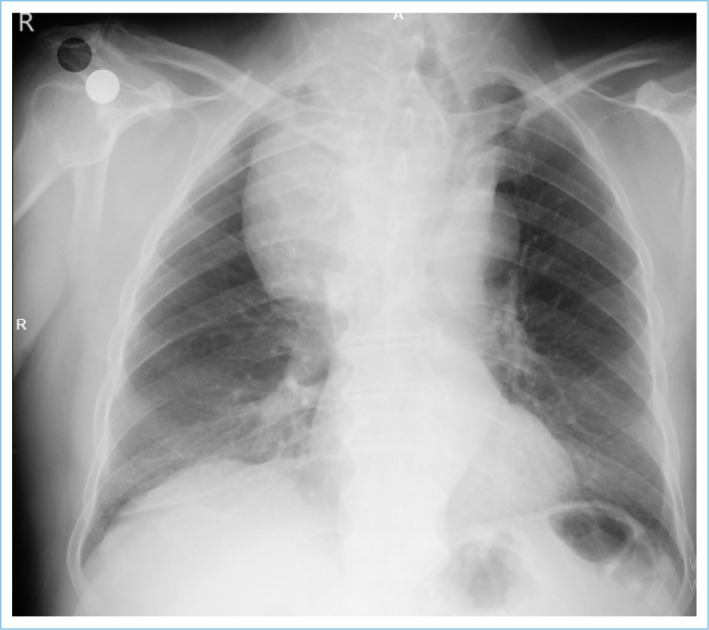
Tracheal deviation due to SG on PA chest X-ray and a mass pushing the trachea and extending to the mediastinum.
Ultrasonography (USG)
USG is used as the first-line imaging method in goiter patients in many clinics. The advantages of this method are that it is cheap, non-invasive, radiation-free, and iodine-free.[1] In addition, if suspicious nodules are detected in the cervical parts of the SGs with the USG method, fine-needle aspiration biopsy (FNAB) can be performed.[43]
Using USG has many advantages especially in cervical goiters.[1] Although the USG method is frequently used in SGs, it has some limitations. Difficulties in sonographic evaluation of retrosternal parts of SGs are one of the most important limiting factors of this method. In the cervical parts of SGs, as in normal cervical goiters, USG is one of the imaging methods frequently used in the determination of the volume and structure of the thyroid tissue, the size, shape, and composition of the nodules, possible metastatic lymph nodes. In addition, it is used as a guide method for histopathological sampling in lesions with suspected malignancy.[44]
Scintigraphy
Scintigraphy is an imaging method, especially suitable for imaging hyperfunctional thyroid tissues. In the presence of autonomic hyperfunctional nodules, only the TSH value may not always be sufficient.[3] Therefore, scintigraphy is considered the best method, especially in demonstrating the presence of an autonomous thyroid nodule.[3]
Although scintigraphy has an important role in demonstrating an ectopic thyroid focus or a hyperfunctional nodule, its sensitivity may decrease in SGs due to the superposition of intrathoracic vascular structures and sternum.[45,46] In addition, SG tissue radioiodine uptake could be low in euthyroid patients and SG may not be seen, because it remains posterior to the sternum and clavicle (Fig. 6).
Figure 6.
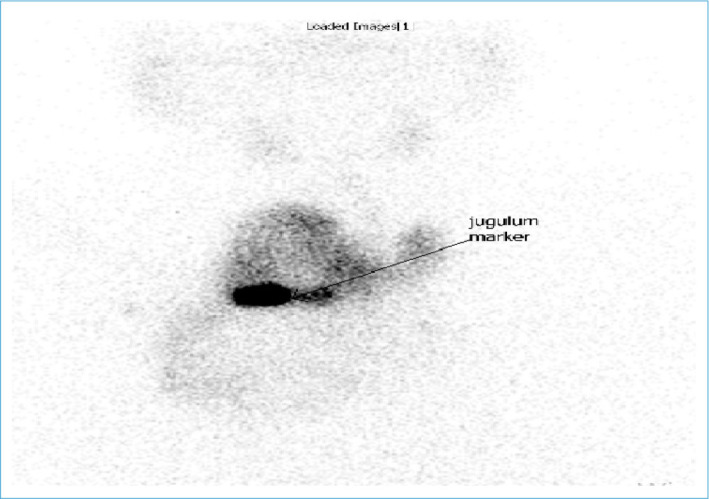
Scintigraphic imaging of SG. The retrosternal part of the thyroid gland appears to shine at a lower attenuation than the cervical part superior to the mark placed on the jugular notch.
Cross-sectional Imaging Methods
CT and MRI are imaging modalities commonly used in SGs. In the present, CT has advantages over MRI in terms of being more accessible, cheaper, easier, and being able to evaluate invasion into major vascular structures. On the other hand, the iodine content of intravenous contrast agents used in CT can cause unwanted side effects, although rarely, especially in patients with subclinical hyperthyroidism (Jod-Basedow Phenomenon).[1] In addition, the radiation exposure of the CT imaging method is also one of its disadvantages compared to MRI. Although both imaging methods are used in SG management today, CT is used more frequently (Fig. 7, Fig. 8a-c).
Figure 7.
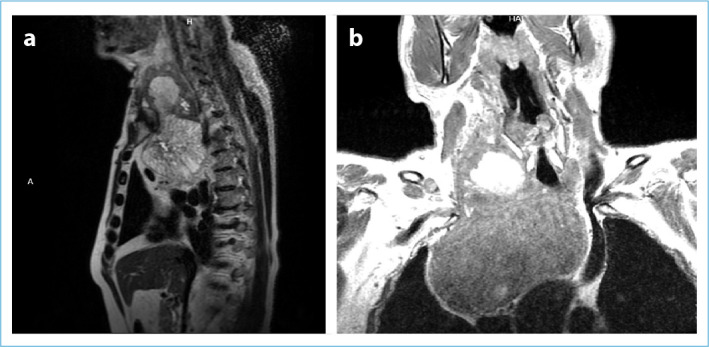
The appearance of SG in sagittal and coronal sections in MRI. (a) Sagittal sections, (b) Coronal Sections. Coronal sections show that the SG is enlarged in the pear-shaped mediastinum.
Figure 8.
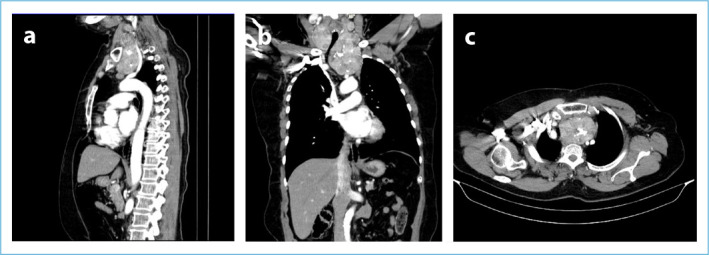
CT images with SG contrast. (a) In the sagittal section, it is seen that the anterior mediastinal SG springs the arterial structures. (b) Tracheal deviation in the coronal section again shows a relationship with the major vascular structures. (c) In the transverse section, SG deviates the trachea to the right in the thorax.
In a study by Cho et al. including 70 patients, it was emphasized that CT was the best method to show the relationship between mediastinal structures and SGs.[47] In addition, CT is of great importance in terms of predicting the need for sternotomy in the pre-operative period in SGs[38,48] (Fig. 8a-c).
FNAB
FNAB is one of the most appropriate methods in the evaluation of thyroid nodules and in the differentiation of benign/malignant nodules. However, in some studies, it has been stated that this method has a 15–30% failure rate in the diagnosis of malignancy.[49] Although the usage of FNAB in SGs is similar to cervical goiter patients, the limitations of the method are more for SG compared to cervical goiter. Even though the benefits of this method in the evaluation of the cervical region in SGs are significantly high, it is very difficult and dangerous to use in the retrosternal region. Due to the difficulty of accessing the retrosternal region with FNAB method and the complications that may develop related to intrathoracic major vascular structures and close adjacents structures, the rate of use of the method decreases in SGs. In addition, it is known that pneumothorax that may develop due to pleural damage or hemothorax that may develop due to damage to major vascular structures can be mortal. For these reasons, FNAB is not routinely used in SGs.[38]
Other Imaging Methods
The majority of SGs are also multinodular. The use of molecular tests has recently been recommended in nodules with suspicious FNAB results (Bethesda III, Bethesda IV).[50] In this way, it may even be possible to save patients from an unnecessary sternotomy. These molecular tests, which are not routinely performed, yet, because they are applied in a small number of centers and are expensive, may be one of the frequently used methods in the future.
Although its feasibility with FNAB is debatable, bronchoscopy is the only invasive method that can be used diagnostically in SGs.[51]
In addition, there are studies in the literature recommending endoscopy in the presence of symptoms of airway obstruction or in the presence of irregularities in the tracheal mucosa during bronchoscopy. Symptoms such as hoarseness, invasion of the trachea or esophagus, dyspnea, and dysphonia suggest malignancy in SGs as well as in cervical goiter.[38]
Airway obstructions, one of the most important indications of surgery in SGs, occur due to the compression of the retrosternal mass on the trachea. Narrowing due to compression of the trachea can be detected by methods such as CT and MRI. However, cross-sectional images are not sufficient to evaluate tracheal airflow. There are studies in the literature suggesting the use of the flow volume loops to adequately evaluate upper airway obstruction in SGs. Furthermore, some of these studies have reported that, especially, the inspiratory part of the flow volume loop is useful in demonstrating airway obstructions.[52] In addition, there are publications reporting that this method may be superior to conventional cross-sectional imaging methods.[53] Again in the literature, it was stated in the study of Albareda et al. that the flow volume loop is the most sensitive and effective method in showing airway obstructions.[54] These results suggest that this method can be used more frequently in the future.
Surgical Indications and Treatment
Although the surgical treatment of substernal goiter will not be examined in detail in this review, studies on the indications and the need for sternotomy will be briefly mentioned.
Surgical indications in SGs have been one of the most debatable issue from past to present. There are studies recommending surgery when detected incidentally in asymptomatic SGs in the literature.[55] In these studies, the respiratory problems that patients may experience in the future due to SG, the possible risk of malignancy, and the possibility of intubation problems in any possible surgery are shown as the necessity of the surgical method. There is a general consensus on the necessity of surgery in symptomatic patients. Surgery is preferred in respiratory and gastrointestinal compression symptoms caused by enlarged SG, compression on major vascular structures, suspected malignancy, and cosmetic problems.[18]
Although it is one of the fearful dreams of endocrine surgeons, the need for sternotomy or thoracotomy in SGs is not as high as it is thought. In fact, there are studies reporting that 95% of SGs can be completely removed with cervical incision. To prevent recurrence and treat possible malignancies, total thyroidectomy should be preferred over subtotal thyroidectomy in SGs.[56] If malignancy is suspected, in the presence of a previous cervical operation and in the presence of an extremely large retrosternal part, a sternotomy or thoracotomy may be required for complete resection.[18] Many studies have been conducted for the preoperative prediction of extracervical approaches such as sternotomy or thoracotomy. In the study of Sormaz et al., it was determined that the need for extra cervical intervention would increase significantly if the volume of the thyroid gland under the thoracic inlet, calculated with the help of tomography, was ≥162 cm3.[57]
There are also some treatment methods that have been tried and are being tried other than surgery. In the literature, there are some studies investigating levothyroxine treatment in SG patients. However, levothyroxine treatment in SG patients is still controversial. Levothyroxine treatment cannot provide sufficient reduction in thyroid volume, especially in large SGs.[58] Furthermore, this treatment could be harmful due to side effects, especially in subclinical hyperthyroidism patients.
Radioactive iodine treatment is another non-surgical alternative method considered for SG patients. The reduction in thyroid volume with radioiodine is only moderate. Furthermore, there are some concerns that radioiodine may acutely worsen obstruction or an overlooked malignancy.[3]
In our opinion, due to the side effects mentioned above, non-surgical treatment methods should only be applied to SG patients with a high risk of surgery.
Footnotes
Please cite this article as ”Unlu MT, Aygun N, Kostek M, Isgor A, Uludag M. Substernal Goiter: From Definitions to Treatment. Med Bull Sisli Etfal Hosp 2022;56(2):167–176”.
Disclosures
Peer-review:
Externally peer-reviewed.
Conflict of Interest:
None declared.
Authorship Contributions
Concept – M.T.U., M.U.; Design – M.T.U., M.K., N.A.; Supervision – A.I., M.U.; Data collection and/or processing – M.T.U., M.K.; Analysis and/or interpretation – N.A., M.U.; Literature review – M.T.U., M.U., A.I.; Writing – M.T.U., M.K.; Critical review – N.A., A.I., M.U.
References
- 1.Unlu MT, Kostek M, Aygun N, Isgor A, Uludag M. Non-toxic multinodular goiter: from etiopathogenesis to treatment. Sisli Etfal Hastan Tip Bul. 2022;56:21–40. doi: 10.14744/SEMB.2022.56514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White ML, Doherty GM, Gauger PG. Evidence-based surgical management of substernal goiter. World J Surg. 2008;32:1285–300. doi: 10.1007/s00268-008-9466-3. [DOI] [PubMed] [Google Scholar]
- 3.Knobel M. An overview of retrosternal goiter. J Endocrinol Invest. 2021;44:679–91. doi: 10.1007/s40618-020-01391-6. [DOI] [PubMed] [Google Scholar]
- 4.Liddy W, Netterville JL, Randolph GW. Surgery of cervical and substernal goiter. In: Randolph GW, editor. Surgery of the Thyroid and Parathyroid Glands. 3rd ed. Philadelphia, PA: Elsevier; 2021. pp. 53–9. [Google Scholar]
- 5.Testini M, Gurrado A, Avenia N, Bellantone R, Biondi A, Brazzarola P, et al. Does mediastinal extension of the goiter increase morbidity of total thyroidectomy? A multicenter study of 19,662 patients. Ann Surg Oncol. 2011;18:2251–9. doi: 10.1245/s10434-011-1596-4. [DOI] [PubMed] [Google Scholar]
- 6.Katlic MR, Wang CA, Grillo HC. Substernal goiter. Ann Thorac Surg. 1985;39:391–9. doi: 10.1016/s0003-4975(10)62645-8. [DOI] [PubMed] [Google Scholar]
- 7.Haller A. Disputationes anatomica selectae. Gottingen: Vandenhoeck. 1749:96. [Google Scholar]
- 8.Klein: Ueber die Austrottung verschiedener geschwiilste besonders jener der Ohrspercheldruse und der Schilddriise; Aussachalung der Schilddriise. J Chir. 1820 Augen-Heilk;120:106. [Google Scholar]
- 9.Ríos A, Rodríguez JM, Balsalobre MD, Tebar FJ, Parrilla P. The value of various definitions of intrathoracic goiter for predicting intra-operative and postoperative complications. Surgery. 2010;147:233–8. doi: 10.1016/j.surg.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Hsu B, Reeve TS, Guinea AI, Robinson B, Delbridge L. Recurrent substernal nodular goiter: incidence and management. Surgery. 1996;120:1072–5. doi: 10.1016/s0039-6060(96)80057-2. [DOI] [PubMed] [Google Scholar]
- 11.Modlin IM. Surgical triumvirate of Theodor Kocher, Harvey Cushing, and William Halsted. World J Surg. 1998;22:103–13. doi: 10.1007/s002689900357. [DOI] [PubMed] [Google Scholar]
- 12.Torre G, Borgonovo G, Amato A, Arezzo A, Ansaldo G, De Negri A, et al. Surgical management of substernal goiter: analysis of 237 patients. Am Surg. 1995;61:826–31. [PubMed] [Google Scholar]
- 13.Lahey FH. Diagnosis and management of intrathoracic goiter. JAMA. 1920;75:163–6. [Google Scholar]
- 14.Crile G. Intrathoracic goiter. Cleve Clin Q. 1939;6:313–22. [Google Scholar]
- 15.Goldenberg IS, Lindskog GE. Differential diagnosis, pathology, and treatment of substernal goiter. J Am Med Assoc. 1957;163:527–9. doi: 10.1001/jama.1957.02970420009004. [DOI] [PubMed] [Google Scholar]
- 16.Netterville JL, Coleman SC, Smith JC, Smith MM, Day TA, Burkey BB. Management of substernal goiter. Laryngoscope. 1998;108:1611–7. doi: 10.1097/00005537-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Dahan M, Gaillard J, Eschapasse H. Surgical treatment of goiters with intrathoracic development. In: Delarue NC, Eschapasse H, editors. International Trends in General Thoracic Surgery. Vol 5. Thoracic Surgery, Frontiers and Uncommon Neoplasms St. Louis; Mosby. 1989. pp. 240–6. [Google Scholar]
- 18.Chen AY, Bernet VJ, Carty SE, Davies TF, Ganly I, Inabnet WB, 3rd, et al. Surgical Affairs Committee of the American Thyroid Association American Thyroid Association statement on optimal surgical management of goiter. Thyroid. 2014;24:181–9. doi: 10.1089/thy.2013.0291. [DOI] [PubMed] [Google Scholar]
- 19.Mack E. Management of patients with substernal goiters. Surg Clin North Am. 1995;75:377–94. doi: 10.1016/s0039-6109(16)46628-4. [DOI] [PubMed] [Google Scholar]
- 20.Sackett WR, Reeve TS, Barraclough B, Delbridge L. Thyrothymic thyroid rests: incidence and relationship to the thyroid gland. J Am Coll Surg. 2002;195:635–40. doi: 10.1016/s1072-7515(02)01319-4. [DOI] [PubMed] [Google Scholar]
- 21.Uludag M, Isgor A, Yetkin G, Citgez B. Ectopic mediastinal thyroid tissue: cervical or mediastinum originated? BMJ Case Rep. 2009;2009:bcr09.2008.1004. doi: 10.1136/bcr.09.2008.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huins CT, Georgalas C, Mehrzad H, Tolley NS. A new classification system for retrosternal goitre based on a systematic review of its complications and management. Int J Surg. 2008;6:71–6. doi: 10.1016/j.ijsu.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JP, Cho HT. Surgery for substernal goiters. Oper Techn Otolaryngol Head Neck Surg. 1994;5:118–25. [Google Scholar]
- 24.Kumar A, Pulle MV, Asaf BB, Puri HV, Bishnoi S, Shah SC. Retro-sternal goitre: an overview. Indian J Surg Oncol. 2022;13:115–20. doi: 10.1007/s13193-021-01402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan A, Serpell JW. Thyroidectomy is safe and effective for retrosternal goitre. ANZ J Surg. 2006;76:238–42. doi: 10.1111/j.1445-2197.2006.03699.x. [DOI] [PubMed] [Google Scholar]
- 26.Shahian DM, Rossi RL. Posterior mediastinal goiter. Chest. 1988;94:599–602. doi: 10.1378/chest.94.3.599. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Sidhu SB. Surgical management of recurrent and retrosternal goiters. In: Clark OH, Duh QY, Kebebew E, Gosnell JE, Shen WT, editors. Textbook of Endocrine Surgery. 3rd ed. New Delhi: Jaypee Brothers Medical Publishers; 2016. pp. 585–602. [Google Scholar]
- 28.Bizakis J, Karatzanis A, Hajiioannou J, Bourolias C, Maganas E, Spanakis E, et al. Diagnosis and management of substernal goiter at the University of Crete. Surg Today. 2008;38:99–103. doi: 10.1007/s00595-006-3572-3. [DOI] [PubMed] [Google Scholar]
- 29.Zambudio AS, Sitges-Serra A. Substernal Guar. In: İşgör A, Uludag M, editors. 1st ed. Istanbul: Nobel Tıp Kitabevleri; 2013. pp. 263–74. [Google Scholar]
- 30.Hashmi SM, Premachandra DJ, Bennett AM, Parry W. Management of retrosternal goitres: results of early surgical intervention to prevent airway morbidity, and a review of the English literature. J Laryngol Otol. 2006;120:644–9. doi: 10.1017/S0022215106000995. [DOI] [PubMed] [Google Scholar]
- 31.Al-Bazzaz F, Grillo H, Kazemi H. Response to exercise in upper airway obstruction. Am Rev Respir Dis. 1975;111:631–40. doi: 10.1164/arrd.1975.111.5.631. [DOI] [PubMed] [Google Scholar]
- 32.Torres A, Arroyo J, Kastanos N, Estopá R, Rabaseda J, Agustí-Vidal A. Acute respiratory failure and tracheal obstruction in patients with intrathoracic goiter. Crit Care Med. 1983;11:265–6. doi: 10.1097/00003246-198304000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Gauger PG, Guinea AI, Reeve TS, Delbridge LW. The spectrum of emergency admissions for thyroidectomy. Am J Emerg Med. 1999;17:591–3. doi: 10.1016/s0735-6757(99)90204-8. [DOI] [PubMed] [Google Scholar]
- 34.Sabaretnam M, Mishra A, Chand G, Agarwal G, Agarwal A, Verma AK, et al. Assessment of swallowing function impairment in patients with benign goiters and impact of thyroidectomy: a case control study. World J Surg. 2012;36:1293–9. doi: 10.1007/s00268-012-1562-8. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues MG, Araujo VJF, Filho Matos LL, Hojaij FC, Simões CA, Araujo VJF Neto, et al. Substernal goiter and laryngopharyngeal reflux. Arch Endocrinol Metab. 2017;61:348–53. doi: 10.1590/2359-3997000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin JJ, Grillo HC, Mathisen D, Katlic MR, Zurakowski D, Kamani D, et al. The surgical management of goiter: Part I. Preoperative evaluation. Laryngoscope. 2011;121:60–7. doi: 10.1002/lary.21084. [DOI] [PubMed] [Google Scholar]
- 37.Lucchini R, Santoprete S, Triola R, Polistena A, Monacelli M, Avenia S, et al. Tracheal varices caused by mediastinal compression of a large intrathoracic goiter: report of a case. G Chir. 2015;36:26–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Di Crescenzo V, Vitale M, Valvano L, Napolitano F, Vatrella A, Zeppa P, et al. Surgical management of cervico-mediastinal goiters: Our experience and review of the literature. Int J Surg. 2016;28(Suppl 1):S47–53. doi: 10.1016/j.ijsu.2015.12.048. [DOI] [PubMed] [Google Scholar]
- 39.Torre G, Borgonovo G, Amato A, Arezzo A, Ansaldo G, De Negri A, et al. Surgical management of substernal goiter: analysis of 237 patients. Am Surg. 1995;61:826–31. [PubMed] [Google Scholar]
- 40.Derwahl M, Studer H. Multinodular goitre: 'much more to it than simply iodine deficiency'. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:577–600. doi: 10.1053/beem.2000.0104. [DOI] [PubMed] [Google Scholar]
- 41.Polistena A, Monacelli M, Lucchini R, Triola R, Conti C, Avenia S, et al. Surgical management of mediastinal goiter in the elderly. Int J Surg. 2014;12(Suppl 2):S148–52. doi: 10.1016/j.ijsu.2014.08.360. [DOI] [PubMed] [Google Scholar]
- 42.Brenet E, Dubernard X, Mérol JC, Louges MA, Labrousse M, Makeieff M. Assessment and management of cervico-mediastinal goiter. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134:409–13. doi: 10.1016/j.anorl.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Galiñanes EL, Caron N. Remnant large retrosternal thyroid goiter after thyroidectomy. Am Surg. 2012;78:E222–3. [PubMed] [Google Scholar]
- 44.Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH. Korean Society of Thyroid Radiology (KSThR); Korean Society of Radiology. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the korean society of thyroid radiology. Korean J Radiol. 2015;16:391–401. doi: 10.3348/kjr.2015.16.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandler MP, Patton JA, Sacks GA, Shaff MI, Kulkarni MV, Partain CL. Evaluation of intrathoracic goiter with I-123 scintigraphy and nuclear magnetic resonance imaging. J Nucl Med. 1984;25:874–6. [PubMed] [Google Scholar]
- 46.Di Crescenzo V, Laperuta P, Napolitano F, Carlomagno C, Garzi A, Vitale M. An unusual case of primary choriocarcinoma of the lung. BMC Surg. 2013;13(Suppl 2):S33. doi: 10.1186/1471-2482-13-S2-S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho HT, Cohen JP, Som ML. Management of substernal and intrathoracic goiters. Otolaryngol Head Neck Surg. 1986;94:282–7. doi: 10.1177/019459988609400304. [DOI] [PubMed] [Google Scholar]
- 48.Noma S, Kanaoka M, Minami S, Sagoh T, Yamashita K, Nishimura K, et al. Thyroid masses: MR imaging and pathologic correlation. Radiology. 1988;168:759–64. doi: 10.1148/radiology.168.3.3406406. [DOI] [PubMed] [Google Scholar]
- 49.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–65. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 50.Muzza M, Colombo C, Pogliaghi G, Karapanou O, Fugazzola L. Molecular markers for the classification of cytologically indeterminate thyroid nodules. J Endocrinol Invest. 2020;43:703–16. doi: 10.1007/s40618-019-01164-w. [DOI] [PubMed] [Google Scholar]
- 51.Blansfield JA, Sack MJ, Kukora JS. Recent experience with preoperative fine-needle aspiration biopsy of thyroid nodules in a community hospital. Arch Surg. 2002;137:818–21. doi: 10.1001/archsurg.137.7.818. [DOI] [PubMed] [Google Scholar]
- 52.Sørensen JR, Hegedüs L, Kruse-Andersen S, Godballe C, Bonnema SJ. The impact of goitre and its treatment on the trachea, airflow, oesophagus and swallowing function. A systematic review. Best Pract Res Clin Endocrinol Metab. 2014;28:481–94. doi: 10.1016/j.beem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Geraghty JG, Coveney EC, Kiernan M, O'Higgins NJ. Flow volume loops in patients with goiters. Ann Surg. 1992;215:83–6. doi: 10.1097/00000658-199201000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albareda M, Viguera J, Santiveri C, Lozano P, Mestrón A, Bengoa N, et al. Upper airway obstruction in patients with endothoracic goiter enlargement: no relationship between flow-volume loops and radiological tests. Eur J Endocrinol. 2010;163:665–9. doi: 10.1530/EJE-10-0235. [DOI] [PubMed] [Google Scholar]
- 55.Hardy RG, Bliss RD, Lennard TJ. Retrosternal goiter: the case for operation in all patients. Ann R Coll Surg Engl. 2009;91:8–9. doi: 10.1308/003588409X359196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoldas T, Makay O, Icoz G, Kose T, Gezer G, Kismali E, et al. Should subtotal thyroidectomy be abandoned in multinodular goiter patients from endemic regions requiring surgery? Int Surg. 2015;100:9–14. doi: 10.9738/INTSURG-D-13-00275.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sormaz İC, Uymaz DS, İşcan AY, Özgür İ, Salmaslıoğlu A, Tunca F, et a. The value of preoperative volumetric analysis by computerised tomography of retrosternal goiter to predict the need for an extra-cervical approach. Balkan Med J. 2018;35:36–42. doi: 10.4274/balkanmedj.2017.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol. 2014;9:S102–9. doi: 10.1097/JTO.0000000000000294. [DOI] [PubMed] [Google Scholar]


