Abstract
SARS‐CoV‐2 first raised from Wuhan City, Hubei Province in November 2019. The respiratory disorder, cough, weakness, fever are the main clinical symptoms of coronavirus disease 2019 (COVID‐19) patients. Natural Killer (NK) cells as a first defense barrier of innate immune system have an essential role in early defense against pulmonary virus. They kill the infected cells by inducing apoptosis or the degranulation of perforin and granzymes. Collectively, NK cells function are coordinated by the transmitted signals from activating and inhibitory receptors. It is clear that the cytotoxic function of NK cells is disrupted in COVID‐19 patients due to the dysregulation of activating and inhibitory receptors. Therefore, better understanding of the activating and inhibitory receptors mechanism could facilitate the treatment strategy in clinic. To improve the efficacy of immunotherapy in COVID‐19 patients, the functional detail of NK cell and manipulation of their key checkpoints are gathered in current review.
Keywords: immunotherapy, Natural Killer cells, SARS‐CoV‐2
Significance statement
Natural Killer (NK) cells as a major innate immunity compartment have a substantial role in the control of infection in coronavirus disease 2019 (COVID‐19) patients. Despite the important role of NK cells in viral diseases, the function of these cells is disrupted in COVID‐19 patients. Dysregulation of the activating and inhibitory receptors and cytokine storm in respiratory air‐way followed by accumulation of disarming NK cells, are major factors in disease severity in COVID‐19 patients. Therefore, it seems that the manipulating of immune checkpoints, the control of excessive secretion of cytokines (anticytokine therapy) and inhibitory receptors targeting by the monoclonal antibodies would be helpful to restore NK cell function
1. INTRODUCTION
In early December 2019, an outbreak of novel virus was reported in Wuhan City, Hubei Province, China. 1 Coronavirus disease 2019 (COVID‐19) pandemic led to the economic problems and disastrous deaths in both undeveloped and developing countries. Acute respiratory disorder, cough, weakness, fever, and spectrum are the main clinical features in COVID‐19 patients. In addition, sore throat, rhinorrhea, hemoptysis, lymphopenia, and diarrhea as a less common symptoms, was reported in some patients. 2 Based on clinical observations, the high mortality, disease severity, and poor clinical outcomes are commonly observed among the elderly patients with underlying disease. 3 Natural Killer (NK) cells with CD56+CD3− phenotype play a pivotal role in viral clearance and immunomodulation. 4 , 5 , 6 Moreover, based on the expression level of the CD56, two subsets of NK cells: CD56bright and CD56dim have been introduced. CD56bright with cytokine production only compose 10% of peripheral blood NK (pNK) cells, whereas CD56dim with a totally mature feature like as short telomere lengths have high cytotoxic activity and represent around 90% of pNK cells. 7 Collectively, NK cells function are regulated by the balance between their activating (NKP46, NKP30, NKP44, NKG2D, DNAM1, CD16, killer cell immunoglobulin like receptors [KIRs]) and inhibitory receptors (KIRs and NKG2A). 4 , 8 , 9 Under homeostatic conditions, healthy cells escape from NK cells killing via the inhibitory receptors–ligands interaction. It has been reported that, NK cell function is disrupted in COVID‐19 patients with dysregulation of activating and inhibitory receptors. 10 In other words, upregulation of inhibitory receptors could dampen NK cell cytotoxicity against virus. Also, the reduction of NK cell count in circulation and cytokine storm are another reason for poor treatment outcome. 11 In other word, dysregulation of NK cell receptors alter the clinical outcome in COVID‐19 patients. Therefore, it seems that the employing appropriate methods to produce large numbers of fully functional NK cells would be a plausible solution for COVID‐19 patient's treatment. Based on knowledge, the present review focused on the several important factors involved in immune cell dysfunction. In this line, manipulating of immune checkpoints, the control of excessive secretion of cytokines (anticytokine therapy), inhibitory receptors targeting by the monoclonal antibodies in COVID‐19 immunotherapy was gathered in this paper.
2. COVID‐19
SARS‐CoV‐2 which is the causal agent of COVID‐19, first emerged from China in November 2019, where it rapidly spread across the world. 12 The fever, cough, dyspnea, fatigue, and myalgia are the main clinical symptoms of COVID‐19 patients. 13 In addition, the involvement of spleen, lymph nodes, and circulating lymphocytes were observed in these patients. 14 There are several risk factors including age, gender of patients, and having an underlying disease such as diabetes, cardiovascular, or respiratory system diseases that stimulates the COVID‐19 severities. Approximately, 20% of the patients experience severe symptoms and have acute respiratory distress syndrome (ARDS). 15 Note, the virus enters the cells through the angiotensin‐converting enzyme 2 and stimulates the body's humoral and cellular immunity. Thus, the further investigation about the immune cell reaction could improve therapeutic strategies. Meanwhile, immune cell response which are mediated by virus‐specific B, T, and NK cells are the first immune system reaction against the virus. 16 Among these, NK cells have a pivotal role in early defense against pulmonary virus infections. 17 However, the reduction of circulating NK cells and high expression of their inhibitory receptors were the most important findings in COVID‐19 patients. Therefore, the further analytical and review points on NK cells function seemingly is useful for improving the clinical outcome in COVID‐19 patients.
3. NK CELLS
NK cells as granular effector cells first identified by Kiesslling and Herberman 30 years ago. 18 They are characterized by the CD56+CD3− phenotype and constitute 10%–15% of total peripheral blood leukocytes. 19 , 20 In humans, a population of CD34+ hematopoietic precursor cells develop into NK cells in lymph nodes. 21 , 22 NK cells can be developed in the presence of IL‐7, IL‐15, stem cell factor, and FLT3 ligand. Based on the sequential acquisition of NK cell specific markers, there are different stages in NK cell development. For example, CD122 positive NK cells are earliest NK cell progenitor. 23 The development stages of tissue‐resident NK Cells in healthy people are gathered recently by Hashemi and Malarkannan. 24 NK cells lyse target cells via the secretion of cytolytic granules including perforin and granzymes. 25 Collectively, NK cells' behavior against the transformed and tumor cells depends on the transmitted signals through the activating and inhibitory receptors after ligand engagement. 4 The NKG2D, DNAM1 (also known as CD226), CD16 (Fc γ‐RIIIa), C‐type lectin, UL16 (unique long 16), SLAM family receptor, and natural cytotoxicity receptors including (NKp46, NKp30, and NKp44) along with some KIRs (KIR 2DL4, 2DS1, 2DS2, 2DS3, 2DS4, 2DS5, 3DS1) are considered the major activation receptors involved in NK cytotoxicity. 8 , 9 NK cells also express inhibitory receptors including KIRs (KIR 2DL1, 2DL2, 2DL3, 2DL5, 3DL1, 3DL2, 3DL3) and NKG2A 26 that recognizes HLA class I (A, B, C) molecules on healthy cells. In summary, the high expression of activating ligands on unhealthy cells following DNA damage stimulate NK cell cytotoxicity upon the activating receptor–ligand engagement. Conversely, the interaction of inhibitory receptors with MHC class I on normal healthy cells could dampen NK cell cytotoxicity. Although NK cells play an important role in the immune surveillance, it is now appreciated that the suppressive components in the tumor microenvironment, including regulatory T cells (Treg), may limit NK‐cell efficacy in cancer patients. 27 In line with this, the researchers showed that, Treg frequencies are highly correlative with impaired NK‐cell function in patients with cancer. 28 Tregs can secrete immune suppressive cytokines (e.g., TGFb, IL10, IL35, and IL37) and express inhibitory proteins (e.g., CTLA‐4 and PD‐L1) on their cell surfaces. 29 In the other word, Treg‐mediated suppression was associated with canonical NK‐cell downregulation of TIM3, a receptor that activates NK‐cell IFN γ production, and upregulation of the NK‐cell inhibitory receptors. However, according to the other studies, a subset of NK cells, termed “adaptive” NK cells (ANK), are highly resistant to Treg‐mediated suppression. ANKs expressed a higher density of mucin‐domain containing‐3 (TIM3), which is an activating receptor on NK cells, resulting in heightened IFNg production. 30 The researchers showed that IL37 produced by Tregs led to downregulation of TIM3 expression on canonical, but not ANK. 30 According to the previous results, the development of NKG2C (C‐lectin type activating receptor) expressing ANK cells were increased following CMV exposure. 31 , 32 In this regard, high expression of NKG2C along with downregulation of NKG2A+ inhibitory NK cells were observed in heat killed Mycobacterium groups. 33 The upregulation of NKG2C stimulates cytotoxic function of ANK cells against the COVID‐19 through antibody‐86 dependent cellular myotoxicity pathway. Collectively, these results showed that ANK have the capacity to maintain normal function in the tumor microenvironment even when suppressor cells are present.
4. NK CELL DYSFUNCTION IS LINKED WITH INCREASED VIRAL SUSCEPTIBILITY
Although, the main immune effector cells in the antiviral response are NK cells, the studies have shown that NK cells cytotoxic function is disrupted in viral infections diseases by different strategies. Recent studies have shown that Cytomegalovirus (CMV) infection can modulate the expression of NKG2D ligands on the surface of infected cells. NKG2D is an activating receptor that is expressed on human and mouse NK cells. Upregulation of NKG2A 34 and downregulation of NKG2D could dampen NK cell cytotoxicity against the CMV. 35 The others showed that Poxviruses, such as molluscum contagiosum virus, evade from NK cell immune surveillance by producing the IL‐18‐binding protein homologs MC53L and MC54L. These viruses might impair NK cell function by interfering with the biological activity of host IL‐18. 36 In this line, the researchers showed that the presence of specific combination of NK cell receptors and their ligands alter the outcome of human immunodeficiency virus (HIV)‐1. 37 , 38 Therefore, dysregulation of NK cell receptors and discovering how viruses evade or modulate immune surveillance may be helpful for immune‐control mechanisms. NK cells have an essential role in control of infection in COVID‐19 patients. 11 They can kill infected viral cells by inducing apoptosis via the variety of mechanisms including perforin and granzymes degranulation. As well as, they can lyse the target cells by receptor mediated apoptosis FAS ligand and tumor necrosis factor (TNF)‐related apoptosis‐inducing ligand. 39 In addition, NK cells interaction with dendritic and T cells can help to adaptive immunity against COVID‐19 infection. 1 There is some evidence that the NK cells function is disrupted in COVID‐19 patients with dysregulation of activating and inhibitory receptors. Recurrent observation indicates that the reduction of circulating NK cells is another reason for the rapid progression of disease. 39 In this line, lower lymphocyte and NK numbers in peripheral blood was reported in COVID‐19 patients. 39 A similar reduction in NK cell counts were observed in severe COVID‐19 patients. 40 , 41 , 42 It seems that, NK cell migration to the lungs and apoptosis of them are the main reason for these reports. In support of these, low numbers of pNK cells 43 , 44 following their migration to the lungs 45 were reported in severe COVID‐19 patients. The studies on murine model indicated that the higher production of chemokines stimulates NK cells migration into the lungs at the initial phases of the COVID‐19 disease. As well as, different studies on COVID‐19 patients reported that chemokine storm can led to the penetration of immune cells into lungs. 39 Also, it has been reported that there is direct relationship between high expression of CCL3, CCL4, CXCL9, CXCL10, and CXCL11 in lungs and disease severity in COVID‐19 patients. 11 As well as, a cohort study in USA confirmed the higher concentrations of IL‐6, IL‐1Ra, CCL2, CCL8, CXCL2, CXCL8, CXCL9, and CXCL16 in COVID‐19 patients. 46 Based on the above research results and the involvement of NK cell receptors in COVID‐19 diseases, manipulating of these checkpoints through various strategies, including inhibitory receptors targeting and increasing the number of NK cells have been proposed to restore NK cell functions. The most important NK cell receptors involved in COVID‐19 diseases are listed in the following sections and Figure 1.
Figure 1.
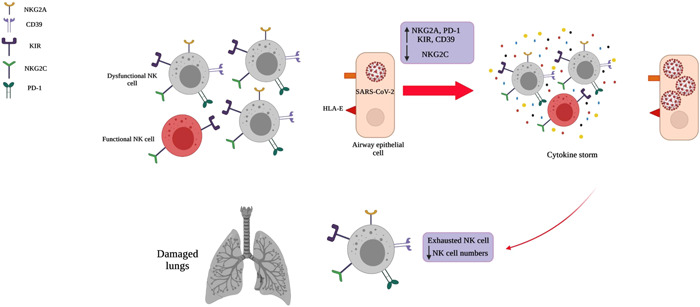
The most important factors involved in disarming of NK cells during coronavirus pathogenesis. The immune system function could dampen with accumulation of disarmed NK cells and cytokine storm in the airway microenvironment. Downregulation of activating receptor (NKG2C) along with upregulation of their inhibitory ones (NKG2A, CD39, KIR, PD‐1) is associated with exhausted phenotype of NK cells and severe lung damage. In addition, the accumulation of cytokines (cytokine storm) including TNF‐α, IL‐1, IL‐6, IL‐18, IL‐8, and IL‐10 may impede viral clearance. KIR, killer cell immunoglobulin like receptor; NK, Natural Killer.
4.1. NKG2C
As a mentioned above NK cells monitor different ligands expression on target cells with the expression of various receptors. The interaction of the ligands with their cognate receptors can either activate or inhibit NK cell mediated cytotoxicity. 47 CD94/NKG2 as a C‐type lectin‐like receptors are expressed on NK and CD8+T cells. There are five different molecular species of NKG2 including NKG2A, B, C, E, and H that related to CD94 with disulfide‐linked heterodimers. 48 Among these, the interaction of NKG2C/CD94 with HLA‐E ligand stimulate the NK cell responses against virus‐infected cells. 49 , 50 , 51 The reliable evidence indicated that there are a direct relationship between the absence of NKG2C receptor with virus infections severity. 52 The similar result was reported by Jordier et al. in 2020. They showed that the high level of HLA‐ E on the respiratory tract epithelial cells stimulate the NKG2C‐mediated cytotoxicity against COVID‐19 infected cells. 10 In this regard, the high expression of HLA‐E was reported in BAL fluid of severity COVID‐19 patients. 40 , 53 Conversely, higher frequencies of NKG2C+CD57+ CD56dim NK cells were detected in severe COVID‐19 patients. The severity of disease, age, gender of patients, having an underlying disease such as diabetes, cardiovascular, or respiratory system diseases and the sample size might affect the study results. In addition, genetic and epigenetic heterogeneity among different populations throughout the world 54 and differences in laboratory equipment and procedures are main explanation for such as conflicting results. Although, these results indicated that NKG2C+ NK cells are functionally active, further studies on NKG2C receptors to improve the efficacy of immunotherapy seemingly are required.
4.2. NKG2A
NKG2A as a c‐type lectin‐like inhibitory receptor expressed by cytotoxic T lymphocytes and NK cells. HLA‐E and Qa‐1 molecules are their ligands in humans and mice, respectively. 55 , 56 The inhibitory signaling cascade initiate upon NKG2A‐HLA‐E interaction 47 and then phosphorylation of immunoreceptor tyrosine‐based inhibitory motifs (ITIMs) by Src family kinase inhibit the NK cell cytotoxcic function. 57 The evidence indicated that, there are a direct relationship between the high level of NKG2A inhibitory receptors with functional exhaustion of NK cells in different diseases. 58 In line with this study, the upregulation of HLA‐E was reported in 50%–80% of patients with leukemia/lymphoma. 59 A recent study has revealed that high level of NKG2A in the sever COVID‐19 patients reduce NK cells function through the HLA‐E/NKG2A pathway. 58 , 60 , 61 The engagement of theses receptors with their ligands, PD‐L1 and HLA‐E inhibit the cytotoxcic function of NK cells. Interestingly, the normal cell count and low expression of NKG2A was reported in improved patients. 58 The presence of NK cells with NKG2A, PD‐1, and CD39 phenotype 62 confirmed the high expression of inhibitory receptors on NK cells in COVID‐19 patients. 63 Overall, these results lead to hypothesize that the upregulation of NKG2A and HLA‐E were associated with a poor clinical outcome in COVID‐19 patients.
4.3. CD39
CD39, a member of the ectonucleotidase triphosphate diphosphohydrolase family (ENTPD), also referred to as ENTPD‐1 (EC 3.6.1.5), is the dominant immune system ectonucleotidase that hydrolyzes extracellular adenosine triphosphate and adenosine diphosphate into adenosine monophosphate (AMP) at the sites of immune activation. CD73 is an ecto‐59‐nucleotidase (59NT) that exists in a soluble or membrane‐bound form and catalyzes the dephosphorylation of AMP to adenosine. 64 Despite the pivotal role of NK cells in infection clearance, the researchers showed that NK cell function is disrupted with high percentage of CD39‐expressing NK cell population. 63 In this line, dysregulation of CD39 on various immune cell was reported by the researcher in the viral infections. 65 , 66 , 67 In support of this, high level of IL‐6 and CD39 are seen in COVID‐19 patients. 68 It is highly possible that CD39‐based immunotherapy can be helpful to improve clinical outcome in COVID‐19 patients.
4.4. Programmed cell death protein 1 (PD‐1)
PD‐1 (also called CD279) was initially detected on T, B, and myeloid cells. 69 Although there are conflicting reports about the PD‐1 expression in human and mouse NK cells, 70 PD‐1 expression were detected on healthy individuals NK cells in the recent years. 71 , 72 The PD‐L1 and PD‐L2 are their ligands and expressed on the majority of infected and tumor cells. 73 The interaction of PD‐1–PD‐L1 inhibit NK cell‐mediated antitumor immunity. 74 The presence of NK cells expressing PD‐1 was reported in Kaposi sarcoma or ovarian carcinoma patients. 75 , 76 In this regard, the high frequency of PD‐1+ NK cells was detected in the COVID‐19 patients peripheral blood. 62 According to Terme et al. 77 study, IL‐18 stimulate PD‐1 expression on NK cells. Upon the PD‐1 receptor–ligand interaction, the tyrosine residue in ITIMs domain becomes phosphorylated by Src family kinase. 78 Then, the recruitment of Src homology domain‐containing tyrosine phosphatase (SHP‐1, SHP‐2) and de‐phosphorylation of Fyn and zeta‐chain‐associated protein kinase 70 (Zap70), and Vav1 by them inhibits the NK cell cytotoxicity 79 (Figure 2).
Figure 2.
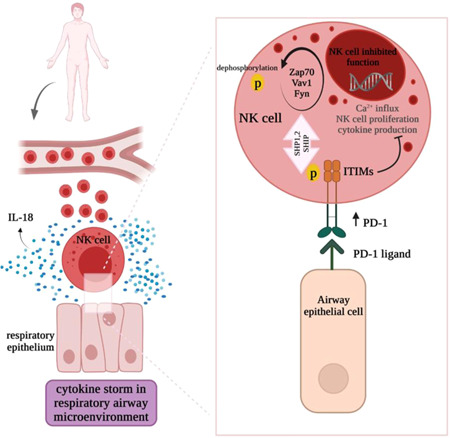
Disarming of NK cells in respiratory airway microenvironment. Over secession of IL‐18 in respiratory airway stimulate PD‐1 expression on NK cells. The interaction of inhibitory PD‐1 receptor with their cognate ligands on the epithelial cells stimulates the phosphorylation of ITIM by the Src family kinase. The phosphorylated ITIM activates the recruitment of SHP‐1, SHP‐2, and SHIP. Finally, Fyn, Zap70, and vav1 molecules dephosphorylation inhibits the Ca2+ influx, NK cell proliferation, and cytotoxic function of NK cells. ITIM, immunoreceptor tyrosine‐based inhibitory motif; NK, Natural Killer; SHIP, SH2 domain‐containing inositol‐5‐phosphatas; SHP, Src homology domain‐containing tyrosine phosphatase; Zap70, zeta‐chain‐associated protein kinase 70.
4.5. KIRs
KIRs with both activating and inhibitory function are expressed on NK cells. Notably, genetic and epigenetic heterogeneity among different populations throughout the world affected KIR expression on NK cells. 80 , 81 In addition, it has been demonstrated that KIRs and various KIR‐HLA combination affected the susceptibility or prevention the diseases. In this line, the expression of KIRs and their related ligand in different disease were summarized by Dizaji Asl et al. 47 In this section, we evaluated the KIR positive NK cells involvement in pathogenesis of COVID‐19 infection. The reliable evidence indicated that the high expression of KIR2DL1/S1 on the ARDS in COVID‐19 patients. 63 Other researcher showed that KIR2SD4 and KIR3DS1‐HLA‐B*15:01 are associated with severe and moderate COVID‐19, respectively. 82 In this line, the protective effect of KIR3DS1/Bw4 was reported against the CMV and HIV infection. 83 , 84 , 85 Collectively, these results showed that susceptibility and mortality to COVID‐19 affected with different KIRs. Furthermore, different geographical area, genetic, and epigenetic heterogeneity among different populations throughout the world affected the response to each pandemic in the various countries.
5. CYTOKINE STORM
Cytokine storm is a clear sign of immune system deviation in the effective response to infection, which occurs when the virus enters affected cells, including macrophages, forcing them to produce more and more inflammatory mediators. Such an imbalance production of inflammatory cytokines not only does not support an effective fight against the virus, but also ultimately leads to widespread inflammatory damage, especially in the lungs, which is one of the major complications of the disease and one of the leading causes of death in patients. 86 , 87 The evidence indicated that cytokine storm is another reason for the reduction of NK cell function in COVID‐19 patients. Under normal conditions, NK cells are expected to effectively destroy infected macrophages and other infected cells which are responsible for causing cytokine storms, their lower circulating counts and exhausted phenotype. 88 The studies have shown that the elevation of TNF‐α, IL‐1, IL‐6, IL‐18, IL‐8, and IL‐10 in COVID‐19 patients impaired NK‐cell cytotoxicity and leading to damage the pulmonary tissue. 89 In agreement with these results, Cifaldi et al. 90 showed that high level of IL‐6 cytokine inhibits the perforin and granzyme B expression in NK cells. IL‐6 and IL‐10 91 reduce NK cell cytotoxicity in COVID‐19 infection through the down regulation of perforin, granzyme B and IFN‐γ. 87 , 90 , 92 IFN‐α directly suppresses IFN‐γ production by NK cells. In addition, T cell activation decreases with high serum levels of IL‐6, IL‐10, and TNF‐a cytokines. 93 IL‐6 may also diminish the expression of NKG2D as an important NK cell receptor which plays a crucial role in the elimination of virally infected cells. 94 The negative effect of IL‐18 on NK cell function was reported with the other researcher. 95 Although the exact mechanism is not known, It is postulated that the cytokine storm has a negative effect on NK cell activating receptors expression. In addition, high level of cytokines in serum are inversely related to the total lymphocyte and cytotoxic T cells count. 96 Collectively, these results indicate that disarming of NK cell can lead to a critical inflammatory phenotype in COVID‐19 infection. 97 Therefore, it is conceivable that the find of the effective ways to reduction of cytokines can improve the clinical outcomes COVID‐19 patients.
6. NK CELL‐BASED IMMUNOTHERAPY
NK cell‐based immunotherapy were used in different diseases. 20 , 47 , 98 According to the above‐mentioned results, NK cells cytotoxic function are disrupted in COVID‐19 patients by the various factors. For example, the down regulation of activating receptor along with the high level of inhibitory receptors, and cytokine storm, lead to the disarming of NK cells. Hence, it seems that manipulating of these checkpoints through the various procedure, including the cytokine‐based therapy, the control of excessive secretion of cytokines (anticytokine therapy), inhibitory receptors targeting by the monoclonal antibodies, can be used to restore NK cell functions. In this regard, NK cells‐based therapy in COVID‐19 patients are gathered in the following section (Figure 3).
Figure 3.
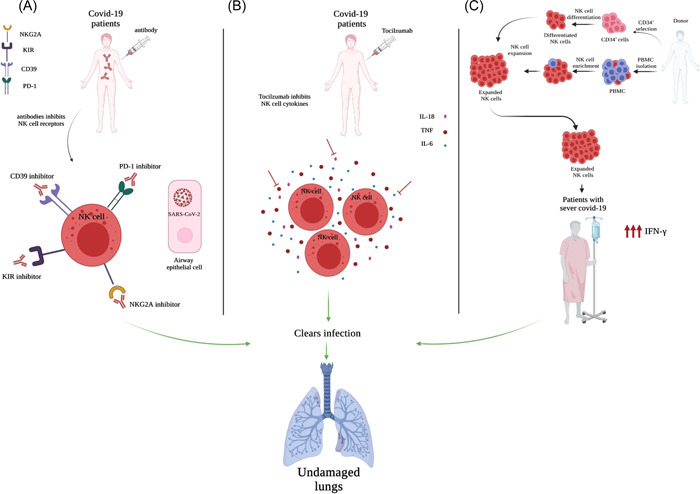
Therapeutic intervention based on the manipulation of the key checkpoints. (A) The injection of human monoclonal antibody directed against the inhibitory receptors stimulate NK cells function by inhibitory signaling pathway disruption. (B) The Tocilzumab antibody directed against IL‐8, IL‐6, and TNF enhance NK cell cytotoxicity and clear infection in SARS‐CoV‐2 patients. (C) The armed NK cells can be obtained from cord blood CD34+ and peripheral blood mononuclear cells (PBMCs) differentiation. The expansion and perfusion of differentiated NK cells clear infection in COVID‐19 patients with severe disease. NK, Natural Killer.
6.1. Inhibitory receptor blocking by specific monoclonal antibody
As previously mentioned, NK cell function are controlled by the balance between the activating and inhibitory receptors. Under homeostatic conditions, normal healthy cells escape from NK cells killing through the inhibitory receptor–ligand interaction. 99 Based on the previous studies, the invasive cells in COVID‐19 patients escape from NK cells mediated killing by the upregulation of inhibitory receptors. Therefore, it is reasonable to imagine that the inhibitory receptor blocking may improve antitumor immune response in NK cells. In support of this hypothesis, Demaria et al. 63 showed that the incubation of NK cells with monalizumab as an anti‐NKG2A mAb can improve NK cell cytotoxic functions in COVID‐19 patients. The targeting of PD‐1, 100 NKG2A, 101 and CD39 102 receptors with monoclonal antibodies have been developed for cancer therapies. POM‐1 is the most applicable antibody for targeting of CD39. The results showed that POM‐1 enhance T cells and NK cells' function, as well as decreased Treg‐mediated suppression of T cell proliferation. 103 The studies showed that PD‐1 inhibition with specific antibody are now being used to treat advanced solid tumors and improve pathogen elimination in viral infection models. 104
6.2. Cytokine‐based immunotherapy
As previously mentioned, cytokine storm lead to the disarming of NK cells in COVID‐19 patients. in this line, excessive secretion of TNF‐α, IL‐1, IL‐6, IL‐18, IL‐8, IL‐10, MCP‐1, and MIP‐1A is extremely dependent on the failure of NK cell cytotoxic function. In this regard, cytokine‐based therapy showed acceptable results in viral suppression. 105 , 106 IL6 and TNF blocking with tocilizumab antibody is another therapeutic strategy to enhanced NK‐cell functions in COVID‐19 patients. 90 Tocilizumab binds to membrane‐bound and soluble IL‐6‐Rs and showed beneficial outcomes in COVID‐19 patients hospitalized in ICU. 107 , 108 , 109 , 110 , 111 , 112 Overall, the results showed that the expression of granzyme A and perforin were increased after tocilizumab treatment. 113 The reliable evidence indicated that IL‐18 could upregulate PD‐1 expression on NK cells and stimulate NK cell disarming in a PD‐1‐dependent manner. 77 Taken together, these data indicated that blockade of these cytokines can rescue NK cells activity among COVID‐19 patients and might help to improve treatment strategies.
6.3. Ex vivo expansion of NK cells
Given that the reduction of NK cells number in COVID‐19 patients, it seems that the restoration of NK cells could maintain the normal NK cell counts in these patients. In this regard, the expansion of NK cells from various several sources, including cord blood CD34+ cells, PBMC NK cells, or NK cell lines 114 has been attention by the researchers. The acceptable results were obtained in Phases I–II trial by expansion and infusion of CD34+ derived NK cells to treat symptomatic patients with mild to moderate COVID‐19 infection. 115 Also, these promising results were supported by Phase I–II trial. In this study, primary NK cells from healthy donors transfer to COVID‐19 patients. 39 Although this procedure is useful for obtaining healthy NK cell count, the high expression of cytokines in inhibits the expanded NK cells function in infection site. Considering the cytokine storm and unsuitable microenvironment in COVID‐19 patients. Therefore, cytokine‐based immunotherapy combined with NK cell transfer may decrease disease severity and mortality rate by the partial activation of immune system.
7. CONCLUSION
NK cells as a major innate immunity compartment have a substantial role in the control of infection in COVID‐19 patients. Despite the important role of NK cells in viral diseases, the function of these cells is disrupted in COVID‐19 patients. Dysregulation of the activating and inhibitory receptors and cytokine storm in respiratory air‐way followed by accumulation of disarming NK cells, are major factors in disease severity in COVID‐19 patients. Therefore, it seems that the manipulating of immune checkpoints, the control of excessive secretion of cytokines (anticytokine therapy) and inhibitory receptors targeting by the monoclonal antibodies would be helpful to restore NK cell function.
AUTHOR CONTRIBUTIONS
Khadijeh Dizaji Asl and Ali Rafat devised the main conceptual ideas. Khadijeh Dizaji Asl, Ali Rafat, Zeinab Mazloumi, Hossein Kalarestaghi, and Shahnaz Sabetkam wrote the manuscript. Khadijeh Dizaji Asl prepared the figures. Ghazal Majidi revised the manuscript. Ali Rafat edited the manuscript for important intellectual content and supervised the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors are especially grateful to Tabriz Valiasr hospital, Tabriz University of Medical Sciences for helpful discussion and technical assistance. This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Dizaji Asl K, Mazloumi Z, Majidi G, Kalarestaghi H, Sabetkam S, Rafat A. NK cell dysfunction is linked with disease severity in SARS‐CoV‐2 patients. Cell Biochem Funct. 2022;40:559‐568. 10.1002/cbf.3725
DATA AVAILABILITY STATEMENT
NA.
REFERENCES
- 1. Bao C, Tao X, Cui W, et al. Natural killer cells associated with SARS‐CoV‐2 viral RNA shedding, antibody response and mortality in COVID‐19 patients. Exp Hematol Oncol. 2021;10(1):5. 10.1186/s40164-021-00199-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cascella M, Rajnik M, Aleem A, Dulebohn S, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID‐19). StatPearls; 2021. [PubMed] [Google Scholar]
- 3. Rafat A, Dizaji Asl K, Mazloumi Z, et al. Telomerase‐based therapies in haematological malignancies. Cell Biochem Funct. 2022;40(2):127‐140. [DOI] [PubMed] [Google Scholar]
- 4. Pende D, Falco M, Vitale M, et al. Killer Ig‐like receptors (KIRs): their role in NK cell modulation and developments leading to their clinical exploitation. Front Immunol. 2019;10:1179. 10.3389/fimmu.2019.01179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prager I, Liesche C, Van Ooijen H, et al. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J Exp Med. 2019;216(9):2113‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee S‐H, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28(6):252‐259. [DOI] [PubMed] [Google Scholar]
- 7. Fan Y‐y, Yang B‐y, Wu C‐y. Phenotypically and functionally distinct subsets of natural killer cells in human PBMCs. Cell Biol Int. 2008;32(2):188‐197. [DOI] [PubMed] [Google Scholar]
- 8. Pende D, Parolini S, Pessino A, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190(10):1505‐1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moretta A, Sivori S, Vitale M, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA‐C molecules in human natural killer cells. J Exp Med. 1995;182(3):875‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jordier F, Gras D, De Grandis M, et al. HLA‐H: transcriptional activity and HLA‐E mobilization. Front Immunol. 2020;10:2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Björkström NK, Strunz B, Ljunggren H‐G. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2021;22:112‐123. 10.1038/s41577-021-00558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shibata S, Arima H, Asayama K, et al. Hypertension and related diseases in the era of COVID‐19: a report from the Japanese Society of Hypertension Task Force on COVID‐19. Hypertension Res. 2020;43(10):1028‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: a systematic review and meta‐analysis. J Infect. 2020;80(6):656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu J, Taylor CR. Acute immunodeficiency, multiple organ injury, and the pathogenesis of SARS. Appl Immunohistochem Mol Morphol. 2003;11:281‐282. [DOI] [PubMed] [Google Scholar]
- 15. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal. 2020;10(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hammer Q, Rückert T, Romagnani C. Natural killer cell specificity for viral infections. Nature Immunol. 2018;19(8):800‐808. [DOI] [PubMed] [Google Scholar]
- 18. Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16(2):216‐229. [DOI] [PubMed] [Google Scholar]
- 19. Mandal A, Viswanathan C. Natural killer cells: in health and disease. Hematol Oncol Stem Cell Ther. 2015;8:47‐55. [DOI] [PubMed] [Google Scholar]
- 20. Asl KD, Rafat A, Movassaghpour AA, Charoudeh HN, Nasrabadi HT, Nasrabadi HT. The effect of telomerase inhibition on NK cell activity in acute myeloid leukemia. Adv Pharm Bull. Forthcoming 2021. [DOI] [PMC free article] [PubMed]
- 21. Freud AG, Becknell B, Roychowdhury S, et al. A human CD34 (+) subset resides in lymph nodes and differentiates into CD56brightNatural killer cells. Immunity. 2005;22(3):295‐304. [DOI] [PubMed] [Google Scholar]
- 22. Rafat A, Dizaji Asl K, Mazloumi Z, et al. Bone marrow CD34 positive cells may be suitable for collection after death. Transfus Apher Sci. 2022:103452. [DOI] [PubMed]
- 23. Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257‐286. [DOI] [PubMed] [Google Scholar]
- 24. Hashemi E, Malarkannan S. Tissue‐resident NK cells: development, maturation, and clinical relevance. Cancers. 2020;12(6):1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudd‐Schmidt JA, Trapani JA, Voskoboinik I. Distinguishing perforin‐mediated lysis and granzyme‐dependent apoptosis. Methods Enzymol. 2019;629:291‐306. [DOI] [PubMed] [Google Scholar]
- 26. Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol. 2019;16(5):430‐441. 10.1038/s41423-019-0206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vacchelli E, Semeraro M, Enot DP, et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post‐radiochemotherapy. Oncotarget. 2015;6(25):20840‐20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+ CD25+ T regulatory cells suppress NK cell‐mediated immunotherapy of cancer. J Immunol. 2006;176(3):1582‐1587. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarhan D, Hippen KL, Lemire A, et al. Adaptive NK cells resist regulatory T‐cell suppression driven by IL37. Cancer Immunol Res. 2018;6(7):766‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jaiswal SR, Malhotra P, Mitra DK, Chakrabarti S. Focusing on a unique innate memory cell population of natural killer cells in the fight against COVID‐19: harnessing the ubiquity of cytomegalovirus exposure. Mediterr J Hematol Infect Dis. 2020;12(1):e2020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopez‐Vergès S, Milush JM, Schwartz BS, et al. Expansion of a unique CD57+ NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108(36):14725‐14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaiswal SR, Arunachalam J, Saifullah A, et al. Impact of an immune modulator mycobacterium‐w on adaptive natural killer cells and protection against COVID‐19. medRxiv. 2021;13:887230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leong CC, Chapman TL, Bjorkman PJ, et al. Modulation of natural killer cell cytotoxicity in human cytomegalovirus infection: the role of endogenous class I major histocompatibility complex and a viral class I homolog. J Exp Med. 1998;187(10):1681‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17(1):19‐29. [DOI] [PubMed] [Google Scholar]
- 36. Xiang Y, Moss B. IL‐18 binding and inhibition of interferon γ induction by human poxvirus‐encoded proteins. Proc Natl Acad Sci USA. 1999;96(20):11537‐11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin MP, Naranbhai V, Shea PR, et al. Killer cell immunoglobulin–like receptor 3DL1 variation modifies HLA‐B* 57 protection against HIV‐1. J Clin Invest. 2018;128(5):1903‐1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin MP, Gao X, Lee J‐H, et al. Epistatic interaction between KIR3DS1 and HLA‐B delays the progression to AIDS. Nature Genet. 2002;31(4):429‐434. [DOI] [PubMed] [Google Scholar]
- 39. Ahmed F, Jo D‐H, Lee S‐H. Can natural killer cells be a principal player in anti‐SARS‐CoV‐2 immunity? Front Immunol. 2020;11(3246):586765. 10.3389/fimmu.2020.586765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maucourant C, Filipovic I, Ponzetta A, et al. Natural killer cell immunotypes related to COVID‐19 disease severity. Sci Immunol. 2020;5(50):eabd6832. 10.1126/sciimmunol.abd6832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He Z, Zhao C, Dong Q, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9(6):323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang Y, Wei X, Guan J, et al. COVID‐19 pneumonia: CD8+ T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin Immunol. 2020;218:108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Osman M, Faridi RM, Sligl W, et al. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID‐19. Blood Adv. 2020;4(20):5035‐5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruetsch C, Brglez V, Crémoni M, et al. Functional exhaustion of type I and II interferons production in severe COVID‐19 patients. Front Med. 2020;7: 603961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chua RL, Lukassen S, Trump S, et al. COVID‐19 severity correlates with airway epithelium–immune cell interactions identified by single‐cell analysis. Nature Biotechnol. 2020;38(8):970‐979. [DOI] [PubMed] [Google Scholar]
- 46. McKechnie JL, Blish CA. The innate immune system: fighting on the front lines or fanning the flames of COVID‐19? Cell Host Microbe. 2020;27(6):863‐869. 10.1016/j.chom.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dizaji Asl K, Velaei K, Rafat A, et al. The role of KIR positive NK cells in diseases and its importance in clinical intervention. Int Immunopharmacol. 2021;92: 107361. [DOI] [PubMed] [Google Scholar]
- 48. Lazetic S, Chang C, Houchins JP, Lanier LL, Phillips JH. Human natural killer cell receptors involved in MHC class I recognition are disulfide‐linked heterodimers of CD94 and NKG2 subunits. J Immunol. 1996;157(11):4741‐4745. [PubMed] [Google Scholar]
- 49. Hervier B, Russick J, Cremer I, Vieillard V. NK cells in the human lungs. Front Immunol. 2019;10:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)‐E complexed with HLA class I signal sequence–derived peptides by CD94/NKG2 confers protection from natural killer cell–mediated lysis. J Exp Med. 1998;187(5):813‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jaiswal SR, Arunachalam J, Bhardwaj A, et al. Impact of adaptive natural killer cells, KLRC2 genotype and cytomegalovirus reactivation on late mortality in patients with severe COVID‐19 lung disease. Clin Transl Immunol. 2022;11(1):e1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vietzen H, Pollak K, Honsig C, Jaksch P, Puchhammer‐Stöckl E. NKG2C deletion is a risk factor for human cytomegalovirus viremia and disease after lung transplantation. J Infect Dis. 2018;217(5):802‐806. [DOI] [PubMed] [Google Scholar]
- 53. Maucourant C, Filipovic I, Ponzetta A, et al. Natural killer cell activation related to clinical outcome of COVID‐19. medRxiv. 2020. 10.1101/2020.07.07.20148478 [DOI]
- 54. Norman PJ, Abi‐Rached L, Gendzekhadze K, et al. Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nature Genet. 2007;39(9):1092‐1099. [DOI] [PubMed] [Google Scholar]
- 55. Mamessier E, Sylvain A, Thibult M‐L, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121(9):3609‐3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yazdi MT, van Riet S, van Schadewijk A, et al. The positive prognostic effect of stromal CD8+ tumor‐infiltrating T cells is restrained by the expression of HLA‐E in non‐small cell lung carcinoma. Oncotarget. 2016;7(3):3477‐3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol. 2006;177(6):3590‐3596. [DOI] [PubMed] [Google Scholar]
- 58. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Platonova S, Cherfils‐Vicini J, Damotte D, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412‐5422. [DOI] [PubMed] [Google Scholar]
- 60. Bortolotti D, Gentili V, Rizzo S, Rotola A, Rizzo R. SARS‐CoV‐2 spike 1 protein controls natural killer cell activation via the HLA‐E/NKG2A pathway. Cells. 2020;9(9):1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell‐produced IFN‐γ and TNF‐α induce target cell cytolysis through up‐regulation of ICAM‐1. J Leukoc Biol. 2012;91(2):299‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carvelli J, Demaria O, Vély F, et al. Identification of immune checkpoints in COVID‐19. Nat Res. 2020;1(1). 10.21203/rs.3.rs-27340/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Demaria O, Carvelli J, Batista L, et al. Identification of druggable inhibitory immune checkpoints on Natural Killer cells in COVID‐19. Cell Mol Immunol. 2020;17(9):995‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bours M, Swennen E, Di Virgilio F, Cronstein B, Dagnelie P. Adenosine 5′‐triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358‐404. [DOI] [PubMed] [Google Scholar]
- 65. Kim ES, Ackermann C, Tóth I, et al. Down‐regulation of CD73 on B cells of patients with viremic HIV correlates with B cell activation and disease progression. J Leukoc Biol. 2017;101(5):1263‐1271. [DOI] [PubMed] [Google Scholar]
- 66. Schulze zur Wiesch J, Thomssen A, Hartjen P, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. 2011;85(3):1287‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tóth I, Le AQ, Hartjen P, et al. Decreased frequency of CD73+ CD8+ T cells of HIV‐infected patients correlates with immune activation and T cell exhaustion. J Leukoc Biol. 2013;94(4):551‐561. [DOI] [PubMed] [Google Scholar]
- 68. Zheng Y, Li Y, Tang B, et al. IL‐6‐induced CD39 expression on tumor‐infiltrating NK cells predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2020;69(11):2371‐2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riley JL. PD‐1 signaling in primary T cells. Immunol Rev. 2009;229(1):114‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Judge SJ, Dunai C, Aguilar EG, et al. Minimal PD‐1 expression in mouse and human NK cells under diverse conditions. J Clin Invest. 2020;130(6):3051‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pesce S, Greppi M, Tabellini G, et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J Allergy Clin Immunol. 2017;139(1):335‐346.e3. [DOI] [PubMed] [Google Scholar]
- 72. Della Chiesa M, Pesce S, Muccio L, et al. Features of memory‐like and PD‐1+ human NK cell subsets. Front Immunol. 2016;7:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Concha‐Benavente F, Srivastava RM, Trivedi S, et al. Identification of the cell‐intrinsic and‐extrinsic pathways downstream of EGFR and IFNγ that induce PD‐L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hsu J, Hodgins JJ, Marathe M, et al. Contribution of NK cells to immunotherapy mediated by PD‐1/PD‐L1 blockade. J Clin Invest. 2018;128(10):4654‐4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Beldi‐Ferchiou A, Lambert M, Dogniaux S, et al. PD‐1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget. 2016;7(45):72961‐72977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Iraolagoitia XL, Spallanzani RG, Torres NI, et al. NK cells restrain spontaneous antitumor CD8+ T cell priming through PD‐1/PD‐L1 interactions with dendritic cells. J Immunol. 2016;197(3):953‐961. [DOI] [PubMed] [Google Scholar]
- 77. Terme M, Ullrich E, Aymeric L, et al. IL‐18 induces PD‐1–dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393‐5399. [DOI] [PubMed] [Google Scholar]
- 78. Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84‐89. [DOI] [PubMed] [Google Scholar]
- 79. Matalon O, Fried S, Ben‐Shmuel A, et al. Dephosphorylation of the adaptor LAT and phospholipase C–γ by SHP‐1 inhibits natural killer cell cytotoxicity. Sci Signal. 2016;9(429):ra54. [DOI] [PubMed] [Google Scholar]
- 80. Uhrberg M, Valiante NM, Shum BP, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7(6):753‐763. [DOI] [PubMed] [Google Scholar]
- 81. Ashouri E, Farjadian S, Reed EF, Ghaderi A, Rajalingam R. KIR gene content diversity in four Iranian populations. Immunogenetics. 2009;61(7):483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bernal E, Gimeno L, Alcaraz MJ, et al. Activating killer‐cell immunoglobulin‐like receptors are associated with the severity of COVID‐19. J Infect Dis. 2021;224:229‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jackson E, Zhang CX, Kiani Z, et al. HIV exposed seronegative (HESN) compared to HIV infected individuals have higher frequencies of telomeric killer immunoglobulin‐like receptor (KIR) B motifs; contribution of KIR B motif encoded genes to NK cell responsiveness. PLoS One. 2017;12(9):e0185160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ivarsson MA, Michaëlsson J, Fauriat C. Activating killer cell Ig‐like receptors in health and disease. Front Immunol. 2014;5:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stern M, Hadaya K, Hönger G, et al. Telomeric rather than centromeric activating KIR genes protect from cytomegalovirus infection after kidney transplantation. Am J Transplant. 2011;11(6):1302‐1307. [DOI] [PubMed] [Google Scholar]
- 86. Pedersen SF, Ho Y‐C. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130(5):2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS. Intrahepatic IL‐10 maintains NKG2A+ Ly49− liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184(5):2693‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wilk AJ, Rustagi A, Zhao NQ, et al. A single‐cell atlas of the peripheral immune response in patients with severe COVID‐19. Nature Med. 2020;26(7):1070‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cifaldi L, Prencipe G, Caiello I, et al. Inhibition of natural killer cell cytotoxicity by interleukin‐6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 2015;67(11):3037‐3046. [DOI] [PubMed] [Google Scholar]
- 91. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID‐19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Szkaradkiewicz A, Karpiński TM, Drews M, Borejsza‐Wysocki M, Majewski P, Andrzejewska E. Natural killer cell cytotoxicity and immunosuppressive cytokines (IL‐10, TGF‐1) in patients with gastric cancer. J Biomed Biotechnol. 2010;2010:901564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chiappelli F, Khakshooy A, Greenberg G. CoViD‐19 immunopathology and immunotherapy. Bioinformation. 2020;16(3):219‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ge X, Li CR, Yang J, Wang GB. Aberrantly decreased levels of NKG 2D expression in children with Kawasaki disease. Scand J Immunol. 2013;77(5):389‐397. [DOI] [PubMed] [Google Scholar]
- 95. Shibatomi K, Ida H, Yamasaki S, et al. A novel role for interleukin‐18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis Rheum. 2001;44(4):884‐892. [DOI] [PubMed] [Google Scholar]
- 96. Caso F, Costa L, Ruscitti P, et al. Could Sars‐coronavirus‐2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5): 102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Osman MS, van Eeden C, Tervaert JWC. Fatal COVID‐19 infections: is NK cell dysfunction a link with autoimmune HLH? Autoimmun Rev. 2020;19(7): 102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rafat A, Dizaji Asl K, Mazloumi Z, et al. Telomerase inhibition on acute myeloid leukemia stem cell induced apoptosis with both intrinsic and extrinsic pathways. Life Sci. 2022;295: 120402. [DOI] [PubMed] [Google Scholar]
- 99. Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu JX, Hodge JP, Oliva C, Neftelinov ST, Hubbard‐Lucey VM, Tang J. Trends in clinical development for PD‐1/PD‐L1 inhibitors. Nat Rev Drug Discov. 2020;19(3):163‐164. [DOI] [PubMed] [Google Scholar]
- 101. André P, Denis C, Soulas C, et al. Anti‐NKG2A mAb is a checkpoint inhibitor that promotes anti‐tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731‐1743.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Perrot I, Michaud H‐A, Giraudon‐Paoli M, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019;27(8):2411‐2425.e9. [DOI] [PubMed] [Google Scholar]
- 103. Bastid J, Regairaz A, Bonnefoy N, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res. 2015;3(3):254‐265. [DOI] [PubMed] [Google Scholar]
- 104. Rao M, Valentini D, Dodoo E, Zumla A, Maeurer M. Anti‐PD‐1/PD‐L1 therapy for infectious diseases: learning from the cancer paradigm. Int J Infect Dis. 2017;56:221‐228. [DOI] [PubMed] [Google Scholar]
- 105. Vaninov N. In the eye of the COVID‐19 cytokine storm. Nat Rev Immunol. 2020;20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Panigrahy D, Gilligan MM, Huang S, et al. Inflammation resolution: a dual‐pronged approach to averting cytokine storms in COVID‐19? Cancer Metastasis Rev. 2020;39(2):337‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2‐mesenchymal stem cells improves the outcome of patients with COVID‐19 pneumonia. Aging Dis. 2020;11(2):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin‐8 (IL‐8) in acute inflammation. J Leukoc Biol. 1994;56(5):559‐564. [PubMed] [Google Scholar]
- 110. Schulert GS, Yasin S, Carey B, et al. Systemic juvenile idiopathic arthritis–associated lung disease: characterization and risk factors. Arthritis Rheum. 2019;71(11):1943‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Michot J‐M, Albiges L, Chaput N, et al. Tocilizumab, an anti‐IL‐6 receptor antibody, to treat COVID‐19‐related respiratory failure: a case report. Ann Oncol. 2020;31(7):961‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Campochiaro C, Della‐Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID‐19 patients: a single‐centre retrospective cohort study. Eur J Intern Med. 2020;76:43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mazzoni A, Salvati L, Maggi L, et al. Impaired immune cell cytotoxicity in severe COVID‐19 is IL‐6 dependent. J Clin Invest. 2020;130(9):4694‐4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discovery. 2020;19(3):200‐218. [DOI] [PubMed] [Google Scholar]
- 115. Casper C, Groysman L, Malhotra V, et al. Abstract CT201: early report of a phase I/II study of human placental hematopoietic stem cell derived natural killer cells (CYNK‐001) for the treatment of adults with COVID‐19 (NCT04365101). Am Assoc Cancer Res. 2021;81:CT201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA.


