Abstract
Vaccinations against the severe acute respiratory syndrome coronavirus 2 which causes COVID‐19 have been administered worldwide. We aimed to investigate associations of COVID‐19 vaccination with the occurrence of Guillain‐Barré syndrome (GBS). We explored potential safety signals regarding the development of GBS using disproportionality analyses to compare COVID‐19 vaccination with all adverse drug reaction (ADR) reports and influenza vaccines reported to VigiBase. As of October 15, 2021, a total of 2163 cases (0.13%) of GBS and its variants (including 46 cases of Miller‐Fisher syndrome and 13 cases of Bickerstaff's encephalitis) were identified in entire ADR database after vaccination with the ChAdOx1 nCoV‐19 (AstraZeneca, Cambridge, UK) or the two messenger RNA‐based COVID‐19 (BNT162b2; Pfizer and BioNTech) or mRNA‐1273; Moderna) vaccines. The median time to onset of GBS after vaccination was around 2 weeks. The ChAdOx1 nCoV‐19 and two messenger RNA‐based COVID‐19 vaccines demonstrated a higher risk for GBS against entire database (information component [IC]025 = 1.73 reporting odds ratio [ROR]025 = 3.51; IC025 = 1.07, ROR025 = 2.22, respectively). When compared with influenza vaccines, neither the ChAdOx1 nCoV‐19 nor mRNA‐based vaccines were found to be associated with greater risks of GBS (IC025 = −1.84, ROR025 = 0.11; IC025 = −1.86, ROR025 = 0.06, respectively). Although potential safety signals associated with GBS COVID‐19 vaccines have been identified, the risk of GBS from COVID‐19 vaccines were low and did not surpass those of influenza vaccines; however, because of the heterogeneity of the sources of information in the WHO pharmacovigilance database, further epidemiological studies are warranted to confirm these observations.
Keywords: COVID‐19, Guillain‐Barré syndrome, Guillain‐Barré syndrome variants, SARS‐CoV‐2, vaccination
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which causes coronavirus disease 2019 (COVID‐19), is still spreading around the world. Although the severity decreased after the omicron variation became the main type, people with comorbidities still require respiratory care or may even die. 1 , 2 , 3 , 4 Accordingly, achieving herd immunity is important to limit COVID‐19 cases as much as possible, and vaccination is one available strategy to attain herd immunity. 5 At this time, the available COVID‐19 vaccines include messenger RNA (mRNA)‐based vaccines, recombinant adenoviral vector vaccines, and inactivated whole‐virus vaccines. Among these, a recombinant adenoviral vector vaccine (ChAdOx1 nCoV‐19; AstraZeneca) and two mRNA‐based vaccines, BNT162b2 (Pfizer and BioNTech) and mRNA‐1273 (Moderna) have been widely used worldwide. 6 , 7
Guillain‐Barré syndrome (GBS) is the most frequent cause of acute flaccid paralysis worldwide, with an incidence of 0.44 to 1.91 cases per 100 000 person‐years. 8 , 9 , 10 Immune‐mediated attacks on myelin and axons (nodes and paranodes) are key mechanisms for GBS, and vaccines had been suggested as possible immune‐mediated triggers of GBS. 10 In 1976, the H1N1 influenza (swine flu) vaccine was reported to increase the risk of GBS (roughly one GBS case per 100 000 doses), leading to global monitoring of vaccination‐related GBS. 11 However, follow‐up studies of other influenza and rabies vaccines failed to reveal any association between GBS and those vaccines, which make the issue controversial. 12
Recently, several case reports of new‐onset GBS after COVID‐19 vaccination have been reported. 6 , 13 , 14 , 15 , 16 , 17 One hundred and thirty presumptive GBS cases following Ad26.CoV2.S (Janssen/Johnson & Johnson) vaccination have been reported in the US Vaccine Adverse Event Reporting System (VAERS). 18 The European Medicines Agency announced a possible causal relationship between ChAdOx1 nCoV‐19 vaccination and GBS. 19 Therefore, there are potential concerns about whether the COVID‐19 vaccine increases the risk of GBS, and these concerns may be one of the factors hindering the general public's acceptance of COVID‐19 vaccination.
Because most of the relevant reports are single cases or case series, it is difficult to determine the relationship between COVID‐19 vaccines and GBS. Furthermore, the aforementioned GBS reports in VAERS dealt only with specific vaccine, and other types of vaccines, such as the ChAdOx1 nCoV‐19, were not included. For these reasons, it is desirable to evaluate the association of COVID‐19 vaccination with the occurrence of GBS in the “real‐world.” We hypothesized that COVID‐19 vaccines would be associated with an increased risk of GBS. Here, therefore, we conducted a disproportionality analysis to assess GBS as a potential safety concern associated with the BNT162b2, mRNA‐1273, and ChAdOx1 nCoV‐19 vaccines using VigiBase, the World Health Organization's (WHO) global pharmacovigilance database of case safety reports.
2. MATERIALS AND METHODS
2.1. Study design and data sources
Disproportionality analyses of adverse drug reactions (ADRs) resulting from BNT162b2, mRNA‐1273, or ChAdOx1 nCoV‐19 vaccination was conducted using personal case safety reports from VigiBase, the WHO's global deduplicated database, which includes reports in more than 130 nations. 20 , 21 , 22 VigiBase is operated by the Uppsala Monitoring Centre (UMC) and has gathered the details of suspected medication‐related ADRs from the national pharmacovigilance centers of each participating country since 1967. This information originates from many different sources, including patients, physicians, pharmaceutical companies, healthcare professionals, and post‐marketing surveillance studies. Our study used the anonymized database and approved by the institutional review board of the studied center (EUMC‐2021‐08‐021).
2.2. Procedures
Our study was conducted in the format of an observation case control study. We extracted all cases of GBS reported as ADR resulting from BNT162b2, mRNA‐1273, or ChAdOx1 nCoV‐19 vaccination by October 15, 2021, in VigiBase using the following Medical Dictionary for Drug Regulatory Activities (MedDRA) preferred terms (PTs). 21 , 22 , 23 We defined the GBS as narrow definitions: “Guillain‐Barre syndrome” and as broad definitions: “Guillain‐Barre syndrome,” “Miller‐Fisher syndrome,” “Acute motor‐sensory axonal neuropathy,” “Acute motor axonal neuropathy,” “Bickerstaff's encephalitis.” Data on sex, age group, type of vaccination, reporting nation, time to onset of GBS, outcomes (seriousness and final) were acquired. Serious ADRs were defined as causing significant disability, hospitalization, life‐threatening conditions, or death in accordance with the ICH E2B criteria. 24 Final outcomes were categorized as recovered, recovered with sequelae, recovering, not recovered, death, or unknown. 24 Sequelae were defined as any permanent injuries or complications that resulted from the ADR. 24 For the sensitivity analysis for GBS, two separate analyses were performed using PTs for GBS (narrow definition) and using PTs for GBS, including their variants (broad definition). Among the reported ADRs, cases of GBS that were highly likely to be associated with BNT162b2, mRNA‐1273, and ChAdOx1 nCoV‐19 (symptoms occurring within 6 weeks after vaccination, a cut‐off period generally considered to have a vaccine‐related temporal relationship) were included in the analyses. 25
2.3. Disproportionality analyses
Disproportionality is analyzed by estimating the information component (IC) or reporting the odds ratio (ROR) by setting the entire database or influenza vaccines as a comparison. The IC is a logarithmic measure of the strength of the association between drug administration and ADRs. Detailed methods to calculate the IC value have been described in previous reports. 21 , 22 , 26 , 27 , 28 To obtain IC values, the entire VigiBase database or all drugs in the category should be defined as the denominator, and data comparisons between drugs or between vaccines cannot be completed. 26 , 27 , 28 We used Bayesian confidence propagation neural network, which was validated by UMC, to calculate IC. 20 , 26 In this way, ADR signals of a selected drug can be compared to those of entire database (including all vaccines and medications) or selected drug class (eg, influenza vaccines) that was expected to have no association with events. The IC025 indicated lower boundary of the 95% confidence interval (CI) of IC. The IC025 value more than 0 was considered statistically significant and regarded to have difference in ADR signal between the two comparison subjects. 29 We also estimated the ROR values for sensitivity analysis. 30 The lower boundary of the 95% CI of ROR (= ROR025) was also evaluated. A ROR025 value more than 1 was considered to have significant difference in ADR signal between the two comparison subjects. 31 Subgroup analyses (mRNA‐based COVID‐19 vaccines and the ChAdOx1 nCoV‐19 vaccine) were further performed. The R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and SAS 9.4 version (SAS Inc, Cary, NC) software programs were used for statistical analyses. Qualitative variables were presented as frequency (%) values, and quantitative variables were presented as median (interquartile range [IQR]) values. The time to the onset and the outcomes of GBS were compared between each vaccine type using the Kruskal‐Wallis test with a subsequent Mann‐Whitney U test. Bonferroni correction was performed for multiple comparisons and P values were adjusted by multiplying by 3, which was the number of comparisons. Adjusted P values less than .05 were considered significant. The odds ratio was calculated using χ 2 tests to determine whether each type of vaccine was a protective or risk factor for mortality or residual neurological sequelae of GBS. Reports with missing outcomes data were excluded from the analyses.
3. RESULTS
As of October 15, 2021, there had been a total of 27 370 413 ADRs reported to VigiBase. Of these, 1 731 147 were ADR reports involving COVID‐19 vaccines (including 1 154 023 cases associated with mRNA‐based vaccination [BNT162b2 and mRNA‐1273] and 577 124 cases associated with ChAdOx1 nCoV‐19 vaccination). Among all ADR reports associated with COVID‐19 vaccines (BNT162b2, mRNA‐1273, and ChAdOx1 nCoV‐19), we found a total of 2163 (0.13%) cases of GBS and its variants consistent with the broad definition, including 2075 cases of GBS, 46 cases of Miller‐Fisher syndrome, 15 cases of acute motor‐sensory axonal neuropathy, 14 cases of acute motor axonal neuropathy, and 13 cases of Bickerstaff's encephalitis. Among these GBS ADR cases, 919 reports were from patients who received BNT162b2, 280 were from patients who received mRNA‐1273, and 964 were from patients who received ChAdOx1 nCoV‐19 (Table 1).
TABLE 1.
Characteristics of COVID‐19 vaccine‐related Guillain‐Barré syndrome cases reported in the WHO Pharmacovigilance Database
| GBS—narrow definition (n = 2075) | GBS—broad definition (n = 2163) | |||||
|---|---|---|---|---|---|---|
| ChAdOx1 nCoV‐19 (n = 927) | BNT162b2 (n = 884) | mRNA‐1273 (n = 264) | ChAdOx1 nCoV‐19 (n = 964) | BNT162b2 (n = 919) | mRNA‐1273 (n = 280) | |
| Age, years | ||||||
| ≤11 | 0 (0.0) | 2 (0.2) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 0 (0.0) |
| 12‐17 | 0 (0.0) | 17 (1.9) | 0 (0.0) | 0 (0.0) | 17 (1.8) | 0.0 (0.0) |
| 18‐44 | 123 (13.3) | 204 (23.1) | 64 (24.2) | 132 (13.7) | 215 (23.4) | 66 (23.6) |
| 45‐64 | 487 (52.5) | 195 (22.1) | 98 (37.1) | 502 (52.1) | 201 (21.9) | 107 (38.2) |
| 65‐74 | 158 (17.0) | 128 (14.5) | 52 (19.7) | 167 (17.3) | 132 (14.4) | 55 (19.6) |
| ≥75 | 77 (8.3) | 118 (13.3) | 41 (15.5) | 78 (8.1) | 122 (13.3) | 43 (15.4) |
| Unknown | 82 (8.8) | 220 (24.9) | 9 (3.4) | 85 (8.8) | 230 (25.0) | 0 (0.0) |
| Sex | ||||||
| Male | 487 (52.5) | 428 (48.4) | 133 (50.4) | 508 (52.7) | 449 (48.9) | 141 (50.4) |
| Female | 419 (45.2) | 455 (51.5) | 131 (49.6) | 435 (45.1) | 469 (51) | 139 (49.6) |
| Unknown | 21 (2.3) | 1 (0.1) | 0 (0.0) | 21 (2.2) | 1 (0.1) | 0 (0.0) |
| Continentals | ||||||
| Africa | 4 (0.4) | 1 (0.1) | 0 (0.0) | 4 (0.4) | 1 (0.1) | 0.0 (0.0) |
| Americas | 76 (8.2) | 584 (66.1) | 229 (86.7) | 77 (8.0) | 604 (65.7) | 241(86.1) |
| Asia | 12 (1.3) | 3 (0.3) | 0.0 (0.0) | 12 (1.2) | 3 (0.3) | 0 (0.0) |
| Europe | 726 (78.3) | 285 (32.2) | 35 (13.3) | 762 (79) | 300 (32.6) | 39 (13.9) |
| Oceania | 109 (11.8) | 11 (1.2) | 0 (0.0) | 109 (11.3) | 11 (1.2) | 0 (0.0) |
| Seriousness | ||||||
| Yes | 875 (94.4) | 743 (84.0) | 226 (85.6) | 907 (94.1) | 776 (84.4) | 240 (85.7) |
| No | 52 (5.6) | 141 (16.0) | 38 (14.4) | 57 (5.9) | 143 (15.6) | 40 (14.3) |
| Outcome | ||||||
| Recovered | 53 (5.7) | 170 (19.2) | 49 (18.6) | 56 (5.8) | 175 (19.0) | 52 (18.6) |
| Recovered with sequelae | 58 (6.3) | 133 (15.0) | 77 (29.2) | 60 (6.2) | 138 (15.0) | 85 (30.4) |
| Recovering | 389 (42.0) | 378 (42.8) | 121 (45.8) | 402 (41.7) | 396 (43.1) | 125 (44.6) |
| Not recovered | 385 (41.5) | 159 (18.0) | 15 (5.7) | 402 (41.7) | 166 (18.1) | 16 (5.7) |
| Death | 12 (1.3) | 28 (3.2) | 2 (0.8) | 13 (1.3) | 28 (3.0) | 2 (0.7) |
| Unknown | 30 (3.2) | 16 (1.8) | 0 (0.0) | 31 (3.2) | 16 (1.7) | 0 (0) |
Note: Data are presented as number (%). Seriousness indicates adverse drug reaction that causes significant disability, hospitalization, life‐threatening, and fatal. Sequelae was defined as any permanent injuries or complications that resulted from the ADR.
The ADR of GBS (defined both by the broad and narrow definitions) associated with ChAdOx1 nCoV‐19 or mRNA‐1273 vaccination were reported to be most frequent in the 45 to 64 years age group. In the case of BNT162b2, the most frequently affected age group was those aged 18 to 44 years, closely followed by those aged 45 to 64 years. The region with the most reports of GBS related to ChAdOx1 nCoV‐19 was Europe, whereas that with the most reports of GBS related to BNT162b2 or mRNA‐1273 was the Americas.
The median time to the onset of GBS as defined by the broad definition was 15 days (IQR, 11‐20 days) for ChAdOx1 nCoV‐19, 13 days (IQR, 4‐20 days) for BNT162b2, and 12 days (IQR, 8‐22 days) for mRNA‐1273 (adjusted P < .0001 for the Kruskal‐Wallis test; ChAdOx1 nCoV‐19 vs BNT162b2, adjusted P < .0001 and ChAdOx1 nCoV‐19 vs mRNA‐1273, adjusted P = .0006; otherwise all adjusted P values were non‐significant) (Figure 1). The median time to the onset of GBS defined by the narrow definition was 15 days (IQR, 11‐20 days) for ChAdOx1 nCoV‐19, 13 days (IQR, 4‐20 days) for BNT162b2, and 12 days (IQR, 8‐22 days) for mRNA‐1273 (adjusted P < .0001 for the Kruskal‐Wallis test; ChAdOx1 nCoV‐19 vs BNT162b2, adjusted P < .0001 and ChAdOx1 nCoV‐19 vs mRNA‐1273, adjusted P = .0053; otherwise all adjusted P values were non‐significant). The time to the onset of GBS among patients who developed the disease within 6 weeks after vaccination is illustrated in Figure 1 for each vaccine type. Differences in outcomes, including mortality and the development of persistent neurological sequelae (not recovered, recovered with sequelae and death), between each vaccine group are presented in Table 2. More than one third of patients who developed GBS had not fully recovered at their last follow‐up visit, and neurological sequelae appeared to occur more frequently in those who received ChAdOx1 nCoV‐19 than either of the mRNA‐based vaccines. However, the mortality rate of BNT162b2 vaccination was significantly higher than that of ChAdOx1 nCoV‐19 and mRNA‐1273 vaccination among patients who developed GBS as defined by both definitions.
FIGURE 1.
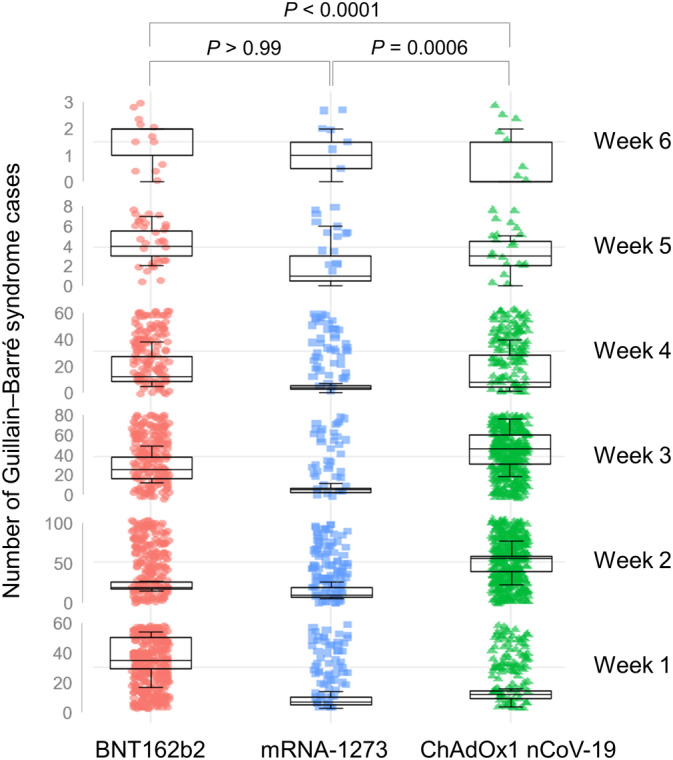
The time interval from vaccination to the onset of neurological symptoms of Guillain‐Barré syndrome. Only data from patients who developed the disease within 6 weeks of vaccination were used for analyses. The median time to the onset of Guillain‐Barré syndrome (defined by broad definitions) was around 2 weeks from vaccination. Box plot indicates median number of Guillain‐Barré syndrome cases (one case per filled point) and interquartile range per week depending on the type of vaccine. The time to onset of Guillain‐Barré syndrome in patients who were vaccinated with ChAdOx1 nCoV‐19 was significantly prolonged relative to that among those who received BNT162b2 or mRNA‐1273
TABLE 2.
Outcome of Guillain‐Barré syndrome developed after COVID‐19 vaccination
| ChAdOx1 nCoV‐19 | BNT162b2 | mRNA‐1273 | P value | ChAdOx1 nCoV‐19 vs BNT162b2 | ChAdOx1 nCoV‐19 vs mRNA‐1273 | BNT162b2 vs mRNA‐1273 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | Odds ratio (95% CI) | Adjusted P value* | Odds ratio (95% CI) | Adjusted P value* | Odds ratio (95% CI) | Adjusted P value* | ||
| Mortality of GBS | ||||||||||
| Narrow definition (n = 2029) | 12 (1.34%) | 28 (3.23%) | 2 (0.76%) | .01 | 0.41 (0.21‐0.79) | .02 | 1.78 (0.45‐8.02) | >.99 | 4.37 (1.13‐18.73) | .04 |
| Broad definition (n = 2116) | 13 (1.39%) | 28 (3.10%) | 2 (0.71%) | .01 | 0.44 (0.22‐0.83) | .03 | 1.96 (0.52‐8.80) | >.99 | 4.45 (1.15‐19.07) | .04 |
| Not recovered/recovered with sequelae/death (vs recovered) in GBS | ||||||||||
| Narrow definition (n = 2029) | 455 (50.72%) | 320 (36.87%) | 94 (35.61%) | <.0001 | 1.72 (1.42‐2.09) | <.0001 | 1.82 (1.37‐2.41) | <.0001 | 1.06 (0.80‐1.41) | >.99 |
| Broad definition (n = 2116) | 475 (50.91%) | 332 (36.77%) | 103 (36.79%) | <.0001 | 1.78 (1.48‐2.15) | <.0001 | 1.78 (1.36‐2.33) | <.0001 | 1.00 (0.76‐1.32) | >.99 |
Note: Patients with unreported outcome of Guillain‐Barré syndrome were not included for analysis. Total analyzed adverse drug reaction reports of narrow definition of Guillain‐Barré syndrome and broad definition of Guillain‐Barré syndrome included 2029 patients (ChAdOx1 nCoV‐19, n = 897; BNT162b2, n = 868; mRNA‐1273, n = 264) and 2116 patients (ChAdOx1 nCoV‐19, n = 933; BNT162b2, n = 903; mRNA‐1273, n = 280), respectively.
Abbreviations: CI, confidence interval; GBS, Guillain‐Barré syndrome.
Bonferroni multiple testing methods were performed and P values are adjusted by multiplying by 3.
When compared with the entire database, a significant signal of disproportionality of GBS, which means a greater risk of GBS, was noted for both the ChAdOx1 nCoV‐19 and mRNA‐based (BNT162b2 and mRNA‐1273) COVID‐19 vaccine groups (GBS broad definition [IC025 = 1.35, ROR025 = 2.87] and GBS narrow definition [IC025 = 1.21, ROR025 = 2.54]). The ChAdOx1 nCoV‐19 vaccine showed significant greater risk of GBS compared with the entire database (GBS broad definition [IC025 = 1.73, ROR025 = 3.51] and GBS narrow definition [IC025 = 1.64, ROR025 = 3.32]; see Figure 2). Also, the mRNA‐based COVID‐19 vaccines revealed a weak but significant signal of GBS (GBS broad definition [IC025 = 1.07, ROR025 = 2.22] and GBS narrow definition [IC025 = 1.03, ROR025 = 2.10]). However, in the disproportionality analyses against influenza vaccines, no potential safety signals of disproportionality on COVID‐19 vaccines with respect to the development of GBS were detected (GBS broad definition [IC025 = −1.36, ROR025 = 0.08] and GBS narrow definition [IC025 = −1.39, ROR025 = 0.08]; Figure 2).
FIGURE 2.
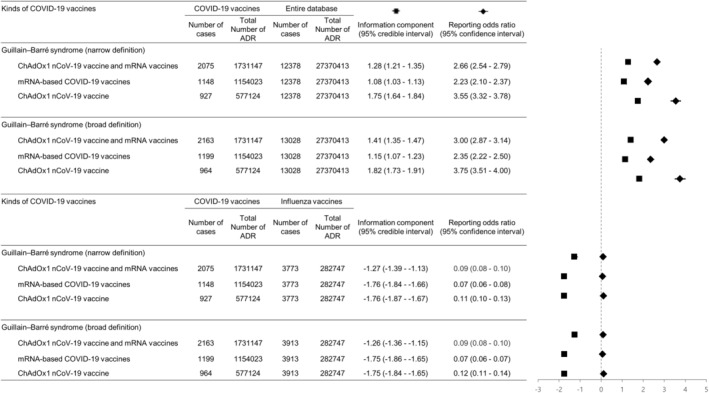
Forest plot with the odds ratios and information component values of mRNA‐based (BNT162b2, mRNA‐1273) and ChAdOx1 nCoV‐19 vaccine‐related Guillain‐Barré syndrome vs those of the entire VigiBase database and influenza vaccines. Compared with the entire database, COVID‐19 vaccines, mRNA‐based vaccines, and ChAdOx1 nCoV‐19 vaccine showed significantly positive associations with Guillain‐Barré syndrome. However, there were no significant positive associations in comparison with influenza vaccines. ADR, adverse drug reaction
4. DISCUSSION
The key findings of our study are that the ChAdOx1 nCoV‐19 vaccine as well as both mRNA‐based COVID‐19 vaccines that were analyzed have a positive potential signal for the occurrence of GBS in real‐world data from the WHO VigiBase collected from 130 countries. However, the association of GBS after the ChAdOx1 nCoV‐19 vaccine and mRNA‐based 209 COVID‐19 vaccines is not greater than that associated with other vaccines like seasonal influenza vaccines.
The occurrence of GBS has been reported after the use of vaccines for other conditions, including polio, tetanus, rabies, hepatitis B, and meningococcal disease. 8 Several GBS cases after COVID‐19 vaccination (both with the ChAdOx1 nCoV‐19 vaccine and the mRNA‐based COVID‐19 vaccines) have been reported in the literature. 6 , 13 , 14 , 18 , 32 In India, the frequency of GBS development following ChAdOx1 nCoV‐19 vaccination was 1.4‐ to 10‐fold greater compared to similar annual periods before vaccination. 14 The US Centers for Disease Control and Prevention reported that the overall estimated observed‐to‐expected rate ratio was 4.18, meaning an absolute increase of 6.36 cases per 100 000 person‐years for the Ad26.COV2.S vaccine. 18 Analysis of the linked National Immunoglobulin Database and the National Immunization Management System dataset in the United Kingdom revealed that a first dose of ChAdOx1 nCoV‐19 vaccine may be associated with 0.576 excess cases of GBS per 100 000 doses. 32 Compared with classical GBS, patients with GBS related to COVID‐19 vaccination show unique clinical features, which include a more severe manifestation of the syndrome and more frequent bifacial diplegia. 14 Our study results are consistent with those of previous reports that suggested ChAdOx1 nCoV‐19 vaccine and mRNA‐based COVID‐19 vaccines had a plausible relationship with GBS, which may not have a favorable prognosis. However, it is noteworthy that in our study the risk of developing GBS after vaccination with ChAdOx1 nCoV‐19 vaccine and mRNA‐based COVID‐19 vaccines was found to be similar to the risk of GBS after other influenza vaccines. Recent studies analyzing US VAERS data found that the frequency of serious neurological events including GBS, myelitis, acute disseminated encephalomyelitis and cerebral venous thrombosis was less than 1 per 1 000 000 doses for mRNA‐based COVID‐19 (BNT162b2 and mRNA‐1273) and Ad26.COV2.S vaccines. 33 These researchers reported that the risk of developing GBS after acute SARS‐CoV‐2 infection was 617 times greater than the risk of developing GBS associated with the COVID‐19 vaccine, noting that the profits of vaccination outweigh the risks. 33 Several studies have aimed to assess the clinical phenotypes and risks of GBS related to COVID‐19 vaccine or SARS‐CoV‐2 infection, but many of the results are controversial. 32 , 33 , 34 , 35 Currently, causality between vaccination and GBS cannot be confirmed, and it is therefore necessary to continue researching this issue using diverse approaches. Our study is meaningful in that we applied disproportionality analyses, an approach that has rarely been attempted in this topic before, to a VigiBase database. Further large‐scale, systematic, prospective and global‐level post‐vaccination surveillance that can supplement the self‐reporting system is warranted.
Postvaccine GBS appeared around 2 weeks following first‐dose COVID vaccination in our study. The onset of GBS after vaccination seemed to be slightly later in the group vaccinated with ChAdOx1 nCoV‐19 compared with the group vaccinated with mRNA‐based COVID‐19 vaccines. It is unclear why there was a difference in the time to GBS onset between the two vaccine types; however, the differences in the manner of presenting the native‐antigen viral spike protein (S) of SARS‐CoV‐2 to the immune system between the ChAdOx1 nCoV‐19 vaccine and mRNA‐based COVID‐19 vaccines might be one possible reason. 36 It has been reported that ChAdOx1 nCoV‐19 induces greater T‐cell responses, while the mRNA vaccines induce antibody titers to a greater degree. 36 The timing of GBS onset after vaccination identified in our study coincides with the well‐known point at which the immune response is maximized after infection or vaccination. Previous studies of GBS cases diagnosed following COVID‐19 vaccination reported a similar time frame to onset (range, 10‐22 days). 13 , 14 These results support the temporal relationship between vaccines and their pathogenesis, and the involvement of autoimmune mechanisms in the pathogenesis of GBS. Possible mechanisms for GBS development after COVID‐19 vaccination may be a direct reaction to the SARS‐CoV‐2 spike protein or a less specific immune cascade triggered by the adenovirus vector to generate host antibodies and cross‐reactions with proteins on nerves. 14
Although our results were not gathered based on a direct comparison of the ChAdOx1 nCoV‐19 vaccine and the mRNA‐based COVID‐19 vaccines, GBS tended to be more strongly associated with the ChAdOx1 nCoV‐19 vaccine (rather than the mRNA‐based COVID‐19 vaccines) in our study. Previous studies have reported GBS related to COVID‐19 vaccination is more often related to ChAdOx1 nCoV‐19 or Ad26.COV2.S vaccine than the mRNA vaccines. 13 , 14 , 33 For this reason, American and European regulatory agencies have warned of the increased risk of GBS following viral‐vector‐associated COVID‐19 vaccination (ie, ChAdOx1 nCoV‐19 from AstraZeneca and Ad26.COV2.S from Johnson & Johnson). The reason for this phenomenon is unclear. Antibodies generated from the components of the adenovirus vector used in the ChAdOx1 nCoV‐19 vaccine might be possible culprits of this disproportional GBS development profile.
The neurological outcome of vaccine‐associated GBS seemed to be unfavorable in our analyses in that more than one third of cases had unresolved neurological complications at last follow‐up. Classical GBS is known to lead to persistent disability in 14% of patients followed for 1 year. 37 The mortality rate in the first year of disease was reported to be around 4%, which is similar to our results. 36 Because follow‐up periods were not uniformly controlled in VigiBase, we cannot simply compare outcomes of COVID‐19 vaccine‐associated GBS and classical GBS. However, our results and other case reports that mention frequent severe manifestations with respiratory failure and partial recovery during the follow‐up periods suggest that COVID‐19 vaccine‐associated GBS may not have a good prognosis and is not a negligible minor side effect. 13 , 14
There are some limitations in our study. First, if the drug monitoring center of certain country does not report ADR, these cases may be omitted from VigiBase data. However, despite some risk of data omission in some regions, VigiBase is the largest ADR database that systematically receives ADR reports from 130 countries around the world. In addition, there may be differences in the certainty of the GBS diagnosis depending on the degree of access to medical resources, and the availability of particular vaccines types may vary by region; these factors may have influenced the high rate of ADR reports in United States and Europe in our results. Second, VigiBase data can be contributed by various participants as well as doctors—in other words, the sources of reports can be inhomogeneous and may have reporting biases. Third, VigiBase does not offer clinical informations including laboratory/radiologic findings and diagnostic certainty. Also, an alternative etiology of GBS as preceding infection or vaccination may not sufficiently excluded. However, reported ADR cases underwent primary review and causality assessment by national drug monitoring centers before sending the data to the UMC. Incoming reports from each national center are rechecked according to pre‐defined quality criteria and then registered in Vigibase. Fourth, in VigiBase, it is not possible to check whether the first or second dose of vaccination was the causative factor, so further analysis in this regard is not possible. Fifth, in VigiBase, it is hard to compare outcome parameters between each type of vaccines directly. Sixth, disproportionality analysis is prone to reference bias and it is not unlikely that because of the public concerns about a specific risk of GBS associated with these vaccines, more of these cases may have been included in the database than for other diseases or other vaccines. Conversely, because GBS is a rare disease and the diagnosis may not be straightforward, there may also have a bias toward underreporting of the condition. Seventh, VigiBase is primarily design to detect unusual safety signals that might be related to vaccines, but does not address causality. In disproportionality analysis, denominator is number of the ADRs of given categories and detailed incidence cannot be calculated.
In conclusion, our study suggests a small but significant association of COVID‐19 vaccines (two mRNA‐based vaccines [Pfizer and Moderna] and one adenoviral vector vaccine [Astrazenecca ChAdOx1 nCoV‐19]) with the occurrence of GBS using data from the WHO pharmacovigilance database, VigiBase. However, the risk of GBS after COVID‐19 vaccination is likely low and does not appear to surpass the risks associated with influenza vaccines. Because of the heterogeneity of the sources of information and the use of a pharmacovigilance database with somewhat weak internal validity, associations between COVID‐19 vaccine and GBS must be confirmed in clinical epidemiological studies.
AUTHOR CONTRIBUTIONS
Tae‐Jin Song had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Jee‐Eun Kim, Jin Park, Young Gi Min, Yoon‐Ho Hong and Tae‐Jin Song. Acquisition, analysis, or interpretation of data: Jee‐Eun Kim, Jin Park, Young Gi Min, Yoon‐Ho Hong, and Tae‐Jin Song. Drafting of the manuscript: Jee‐Eun Kim, Jin Park, Young Gi Min, Yoon‐Ho Hong, and Tae‐Jin Song. Critical revision of the manuscript for important intellectual content: Jee‐Eun Kim, Jin Park, Yoon‐Ho Hong, and Tae‐Jin Song. Statistical analysis: Tae‐Jin Song. Administrative, technical, or material support: Tae‐Jin Song. Supervision: Tae‐Jin Song.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank VigiBase for giving us access to the data. The data supplied to VigiBase come from a variety of sources, and the likelihood of a causal relationship is not the same in all reports. This project was supported by grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1F1A1048113 to Tae‐Jin Song).
Kim J‐E, Park J, Min YG, Hong Y‐H, Song T‐J. Associations of Guillain‐Barré syndrome with coronavirus disease 2019 vaccination: Disproportionality analysis using the World Health Organization pharmacovigilance database. J Peripher Nerv Syst. 2022;1‐9. doi: 10.1111/jns.12507
Jee‐Eun Kim and Jin Park contributed equally to this study.
Funding information Basic Science Research Program through the National Research Foundation of Korea, Grant/Award Number: 2021R1F1A1048113
DATA AVAILABILITY STATEMENT
Anonymized data used in this study are publicly shared by the Uppsala Monitoring Centre or the World Health Organization and can be obtained from VigiBase.
REFERENCES
- 1. Chang Y, Jeon J, Song TJ, Kim J. Association between the fatty liver index and the risk of severe complications in COVID‐19 patients: a nationwide retrospective cohort study. BMC Infect Dis. 2022;22(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoo J, Kim JH, Jeon J, Kim J, Song TJ. Risk of COVID‐19 infection and of severe complications among people with epilepsy: a nationwide cohort study. Neurology. 2022;98:e1886‐e1892. doi: 10.1212/WNL.0000000000200195 [DOI] [PubMed] [Google Scholar]
- 3. Park J, Shin JI, Kim DH, et al. Association of atrial fibrillation with infectivity and severe complications of COVID‐19: a nationwide cohort study. J Med Virol. 2022;94(6):2422‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim HJ, Park MS, Shin JI, et al. Associations of heart failure with susceptibility and severe complications of COVID‐19: a nationwide cohort study. J Med Virol. 2022;94:1138‐1145. doi: 10.1002/jmv.27435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omer SB, Yildirim I, Forman HP. Herd immunity and implications for SARS‐CoV‐2 control. JAMA. 2020;324(20):2095‐2096. [DOI] [PubMed] [Google Scholar]
- 6. Marquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain‐Barré syndrome in the placebo and active arms of a COVID‐19 vaccine clinical trial. Temporal associations do not imply causality. Neurology. 2021;96:1052‐1054. doi: 10.1212/WNL.0000000000011881 [DOI] [PubMed] [Google Scholar]
- 7. Pascual‐Iglesias A, Canton J, Ortega‐Prieto AM, Jimenez‐Guardeno JM, Regla‐Nava JA. An overview of vaccines against SARS‐CoV‐2 in the COVID‐19 pandemic era. Pathogens. 2021;10(8):1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain‐Barre syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32(2):150‐163. [DOI] [PubMed] [Google Scholar]
- 9. Matsui N, Nodera H, Kuzume D, et al. Guillain‐Barre syndrome in a local area in Japan, 2006‐2015: an epidemiological and clinical study of 108 patients. Eur J Neurol. 2018;25(5):718‐724. [DOI] [PubMed] [Google Scholar]
- 10. Shahrizaila N, Lehmann HC, Kuwabara S. Guillain‐Barre syndrome. Lancet. 2021;397(10280):1214‐1228. [DOI] [PubMed] [Google Scholar]
- 11. Schonberger LB, Bregman DJ, Sullivan‐Bolyai JZ, et al. Guillain‐Barre syndrome following vaccination in the national influenza IMMUNIZATION PROGRAM, United States, 1976–1977. Am J Epidemiol. 1979;110(2):105‐123. [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Zhang J, Chu X, Xu Y, Ma F. Vaccines and the risk of Guillain‐Barre syndrome. Eur J Epidemiol. 2020;35(4):363‐370. [DOI] [PubMed] [Google Scholar]
- 13. Allen CM, Ramsamy S, Tarr AW, et al. Guillain‐Barre syndrome variant occurring after SARS‐CoV‐2 vaccination. Ann Neurol. 2021;90(2):315‐318. [DOI] [PubMed] [Google Scholar]
- 14. Maramattom BV, Krishnan P, Paul R, et al. Guillain‐Barre syndrome following ChAdOx1‐S/nCoV‐19 vaccine. Ann Neurol. 2021;90(2):312‐314. [DOI] [PubMed] [Google Scholar]
- 15. Bonifacio GB, Patel D, Sook S, et al. Bilateral facial weakness with paraesthesia variant of Guillain‐Barre syndrome following Vaxzevria COVID‐19 vaccine. J Neurol Neurosurg Psychiatry. 2022;93(3):341‐342. [DOI] [PubMed] [Google Scholar]
- 16. Shao SC, Wang CH, Chang KC, Hung MJ, Chen HY, Liao SC. Guillain‐Barré syndrome associated with COVID‐19 vaccination. Emerg Infect Dis. 2021;27(12):3175‐3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bouattour N, Hdiji O, Sakka S, et al. Guillain‐Barre syndrome following the first dose of Pfizer‐BioNTech COVID‐19 vaccine: case report and review of reported cases. Neurol Sci. 2022;43(2):755‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woo EJ, Mba‐Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of receipt of the Ad26.COV2.S COVID‐19 vaccine with presumptive Guillain‐Barre syndrome, February‐July 2021. JAMA. 2021;326(16):1606‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Medicine Agency . COVID‐19 vaccine safety update ‐ VAXZEVRIA, AstraZeneca; 2021. https://www.ema.europa.eu/en/documents/covid‐19‐vaccine‐safety‐update/covid‐19‐vaccine‐safetyupdate‐vaxzevria‐previously‐covid‐19‐vaccine‐astrazeneca‐8‐september‐2021_en.pdf. Accessed March 26, 2022.
- 20. Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Ther Innov Regul Sci. 2008;42(5):409‐419. [Google Scholar]
- 21. Park J, Kim D, Song TJ. A disproportionality analysis for Association of Systemic Capillary Leak Syndrome with COVID‐19 vaccination using the World Health Organization pharmacovigilance database. Vaccine. 2022;10(6):835‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park J, Park MS, Kim HJ, Song TJ. Association of Cerebral Venous Thrombosis with mRNA COVID‐19 vaccines: a disproportionality analysis of the World Health Organization pharmacovigilance database. Vaccine. 2022;10(5):799‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109‐117. [DOI] [PubMed] [Google Scholar]
- 24. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . Maintenance of the ICH Guideline on Clinical Safety Data Management: Data Elements for Transmission of Individual Case Safety Reports E2B(R2). https://admin.ich.org/sites/default/files/inline-files/E2B_R2_Guideline.pdf. Accessed March 9, 2021.
- 25. Butler M, Tamborska A, Wood GK, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS‐CoV‐2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry. 2021;92(11):1144‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315‐321. [DOI] [PubMed] [Google Scholar]
- 27. Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with Ibrutinib. J Am Coll Cardiol. 2019;74(13):1667‐1678. [DOI] [PubMed] [Google Scholar]
- 28. Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norén GN, Hopstadius J, Bate A. Shrinkage observed‐to‐expected ratios for robust and transparent large‐scale pattern discovery. Stat Methods Med Res. 2013;22:57‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519‐523. [DOI] [PubMed] [Google Scholar]
- 31. De Bruin ML, Pettersson M, Meyboom RHB, Hoes AW, Leufkens HGM. Anti‐HERG activity and the risk of drug‐induced arrhythmias and sudden death. Eur Heart J. 2005;26(6):590‐597. [DOI] [PubMed] [Google Scholar]
- 32. Keh RYS, Scanlon S, Datta‐Nemdharry P, et al. COVID‐19 vaccination and Guillain‐Barre syndrome: analyses using the National Immunoglobulin Database. Brain. 2022. [Online ahead of print]. doi: 10.1093/brain/awac067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frontera JA, Tamborska AA, Doheim MF, et al. Neurological events reported after COVID‐19 vaccines: an analysis of VAERS. Ann Neurol. 2022;91(6):756‐771. doi: 10.1002/ana.26339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID‐19 and Guillain‐Barre syndrome. Brain. 2021;144(2):682‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barre syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heinz FX, Stiasny K. Distinguishing features of current COVID‐19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rajabally YA, Uncini A. Outcome and its predictors in Guillain‐Barre syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711‐718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data used in this study are publicly shared by the Uppsala Monitoring Centre or the World Health Organization and can be obtained from VigiBase.


