Abstract
Shigella spp. are the major cause of bacillary dysentery worldwide. The pathogenic process involves bacterial invasion and lysis of the phagocytic vacuole, followed by replication and movement within the cell cytoplasm and, ultimately, spread directly into adjacent cells. This study demonstrates that S. flexneri cytochrome bd expression is necessary for normal intracellular survival and virulence. Cytochrome bd is one of two terminal oxidases in the bacterial respiratory chain that reduce molecular oxygen to water, utilizing intermediates shuttled through the electron transport chain. S. flexneri mutants that contain a disruption in the cydC locus, which leads to defective cytochrome bd expression, or in the riboflavin (ribE) or ubiquinol-8 (ubiH) biosynthetic pathway, which leads to elevated cytochrome bd expression, were evaluated in intracellular survival and virulence assays. The cydC mutant formed significantly smaller plaques, had significantly decreased intracellular survival, and had a 100-fold increase in lethal dose for mice compared with the wild type. The ribE and ubiH mutants each formed significantly larger plaques and had a 10-fold decrease in lethal dose for mice compared with the wild type. The data indicate that expression of cytochrome bd is required for S. flexneri intracellular survival and virulence.
Shigellosis is an invasive disease of the human intestinal tract that is responsible for 200 million cases of bacillary dysentery and 650,000 fatalities annually (17). Following invasion of colonic epithelial cells, shigellae escape the endocytic vacuole and enter the host cell cytoplasm (3, 15, 36). Within the cytoplasm, shigellae replicate and incorporate host actin into an organized tail on one pole of the bacterial surface. These actin tails are required for Shigella intra- and intercellular motility (4, 23, 28). Bacterial division within the cell cytoplasm eventually leads to host cell death. The ability of shigellae to survive within host cells and to spread from an infected cell into adjacent cells is required for pathogenesis (35).
Many bacterial pathogens are adapted to survive within mammalian cells. These organisms can be grossly divided into two groups: those that reside predominantly within the phagosomal compartment, such as Salmonella spp., Legionella pneumophila, Yersinia pseudotuberculosis, and Mycobacteria spp., and those that lyse the phagosome and reside predominantly within the cytoplasm, such as Listeria monocytogenes, Rickettsia spp. and Shigella spp. In whichever compartment the organism resides, it must be able to acquire nutrients and resist killing by host cell factors to thrive. The mechanisms necessary for resisting host cell killing undoubtedly differ for different types of host cells, for example professionally nonphagocytic and phagocytic cells. Organisms that reside predominantly within phagosomes or phagolysosomes have been shown to either impede phagolysosomal fusion, e.g., L. pneumophilia (16), slow and adapt to the acidification of the phagolysosome, e.g., Salmonella typhimurium (2), or establish specific mechanisms for acquiring nutrients across the phagosomal membrane, e.g., L. pneumophila (39). The mechanisms by which intracytoplasmic pathogens are adapted to their niches are less well understood. We have recently demonstrated that the ability of Shigella flexneri to survive within the cytoplasm of either fibroblasts or macrophages is inhibited by activation of host cells with gamma interferon, which suggests that specific host cell factors involved in bacterial killing are up-regulated by gamma interferon (42).
Shigella invasion, intracellular viability, and intercellular spread can be studied in vitro by infecting confluent monolayers of mammalian cells. Following cellular invasion, pathogenic shigellae are able to spread from an infected cell into adjacent cells, thereby forming bacterial plaques within the cell monolayer, while Shigella mutants with disruption of any step in the pathogenic process form smaller plaques or are unable to form plaques. Many Shigella genes and gene products responsible for initial cellular invasion and intercellular spread have been defined by using this in vitro pathogenesis assay (1, 3, 4, 7, 15, 23, 25, 28, 37).
In this study, we examine the role of S. flexneri cytochrome bd terminal oxidase expression on intracellular survival and virulence. S. flexneri mutants that either overexpress or do not express the cytochrome bd terminal oxidase were identified in a screen originally designed to identify genes involved in the inability of galU S. flexneri to form plaques. A positive correlation was observed between cytochrome bd expression and S. flexneri virulence as represented by size of bacterial plaques formed, intracellular survival, and lethal doses in intranasally infected mice.
MATERIALS AND METHODS
Bacterial strains and media.
S. flexneri and E. coli strains used in this study are described in Table 1. E. coli strains were grown in L broth, and S. flexneri strains were grown in tryptic soy broth.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| S. flexneri strains | ||
| 2457T | Wild-type serotype 2a | 10 |
| BS109 | 2457T galU1::Tn10 | 34 |
| SS100 | 2457T ΔgalU2 | This work |
| MBG283 | 2457T icsA1 | 38 |
| SSW201 | SS100 cydC1::Tn10 | This work |
| SSW301 | SS100 pepP1::Tn10 | This work |
| SSW401 | SS100 ribE1::Tn10 | This work |
| SSW202 | 2457T cydC1::Tn10 | This work |
| SSW302 | 2457T pepP1::Tn10 | This work |
| SSW402 | 2457T ribE1::Tn10 | This work |
| SSW203 | MBG283 cydC1::Tn10 | This work |
| SSW303 | MBG283 pepP1::Tn10 | This work |
| SSW403 | MBG283 ribE1::Tn10 | This work |
| E. coli DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) f80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ−rpsL nupG | GibcoBRL |
| Plasmids | ||
| LITMUS38 | Cloning vector: Amr | New England Biolabs |
| pSSW204 | LITMUS38 containing intact S. flexneri cydDC; Amr | This work |
| pMBG462 | pSSW204 with two frame-shift mutations introduced into cydD, such that cydC is in the native reading frame | This work |
Cell lines.
Rat lung fibroblast L2 cells were obtained from the American Type Culture Collection and were maintained in minimal essential medium (MEM) supplemented with 10% fetal calf serum and 1× nonessential amino acids.
In vitro infections.
For infections of L2 cells, S. flexneri, diluted in MEM, were centrifuged (at 700 × g, for 10 min) onto confluent monolayers in either 24-well dishes or 60-mm-diameter tissue culture plates at a multiplicity of infection of 0.05 to 0.1. At 80 min postcentrifugation, the bacterial suspension overlying the cell monolayer was replaced with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and gentamicin (25 μg/ml). Gentamicin kills extracellular, but not intracellular, bacteria. Assays of S. flexneri plaque formation were performed as previously described (27). For complementation experiments, plaque assays were performed in the presence of ampicillin (100 μg/ml).
To quantitate the viable intracellular S. flexneri, adherent cells were washed three times with MEM at the indicated times postinfection and lysed with 0.1% sodium deoxycholate in phosphate-buffered saline (PBS). Serial dilutions of the cell lysate were then plated onto tryptic soy agar containing Congo red (0.01%). Invasion efficiencies were calculated as the number of intracellular bacteria at 1 h postinfection/the number of bacteria used to infect the monolayer. The concentration of sodium deoxycholate used to lyse the cell monolayer (0.1%) was not bactericidal for any of the strains studied.
Fusaric acid selection.
Strain SS100, a tetracycline-sensitive derivative of BS109, was generated by fusaric acid selection as previously described (5) and confirmed to maintain the galU phenotype by failure to grow on MacConkey agar.
Molecular and genetic techniques.
DNA analysis, including DNA preparation, restriction enzyme digestion, agarose gel electrophoresis, ligation, bacterial transformation, and P1 phage transduction, was performed according to standard procedures (32). Genetic techniques utilized to generate the miniTn10tet transposon library within SS100 were as described previously (20). Strain SS100, transformed with pSM5, which caries the Escherichia coli lamB gene, was transfected with lambda phage NK1098, which contains a miniTn10tet transposon (41). Selection of mutants with transposition onto the S. flexneri genome was performed by plating onto tryptic soy agar containing tetracycline (12.5 μg/ml).
S. flexneri DNA flanking the site into which the transposon inserted in each mutant was sequenced by using primers MBG121 (5′ GCTGTTGACAAAGGGAATC 3′) and MBG122 (5′ GGTCACCAACGCTTTTC 3′), which correspond to bases 653 to 635 and bases 3382 to 3398, respectively, of the TRN10TETR locus (GenBank accession no. J01830, K00615, K01493, and X00694). The TRN10TETR locus spans the entire 2.8-kb sequence corresponding to the miniTn10tet transposon within lambda NK1098 used in this study. The S. flexneri sequences corresponding to the ECOCYDD, ECOPUV, and ECNUSB loci in E. coli are listed in GenBank under accession no. AF040718, AF041033, and AF002857, respectively.
LPS analysis.
Lipopolysaccharide (LPS) was prepared by proteinase K treatment of late-logarithmic-phase cultures. It was separated on sodium dodecyl sulfate–12.5% polyacrylamide gels in the presence of 4 M urea and was analyzed by immunoblotting using S. flexneri serotype 2 antiserum (Difco Laboratories, Detroit, Mich.).
Levels of cytochrome bd expression.
S. flexneri membrane proteins were prepared from aerobically grown cultures as previously described for E. coli (6). Cytochrome bd levels in S. flexneri membrane preparations were quantitated by using sodium dithionite-reduced minus potassium ferricyanide-oxidized difference spectra. All samples contained 300 μg of membrane protein per ml. Spectra were recorded at room temperature on a Cary 14 spectrophotometer modified by Aviv Associates (Lakewood, N.J.). Concentrations of cytochrome d were calculated by using the extinction coefficient E630–615 = 8.5 mM−1 cm−1 (14).
MIC.
Determination of the MIC of gentamicin for S. flexneri strains was performed as described previously (9).
Mice.
C57BL/6 mice at 6 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, Maine) and housed in a pathogen-free environment in the Animal Institute at the Albert Einstein College of Medicine. Care of animals was in accordance with guidelines set by the American Association for Accreditation of Laboratory Animal Care and the Albert Einstein College of Medicine.
Mouse infections.
Murine intranasal S. flexneri infections were performed as previously described (24, 40, 42). S. flexneri grown to midexponential phase was washed with PBS and adjusted to the appropriate concentration prior to inoculation. The inoculum, resuspended in 30 μl of PBS, was introduced dropwise into the nares of mice that had been anesthetized intramuscularly with a mixture of 0.6 mg of ketamine hydrochloride (Ketaset; Aveco Co., Fort Dodge, Iowa) and 0.18 mg of xylazine hydrochloride (Rompun; Mobay Corp., Shawnee, Kans.) in 100 μl of saline. The number of bacteria in each inoculum was confirmed by plating serial dilutions of the inoculum. Mice were examined 2 to 4 h after infection to confirm recovery from anesthetic and were monitored daily thereafter for survival. Lethal doses were determined by infecting groups of 6 to 8 C57BL/6 mice with serial 10-fold dilutions of each S. flexneri strain. The lethal dose represents the S. flexneri inoculum size that caused 75 to 100% mortality.
Statistics.
Means between groups were evaluated by using the independent Student t test, with P values of <0.05 taken as significant.
RESULTS
Identification of mutants that restore the plaque-forming phenotype to galU S. flexneri in tissue culture monolayers.
galU S. flexneri mutants are defective in outer core LPS biosynthesis. The inability of S. flexneri mutants with a variety of LPS defects to form plaques on confluent fibroblast monolayers has been previously attributed to the abnormal distribution of IcsA on the surface of these mutants and resultant defects in actin-based motility (34). S. flexneri galU mutants are invasive and are able to lyse the phagocytic vacuole (34). The molecular mechanisms underlying the inability of these mutants to form plaques in L2 cell monolayers are not fully understood. Random transposon mutagenesis was performed on the genome of a ΔgalU2 S. flexneri strain (SS100) using a miniTn10tet transposon; in a screen to identify genes involved in actin-based motility and cell-to-cell spread, the transposon insertion library was used to infect confluent monolayers of L2 cells. Of 38,000 independent members of the transposon library screened, 50 mutants that were able to form plaques were identified. The plaques formed by these mutants were significantly smaller than plaques formed by wild-type S. flexneri (Fig. 1). Genomic DNAs from 26 of these mutants, chosen randomly, were analyzed by Southern blot analysis; banding patterns suggested that sites of transposon insertion in all of these 26 suppressor mutants fell into only three distinct loci (data not shown). One mutant from each of the three loci was selected and used for all further studies.
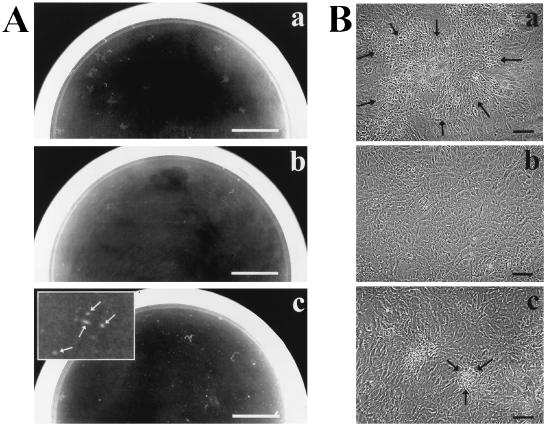
FIG. 1.
Screen for suppressor mutants of galU S. flexneri. Shown are macroscopic (A) and microscopic (B) views of plaque formation by S. flexneri strains 2457T (wild-type) (a), SS100 (ΔgalU2) (b), and SSW301 (ΔgalU2 pepP1) (c) on L2 cell monolayers. Plaques formed by SSW201 (ΔgalU2 cydC1) and SSW401 (ΔgalU2 ribE1) are identical in size and morphology to those formed by SSW301 (data not shown). (A) Plaques were photographed at 72 h postinfection. The inset in panel c is magnified 4×; arrows in panel c indicate plaques. Bar, 1 cm. (B) Plaques were photographed at 48 h postinfection. Arrows indicate the periphery of a single plaque in each panel. Bar, 100 μm.
To confirm that plaque formation in these mutants was the result of a single site of transposon insertion within the ΔgalU2 S. flexneri genome, the tetracycline-resistant locus from the mutant selected from each class of suppressors was transduced back into the parental ΔgalU2 S. flexneri genome. The ability of the resultant strains to form plaques was confirmed on L2 cell monolayers; each formed plaques that were of the same small size as was formed by the original isolate (data not shown). That the transposon insertion site of the transductant from each class of suppressors was the same as in its parent stain was verified by the presence of the same banding pattern on Southern blot analysis (data not shown). Subsequent experiments were carried out with these transductants (SSW201, SSW301, and SSW401) or their derivatives (described below).
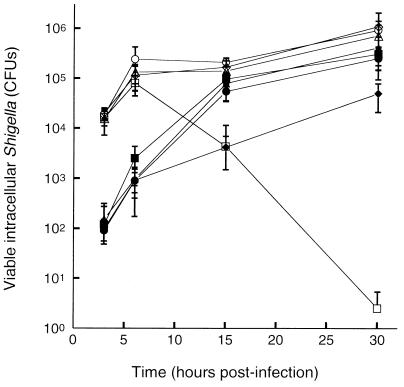
To determine whether plaque formation by the galU suppressor mutants was associated with increases in viable bacteria within the monolayer, the survival of SSW201, SSW301, SSW401, as well as the parental ΔgalU2 strain SS100 was determined in L2 cell monolayers over time (Fig. 2). While no differences were observed in the efficiency of cellular invasion between any suppressor mutant and SS100, significant differences in recovery of bacteria from the monolayer were observed among the suppressors and SS100 beginning at 6 h postinfection and extending up to 30 h postinfection. By 30 h postinfection, SS100 was completely eradicated from the cell monolayer, while the numbers of intracellular organisms for SSW201, SSW301, and SSW401 were still increasing. There were no significant differences in the recovery of bacteria among the different suppressors.
FIG. 2.
Intracellular survival of suppressor mutants in the galU (open symbols) and wild-type (solid symbols) backgrounds in L2 cell monolayers. Shown are S. flexneri strain SS100 (ΔgalU2) (open squares), suppressor mutants SSW201 (ΔgalU2 cydC1) (open diamonds), SSW301 (ΔgalU2 pepP1) (open circles), SSW401 (ΔgalU2 ribE1) (open triangles), 2457T (wild-type) (solid squares), SSW202 (cydC1) (solid diamonds), SSW302 (pepP1) (solid circles), and SSW402 (ribE1) (solid triangles). Each data point represents the geometric mean of five or six independent determinations. Error bar, 1 standard deviation.
Disruption of the cydC, pepP, or ribE locus restores the ability of galU S. flexneri to form plaques.
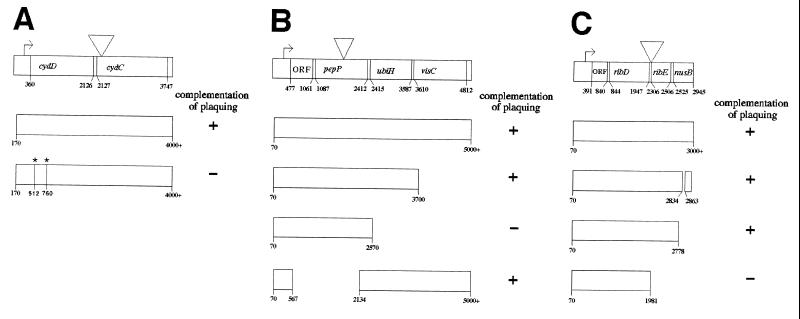
To identify the sites into which the transposon inserted in each strain, genomic DNA from each suppressor mutant was cloned into a LITMUS plasmid. S. flexneri DNA flanking the site of transposon insertion was analyzed by sequence analysis using primers oriented outwards on either end of the transposon. The sites of transposon insertion in the three suppressor mutants were determined to be regions of the S. flexneri chromosome that have significant sequence similarity to the open reading frames of the cydC and pepP genes of E. coli and to the promoter region of the ribE gene of E. coli (Fig. 3). Specifically, for mutant SSW201, the site of transposon insertion is immediately 3′ of nucleotide 2141 in the E. coli cydDC locus (ECOCYDD) (accession no. L21749) (Fig. 3A) with duplication of nucleotides 2131 to 2141, thereby placing it just after the 15th base of the coding sequence of cydC. The locus in S. flexneri has 99.2% nucleotide sequence identity and 100% amino acid sequence identity with nucleotides 1772 to 2514 of the E. coli cydDC locus. For SSW301, the site of transposon insertion is immediately 3′ to nucleotide 1940 in the E. coli pepP locus (ECOPUV) (accession no. D90281) (Fig. 3B), thereby placing the insertion within the coding sequence of pepP. The locus in S. flexneri has 98.6% nucleotide sequence identity and 99.3% amino acid sequence identity with nucleotides 1320 to 2580 of the E. coli pepP locus. For SSW401, the site of transposon insertion is immediately 3′ to nucleotide 1975 in the E. coli ribE locus (ECNUSB) (accession no. X64395 and S47077) (Fig. 3C) with duplication of nucleotides 1965 to 1975, thereby placing the insertion within the promoter of ribE. The locus in S. flexneri has 99.2% nucleotide sequence identity and 99.0% amino acid sequence identity with nucleotides 1514 to 2431 of the E. coli ribE locus.
FIG. 3.
Complementation in trans of altered plaque phenotype for mutants SSW202 (cydC1) (A), SSW302 (pepP1) (B), and SSW402 (ribE1) (C). Inverted triangles indicate the site of transposon insertion. Asterisks indicate location of frame-shift mutations introduced into cydD; introduction of the two mutations restores the native reading frame to sequences downstream of the second mutation. Nucleotide numbering corresponds to sequences in the ECOCYDD, ECOPUV, and ECNUSB loci, respectively. ORF, open reading frame.
S. flexneri with disruption of cydC, ribE, or pepP locus exhibits altered plaque phenotype.
To evaluate the effects of each of the disrupted loci in the wild-type background, the tetracycline-resistant locus in each mutant was transduced into the wild-type S. flexneri strain 2457T. In the wild-type background, the mutant with a transposon insertion within the pepP or ribE locus, SSW302 or SSW402, respectively, formed plaques that are larger and more circular in appearance than plaques formed by wild-type S. flexneri (compare Fig. 4c and a). Notably, the strain carrying a transposon insertion within the cydC locus, SSW202, formed plaques that are significantly smaller than plaques formed by wild-type S. flexneri (compare Fig. 4b and a).
FIG. 4.
Plaque formation by strains carrying suppressor mutations in the wild-type S. flexneri background on L2 cell monolayers. (a) 2457T (wild-type); (b) SSW202 (cydC1); and (c) SSW402 (ribE1). Plaques formed by SSW302 (pepP1) are identical in size and morphology to those formed by SSW402 (data not shown). The Inset in panel b is magnified 4×. Arrows indicate individual plaques. Plaques were photographed at 72 h postinfection. Bars, 1 cm.
PCR products containing regions from each of the three disrupted operons were provided in trans to the corresponding mutant to determine the gene responsible for the altered plaque phenotype. For SSW202 (cydC1), the operon in which the transposon is inserted contains two open reading frames; the transposon is inserted within the second open reading frame. Complementation in trans with a plasmid that contains cydDC restored the plaque phenotype to wild-type (Fig. 3A). Complementation in trans with a plasmid that contains only a translatable cydC (by virtue of the introduction of two frame-shift mutations in the upstream portion of cydD such that the native reading frame is returned to the downstream portion of cydD and to cydC) did not restore the plaque phenotype to wild type; we hypothesize that the lack of complementation by cydC alone is possibly due to instability of the chromosomally encoded cydD transcript that results from the location of the Tn10 transposon only 16 bases 3′ of the cydD stop codon. The cydDC locus encodes both subunits of a heterodimeric membrane protein with significant sequence similarity to known ABC-type transporters (29, 30). The cydDC gene products are required for the proper membrane assembly of the cytochrome bd complex (11, 31).
For SSW302 (pepP1), the operon in which the transposon is inserted contains four open reading frames; the transposon is inserted within the second open reading frame. Complementation in trans with a plasmid containing either the full-length operon or the first three open reading frames restored the plaque phenotype to that of wild type; however, a plasmid containing only the first two open reading frames did not complement the altered plaque phenotype. Furthermore, a plasmid containing only the promoter region followed by the third and fourth open reading frames was able to complement the plaque phenotype (Fig. 3B). This demonstrates that the third open reading frame, the ubiH gene, is responsible for the observed altered plaque phenotype of SSW302. The ubiH gene product, UbiH, catalyzes the oxidation of 2-octaprenyl-6-methoxyphenol to 2-octaprenyl-6-methoxy-1,4-benzoquinol in the ubiquinol-8 biosynthetic pathway (43).
For SSW402 (ribE1), the operon in which the transposon is inserted contains four open reading frames. The transposon is inserted within the promoter region for the third open reading frame. Complementation in trans with plasmids containing either the full-length operon or the first three open reading frames restored the plaque phenotype to that of wild-type, while a plasmid containing only the first two open reading frames did not (Fig. 3C). This demonstrates that the third open reading frame, the ribE gene, is responsible for the observed altered plaque phenotype of SSW402. The ribE gene encodes 6,7-dimethyl-8-ribityllumazine synthase, which catalyzes the condensation of 5-amino-6-ribitylamino-2,4(1H, 3H)-pyrimidinedione with 3,4-dihydroxy-2-butanone phosphate in the riboflavin biosynthetic pathway (18, 26).
The MICs of gentamicin for SSW202, SSW302, SSW402, and 2457T were determined to address whether alterations in sensitivity to gentamicin, which is placed in the agar overlaid on the monolayer to kill extracellular organisms, could account for the observed plaque phenotypes. Each Shigella mutant strain demonstrated the same sensitivity to gentamicin as did the wild type (MIC = 5.0 μg/ml).
Intracellular survival of S. flexneri mutants with disruptions in cydC, ribE, and pepP.
To determine whether the observed differences in plaque phenotypes of SSW202 (cydC1), SSW302 (pepP1), and SSW402 (ribE1) are associated with their ability to survive within the cell monolayer, the numbers of viable intracellular bacteria from L2 cell monolayers infected with each of these strains or 2457T (wild type) were quantified at multiple times from 3 to 30 h postinfection (Fig. 2). At all times after 6 h, significantly reduced numbers of viable bacteria were recovered from monolayers infected with SSW202 (cydC1) as compared with SSW302 (pepP1), SSW402 (ribE1), or 2457T (wild-type) (P < 0.05). No significant differences were observed among numbers of viable bacteria recovered from monolayers infected with SSW302 (pepP1), SSW402 (ribE1), or 2457T (wild-type). Similar numbers of each of these strains were present at early times after infection.
Comparison of the intracellular survival of SSW202 (cydC1), SSW302 (pepP1), SSW402 (ribE1), and 2457T (wild-type) to each of these in the galU background reveal that significantly fewer of each mutant in the wild-type background were present at early times after infection (Fig. 2); we have previously observed a higher invasion efficiency of galU S. flexneri (31a). The initial rates of intracellular growth of the mutants were similar in the two backgrounds. After 3 h, the growth of the mutants in the galU background dropped off compared to that of the mutants in the wild-type background. Growth of the cydC mutant was significantly reduced compared to that of the other mutants in the wild-type background, but not in the galU background, and growth of the galU2 cydC1 mutant was better than that of the galU mutant, suggesting that the effects of the cydC mutation on intracellular survival are suppressed by the galU mutation, and vice versa.
Actin-based motility and LPS profiles of S. flexneri mutants with disruptions in cydC, ribE, and pepP.
The observed differences in bacteria recoverable from monolayers infected with SSW202 (cydC1) and 2457T (wild-type) could be due either to a decrease in intracellular survival of SSW202 relative to 2457T or to an inability of SSW202 to spread normally through the monolayer, leading to lysis of cells infected with SSW202 and release of bacteria into the gentamicin-containing medium that overlies the cells. To test these possibilities, the following series of experiments were performed.
Wild-type S. flexneri utilizes actin-based motility to move within the host cell cytoplasm and to spread from cell to cell (4). Examination of cells infected with SSW202 (cydC1), SSW302 (pepP1), SSW402 (ribE1), or 2457T (wild-type) showed no differences in actin tail or cell surface protrusion formation (data not shown). Actin tails and protrusions were morphologically indistinguishable and present in approximately similar numbers among the strains. These data suggest that the mutants were able to utilize actin-based motility normally.
The outer membrane protein IcsA is essential to actin-based motility (13, 21); strains with mutations in icsA do not form bacterial plaques, actin tails, or cell surface protrusions within infected cell monolayers (4). On wild-type S. flexneri, IcsA is located unipolarly on the bacterial surface (12). The localization of IcsA on the surface of SSW202 (cydC1), SSW302 (pepP1), and SSW402 (ribE1) was not different from its localization on the surface of 2457T (wild-type) (data not shown). On galU S. flexneri, IcsA is delocalized from the pole, extending down the sides of the bacterium (34). The localization of IcsA on the surface of SSW201 (galU2 cydC1), SSW301 (galU2 pepP1), and SSW401 (galU2 ribE1) was not different from its localization on the surface of SSW100 (galU2) (data not shown).
To further assess the intracellular survival of SSW202 (cydC1) as compared with SSW302 (pepP1), SSW402 (ribE1), and 2457T (wild-type), the sites of transposon insertion in these mutants were transduced into the icsA strain MBG283. Following infection of L2 cell monolayers, the numbers of viable intracellular bacteria were determined for SSW203 (cydC1 icsA), SSW303 (pepP1 icsA), SSW403 (ribE1 icsA), and MBG283 (icsA) (data not shown). At 6 h postinfection, three- to six-fold decreased numbers of intracellular SSW203 were observed compared to either SSW303, SSW403, or MBG283 (P < 0.05 for SSW302 compared with SSW303, SSW403, or MBG283). This experiment was carried out for only 6 h because, after this time, the number of viable intracellular MBG283 cells (icsA) decreases relative to 2457T (wild type) (data not shown); this decrease is likely due to lysis of the MBG283-infected cells (now packed with organisms as a result of not being able to spread into adjacent cells). Further, as previously described for icsA mutants (4), SSW203, SSW303, SSW403, and MBG283 did not form plaques in cell monolayers (data not shown). Taken together, these data suggest that the small size of plaques formed by SSW202 (cydC1) is due to the strain’s decreased ability to survive within infected cells and not to lysis of infected cells or the inability to utilize actin-based motility.
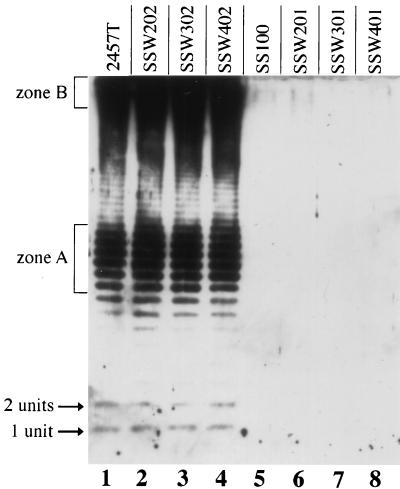
Shigella spp. that carry mutations in the biosynthetic pathway of LPS show decreased ability to form plaques on mammalian cell monolayers (33, 34). Among these, galU S. flexneri is unable to form plaques on mammalian cell monolayers (34). To determine whether the suppressor mutations cydC1, pepP1, or ribE1 caused alterations in LPS, LPSs of each mutant in both the galU and the wild-type backgrounds were examined by immunoblotting (Fig. 5). The LPS profile of each mutant was no different than that of the parent strain (Fig. 5, compare lanes 2 to 4 with lane 1 and lanes 6 to 8 with lane 5). Wild-type strain 2457T (Fig. 5, lane 1) and each of the mutants in the wild-type background (SSW202, SSW302, and SSW402 [Fig. 5, lanes 2 to 4, respectively]) had full O antigen, while galU S. flexneri (SSW100 [Fig. 5, lane 5]) and each of the mutants in this background (SSW201, SSW301, and SSW401 [Fig. 5, lanes 6 to 8) had undetectable levels of O antigen, as has been shown previously for galU S. flexneri (34). These data suggest that the cydC, pepP, and ribE mutations do not effect LPS biosynthesis.
FIG. 5.
LPS analysis of strains carrying suppressor mutations cydC1, pepP1, or ribE1. Shown is an immunoblot using S. flexneri serotype 2 antiserum. Lanes: 1, 2457T (wild-type); 2, SSW202 (cydC1); 3, SSW302 (pepP1); 4, SSW402 (ribE1); 5, SS100 (galU2); 6, SSW201 (galU2 cydC1); 7, SSW301 (galU2 pepP1); 8, SSW401 (galU2 ribE1). 1 unit or 2 units, LPS molecules with one or two O-antigen repeating units attached, respectively; zone A, LPS molecules with 11 to 17 O-antigen repeating units attached; zone B, LPS molecules with long O-antigen chains.
Levels of cytochrome bd expression correlate with the size of plaques in cydC1, pepP1, and ribE1 S. flexneri mutants.
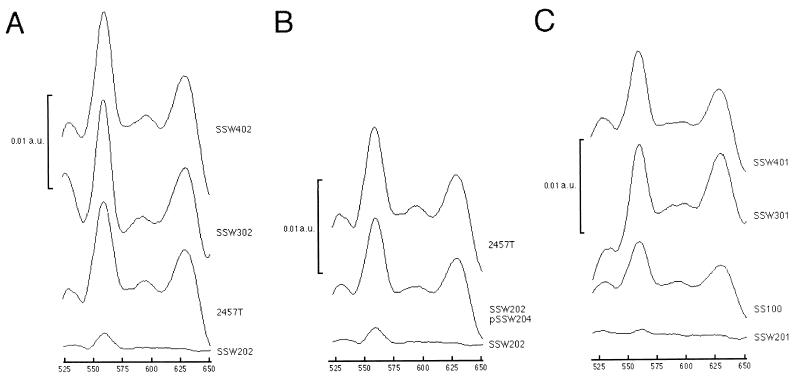
The cydDC gene products are required for proper membrane assembly of the cytochrome bd complex. E. coli mutants with disruptions in either the cydD or cydC locus do not express cytochrome bd in the bacterial membrane as measured by difference spectroscopy (11, 31). Since each of our mutants appeared to be linked to pathways of bacterial respiration, we determined the levels of cytochrome bd expression by measuring sodium dithionite-reduced minus potassium ferricyanide-oxidized difference spectra of membrane protein preparations from aerobically grown SSW202 (cydC1), SSW302 (pepP1), SSW402 (ribE1), and 2457T (wild-type). Since the spectral peak of the cytochrome b component of cytochrome bd overlies that of cytochrome bo, cytochrome bd levels are determined by measuring levels of the cytochrome d component, which corresponds to an absorbance peak at 630 nm (Fig. 6A). As expected, SSW202 expressed only baseline levels of cytochrome d (SSW202 vs. 2457T, P = 0.0011) (Fig. 6A and Table 2), and complementation of SSW202 in trans with pSSW204, which contains intact cydDC, led to a return to wild-type levels of cytochrome d expression (Fig. 6B and Table 2). Interestingly, SSW302 and SSW402, the two mutants that form larger plaques than the wild type does, had significantly increased levels of cytochrome d expression as compared with wild-type (SSW302 vs. 2457T, P = 0.04; SSW402 vs. 2457T, P = 0.033) (Table 2).
FIG. 6.
Sodium dithionite-reduced minus potassium ferricyanide-oxidized difference spectra of membrane protein preparations. (A) Suppressor mutants in the wild-type background (from top to bottom): SSW402 (ribE1), SSW302 (pepP1), 2457T (wild-type), and SSW202 (cydC1); (B) complementation of SSW202 by pSSW204 (from top to bottom), 2457T (wild-type), SSW202 (pSSW204) (cydC1 complemented in trans with cydDC), and SSW202 (cydC1); (C) suppressor mutants in the galU background (from top to bottom): SSW401 (galU2 ribE1), SSW301 (galU2 pepP1), SS100 (galU2), and SSW201 (galU2 cydC1). The magnitude of the absorbance peak at 630 nm directly corresponds to the amount of cytochrome d. Data represent results from three spectra per strain. a.u., absorbance units.
TABLE 2.
Levels of cytochrome d expression by S. flexneri strains
| Strain | Cytochrome d (nM/mg of protein ± 1 SD) |
|---|---|
| 2457T | 1.40 ± 0.26 |
| SSW202 | 0.09 ± 0.06 |
| SSW302 | 1.91 ± 0.13 |
| SSW402 | 2.10 ± 0.27 |
| SSW202(pSSW204) | 1.18 ± 0.07 |
| SS100 | 0.61 ± 0.06 |
| SSW201 | 0.07 ± 0.04 |
| SSW301 | 1.53 ± 0.58 |
| SSW401 | 1.84 ± 0.19 |
To determine the effect of the galU mutation on cytochrome levels, spectra were also measured for SSW201 (galU2 cydC1), SSW301 (galU2 pepP1), SSW401 (galU2 ribE1), and SS100 (galU2). The levels of cytochrome d expression of each of the suppressor mutants in the galU background was not significantly different from that of each in the wild-type background (Fig. 6C and Table 2). Yet, the level of cytochrome d expression of SS100 (galU2) was approximately half that of 2457T (wild-type) (P = 0.0071) (Fig. 6A and C and Table 2).
S. flexneri virulence in intranasally infected mice correlates with cytochrome bd expression.
The murine intranasal model of infection provides an in vivo model system in which to study Shigella pathogenesis. Following infection with a lethal inoculum of S. flexneri, mice die of an acute pneumonitis with bacterial invasion of both the bronchiolar and alveolar epithelium (24, 40, 42). Mice infected with sublethal inocula are able to clear the infection and resolve the inflammation (42). To assess whether the observed differences in plaque size and cytochrome bd expression in SSW202, SSW302, SSW402, and 2457T bear a relationship with the virulence of these strains in vivo, the lethal dose of each strain in C57BL/6 mice was determined. The lethal dose of wild-type S. flexneri strain 2457T was 107 CFU, as has been previously reported (40, 42). The lethal dose of SSW202 (cydC1) was 2 orders of magnitude higher, and that of SSW302 (pepP1) or SSW402 (ribE1) was 1 order of magnitude lower than that of 2457T (wild-type) (Table 3).
TABLE 3.
Plaque phenotype and lethal dose of S. flexneri strains
| Strain | Mutation | Gene responsible for plaque phenotype | Plaque phenotyped | Lethal dose (CFU) |
|---|---|---|---|---|
| 2457T | WTc | NAa | WT | 107 |
| SS100 | galU2 | galU | No plaques | NDb |
| SSW202 | cydC1 | cydC | <WT | 109 |
| SSW302 | pepP1 | ubiH | >WT | 106 |
| SSW402 | ribE1 | ribE | >WT | 106 |
NA, not applicable.
ND, not determined.
WT, wild type.
< and >, smaller and larger than indicated phenotype.
DISCUSSION
In this study, we describe three classes of S. flexneri mutants that are able to restore the plaque-forming phenotype to galU S. flexneri: disruption of the cydC, pepP, or ribE homologue in S. flexneri suppresses the inability of galU mutants to form bacterial plaques on mammalian cell monolayers. When these mutations were studied in the wild-type S. flexneri background, two distinct phenotypes were observed. A disruption of cydC (SSW202) led to the formation of smaller plaques and a 100-fold increase in lethal dose compared with the wild type. Either a disruption of pepP, which has polar effects on ubiH (SSW302), or a disruption of ribE (SSW402) led to the formation of larger plaques and a 10-fold decrease in lethal dose as compared with wild-type. The cydC mutant expressed essentially undetectable levels of cytochrome bd, while the pepP (ubiH) and ribE mutants each expressed significantly elevated levels of cytochrome bd as compared with wild-type. Taken together, these data suggest that virulence in these mutants is linked with alterations in cytochrome bd expression. It would be of interest to examine the epistatic relationship between cydC and both ubiH and ribE; we have as yet been unable to generate stable double mutants with which to address this issue, suggesting that such double mutants may not be viable.
Alterations in plaque formation can be due to one or more of the following: (i) altered ability to spread from an infected into an adjacent cell, (ii) secreted bacterial products with toxic effects on the cell monolayer, (iii) altered resistance to gentamicin, or (iv) altered ability to survive within an infected cell. To address whether actin-based motility was altered in the mutants, we examined each one’s ability to form actin tails and cell surface protrusions; there were no detectable differences from the wild-type strain. Since the double mutant in each class (cydC icsA, pepP icsA, or ribE icsA) did not form bacterial plaques, plaque formation by each parental strain was dependent on actin-based motility. Furthermore, none of these strains was cytotoxic for the monolayer, and none of the mutants in the wild-type background had alterations in sensitivity to gentamicin. Thus, actin-based motility, and not cytotoxicity or altered sensitivity to gentamicin, mediates the ability of the mutants to form plaques. Of note, a mutation in the aarD gene of Providencia stuartii, which bears significant sequence similarity to cydDC has been reported to cause a 32-fold increase in resistance to gentamicin (22).
To assess whether the observed differences in plaque size were due to differences in intracellular survival per se, we assessed the recovery of intracellular bacteria for each mutant in the icsA background as well as in the wild-type background. Within each background (icsA and wild-type), the cydC mutant exhibited significantly decreased intracellular survival compared with the pepP, ribE, icsA, or wild-type strain. Taken together, these data indicate that the small plaque formation by SSW202 (cydC1) is due to decreased intracellular survival. Based on the data presented here, it is not possible to distinguish whether the formation of larger-than-wild-type plaques by mutants SSW302 (pepP) and SSW402 (ribE) is due to slight increases in intracellular survival that were not detectable in the assays employed here or to more rapid cell-to-cell spread.
In addition to loss of plaque formation, the galU mutation has been shown to lead to delocalization of IcsA on the bacterial surface (34). The loss of plaque formation was initially postulated to be due to this delocalization of IcsA, which was thought to cause a corresponding decreased ability to assemble actin tails (34). Subsequently, we have shown that E. coli that express IcsA circumferentially on the surface are able to assemble actin tails efficiently (13). This observation indicates that loss of actin tail assembly and plaque formation of galU S. flexneri is unlikely to be due to delocalization of IcsA per se but rather is likely due to other factors. In the present study, we show that the galU strain can no longer be recovered from an infected monolayer 30 h postinfection. Since bacterial plaques are routinely visible beginning at 24 to 48 h postinfection, it seems possible that the marked decrease in intracellular survival of galU S. flexneri is at least in part responsible for its inability to form plaques.
We have previously shown that following intranasal inoculation of mice with wild-type S. flexneri, bacterial replication occurs within the infected mouse lung and that lethality correlates with the extent of bacterial replication (42). Thus, the observed differences in lethal dose among S. flexneri strains SSW202 (cydC1), SSW302 (pepP1), SSW402 (ribE1), and 2457T (wild-type) in intranasally infected mice is likely a result of both differences in intracellular survival of the mutants, leading to net differences in bacterial load within the mouse lung and differences in cell-to-cell spread.
The phenotype of the cydC mutant differs significantly in the galU and wild-type backgrounds: in the galU background, it has increased intracellular survival and forms larger plaques than the galU parental strain (Fig. 1 and 2), whereas in the wild-type background it has decreased intracellular survival and forms smaller plaques than the wild type (Fig. 2 and 4). The galU mutation expresses cytochrome bd at approximately half the level of expression in wild-type (Fig. 6 and Table 2). We hypothesize that the galU mutation affects the electron transport chain so as to lead to the markedly reduced intracellular survival of the galU mutant, and in such a way that either a decrease or an increase in cytochrome bd expression can suppress the intracellular survival defect. While it is apparent how an increase in cytochrome bd expression would lead to suppression of the intracellular survival defect of galU S. flexneri, it is less clear how a further reduction in cytochrome bd expression would also lead to its suppression. The suppression of the galU intracellular survival defect was detected in our screen by the ability of the resultant suppressor strains to form plaques that are significantly smaller than plaques formed by either wild-type or cydC S. flexneri. It appears that the intracellular survival defect of galU S. flexneri is due to more than the decreased level of cytochrome bd expression, since in the wild-type background, the cydC mutation leads to a lesser defect in intracellular survival than the galU cydC double mutant, as evidenced by the slightly more rapid growth (Fig. 2) and slightly larger plaques formed by the cydC mutant (compare Fig. 1 and 4).
The respiratory chain of E. coli contains two terminal oxidases, cytochrome bd and cytochrome bo, each of which functions to reduce molecular oxygen to water by utilizing reducing intermediates shuttled through the electron transport chain. Energy is conserved in this process though the generation of a proton gradient in the bacterial inner membrane. Cytochrome bd terminal oxidase has a relatively higher affinity for molecular oxygen (Km = 0.38 μM) (19) and a lower efficiency of oxygen utilization (H+/e− ratio = 1.0) (8) than cytochrome bo terminal oxidase and is therefore expressed predominantly under microaerophilic conditions. In contrast, cytochrome bo terminal oxidase has a lower affinity for oxygen (Km = 2.9 μM) (19) and a higher efficiency of oxygen utilization (H+/e− ratio = 2.0) (8) than cytochrome bd and is therefore predominantly expressed when oxygen is not limited. Distribution of microflora within the gastrointestinal tract suggests that the gut becomes progressively more limited in oxygen distal to the stomach. Since shigellosis is predominantly a disease of the human colon, in the natural course of S. flexneri infection, up-regulation of the cytochrome bd terminal oxidase as the bacterium transits though the gastrointestinal tract would be predicted to occur. If this is the case, then up-regulation of cytochrome bd in turn might enhance the ability of S. flexneri to survive within host cells of the colon.
The ribE gene encodes 6,7-dimethyl-8-ribityllumazine synthase, which catalyzes the condensation of 5-amino-6-ribitylamino-2,4(1H, 3H)-pyrimidinedione with 3,4-dihydroxy-2-butanone phosphate in the riboflavin biosynthetic pathway (18, 26). Riboflavin in the form of riboflavin 5′-phosphate is required as a cofactor for many metabolic enzymes; one of its functions is to serve as an electron acceptor for dehydrogenases in the respiratory chain. The observation that an S. flexneri mutant defective in riboflavin biosynthesis expresses increased levels of cytochrome bd is consistent with previous studies of E. coli mutants with defects in riboflavin biosynthesis that also overexpress cytochrome bd (6). Here we demonstrate a similarly increased expression of cytochrome bd in an S. flexneri mutant that has a defect in ubiquinol-8 biosynthesis. Ubiquinol-8 is an intermediate in the electron transfer chain that shuttles electrons to the terminal oxidases during aerobic respiration. Since both riboflavin and ubiquinol-8 mutants are defective in the initial steps of respiration, they may induce the compensatory expression of proteins (such as cytochrome bd terminal oxidase) usually expressed under more oxygen limited conditions even while under the relatively oxygen-rich experimental conditions of this study.
Since the terminal oxidases are highly conserved among bacterial species, cytochrome bd may have a role in virulence of other intracellular bacterial pathogens as well. As has been shown here for SSW202 (cydC), mutants of other intracellular pathogens that have reduced expression of cytochrome bd may be markedly attenuated, causing significantly reduced morbidity, yet retaining the ability to invade target cells, thereby inducing an immune response by the natural route of infection. Since bacterial LPS is unaltered in cytochrome bd-deficient mutants, elimination of cytochrome bd would represent a novel form of attenuation in the development of live vaccines for pathogens in which protective immunity is serotype-specific. Further, these genes could be targets for attenuated variants in vaccine design.
ACKNOWLEDGMENTS
We thank T. Maurelli for galU S. flexneri strain BS109; W. Jacobs, B. Bloom, A. Casadevall, and J. Peisach for helpful discussions; T. Pham for technical assistance; and the Analytical Imaging and DNA Sequencing (NCI grant CA13330) Facilities at the Albert Einstein College of Medicine for technical assistance.
This work was supported by NIH grants T32 GM07288 (S.S.W.), GM40168 (R.S.M.), and AI35817 (M.B.G.), the Pew Scholars Program in the Biomedical Sciences (M.B.G.), Established Investigator and Grant-in-Aid Awards from the American Heart Association (M.B.G.), and a Burroughs Wellcome Fund Postdoctoral Fellowship (S.S.).
REFERENCES
- 1.Andrews G P, Hromockyj A E, Coker C, Maurelli A T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect Immun. 1991;59:1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aranda C M A, Swanson J A, Loomis W P, Miller S I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudry B, Maurelli A T, Clerc P, Sadoff J C, Sansonetti P J. Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol. 1987;133:3403–3413. doi: 10.1099/00221287-133-12-3403. [DOI] [PubMed] [Google Scholar]
- 4.Bernardini M L, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner B R, Huang H-C, Schieven G L, Ames B N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980;143:926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogachev A V, Murtazina R A, Skulachev V P. Cytochrome d induction in Escherichia coli growing under unfavorable conditions. FEBS Lett. 1993;336:75–78. doi: 10.1016/0014-5793(93)81612-4. [DOI] [PubMed] [Google Scholar]
- 7.Buysse J M, Stover C K, Oaks E V, Venkatesan M, Kopecko D J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987;169:2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter P A, Chepuri V, Gennis R B, Gunsalus R P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990;172:6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ericsson H M, Sherris J C. Antibiotic sensitivity testing, report of an international collaborative study. Acta Pathol Microbiol Scand. 1971;217:3–90. [PubMed] [Google Scholar]
- 10.Formal S B, Dammin G J, LaBrec E H, Schneider H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958;75:604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgiou C D, Fang H, Gennis R B. Identification of the cydC locus required for expression of the functional form of the cytochrome d terminal complex in Escherichia coli. J Bacteriol. 1987;169:2107–2112. doi: 10.1128/jb.169.5.2107-2112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg M B, Bârzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg M B, Theriot J A. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc Natl Acad Sci USA. 1995;92:6572–6576. doi: 10.1073/pnas.92.14.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green G N, Gennis R B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983;154:1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hale T L. Invasion of epithelial cells by Shigella: microbial invasion of non-phagocytic cells. Ann Inst Pasteur Microbiol. 1986;137A:311–314. doi: 10.1016/s0769-2609(86)80040-0. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz S L. New vaccine development: establishing priorities. II. Disease of importance in developing countries. Washington, D.C: National Academy Press; 1986. The burden of disease resulting from diarrhea. [Google Scholar]
- 18.Kis K, Volk R, Bacher A. Biosynthesis of riboflavin. Studies on the reaction mechanism of 6,7-dimethyl-8-tibityllumazine synthase. Biochemistry. 1995;34:2883–2892. doi: 10.1021/bi00009a019. [DOI] [PubMed] [Google Scholar]
- 19.Kita K, Konishi K, Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. J Biol Chem. 1984;259:3375–3381. [PubMed] [Google Scholar]
- 20.Kleckner N, Bender J, Gottesman S. Uses of transposons with emphasis on Tn10. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- 21.Kocks C, Marchand J-B, Gouin E, d’Hauteville H, Sansonetti P J, Carlier M-F, Cossart P. The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility on Listeria innocua and Escherichia coli, respectively. Mol Microbiol. 1995;18:413–423. doi: 10.1111/j.1365-2958.1995.mmi_18030413.x. [DOI] [PubMed] [Google Scholar]
- 22.Macinga D R, Rather P N. aarD, a Providencia stuartii homologue of cydD: role in 2′-N-acetyltransferase expression, cell morphology and growth in the presence of an extracellular factor. Mol Microbiol. 1996;19:511–520. doi: 10.1046/j.1365-2958.1996.385912.x. [DOI] [PubMed] [Google Scholar]
- 23.Makino S, Sasakawa C, Kamata K, Kurata T, Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986;46:551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- 24.Mallett C P, VanDeVerg L, Collins H H, Hale T L. Evaluation of Shigella vaccine safety and efficacy in an intranasal challenged mouse model. Vaccine. 1993;11:190–196. doi: 10.1016/0264-410x(93)90016-q. [DOI] [PubMed] [Google Scholar]
- 25.Maurelli A T, Baudry B, d’Hauteville H, Hale T L, Sansonetti P J. Cloning of virulence plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuberger G, Bacher A. Biosynthesis of riboflavin. Enzymatic formation of 6,7-dimethyl-8-ribityllumazine by heavy riboflavin synthase from Bacillus subtilis. Biochem Biophys Commun. 1986;139:1111–1116. doi: 10.1016/s0006-291x(86)80292-3. [DOI] [PubMed] [Google Scholar]
- 27.Oaks E V, Wingfield M E, Formal S B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal T, Newland J W, Tall B D, Formal S B, Hale T L. Intracellular spread of Shigella flexneri associated with the kcpA locus and a 140-kilodalton protein. Infect Immun. 1989;57:477–486. doi: 10.1128/iai.57.2.477-486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole R K, Gibson F, Wu G. The cydD gene product, component of a heterodimeric ABC transporter, is required for assembly of periplasmic cytochrome c and of cytochrome bd in Escherichia coli. FEMS Microbiol Lett. 1994;117:217–224. doi: 10.1111/j.1574-6968.1994.tb06768.x. [DOI] [PubMed] [Google Scholar]
- 30.Poole R K, Hatch L, Cleeter M W J, Gibson F, Cox G B, Wu G. Cytochrome bd biosynthesis in Escherichia coli: the sequences of the cydC and cydD genes suggest that they encode the components of an ABC membrane transporter. Mol Microbiol. 1993;10:421–430. [PubMed] [Google Scholar]
- 31.Poole R K, Williams H D, Downie J A, Gibson F. Mutations affecting the cytochrome d-containing oxidase complex of Escherichia coli K12: Identification and mapping of a fourth locus, cydD. J Gen Microbiol. 1989;135:1865–1874. doi: 10.1099/00221287-135-7-1865. [DOI] [PubMed] [Google Scholar]
- 31a.Sallustio, S., S. S. Way, and M. B. Goldberg. Unpublished data.
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Sandlin R C, Goldberg M B, Maurelli A T. Effect of O side chain length and composition on the virulence of Shigella flexneri 2a. Mol Microbiol. 1996;22:63–73. doi: 10.1111/j.1365-2958.1996.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 34.Sandlin R C, Lampel K A, Keasler S P, Goldberg M B, Stolzer A L, Maurelli A T. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in bacterial outer membrane. Infect Immun. 1995;63:229–237. doi: 10.1128/iai.63.1.229-237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sansonetti P J, Arondel J, Fontaine A, d’Hauteville H, Bernardini M L. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 36.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasakawa C, Kamata K, Sakai T, Makino S, Yamada M, Okada N, Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988;170:2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shere K D, Sallustio S, Manessis A, D’Aversa T G, Goldberg M B. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 39.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanDeVerg L L, Mallett C P, Collins H H, Larsen T, Hammack C, Hale T L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63:1947–1954. doi: 10.1128/iai.63.5.1947-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Way J C, Davis M A, Morisato D, Roberts D E, Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984;32:369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- 42.Way S S, Borczuk A C, Dominitz R, Goldberg M B. An essential role for gamma interferon in innate resistance to Shigella flexneri infection. Infect Immun. 1998;66:1342–1348. doi: 10.1128/iai.66.4.1342-1348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young I G, Stroobant P, MacDonald C G, Gibson F. Pathway for ubiquinone biosynthesis on Escherichia coli K-12: gene enzyme relationships and intermediates. J Bacteriol. 1973;114:42–52. doi: 10.1128/jb.114.1.42-52.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]