Abstract
The Coronavirus Disease 2019 (COVID‐19) pandemic has been caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). It is a global problem that humanity has not yet found a definitive solution for it. In this regard, a global effort has been done to find effective or potential adjuvant therapies in order to fight this infection. Genistein is a small, biologically active phytoestrogen flavonoid that is found in high amounts in soy and plants of the Fabaceae family. This important compound is known due to its anti‐cancer, anti‐inflammatory, and antioxidant effects. Additionally, protective effects of genistein have been reported in different pathological conditions through modulating intracellular pathways such as PI3K, Akt, mTOR, NF‐κB, PPARγ, AMPK, and Nrf2. Scientific evidence suggests that genistein could have a potential role to treat COVID‐19 through its anti‐inflammatory and anti‐oxidant effects. Furthermore, it appears to interfere with intracellular pathways involved in viral entry into the cell. This review provides a basis for further research and development of clinical applications of genistein as a potential alternative therapy to decrease inflammation and oxidative stress in COVID‐19 patients.
Practical applications
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the etiological agent for the Coronavirus Disease 2019 (COVID‐19), has brought unprecedented untold hardship to both developing and developed countries. The inflammation, cytokine storm, and oxidative stress have an important role in the pathogenesis of this infection. In this regard, finding plant‐derived compounds with anti‐inflammatory and anti‐oxidative effects would be very beneficial in reducing the mortality induced by this infection. Genistein an isoflavone derived from soy‐rich products possesses versatile biological activities. It has potent anti‐inflammatory and anti‐oxidative and immunomodulatory effects. Furthermore, this compound may prevent viral entry to host cells and reduce SARS‐CoV2‐induced lung injury. Therefore, we suggest further studies on the effects of genistein on SARS‐Cov‐2 infection.
Keywords: anti‐inflammatory, anti‐oxidant, COVID‐19, flavonoid, genistein, SARS‐CoV‐2
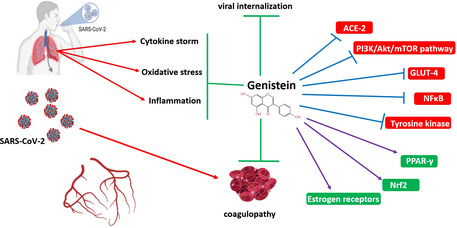
The probable beneficial effects of genistein in COVID‐19.
Abbreviations
- ACE2
angiotensin‐converting enzyme2
- AKT
protein kinase B
- AMPK
AMP‐activated protein kinase
- ARDS
acute respiratory distress syndrome
- COVID‐19
Coronavirus Disease 2019
- COX‐2
cyclooxygenase‐2
- ER
estrogen receptors
- GLUT‐4
glucose transporter‐4
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- mTOR
mechanistic target of rapamycin
- NFκ B
nuclear factor kappa B
- Nrf2
nuclear factor erythroid 2‐related factor 2
- PGE2
Prostaglandin E2
- PI3K
phosphatidylinositol 3‐kinase
- PPARγ
Peroxisome proliferator‐activated receptor γ
- PTEN
phosphatase and tensin homolog
- PTK
protein tyrosine kinase
- ROS
reactive oxygen species
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SOD
superoxide dismutase
- TLR4
toll‐like receptor 4
- TMPRSS2
protease Transmembrane protease serine 2
- TNF‐α
tum or necrosis factor‐ α
- TXA2
Thromboxane A2
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a single‐stranded RNA‐enveloped virus. A large number of glycosylated S proteins cover the surface of SARS‐CoV‐2 and bind to the host cell receptor angiotensin‐converting enzyme 2 (ACE2), mediating viral cell entry. When the S protein binds to the receptor, TM protease serine 2 (TMPRSS2), a type 2 TM serine protease located on the host cell membrane, promotes virus entry into the cell by activating the S protein. Once the virus enters the cell, the viral RNA is released, polyproteins are translated from the RNA genome, and replication and transcription of the viral RNA genome occur via protein cleavage and assembly of the replicase–transcriptase complex. Viral RNA is replicated, and structural proteins are synthesized, assembled, and packaged in the host cell, after which viral particles are released. So, attacks the host using angiotensin‐converting enzyme2 ACE2 receptors have a crucial role in the pathogenesis of SARS‐CoV‐2 (Bian & Li, 2021; Khezri, Yousefi, & Ghasemnejad‐Berenji, 2021). Since these receptors have been broadly distributed on immune cells and various tissues, SARS‐CoV‐2 infection, motivates the immune responses by stimulating the monocytes and macrophages as well as, adaptive T and B cell immune responses and cytokines production, in the lung tissue (Jafarzadeh et al., 2020). Subsequently, the replication of SARS‐CoV‐2 induced programmed cell death (pyroptosis) in the airway epithelial cells. Finally, this process induces vascular leakage and triggers severe destructive inflammatory responses (Wang, Yang, & Xu, 2021). For instance, interleukin (IL‐)1β, which is one of the major inflammatory cytokines released during SARS‐CoV‐2 infection, is involved in viral‐induced pyroptosis (Ferreira et al., 2021; Li et al., 2020). In this regard, a wide range of clinical symptoms from mild forms such as myalgia, cough, and fever to moderate forms such as localized inflammation and pneumonia which require hospitalization could happen(Lechien et al., 2020). It should be mentioned that the cytokine storm which is a main pathological sign of COVID‐19 is an unregulated production of inflammatory cytokines inducing destructive inflammation and causing organ failure such as severe cardiovascular, pulmonary, and kidney injuries in SARS‐CoV‐2 infection (Catanzaro et al., 2020; Ghasemnejad‐Berenji et al., 2021; Robba et al., 2020). In addition, oxidative stress, mitochondrial dysfunction, and apoptosis pathways are involved in this multiple organ failure (de Las Heras et al., 2020). Since in this viral infection various signaling pathways are affected at the molecular level, in the lack of a specific treatment for this novel infection, finding a safe and effective compound that could inhibit the effects of this virus on infected cells could be a valuable achievement in controlling this pandemic (Zhang & Liu, 2020). Genistein is a multifunctional natural isoflavonoid class of flavonoids. Similar to other plant constituents, such as lignans, which possess an estrogenic effect, genistein is a typical example of a phytoestrogen compound (Sharma et al., 2017). The best‐known sources of genistein are soy‐based foods, such as soy cheese or soy drinks (i.e., soy milk and soy‐based beverages). The content of genistein in mature soybeans has been shown to range from 5.6 to 276 mg/100 g, and an average content of 81 mg/100 g is often described for comparative purposes (Spagnuolo et al., 2015). Facile routes for the chemical synthesis of genistein are available, and the production of genistein can be engineered in transgenic plants (Dixon & Ferreira, 2002). To extract the genistein from plants, solvents such as methanol, ethanol, and acetonitrile are normally used (Zhao et al., 2019). Genistein possesses many therapeutic potentials and pharmacologic properties, such as anti‐carcinogenic (Tuli et al., 2019), anti‐microbial (Wang et al., 1956), anti‐viral (LeCher et al., 2019), antioxidant (Borrás et al., 2006), and anti‐inflammatory activity (Ginwala et al., 2019). In vivo and in vitro analyses indicated that genistein can modulate numerous signaling cascades in inflammatory diseases. Furthermore, it has been shown that genistein could inhibit the infectivity of various viruses affecting humans and animals, including adenovirus, herpes simplex virus, HIV, respiratory syndrome virus, and rotavirus (Andres et al., 2009). In this regard, we hypothesized that genistein could exert beneficial effects against SARS‐CoV‐2 infection. So, this review will highlight the promising targets of genistein in pathological manifestations of SARS‐CoV‐2 infection, which can be exploited in preclinical and clinical investigations. To the best of review literature to date, there is no laboratory or clinical investigation which confirms the potential of genistein against SARS‐CoV‐2. However, the vast pharmacological properties of genistein promise the potential of genistein against SARS‐CoV‐2‐induced oxidative stress and inflammation.
2. GENISTEIN STRUCTURE
Genistein [4′,5,7‐trihydroxyisoflavone or 5,7‐dihydroxy‐3‐(4‐hydroxyphenyl) chromen‐4‐one] (C15H10O5) belongs to a multifunctional natural isoflavonoid class of flavonoids with a 15‐carbon skeleton. (Węgrzyn et al., 2010). It has been isolated from Genista tinctoria L. for the first time and its name originated from this plant (Spagnuolo et al., 2015). The isoflavones are structurally characterized by their 3‐phenylchromen‐4‐one backbone, which consists of two benzene rings linked by a heterocyclic pyran ring. In addition to this heterocyclic core, genistein and its related isoflavone family members are polyphenols, in that they contain several hydroxyl groups attached to core phenyl rings. These phenols lend significant antioxidant activity to this class of compounds, with genistein and other related flavonoids, such as epigallocatechin 3‐gallate possessing significant activity against free radicals in tissue (Andersen & Markham, 2005). Importantly, genistein is a recognized protein‐tyrosine kinase inhibitor (Akiyama et al., 1987). This activity is presumed to stem from genistein's C4′ phenolic group, which structurally resembles the phosphoacceptor moiety of tyrosine (Pavese et al., 2010). As shown in Figure 1, there is a similarity between the chemical structure of genistein and estradiol (Figure 1), leading to its binding ability to estrogen receptors (ERs) (Vaya & Tamir, 2004). Genistein shares structural features with the potent estrogen estradiol‐17β (4), particularly the phenolic ring and the distance (11.5 A) between its 4′‐ and 7‐ hydroxyl groups (Figure 1). These features confer the ability to bind estrogen receptors and sex‐hormone binding proteins, and genistein can thus exert both estrogenic and anti‐estrogenic activity, the latter by competing for receptor binding by estradiol (Dixon & Ferreira, 2002). Many epidemiological studies have indicated the beneficial effects of plant‐based foods rich in isoflavones on human health including cancers, cardiovascular diseases, osteoporosis, diabetes, and postmenopausal symptoms (Goldwyn et al., 2000; Pabich & Materska, 2019; Setchell & Cassidy, 1999; Sirtori, 2001). Thus, many studies have been conducted to clarify the effects of these compounds, especially genistein, in human pathological and physiological states (Behloul & Wu, 2013).
FIGURE 1.
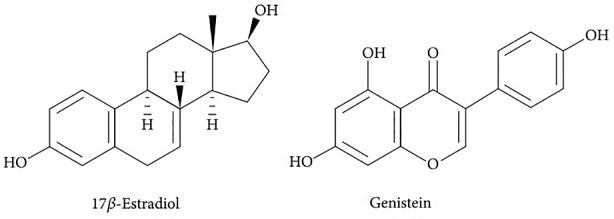
Structural similarity between genistein and 17β‐Estradiol.
3. PHARMACOLOGICAL EFFECTS OF GENISTEIN AS A PROBABLE BENEFICIAL COMPOUND FOR SARS‐COV‐2 INFECTION
3.1. Antiviral effects of genistein
The antiviral effects of genistein against viruses including cytomegalovirus, bovine herpesvirus‐1, HSV‐1 human immunodeficiency virus, African swine fever virus, human papillomavirus, and respiratory syndrome virus have been reported previously (LeCher et al., 2019). It has been shown that genistein could inhibit viral replication with various mechanisms (Arabyan et al., 2018). Furthermore, the inhibitory effect of genistein on human cytomegalovirus via blocking viral immediate‐early protein function has been reported previously (Akula et al., 2002; Arabyan et al., 2018; LeCher et al., 2019; Sauter et al., 2014).
3.2. Genistein effect on estrogen receptors (ERs)
Estrogen has anti‐inflammatory properties by decreasing gene and protein expressions of inflammatory elements (Pelekanou et al., 2016; Suuronen et al., 2005). Studies have indicated that low doses of estrogen could subside persistent inflammation (Samantaray et al., 2016). Genistein has structural similarity to estrogen and it binds to ERs, suggesting that it may show estrogenic function (Ishimi et al., 2000). Protective effects of ERs mediators were also reported in murine models of pulmonary inflammation induced by influenza virus infection (Robinson et al., 2014; Vermillion et al., 2018). Consistently, ER modulators and estrogen hormones such as estradiol have been presently reviewed as beneficial agents for reducing the severity of SARS‐CoV‐2 infection (Ghasemnejad‐Berenji et al., 2020; Suba, 2020; Zhang & Liu, 2020). It has been reported that ERs activation disrupts NF‐κB transactivation and inhibits the transcription factors NF‐IL6 and subsequently suppresses the expression of IL‐6 gene (Liu et al., 2005). Furthermore, Th17 cell differentiation is affected by estradiol (Fuseini et al., 2019). Previous studies have shown that ER‐α activation in immune cells could reduce Th1 and Th17 responses and skew cytokine production (Calderone et al., 2020; Vegeto et al., 2010). Genistein has shown the ability to modulate the estrogen receptors (Sutrisno et al., 2018) and therefore, could subside the inflammatory cascades with these properties (Du et al., 2018). In addition, modulation of ER has been suggested in a murine experimental model of pulmonary inflammation as a beneficial therapeutic strategy. In particular, ER‐α is expressed in infiltrated or resident inflammatory cells of the respiratory system and activation of these receptors by estradiol markedly decreases the biochemical and histological markers of hyper‐inflammation (Calderone et al., 2020; Murphy, 2010). In this regard, it has been reported that estrogen receptor mediators could exert protective effects in murine models of pulmonary inflammation induced by influenza virus infection (Vermillion et al., 2018). As genistein has estrogenic activity in tissues (Kavoosi et al., 2016), considering the role of ERs activation in the prevention of SARS‐CoV‐2 induced hyper‐inflammation, it could be hypothesized that activation of estrogen receptors by genistein inhibits the development of inflammation in COVID‐19.
3.3. Genistein and inhibition of tyrosine kinase
Genistein inhibits protein tyrosine kinase (PTK) at pharmacological dosage (Russo et al., 2016). Observations signify that the tyrosine kinase signaling cascade can be an important target for pharmacologic intervention in order to prevent airway inflammation (Duan et al., 2003). PTK plays a fundamental role in maintaining cellular homeostasis and accommodation to the external environment stress through diverse cellular processes, such as inflammation, proliferation, migration, differentiation, and preservation of cellular barrier integrity (Aschner & Downey, 2018). According to these regulatory effects, PTK dysregulation might play a critical role in the pathogenesis of many inflammatory pulmonary dysfunctions, with an overlap in various disease states, proposing their wide‐range function as potential pharmacologic targets (Assaad & Assaad‐Khalil, 2020). Also, over‐activation of tyrosine kinase has been detected as a potential cascade contributing to the induction of inflammatory cells such as eosinophils, mast cells, neutrophils, and macrophages (Berkow et al., 1989; Dong et al., 1993; Elmarakby et al., 2011). It has been reported that enhanced tyrosine phosphorylation is involved in many destructive diseases such as inflammation‐induced respiratory complications (Aschner & Downey, 2018; Page et al., 2009). Furthermore, PTK has a key role in cytokine production(Hsu et al., 2001). Many cytokines, including IL‐10 and IL‐6 and tumor necrosis factor‐ α (TNF‐α), uses tyrosine kinases in their signaling pathways (Dahle et al., 2004; Page et al., 2009). It has been shown that inhibition of tyrosine kinase by genistein reduces monocyte chemoattractant protein (MCP‐1) excretion via inhibition of TNF‐α‐induced NFκ B activation (Čoma et al., 2021; Kim, 2021). In this regard, genistein‐induced inhibition of tyrosine kinase exerts anti‐inflammatory effects which could be a potential mechanism for preventing SARS‐CoV‐2‐induced lung injury.
3.4. Genistein and PI3K/Akt/mTOR pathway
Protein kinase B (Akt) is a potential pharmacological target for the advanced‐stage SARS‐CoV‐2 infection; its inhibition will effectively suppress the pathological fibroproliferation, cytokine storm, platelet activation, and inflammation associated with COVID‐19 (Basile et al., 2021). Furthermore, this inhibition could prevent scarring and lung injuries (Wang, Lin, et al., 2021). In addition, since inhibition of Akt by pharmacologic inhibitors has also been reported to reduce ACE2 expression, a crucial receptor for the viral entry into the respiratory system cells, targeting Akt for COVID‐19 seems to be a feasible option (Somanath, 2020). The phosphoinositide 3 (PI3)‐kinase/Akt pathway induces hyper‐inflammation in various pathologic states (Khezri, Moloodsouri, et al., 2022; Zhang et al., 2021) and, Akt inhibition could suppress inflammation (Zhang, Ma, et al., 2020). It has been reported that inhibition of Akt could suppress inflammation and alleviate lung tissue injury in acute respiratory distress syndrome (ARDS) (Liu et al., 2020). Furthermore, in ARDS lung wound resolution, vascular regeneration, and limiting scar formation have been observed by this inhibition(Qi et al., 2016). In this regard, the potential therapeutic benefits of Akt inhibitors such as genistein in treating ARDS in advanced‐stage COVID19 patients have been suggested recently (Li & Sarkar, 2002; Somanath, 2020). Interestingly, some of the inhibitors of the PI3K/Akt/mTOR pathway, especially rapamycin, which targets the mechanistic target of rapamycin (mTOR) function, were indicated to suppress the replication of viruses such as the Middle East respiratory syndrome coronavirus (MERS‐CoV) (Kindrachuk et al., 2015) and the influenza virus (Murray et al., 2012). This suggests the potential beneficial effects of genistein in inhibiting the replication of SARS‐CoV‐2. In addition when patients progress to uncontrolled immune responses, targeting this pathway could be a potential therapeutic strategy to inhibit neutrophil recruitment, suppress the cytokine storm, and enhance the suppressor regulatory T cells (Ghasemnejad‐Berenji, 2021b). Furthermore, due to the involvement of this signaling pathway in clot formation (Abu‐Eid & Ward, 2021; Meng et al., 2021), inhibiting this pathway could exert a preventive effect on thrombosis associated with severe COVID‐19 cases. It has been reported that genistein inhibits the PI3K/Akt/mTOR pathway either directly or indirectly (Lee et al., 2016; Malloy et al., 2018; Sahin et al., 2012; Tan et al., 2014). So, the potential beneficial role of genistein in COVID‐19 by inhibiting the PI3K/Akt/mTOR pathway could be explained in three steps. In the early stages of infection, inhibiting this pathway by genistein could prevent the entry and replication of the SARS‐CoV‐2 and decrease the viral load and subsequently ameliorate patient outcomes. It should be mentioned that, during the initial stages of the immune responses, inhibiting PI3K and Akt by genistein could eradicate the virus before the induction of severe immune dysregulation. In addition, the uncontrolled immune responses in the progressive stage of COVID‐19 targeting this pathway by genistein could be a potential pharmacologic strategy to enhance the suppressor regulatory T cells, inhibit neutrophil recruitment, and suppress the cytokine storm. The other probable beneficial effect of genistein that could be related to PI3K/AKT inhibition is the impact of PI3K/AKT on glucose transporter‐4 (GLUT‐4) (Figure 2). GLUT‐4 is the major glucose transporter in cardiac and skeletal muscles and adipose tissue (Langfort et al., 2003). It has been reported that Akt activation, induces rapid translocation of intracellular vesicles which includes GLUT4 to the cell surface, resulting in an enhancement of the cellular glucose transport activity. So, inhibition of glucose uptake by GLUT4 could inhibit glucose transport and viral replication (Yu et al., 2011). In this regard, it has been hypothesized that activation of the PI3K/AKT signaling pathway by SARS‐CoV‐2 contributes to inducing glucose uptake through GLUT‐4 leading to increase glycolysis and viral replication in host cells (Malgotra & Sharma, 2021). Since it has been reported that genistein is a potent and direct inhibitor of GLUT‐4 (Lewicki et al., 2018), the direct inhibition of GLUT‐4 by genistein from one side and inhibition of the PI3K/AKT pathway from another side could disrupt the viral replication (Figure 2). Furthermore, due to the involvement of the PI3K/AKT pathway in clot formation, targeting this pathway by genistein could prevent thrombosis in COVID‐19 patients.
FIGURE 2.
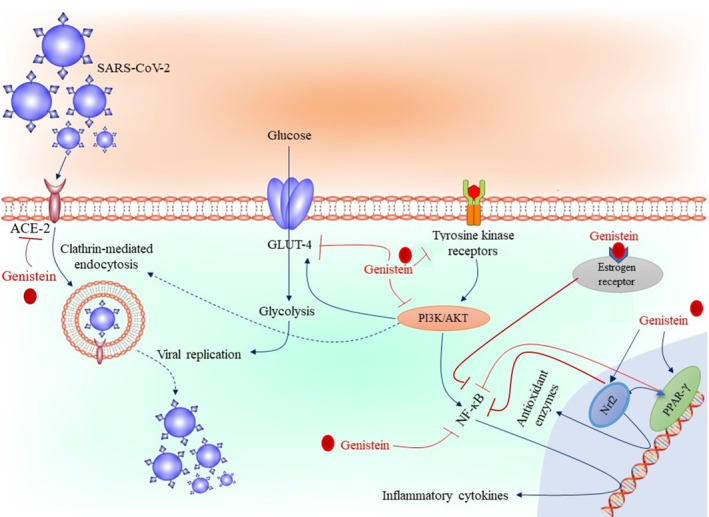
Possible molecular mechanisms of genistein against COVID‐19. ACE‐2: angiotensin‐converting enzyme 2, Nrf2: nuclear factor erythroid 2‐related factor 2, NF‐κB: nuclear factor kappa‐light‐chain‐enhancer of activated B cells, PI3K: phosphoinositide 3 kinase, GLUT‐4: glucose transporters‐4, PPAR‐γ: Peroxisome proliferator‐activated receptor gamma, SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2.
3.5. immunomodulatory and anti‐inflammatory effects of genistein
It has been approved that inflammation plays an essential role in the development of COVID‐19‐induced lung injury (Khezri, Zolbanin, et al., 2021). Thus, suppressing the immune responses with immunomodulatory drugs may be as crucial as addressing SARS_CoV‐2 replication to inhibit the progression of multiorgan injuries (Rizk et al., 2020). Immunologically, viral immunopathogenesis involved in SARS‐CoV2 infection is specified by increased amounts of proinflammatory cytokines, as well as upregulated frequency and over‐function of Th1 and Th17 cells (Alshammary & Al‐Sulaiman, 2021; Tahmasebi et al., 2021). In this regard pharmacological inhibition of IL‐1, IL‐6, and TNF‐α, is a good practical example for the application of inflammatory cytokine suppression strategy (Buonaguro et al., 2020). It has been reported previously that genistein is a highly pleiotropic molecule with the ability to interact with different cellular targets involved in hyper‐inflammation states (Ji et al., 2011; Verdrengh et al., 2003; Zhao et al., 2016). It has been reported that genistein exerts its anti‐inflammatory properties via several mechanisms, for example, it has been shown that genistein could downregulate the NF‐κB, leading to a reduction in the expression of IL‐6, IL‐1, and TNF‐α (Calveley et al., 2010; Mohammad‐Shahi et al., 2011; Sutrisno et al., 2015). In addition, the inhibitory effect of mitogen‐activated protein kinase (MAPK) pathways by this molecule which are the pathways activated by most inflammatory stimuli in humans have been reported in several studies (Li et al., 2008; Linford et al., 2001). Since genistein has been indicated to downregulate cytokine‐induced signal transduction pathways in the immune system cells, we hypnotized that it may also, exert anti‐inflammatory effects on COVID‐19. These immunoregulatory effects of genistein could be attributed to the inhibitory effect of this compound on the production of cytokines and chemokines like IL‐6, TNF‐α, and Macrophage inflammatory protein‐1 alpha (MIP‐1a) (Kim et al., 2004; Verdrengh et al., 2003). TNF induces leukocyte adhesion and MIP‐1a acts as a chemoattractant for T cells, macrophages, and neutrophils, thus recruiting inflammatory immune cells into the inflamed focus (Bhavsar et al., 2015). Furthermore, very high levels of IL‐6 in serum of patients with COVID‐19 have been reported (Ulhaq & Soraya, 2020) which indicates the important role of that in the development of SARS‐Cov‐2 induced ARDS (Magro, 2020). Previously it has been reported that genistein could downregulate the TNF and IL‐6 production by immune cells in in vitro models of lipopolysaccharide (LPS) induced inflammation (Choi et al., 2016; Du et al., 2018; Ji et al., 2012). The other molecular effect of genistein is the ability of this compound to inhibit the secretion of granule enzymes by activated neutrophils and macrophages which leads to the downregulation of the inflammatory responses (Kang et al., 2001; Kim et al., 2001). Furthermore, it has been reported that genistein could inhibit NF‐kB DNA binding by blocking the phosphorylation of the inhibitory protein IkBa, which will inhibit the nuclear translocation of the NF‐kB (Davis et al., 1999; Romier et al., 2008; Shukla et al., 2015). Genistein significantly inhibited NF‐kB activation by the DNA‐damaging agents TNF‐α and H2O2 (Mazumder & Hongsprabhas, 2016). The migration of inflammatory cells is also blocked by genistein by inhibiting the adherence of leukocytes to endothelial cells (McGregor et al., 1994). For instance, the ability of genistein as an anti‐inflammatory agent has been reported in preclinical models of Alzheimer's disease. Furthermore, by induced expression levels of Peroxisome proliferator‐activated receptor γ (PPARγ) in cultured astrocytes, the ability of genistein in preventing inflammation has been reported previously (Devi et al., 2017; Valles et al., 2010). For explaining this effect it should be mentioned that activated PPARγ suppresses the expression levels of NF‐κB (Lim et al., 2012; Mahmoud et al., 2019). In addition, in vitro studies have shown that genistein could downregulate the production of IL‐6 and TNF in cell lines treated with this compound (Spagnuolo et al., 2018). AMP‐activated protein kinase (AMPK) is known to inhibit inflammation, by decreasing NF‐kB levels and pro‐inflammatory markers (Ji et al., 2012). Interestingly, genistein by down‐regulating inflammation, via AMPK activation and subsequent NF‐κB suppression could weaken the pro‐inflammatory responses. Genistein down‐regulated IL‐6 and TNF, among the pro‐inflammatory cytokines secreted by NF‐kB signaling (Bai & Wang, 2019). Furthermore, The AMPK‐dependent increase in ACE2 receptor phosphorylation by genistein could cause a conformational change which could block the ACE2 – viral spike protein binding and inhibition of viral entry into the host cells (Al‐Kuraishy et al., 2021; Liu et al., 2019; Samuel et al., 2021). The other factors which have a central role in inflammation are cyclooxygenase‐2 (COX‐2) and Prostaglandin E2 (PGE2) (Paul et al., 2013; Schoenberger et al., 2012). It has been shown that genistein could inhibit lipopolysaccharide‐induced COX‐2 expression in addition to lipopolysaccharide‐induced PGE2 production in over‐activated macrophages (Dia et al., 2008; Hämäläinen et al., 2011; O'Leary et al., 2004). Since inflammation has an undeniable role in COVID‐19‐induced organ dysfunctions and according to the mentioned anti‐inflammatory effects of this nutraceutical, it could be concluded that genistein could subside the inflammation‐induced tissue injuries in COVID‐19.
3.6. Antioxidant effects of genistein
The pathological role of oxidative stress in Covid‐19‐induced tissue injury has been reported in different studies (Ghasemnejad‐Berenji et al., 2021; Laforge et al., 2020). If physiological buffering capacity in cells couldn't neutralize the excessive reactive oxygen species (ROS) formation, it will induce oxidative stress. This over‐production of ROS could flare the inflammation and exacerbate tissue damage in SARS‐CoV‐2 infection (Zorov et al., 2014). Several studies have also indicated an inverse relationship between the incidence of COVID‐19‐induced ARDS and using antioxidants (Soto et al., 2020). Thus, ROS has been considered a probable promising target for introducing novel therapeutic agents in controlling Covid‐19‐induced injuries (Beltrán‐García et al., 2020). The anti‐oxidative effects of Isoflavones such as genistein have been reported previously (Lee et al., 2005; Mazumder & Hongsprabhas, 2016). It has been observed that genistein protects cells against over‐production of ROS by scavenging free radicals, thereby resulting in the inhibition of NF‐κB activation, which has an essential role in cytokine storm and inflammation (Davis et al., 2001; Li et al., 2013; Nazari‐Khanamiri & Ghasemnejad‐Berenji, 2021). Park et al. have shown that genistein treatment for 24 h increases antioxidant enzyme activities through increased phosphatase and tensin homolog (PTEN) and AMPK expression (Park et al., 2010). So, the ability of genistein to activate cell signaling pathways which could inhibit ROS generation, makes it a beneficial candidate for COVID‐19 treatment.
3.7. Genistein and Nrf2 signaling pathway
Nuclear factor‐erythroid factor 2‐related factor 2 (Nrf2) has a fundamental function in cellular antioxidant defense against oxidant injury (Chen & Kunsch, 2004). So activation of NRF2 has been reported to be involved in maintaining lung architecture in response to inflammation, and therapeutic properties of NRF2 activation have been shown in animal models of various lung disorders, including ARDS and respiratory infections (Yao et al., 2014), hence reinforcing NRF2 could be beneficial as a therapeutic target for pulmonary diseases such as COVID‐19 (Cuadrado et al., 2020; Ghasemnejad‐Berenji, 2021; Khan et al., 2021). NRF2 exerts anti‐inflammatory and antioxidant properties via interactions that occur along various signaling pathways (Ghasemnejad‐Berenji, 2021a; Khan et al., 2017). For instance, it has been reported that Nrf2‐driven PPAR‐γ induction has a protective function in pulmonary oxidant injury (Figure 2) (Cho et al., 2010). Furthermore, activation of Nrf2 inhibits NF‐κB activation and inflammatory cytokines (Jafari et al., 2021; Park & Kim, 2020). Several studies have demonstrated that genistein can effectively induce Nrf2 expression (Guo et al., 2021; Miao et al., 2018; Yi et al., 2021; Zhai et al., 2013). Thus, the properties of genistein could consider as another reason which shows that this compound could subside the organ injuries in COVID‐19 patients.
3.8. Genistein and PPAR‐γ
Peroxisome proliferator‐activated receptor‐gamma (PPAR‐γ) is a transcription factor belonging to the nuclear hormone receptor superfamily. Various evidence indicates that PPAR‐γ activation has a broad range of beneficial effects on respiratory diseases to delay the pathological changes of fibrosis (Ciavarella et al., 2020; Milam et al., 2008). In addition, PPAR‐γ agonism in resident alveolar macrophages limits pulmonary inflammation following respiratory viral infections and significantly promotes host cell recovery (Chen et al., 2016; Huang et al., 2019). It has been reported that PPAR‐γ agonists modulate NF‐kB‐dependent inflammation by upregulating IkBα, a negative regulator of NF‐kB (Scirpo et al., 2015). Furthermore, PPAR‐γ activation is also responsible for the control of cytokine over‐production with consequent amelioration of the tissue damage (Esposito et al., 2020). Several studies have indicated that genistein increases upregulating PPAR‐γ expression (Hall et al., 2019; Valles et al., 2010; Wang et al., 2014). In this regard, it may exert protective effects against SARS‐CoV‐2 induced‐inflammation.
3.9. The probable effect of genistein on SARS‐CoV‐2 cell entry
The SARS‐CoV‐2 has been seen to infect human cells through the S‐protein (envelope spike glycoprotein), which is involved in SARS‐CoV‐2 cell entry and subsequent host‐to‐host cellular transmission (Mittal et al., 2020). During viral infection, this S‐protein cleaves into S1 and S2 (Sasaki et al., 2021). The ectodomain S1 binds to the peptidase domain of the ACE‐2 enzyme, while the S2 is cleaved further by the host cell serine protease Transmembrane protease serine 2 (TMPRSS2) resulting in membrane fusion (Samavati & Uhal, 2020). Both these processes are crucial for viral entry into the host cells. Since ACE2 is the entry receptor for cellular infection by SARS‐CoV‐2, inhibiting viral entry using ACE2‐related therapy could be practical to block the spreading of infection in the whole body, especially in the respiratory system (Dalan et al., 2020). Several studies have reported that genistein could reduce the expression of ACE and TMPRSS2 in cells (Palanisamy & Venkataraman, 2013; Pihlajamaa et al., 2011; Xu et al., 2006). Consequently, it is probable that genistein could inhibit the SARS‐CoV‐2 entry into human cells (Figure 2). Furthermore, this broad‐spectrum tyrosine kinase inhibitor could interfere with endocytosis and subsequent viral entry by inhibiting the internalization of viruses into host cells (Lecot et al., 2005; Rejman et al., 2005). Although the endocytic mechanisms used by SARS‐CoV‐2 have remained unclear it is probable that clathrin‐dependent endocytosis may have an essential role in viral internalization (Bayati et al., 2020). It has been reported that activation of the PI3K signaling pathway leads to clathrin‐dependent endocytosis (Khezri, Ghasemnejad‐Berenji, & Moloodsouri, 2022; Posor et al., 2013; Shimizu et al., 2021; Xu et al., 2020). So, it is probable that inhibition of the PI3K signaling pathway by genistein inhibits the clathrin‐mediated endocytosis and exerts a preventive effect on SARS‐CoV‐2 internalization by indirect inhibiting of clathrin‐mediated endocytosis. Possible molecular mechanisms of genistein against COVID‐19 have been summarized in Figure 1.
3.10. Genistein and its beneficial effects on acute lung injury models
Previous investigations have shown that genistein could inhibit the activation of NF‐κB and the production of TNF‐α in patients with asthma (Hozumi et al., 2001). Furthermore, previous studies have demonstrated that in patients with asthma increased consumption of genistein is related to improved lung function (Bime et al., 2012). Additionally, it has been reported that eosinophilic airway inflammation and eosinophil leukotriene C4 synthesis were reduced by dietary soy isoflavone supplementation in these patients. In this regard, Hirayama, Lee, and Hiramatsu (2010) indicated that there was a positive relationship between the intake of isoflavones including daidzein and genistein, and respiratory system function. Furthermore, a reduction in risk of COPD and breathlessness was observed with the consumption of these nutraceuticals (Hirayama, Lee, Binns, et al., 2010). Furthermore, it has been reported that consuming genistein would likely ameliorate Cystic Fibrosis (Arora et al., 2016). In addition, in experimental animal studies, it has been indicated that genistein could attenuate LPS‐induced acute lung responses through inhibition of NF‐κB activation and alleviating inflammatory response of cells (Kang et al., 2001). Furthermore, the protective effect of Genistein against radiation‐induced lung damage has been reported previously (Liu et al., 2014).
3.11. The probable effect of genistein on SARS‐Cov‐2‐induced coagulopathy
Several studies have indicated that platelets are hyperactivated in COVID‐19 patients (Khezri, Varzandeh, & Ghasemnejad‐Berenji, 2022; Léopold et al., 2021; Rohlfing et al., 2021). These investigations have confirmed that cytokine storm may trigger hypercoagulability and hyperinflammation. Previous studies have reported that fibrinogen level is higher in patients with COVID‐19 (Ranucci et al., 2020), which may exacerbate thrombotic disorder in capillaries by activating platelets (Zhang, Liu, et al., 2020). Therefore, compounds with anti‐platelet activity could reduce the probability of the SARS‐CoV‐2 induced‐coagulopathy. In this regard, several studies have shown that genistein might exert anti‐platelet function through cAMP regulation (Kim et al., 2013), tyrosine kinase (Nakashima et al., 1991), and Thromboxane A2 (TxA2) pathway inhibition (Guerrero et al., 2007; McNicol, 1993). In the study by Kondo et al. on the effect of genistein on the photochemical thrombosis model, it has been reported that genistein could prolong thrombotic vessel occlusion time and suppress the in vitro platelet aggregation induced by collagen in the femoral arteries of mice. Furthermore, genistein has been shown to have an alleviating effect on lipopolysaccharide‐induced disseminated intravascular coagulation by its anticoagulation effects (Chen et al., 2018). In addition, several data suggested that genistein decreases levels of TXA2 in an indirect way mainly related to inhibition of COX‐1 (Faggio et al., 2017). It has been reported that SARS‐CoV‐2‐induced platelet activation via binding of the spike to ACE2 (Chung et al., 2020). Previous studies have indicated that genistein could reduce the expression of ACE in cells (Xu et al., 2006). Therefore, these results suggest that genistein may prevent the progression of SARS‐CoV‐2‐induced coagulopathy.
4. LIMITATIONS OF GENISTEIN IN CLINICAL APPLICATION
The largest technological problem limiting the use of genistein in clinical application is its low water‐solubility and poor oral bioavailability (Garbiec et al., 2022). The solution seems to be to carry out the procedure of encapsulating genistein in zein or zein/carboxymethyl/chitosan nanoparticles (Xiao et al., 2020). The other limitation is the effect of genistein on thyroid function. This concern is raised owing to the fact that genistein has been proved to act as an inhibitor of thyroid peroxidase in vitro (Divi et al., 1997) and shows such inhibition in rats (Chang & Doerge, 2000). Nevertheless, as follows from studies reviewed by Messina and Redmond (2006), isoflavones do not appear to affect thyroid function in healthy adults. Possible adverse effects may be concomitant with iodine deficiency (Hüser et al., 2018).
5. SUMMARY AND OUTLOOK
Collectively, genistein can modulate the events of SARS‐CoV‐2 cellular entry, and molecular cascade manifesting pathophysiological consequences of COVID‐19. Although direct experimental (in vitro as well as in vivo) confirmation of the hypothesized benefits of genistein against SARS‐CoV‐2 are absent, previous experimental evidence indicating its efficacy in respiratory ailments, inflammatory disorders, and coagulopathy, promotes its candidature as an adjuvant drug in the treatment of COVID‐19. This review of genistein and its abilities motivates its clinical investigation as an adjuvant therapeutic agent to improve COVID‐19.
AUTHOR CONTRIBUTIONS
Abbas Jafari: Conceptualization. Zeinab Esmaeilzadeh: Investigation. Mohhamad Rafi Khezri: Writing ‐ review & editing. Hojat Ghaemnejad‐Berenji: Investigation. Sarvin Pashapour: Writing ‐ original draft. Sonia Sadeghpour: Writing ‐ review & editing. Morteza Ghasemnejad‐Berenji: Conceptualization; Supervision; Writing ‐ original draft.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
CODE AVAILABILITY
Not applicable.
6. ACKNOWLEDGMENT
None.
Jafari, A. , Esmaeilzadeh, Z. , Khezri, M. R. , Ghasemnejad‐Berenji, H. , Pashapour, S. , Sadeghpour, S. , & Ghasemnejad‐Berenji, M. (2022). An overview of possible pivotal mechanisms of Genistein as a potential phytochemical against SARS‐CoV‐2 infection: A hypothesis. Journal of Food Biochemistry, 00, e14345. 10.1111/jfbc.14345
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- Abu‐Eid, R. , & Ward, F. J. (2021). Targeting the PI3K/Akt/mTOR pathway: A therapeutic strategy in COVID‐19 patients. Immunology Letters, 240, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama, T. , Ishida, J. , Nakagawa, S. , Ogawara, H. , Watanabe, S.‐I. , Itoh, N. , Shibuya, M. , & Fukami, Y. (1987). Genistein, a specific inhibitor of tyrosine‐specific protein kinases. Journal of Biological Chemistry, 262(12), 5592–5595. [PubMed] [Google Scholar]
- Akula, S. M. , Hurley, D. J. , Wixon, R. L. , Wang, C. , & Chase, C. C. (2002). Effect of genistein on replication of bovine herpesvirus type 1. American Journal of Veterinary Research, 63(8), 1124–1128. [DOI] [PubMed] [Google Scholar]
- Al‐Kuraishy, H. M. , Al‐Gareeb, A. I. , Alblihed, M. , Cruz‐Martins, N. , & Batiha, G. E.‐S. (2021). COVID‐19 and risk of acute ischemic stroke and acute lung injury in patients with type II diabetes mellitus: The anti‐inflammatory role of metformin. Frontiers in Medicine, 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammary, A. F. , & Al‐Sulaiman, A. M. (2021). The journey of SARS‐CoV‐2 in human hosts: A review of immune responses, immunosuppression, and their consequences. Virulence, 12(1), 1771–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, O. M. , & Markham, K. R. (2005). Flavonoids: Chemistry, biochemistry and applications. CRC Press. [Google Scholar]
- Andres, A. , Donovan, S. M. , & Kuhlenschmidt, M. S. (2009). Soy isoflavones and virus infections. The Journal of Nutritional Biochemistry, 20(8), 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabyan, E. , Hakobyan, A. , Kotsinyan, A. , Karalyan, Z. , Arakelov, V. , Arakelov, G. , Nazaryan, K. , Simonyan, A. , Aroutiounian, R. , Ferreira, F. , & Zakaryan, H. (2018). Genistein inhibits African swine fever virus replication in vitro by disrupting viral DNA synthesis. Antiviral Research, 156, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora, K. , Yarlagadda, S. , Zhang, W. , Moon, C. , Bouquet, E. , Srinivasan, S. , Li, C. , Stokes, D. C. , & Naren, A. P. (2016). Personalized medicine in cystic fibrosis: Genistein supplementation as a treatment option for patients with a rare S1045Y‐CFTR mutation. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 311(2), L364–L374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner, Y. , & Downey, G. P. (2018). The importance of tyrosine phosphorylation control of cellular signaling pathways in respiratory disease: pY and pY not. American Journal of Respiratory Cell and Molecular Biology, 59(5), 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad, H. S. , & Assaad‐Khalil, S. (2020). Imatinib a Tyrosine Kinase Inhibitor: A potential treatment for SARS‐COV‐2 induced pneumonia. Alexandria Journal of Medicine, 56(1), 68–72. [Google Scholar]
- Bai, Z. , & Wang, Z. (2019). Genistein protects against doxorubicin‐induced cardiotoxicity through Nrf‐2/HO‐1 signaling in mice model. Environmental Toxicology, 34(5), 645–651. [DOI] [PubMed] [Google Scholar]
- Basile, M. S. , Cavalli, E. , McCubrey, J. , Hernández‐Bello, J. , Muñoz‐Valle, J. F. , Fagone, P. , & Nicoletti, F. (2021). The PI3K/Akt/mTOR pathway: A potential pharmacological target in COVID‐19. Drug Discovery Today, 27(3), 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayati, A. , Kumar, R. , Francis, V. , & McPherson, P. S. (2020). SARS‐CoV‐2 uses clathrin‐mediated endocytosis to gain access into cells. Journal of Biological Chemistry, 296, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behloul, N. , & Wu, G. (2013). Genistein: A promising therapeutic agent for obesity and diabetes treatment. European Journal of Pharmacology, 698(1–3), 31–38. [DOI] [PubMed] [Google Scholar]
- Beltrán‐García, J. , Osca‐Verdegal, R. , Pallardó, F. V. , Ferreres, J. , Rodríguez, M. , Mulet, S. , Sanchis‐Gomar, F. , Carbonell, N. , & García‐Giménez, J. L. (2020). Oxidative stress and inflammation in COVID‐19‐associated sepsis: The potential role of anti‐oxidant therapy in avoiding disease progression. Antioxidants, 9(10), 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow, R. L. , Dodson, R. W. , & Kraft, A. S. (1989). Human neutrophils contain distinct cytosolic and particulate tyrosine kinase activities: Possible role in neutrophil activation. Biochimica et Biophysica Acta (BBA)‐Protein Structure and Molecular Enzymology, 997(3), 292–301. [DOI] [PubMed] [Google Scholar]
- Bhavsar, I. , Miller, C. S. , & Al‐Sabbagh, M. (2015). Macrophage inflammatory protein‐1 alpha (MIP‐1 alpha)/CCL3: As a biomarker. General Methods in Biomarker Research and Their Applications, 1, 223–249. [Google Scholar]
- Bian, J. , & Li, Z. (2021). Angiotensin‐converting enzyme 2 (ACE2): SARS‐CoV‐2 receptor and RAS modulator. Acta Pharmaceutica Sinica B, 11(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bime, C. , Wei, C. Y. , Holbrook, J. , Smith, L. J. , & Wise, R. A. (2012). Association of dietary soy genistein intake with lung function and asthma control: A post‐hoc analysis of patients enrolled in a prospective multicentre clinical trial. Primary Care Respiratory Journal, 21(4), 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrás, C. , Gambini, J. , Gómez‐Cabrera, M. C. , Sastre, J. , Pallardó, F. V. , Mann, G. E. , Vina, J. , Borrás, C. , Gambini, J. , Gómez‐Cabrera, M. C. , & Sastre, J. (2006). Genistein, a soy isoflavone, up‐regulates expression of antioxidant genes: Involvement of estrogen receptors, ERK1/2, and NFκB. The FASEB Journal, 20(12), 2136–2138. [DOI] [PubMed] [Google Scholar]
- Buonaguro, F. M. , Ascierto, P. A. , Morse, G. D. , Buonaguro, L. , Puzanov, I. , Tornesello, M. L. , Bréchot, C. , & Gallo, R. C. (2020). Covid‐19: Time for a paradigm change. Reviews in Medical Virology, 5, e2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone, A. , Menichetti, F. , Santini, F. , Colangelo, L. , Lucenteforte, E. , & Calderone, V. (2020). Selective estrogen receptor modulators in COVID‐19: A possible therapeutic option? Frontiers in Pharmacology, 11, 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calveley, V. L. , Jelveh, S. , Langan, A. , Mahmood, J. , Yeung, I. W. , Van Dyk, J. , & Hill, R. P. (2010). Genistein can mitigate the effect of radiation on rat lung tissue. Radiation Research, 173(5), 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro, M. , Fagiani, F. , Racchi, M. , Corsini, E. , Govoni, S. , & Lanni, C. (2020). Immune response in COVID‐19: Addressing a pharmacological challenge by targeting pathways triggered by SARS‐CoV‐2. Signal Transduction and Targeted Therapy, 5(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H. C. , & Doerge, D. R. (2000). Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicology and Applied Pharmacology, 168(3), 244–252. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Li, H. , Wang, G. , Shen, X. , Zhao, S. , & Su, W. (2016). Atorvastatin prevents advanced glycation end products (AGEs)‐induced cardiac fibrosis via activating peroxisome proliferator‐activated receptor gamma (PPAR‐γ). Metabolism, 65(4), 441–453. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Tan, J. , Yang, M. , Liao, Z.‐K. , Lu, C. , Huang, Y. , & Wu, L.‐C. (2018). Genistein has the function of alleviating and treating disseminated intravascular coagulation caused by lipopolysaccharide. Journal of Natural Medicines, 72(4), 846–856. [DOI] [PubMed] [Google Scholar]
- Chen, X.‐L. , & Kunsch, C. (2004). Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: A new therapeutic approach for the treatment of inflammatory diseases. Current Pharmaceutical Design, 10(8), 879–891. [DOI] [PubMed] [Google Scholar]
- Cho, H.‐Y. , Gladwell, W. , Wang, X. , Chorley, B. , Bell, D. , Reddy, S. P. , & Kleeberger, S. R. (2010). Nrf2‐regulated PPARγ expression is critical to protection against acute lung injury in mice. American Journal of Respiratory and Critical Care Medicine, 182(2), 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, E.‐Y. , Bae, S. H. , Ha, M. H. , Choe, S.‐H. , Hyeon, J.‐Y. , Choi, J.‐I. , Choi, I. S. , & Kim, S.‐J. (2016). Genistein suppresses Prevotella intermedia lipopolysaccharide‐induced inflammatory response in macrophages and attenuates alveolar bone loss in ligature‐induced periodontitis. Archives of Oral Biology, 62, 70–79. [DOI] [PubMed] [Google Scholar]
- Chung, M. K. , Karnik, S. , Saef, J. , Bergmann, C. , Barnard, J. , Lederman, M. M. , Tilton, J. , Cheng, F. , Harding, C. V. , Young, J. B. , & Mehta, N. (2020). SARS‐CoV‐2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. eBioMedicine, 58, 102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavarella, C. , Motta, I. , Valente, S. , & Pasquinelli, G. (2020). Pharmacological (or synthetic) and nutritional agonists of PPAR‐γ as candidates for cytokine storm modulation in COVID‐19 disease. Molecules, 25(9), 2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čoma, M. , Lachová, V. , Mitrengová, P. , & Gál, P. (2021). Molecular changes underlying genistein treatment of wound healing: A review. Current Issues in Molecular Biology, 43(1), 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado, A. , Pajares, M. , Benito, C. , Jiménez‐Villegas, J. , Escoll, M. , Fernández‐Ginés, R. , Yagüe, A. J. G. , Lastra, D. , Manda, G. , Rojo, A. I. , & Dinkova‐Kostova, A. T. (2020). Can activation of NRF2 be a strategy against COVID‐19? Trends in Pharmacological Sciences, 41, 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle, M. K. , Øverland, G. , Myhre, A. E. , Stuestøl, J. F. , Hartung, T. , Krohn, C. D. , Mathiesen, Ø. , Wang, J. E. , & Aasen, A. O. (2004). The phosphatidylinositol 3‐kinase/protein kinase B signaling pathway is activated by lipoteichoic acid and plays a role in Kupffer cell production of interleukin‐6 (IL‐6) and IL‐10. Infection and Immunity, 72(10), 5704–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalan, R. , Bornstein, S. R. , El‐Armouche, A. , Rodionov, R. N. , Markov, A. , Wielockx, B. , Beuschlein, F. , & Boehm, B. O. (2020). The ACE‐2 in COVID‐19: foe or friend? Hormone and Metabolic Research, 52(5), 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. N. , Kucuk, O. , Djuric, Z. , & Sarkar, F. H. (2001). Soy isoflavone supplementation in healthy men prevents NF‐κB activation by TNF‐α in blood lymphocytes. Free Radical Biology and Medicine, 30(11), 1293–1302. [DOI] [PubMed] [Google Scholar]
- Davis, J. N. , Kucuk, O. , & Sarkar, F. H. (1999). Genistein inhibits NF‐kB activation in prostate cancer cells. Nutrition and Cancer, 35(2), 167–174. [DOI] [PubMed] [Google Scholar]
- de Las Heras, N. , Martín Giménez, V. M. , Ferder, L. , Manucha, W. , & Lahera, V. (2020). Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID‐19: Therapeutic effects of vitamin D. Antioxidants, 9(9), 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi, K. P. , Shanmuganathan, B. , Manayi, A. , Nabavi, S. F. , & Nabavi, S. M. (2017). Molecular and therapeutic targets of genistein in Alzheimer's disease. Molecular Neurobiology, 54(9), 7028–7041. [DOI] [PubMed] [Google Scholar]
- Dia, V. P. , Berhow, M. A. , & Gonzalez De Mejia, E. (2008). Bowman− Birk inhibitor and Genistein among Soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide‐induced macrophages. Journal of Agricultural and Food Chemistry, 56(24), 11707–11717. [DOI] [PubMed] [Google Scholar]
- Divi, R. L. , Chang, H. C. , & Doerge, D. R. (1997). Anti‐thyroid isoflavones from soybean: Isolation, characterization, and mechanisms of action. Biochemical Pharmacology, 54(10), 1087–1096. [DOI] [PubMed] [Google Scholar]
- Dixon, R. A. , & Ferreira, D. (2002). Genistein. Phytochemistry, 60(3), 205–211. [DOI] [PubMed] [Google Scholar]
- Dong, Z. , Qi, X. , & Fidler, I. J. (1993). Tyrosine phosphorylation of mitogen‐activated protein kinases is necessary for activation of murine macrophages by natural and synthetic bacterial products. The Journal of Experimental Medicine, 177(4), 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Z.‐R. , Feng, X.‐Q. , Li, N. , Qu, J.‐X. , Feng, L. , Chen, L. , & Chen, W.‐F. (2018). G protein‐coupled estrogen receptor is involved in the anti‐inflammatory effects of genistein in microglia. Phytomedicine, 43, 11–20. [DOI] [PubMed] [Google Scholar]
- Duan, W. , Kuo, I. C. , Selvarajan, S. , Chua, K. Y. , Bay, B. H. , & Wong, W. F. (2003). Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. American Journal of Respiratory and Critical Care Medicine, 167(2), 185–192. [DOI] [PubMed] [Google Scholar]
- Elmarakby, A. A. , Ibrahim, A. S. , Faulkner, J. , Mozaffari, M. S. , Liou, G. I. , & Abdelsayed, R. (2011). Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin‐induced diabetic mice. Vascular Pharmacology, 55(5–6), 149–156. [DOI] [PubMed] [Google Scholar]
- Esposito, G. , Pesce, M. , Seguella, L. , Sanseverino, W. , Lu, J. , Corpetti, C. , & Sarnelli, G. (2020). The potential of cannabidiol in the COVID‐19 pandemic: A hypothesis letter. British Journal of Pharmacology, 177, 4967–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggio, C. , Sureda, A. , Morabito, S. , Sanches‐Silva, A. , Mocan, A. , Nabavi, S. F. , & Nabavi, S. M. (2017). Flavonoids and platelet aggregation: A brief review. European Journal of Pharmacology, 807, 91–101. [DOI] [PubMed] [Google Scholar]
- Ferreira, A. C. , Soares, V. C. , de Azevedo‐Quintanilha, I. G. , Dias, S. D. S. G. , Fintelman‐Rodrigues, N. , Sacramento, C. Q. , Mattos, M. , de Freitas, C. S. , Temerozo, J. R. , Teixeira, L. , & Damaceno Hottz, E. (2021). SARS‐CoV‐2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discovery, 7(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuseini, H. , Cephus, J.‐Y. , Wu, P. , Davis, J. B. , Contreras, D. C. , Gandhi, V. D. , Rathmell, J. C. , & Newcomb, D. C. (2019). ERα signaling increased IL‐17A production in Th17 cells by upregulating IL‐23R expression, mitochondrial respiration, and proliferation. Frontiers in Immunology, 10, 2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbiec, E. , Cielecka‐Piontek, J. , Kowalówka, M. , Hołubiec, M. , & Zalewski, P. (2022). Genistein—opportunities related to an interesting molecule of natural origin. Molecules, 27(3), 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemnejad‐Berenji, M. (2021). Immunomodulatory and anti‐inflammatory potential of crocin in COVID‐19 treatment. Journal of Food Biochemistry, 45(5), e13718. 10.1111/jfbc.13718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemnejad‐Berenji, M. (2021a). Can sulfasalazine as an old drug with immunomodulatory and anti‐inflammatory effects be effective in COVID‐19? Journal of Basic and Clinical Physiology and Pharmacology, 33, 113–115. [DOI] [PubMed] [Google Scholar]
- Ghasemnejad‐Berenji, M. (2021b). mTOR inhibition: A double‐edged sword in patients with COVID‐19? Human Cell, 34(2), 698–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemnejad‐Berenji, M. , Pashapour, S. , & Ghasemnejad‐Berenji, H. (2020). Therapeutic potential for clomiphene, a selective estrogen receptor modulator, in the treatment of COVID‐19. Medical Hypotheses, 145, 110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemnejad‐Berenji, M. , Pashapour, S. , & Sadeghpour, S. (2021). Pentoxifylline: A drug with antiviral and anti‐inflammatory effects to be considered in the treatment of coronavirus disease 2019. Medical Principles and Practice, 30(1), 98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginwala, R. , Bhavsar, R. , Chigbu, D. G. I. , Jain, P. , & Khan, Z. K. (2019). Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti‐inflammatory activity of apigenin. Antioxidants, 8(2), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwyn, S. , Lazinsky, A. , & Wei, H. (2000). Promotion of health by soy isoflavones: Efficacy, benefit and safety concerns. Drug Metabolism and Drug Interactions, 17(1–4), 261–290. [DOI] [PubMed] [Google Scholar]
- Guerrero, J. A. , Navarro‐Nuñez, L. , Lozano, M. L. , Martínez, C. , Vicente, V. , Gibbins, J. M. , & Rivera, J. (2007). Flavonoids inhibit the platelet TxA2 signalling pathway and antagonize TxA2 receptors (TP) in platelets and smooth muscle cells. British Journal of Clinical Pharmacology, 64(2), 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. , Yang, G. , He, Y. , Xu, H. , Fan, H. , An, J. , Zhang, L. , Zhang, R. , Cao, G. , Hao, D. , & Yang, H. (2021). Involvement of α7nAChR in the protective effects of genistein against β‐amyloid‐induced oxidative stress in neurons via a PI3K/Akt/Nrf2 pathway‐related mechanism. Cellular and Molecular Neurobiology, 41(2), 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. M. , Powell, H. R. , Rajic, L. , & Korach, K. S. (2019). The role of dietary phytoestrogens and the nuclear receptor PPAR γ in adipogenesis: An in vitro study. Environmental Health Perspectives, 127(3), 037007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen, M. , Nieminen, R. , Asmawi, M. Z. , Vuorela, P. , Vapaatalo, H. , & Moilanen, E. (2011). Effects of flavonoids on prostaglandin E2 production and on COX‐2 and mPGES‐1 expressions in activated macrophages. Planta Medica, 77(13), 1504–1511. [DOI] [PubMed] [Google Scholar]
- Hirayama, F. , Lee, A. H. , Binns, C. W. , Hiramatsu, N. , Mori, M. , & Nishimura, K. (2010). Dietary intake of isoflavones and polyunsaturated fatty acids associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease: Possible protective effect of traditional Japanese diet. Molecular Nutrition & Food Research, 54(7), 909–917. [DOI] [PubMed] [Google Scholar]
- Hirayama, F. , Lee, A. H. , & Hiramatsu, N. (2010). Dietary nutrients in relation to chronic obstructive pulmonary disease: Emerging epidemiological evidence. Current Respiratory Medicine Reviews, 6(2), 124–132. [Google Scholar]
- Hozumi, A. , Nishimura, Y. , Nishiuma, T. , Kotani, Y. , & Yokoyama, M. (2001). Induction of MMP‐9 in normal human bronchial epithelial cells by TNF‐α via NF‐κB‐mediated pathway. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 281(6), L1444–L1452. [DOI] [PubMed] [Google Scholar]
- Hsu, H.‐Y. , Chiu, S.‐L. , Wen, M.‐H. , Chen, K.‐Y. , & Hua, K.‐F. (2001). Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. Journal of Biological Chemistry, 276(31), 28719–28730. [DOI] [PubMed] [Google Scholar]
- Huang, S. , Goplen, N. P. , Zhu, B. , Cheon, I. S. , Son, Y. , Wang, Z. , Li, C. , Dai, Q. , Jiang, L. , Xiang, M. , & Carmona, E. M. (2019). Macrophage PPAR‐γ suppresses long‐term lung fibrotic sequelae following acute influenza infection. PLoS One, 14(10), e0223430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüser, S. , Guth, S. , Joost, H. , Soukup, S. , Köhrle, J. , Kreienbrock, L. , Diel, P. , Lachenmeier, D. W. , Eisenbrand, G. , Vollmer, G. , & Nöthlings, U. (2018). Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Archives of Toxicology, 92(9), 2703–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi, Y. , Arai, N. , Wang, X. , Wu, J. , Umegaki, K. , Miyaura, C. , Takeda, A. , & Ikegami, S. (2000). Difference in effective dosage of genistein on bone and uterus in ovariectomized mice. Biochemical and Biophysical Research Communications, 274(3), 697–701. [DOI] [PubMed] [Google Scholar]
- Jafari, A. , Sadeghpour, S. , Ghasemnejad‐Berenji, H. , Pashapour, S. , & Ghasemnejad‐Berenji, M. (2021). Potential antioxidative, anti‐inflammatory and immunomodulatory effects of ghrelin, an endogenous peptide from the stomach in SARS‐CoV2 infection. International Journal of Peptide Research and Therapeutics, 27, 1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarzadeh, A. , Chauhan, P. , Saha, B. , Jafarzadeh, S. , & Nemati, M. (2020). Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID‐19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sciences, 257, 118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, G. , Yang, Q. , Hao, J. , Guo, L. , Chen, X. , Hu, J. , Leng, L. , & Jiang, Z. (2011). Anti‐inflammatory effect of genistein on non‐alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. International Immunopharmacology, 11(6), 762–768. [DOI] [PubMed] [Google Scholar]
- Ji, G. , Zhang, Y. , Yang, Q. , Cheng, S. , Hao, J. , Zhao, X. , & Jiang, Z. (2012). Genistein suppresses LPS‐induced inflammatory response through inhibiting NF‐κB following AMP kinase activation in RAW 264.7 macrophages. PLoS One, 7(12), e53101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. L. , Lee, H. W. , Lee, H. S. , Pack, I. S. , Chong, Y. , Castranova, V. , & Koh, Y. (2001). Genistein prevents nuclear factor‐kappa B activation and acute lung injury induced by lipopolysaccharide. American Journal of Respiratory and Critical Care Medicine, 164(12), 2206–2212. [DOI] [PubMed] [Google Scholar]
- Kavoosi, F. , Dastjerdi, M. N. , Valiani, A. , Esfandiari, E. , Sanaei, M. , & Hakemi, M. G. (2016). Genistein potentiates the effect of 17‐beta estradiol on human hepatocellular carcinoma cell line. Advanced Biomedical Research, 5, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, H. , Patel, S. , & Majumdar, A. (2021). Role of NRF2 and sirtuin activators in COVID‐19. Clinical Immunology, 233, 108879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, N. M. , Haseeb, A. , Ansari, M. Y. , Devarapalli, P. , Haynie, S. , & Haqqi, T. M. (2017). Wogonin, a plant derived small molecule, exerts potent anti‐inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radical Biology and Medicine, 106, 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khezri, M. R. , Ghasemnejad‐Berenji, M. , & Moloodsouri, D. (2022). Hesperetin and the PI3K/AKT pathway: Could their interaction play a role in the entry and replication of the SARS‐CoV‐2? Journal of Food Biochemistry, e14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khezri, M. R. , Moloodsouri, D. , Hodaei, D. , & Ghasemnejad‐Berenji, M. (2022). Therapeutic potential of loureirin A against SARS‐CoV‐2 infection. Phytotherapy Research. 10.1002/ptr.7453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khezri, M. R. , Varzandeh, R. , & Ghasemnejad‐Berenji, M. (2022). The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS‐CoV‐2 induced coagulopathy. Cellular & Molecular Biology Letters, 27(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khezri, M. R. , Yousefi, K. , & Ghasemnejad‐Berenji, M. (2021). Angiotensin II: A possible target for therapeutic intervention in COVID‐19. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 139, 111564. [DOI] [PubMed] [Google Scholar]
- Khezri, M. R. , Zolbanin, N. M. , Ghasemnejad‐Berenji, M. , & Jafari, R. (2021). Azithromycin: Immunomodulatory and antiviral properties for SARS‐CoV‐2 infection. European Journal of Pharmacology, 905, 174191. 10.1016/j.ejphar.2021.174191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. P. , Son, K. H. , Chang, H. W. , & Kang, S. S. (2004). Anti‐inflammatory plant flavonoids and cellular action mechanisms. Journal of Pharmacological Sciences, 96, 229–245. [DOI] [PubMed] [Google Scholar]
- Kim, I.‐S. (2021). Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants, 10(7), 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. , Lim, K.‐M. , Shin, H.‐J. , Seo, D.‐B. , Noh, J.‐Y. , Kang, S. , Chung, H. Y. , Shin, S. , Chung, J. H. , & Bae, O.‐N. (2013). Inhibitory effects of black soybean on platelet activation mediated through its active component of adenosine. Thrombosis Research, 131(3), 254–261. [DOI] [PubMed] [Google Scholar]
- Kim, Y. K. , Jang, Y. Y. , Kim, D. H. , Ko, H. H. , Han, E. S. , & Lee, C. S. (2001). Differential regulation of protein tyrosine kinase on free radical production, granule enzyme release, and cytokine synthesis by activated murine peritoneal macrophages. Biochemical Pharmacology, 61(1), 87–96. [DOI] [PubMed] [Google Scholar]
- Kindrachuk, J. , Ork, B. , Mazur, S. , Holbrook, M. R. , Frieman, M. B. , Traynor, D. , Johnson, R. F. , Dyall, J. , Kuhn, J. H. , Olinger, G. G. , & Hensley, L. E. (2015). Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrobial Agents and Chemotherapy, 59(2), 1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge, M. , Elbim, C. , Frère, C. , Hémadi, M. , Massaad, C. , Nuss, P. , Benoliel, J. J. , & Becker, C. (2020). Tissue damage from neutrophil‐induced oxidative stress in COVID‐19. Nature Reviews Immunology, 20(9), 515–516. 10.1038/s41577-020-0407-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort, J. , Viese, M. , Ploug, T. , & Dela, F. (2003). Time course of GLUT4 and AMPK protein expression in human skeletal muscle during one month of physical training. Scandinavian Journal of Medicine & Science in Sports, 13(3), 169–174. [DOI] [PubMed] [Google Scholar]
- LeCher, J. C. , Diep, N. , Krug, P. W. , & Hilliard, J. K. (2019). Genistein has antiviral activity against herpes B virus and acts synergistically with antiviral treatments to reduce effective dose. Viruses, 11(6), 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien, J. R. , Chiesa‐Estomba, C. M. , De Siati, D. R. , Horoi, M. , Le Bon, S. D. , Rodriguez, A. , Dequanter, D. , Blecic, S. , El Afia, F. , Distinguin, L. , & Chekkoury‐Idrissi, Y. (2020). Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): A multicenter European study. European Archives of Oto‐Rhino‐Laryngology, 277(8), 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecot, S. , Belouzard, S. , Dubuisson, J. , & Rouillé, Y. (2005). Bovine viral diarrhea virus entry is dependent on clathrin‐mediated endocytosis. Journal of Virology, 79(16), 10826–10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. H. , Yang, L. , Xu, J. Z. , Yeung, S. Y. V. , Huang, Y. , & Chen, Z.‐Y. (2005). Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chemistry, 90(4), 735–741. [Google Scholar]
- Lee, K. Y. , Kim, J.‐R. , & Choi, H. C. (2016). Genistein‐induced LKB1–AMPK activation inhibits senescence of VSMC through autophagy induction. Vascular Pharmacology, 81, 75–82. [DOI] [PubMed] [Google Scholar]
- Léopold, V. , Pereverzeva, L. , Schuurman, A. R. , Reijnders, T. D. , Saris, A. , de Brabander, J. , Van Linge, C. C. , Douma, R. A. , Chouchane, O. , Nieuwland, R. , & Wiersinga, W. J. (2021). Platelets are hyperactivated but show reduced glycoprotein VI reactivity in COVID‐19 patients. Thrombosis and Haemostasis, 121, 1258–1262. [DOI] [PubMed] [Google Scholar]
- Lewicki, S. , Lewicka, A. , Kalicki, B. , Sobolewska‐Ruta, A. , Debski, B. , Zdanowski, R. , Syryło, T. , Kloc, M. , & Kubiak, J. Z. (2018). Effects of genistein on insulin pathway‐related genes in mouse differentiated myoblast C2C12 cell line: Evidence for two independent modes of action. Folia Histochemica et Cytobiologica, 56(3), 123–132. [DOI] [PubMed] [Google Scholar]
- Li, J. , Gang, D. , Yu, X. , Hu, Y. , Yue, Y. , Cheng, W. , Pan, X. , & Zhang, P. (2013). Genistein: The potential for efficacy in rheumatoid arthritis. Clinical Rheumatology, 32(5), 535–540. [DOI] [PubMed] [Google Scholar]
- Li, S. , Zhang, Y. , Guan, Z. , Li, H. , Ye, M. , Chen, X. , Shen, J. , Zhou, Y. , Shi, Z. L. , Zhou, P. , & Peng, K. (2020). SARS‐CoV‐2 triggers inflammatory responses and cell death through caspase‐8 activation. Signal Transduction and Targeted Therapy, 5(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , & Sarkar, F. H. (2002). Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clinical Cancer Research, 8(7), 2369–2377. [PubMed] [Google Scholar]
- Li, Z. , Li, J. , Mo, B. , Hu, C. , Liu, H. , Qi, H. , Wang, X. , & Xu, J. (2008). Genistein induces cell apoptosis in MDA‐MB‐231 breast cancer cells via the mitogen‐activated protein kinase pathway. Toxicology In Vitro, 22(7), 1749–1753. [DOI] [PubMed] [Google Scholar]
- Lim, H. A. , Lee, E. K. , Kim, J. M. , Park, M. H. , Kim, D. H. , Choi, Y. J. , Ha, Y. M. , Yoon, J. H. , Choi, J. S. , Yu, B. P. , & Chung, H. Y. (2012). PPARγ activation by baicalin suppresses NF‐κB‐mediated inflammation in aged rat kidney. Biogerontology, 13(2), 133–145. [DOI] [PubMed] [Google Scholar]
- Linford, N. J. , Yang, Y. , Cook, D. G. , & Dorsa, D. M. (2001). Neuronal apoptosis resulting from high doses of the isoflavone genistein: Role for calcium and p42/44 mitogen‐activated protein kinase. Journal of Pharmacology and Experimental Therapeutics, 299(1), 67–75. [PubMed] [Google Scholar]
- Liu, G.‐D. , Xia, L. , Zhu, J.‐W. , Ou, S. , Li, M.‐X. , He, Y. , Luo, W. , Li, J. , Zhou, Q. , Yang, X. Q. , & Shan, J. L. (2014). Genistein alleviates radiation‐induced pneumonitis by depressing Ape1/Ref‐1 expression to down‐regulate inflammatory cytokines. Cell Biochemistry and Biophysics, 69(3), 725–733. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Liu, K. , & Bodenner, D. L. (2005). Estrogen receptor inhibits interleukin‐6 gene expression by disruption of nuclear factor κB transactivation. Cytokine, 31(4), 251–257. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Li, X. , Lu, Q. , Ren, D. , Sun, X. , Rousselle, T. , Li, J. , & Leng, J. (2019). AMPK: A balancer of the renin–angiotensin system. Bioscience Reports, 39(9), BSR20181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zhu, J.‐Q. , Jin, X.‐H. , Dong, M.‐P. , & Zheng, J.‐F. (2020). Up‐regulation of miR‐146b‐3p protects septic mice with acute respiratory distress syndrome by inhibiting PI3K/AKT signaling pathway. Journal of Bioenergetics and Biomembranes, 52(4), 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro, G. (2020). SARS‐CoV‐2 and COVID‐19: Is interleukin‐6 (IL‐6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine: X, 2(2), 100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, A. M. , Hussein, O. E. , Abd El‐Twab, S. M. , & Hozayen, W. G. (2019). Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO‐1 signaling and PPARγ, and suppression of NF‐κB/NLRP3 inflammasome axis. Food & Function, 10(8), 4593–4607. [DOI] [PubMed] [Google Scholar]
- Malgotra, V. , & Sharma, V. (2021). 2‐Deoxy‐d‐glucose inhibits replication of novel coronavirus (SARS‐CoV‐2) with adverse effects on host cell metabolism. Clinical Trials, 7, 10. [Google Scholar]
- Malloy, K. M. , Wang, J. , Clark, L. H. , Fang, Z. , Sun, W. , Yin, Y. , Kong, W. , Zhou, C. , & Bae‐Jump, V. L. (2018). Novasoy and genistein inhibit endometrial cancer cell proliferation through disruption of the AKT/mTOR and MAPK signaling pathways. American Journal of Translational Research, 10(3), 784–795. [PMC free article] [PubMed] [Google Scholar]
- Mazumder, M. A. R. , & Hongsprabhas, P. (2016). Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomedicine & Pharmacotherapy, 82, 379–392. [DOI] [PubMed] [Google Scholar]
- McGregor, P. E. , Agrawal, D. K. , & Edwards, J. D. (1994). Attenuation of human leukocyte adherence to endothelial cell monolayers by tyrosine kinase inhibitors. Biochemical and Biophysical Research Communications, 198(1), 359–365. [DOI] [PubMed] [Google Scholar]
- McNicol, A. (1993). The effects of genistein on platelet function are due to thromboxane receptor antagonism rather than inhibition of tyrosine kinase. Prostaglandins, Leukotrienes and Essential Fatty Acids, 48(5), 379–384. [DOI] [PubMed] [Google Scholar]
- Meng, Y. , Yin, Q. , Ma, Q. , Qin, H. , Zhang, J. , Zhang, B. , Pang, H. , & Tian, H. (2021). FXII regulates the formation of deep vein thrombosis via the PI3K/AKT signaling pathway in mice. International Journal of Molecular Medicine, 47(5), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina, M. , & Redmond, G. (2006). Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: A review of the relevant literature. Thyroid, 16(3), 249–258. [DOI] [PubMed] [Google Scholar]
- Miao, Z.‐Y. , Xia, X. , Che, L. , & Song, Y.‐T. (2018). Genistein attenuates brain damage induced by transient cerebral ischemia through up‐regulation of Nrf2 expression in ovariectomized rats. Neurological Research, 40(8), 689–695. [DOI] [PubMed] [Google Scholar]
- Milam, J. E. , Keshamouni, V. G. , Phan, S. H. , Hu, B. , Gangireddy, S. R. , Hogaboam, C. M. , Standiford, T. J. , Thannickal, V. J. , & Reddy, R. C. (2008). PPAR‐γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin‐induced pulmonary fibrosis. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 294(5), L891–L901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal, A. , Manjunath, K. , Ranjan, R. K. , Kaushik, S. , Kumar, S. , & Verma, V. (2020). COVID‐19 pandemic: Insights into structure, function, and hACE2 receptor recognition by SARS‐CoV‐2. PLoS Pathogens, 16(8), e1008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad‐Shahi, M. , Haidari, F. , Rashidi, B. , Saei, A. A. , Mahboob, S. , & Rashidi, M.‐R. (2011). Comparison of the effects of genistein and daidzein with dexamethasone and soy protein on rheumatoid arthritis in rats. BioImpacts: BI, 1(3), 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, A. J. (2010). Molecular mechanisms of estrogen receptor regulation and signaling in human monocytes and macrophages. Dartmouth College. [Google Scholar]
- Murray, J. L. , McDonald, N. J. , Sheng, J. , Shaw, M. W. , Hodge, T. W. , Rubin, D. H. , O'Brien, W. A. , & Smee, D. F. (2012). Inhibition of influenza A virus replication by antagonism of a PI3K‐AKT‐mTOR pathway member identified by gene‐trap insertional mutagenesis. Antiviral Chemistry and Chemotherapy, 22(5), 205–215. [DOI] [PubMed] [Google Scholar]
- Nakashima, S. , Koike, T. , & Nozawa, Y. (1991). Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2‐mediated human platelet responses. Molecular Pharmacology, 39(4), 475–480. [PubMed] [Google Scholar]
- Nazari‐Khanamiri, F. , & Ghasemnejad‐Berenji, M. (2021). Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: An overview. Journal of Food Biochemistry, 45(11), e13972. [DOI] [PubMed] [Google Scholar]
- O'Leary, K. A. , de Pascual‐Tereasa, S. , Needs, P. W. , Bao, Y.‐P. , O'Brien, N. M. , & Williamson, G. (2004). Effect of flavonoids and vitamin E on cyclooxygenase‐2 (COX‐2) transcription. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 551(1–2), 245–254. [DOI] [PubMed] [Google Scholar]
- Pabich, M. , & Materska, M. (2019). Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients, 11(7), 1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, T. H. , Smolinska, M. , Gillespie, J. , Urbaniak, A. M. , & Foxwell, B. M. (2009). Tyrosine kinases and inflammatory signalling. Current Molecular Medicine, 9(1), 69–85. [DOI] [PubMed] [Google Scholar]
- Palanisamy, N. , & Venkataraman, A. C. (2013). Beneficial effect of genistein on lowering blood pressure and kidney toxicity in fructose‐fed hypertensive rats. British Journal of Nutrition, 109(10), 1806–1812. [DOI] [PubMed] [Google Scholar]
- Park, C. E. , Yun, H. , Lee, E.‐B. , Min, B.‐I. , Bae, H. , Choe, W. , Kang, I. , Kim, S. S. , & Ha, J. (2010). The antioxidant effects of genistein are associated with AMP‐activated protein kinase activation and PTEN induction in prostate cancer cells. Journal of Medicinal Food, 13(4), 815–820. [DOI] [PubMed] [Google Scholar]
- Park, J. , & Kim, Y. T. (2020). Erythronium japonicum alleviates inflammatory pain by inhibiting MAPK activation and by suppressing NF‐κB activation via ERK/Nrf2/HO‐1 signaling pathway. Antioxidants, 9(7), 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, A. G. , Chandran, B. , & Sharma‐Walia, N. (2013). Cyclooxygenase‐2‐prostaglandin E2‐eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma‐associated herpes virus associated malignancies. Translational Research, 162(2), 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese, J. M. , Farmer, R. L. , & Bergan, R. C. (2010). Inhibition of cancer cell invasion and metastasis by genistein. Cancer and Metastasis Reviews, 29(3), 465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelekanou, V. , Kampa, M. , Kiagiadaki, F. , Deli, A. , Theodoropoulos, P. , Agrogiannis, G. , Patsouris, E. , Tsapis, A. , Castanas, E. , & Notas, G. (2016). Estrogen anti‐inflammatory activity on human monocytes is mediated through cross‐talk between estrogen receptor ERα36 and GPR30/GPER1. Journal of Leukocyte Biology, 99(2), 333–347. [DOI] [PubMed] [Google Scholar]
- Pihlajamaa, P. , Zhang, F.‐P. , Saarinen, L. , Mikkonen, L. , Hautaniemi, S. , & Jänne, O. A. (2011). The phytoestrogen genistein is a tissue‐specific androgen receptor modulator. Endocrinology, 152(11), 4395–4405. [DOI] [PubMed] [Google Scholar]
- Posor, Y. , Eichhorn‐Gruenig, M. , Puchkov, D. , Schöneberg, J. , Ullrich, A. , Lampe, A. , Müller, R. , Zarbakhsh, S. , Gulluni, F. , Hirsch, E. , & Krauss, M. (2013). Spatiotemporal control of endocytosis by phosphatidylinositol‐3, 4‐bisphosphate. Nature, 499(7457), 233–237. [DOI] [PubMed] [Google Scholar]
- Qi, D. , Tang, X. , He, J. , Wang, D. , Zhao, Y. , Deng, W. , Deng, X. , Zhou, G. , Xia, J. , Zhong, X. , & Pu, S. (2016). Omentin protects against LPS‐induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS‐dependent mechanism. Cell Death & Disease, 7(9), e2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranucci, M. , Ballotta, A. , Di Dedda, U. , Bayshnikova, E. , Dei Poli, M. , Resta, M. , Falco, M. , Albano, G. , & Menicanti, L. (2020). The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. Journal of Thrombosis and Haemostasis, 18(7), 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman, J. , Bragonzi, A. , & Conese, M. (2005). Role of clathrin‐and caveolae‐mediated endocytosis in gene transfer mediated by lipo‐and polyplexes. Molecular Therapy, 12(3), 468–474. [DOI] [PubMed] [Google Scholar]
- Rizk, J. G. , Kalantar‐Zadeh, K. , Mehra, M. R. , Lavie, C. J. , Rizk, Y. , & Forthal, D. N. (2020). Pharmaco‐immunomodulatory therapy in COVID‐19. Drugs, 80, 1267–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba, C. , Battaglini, D. , Pelosi, P. , & Rocco, P. R. (2020). Multiple organ dysfunction in SARS‐CoV‐2: MODS‐CoV‐2. Expert Review of Respiratory Medicine, 14(9), 865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D. P. , Hall, O. J. , Nilles, T. L. , Bream, J. H. , & Klein, S. L. (2014). 17β‐estradiol protects females against influenza by recruiting neutrophils and increasing virus‐specific CD8 T cell responses in the lungs. Journal of Virology, 88(9), 4711–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing, A.‐K. , Rath, D. , Geisler, T. , & Gawaz, M. (2021). Platelets and COVID‐19. Hämostaseologie, 41(5), 379–385. [DOI] [PubMed] [Google Scholar]
- Romier, B. , Van De Walle, J. , During, A. , Larondelle, Y. , & Schneider, Y.‐J. (2008). Modulation of signalling nuclear factor‐κB activation pathway by polyphenols in human intestinal Caco‐2 cells. British Journal of Nutrition, 100(3), 542–551. [DOI] [PubMed] [Google Scholar]
- Russo, M. , Russo, G. L. , Daglia, M. , Kasi, P. D. , Ravi, S. , Nabavi, S. F. , & Nabavi, S. M. (2016). Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chemistry, 196, 589–600. [DOI] [PubMed] [Google Scholar]
- Sahin, K. , Tuzcu, M. , Basak, N. , Caglayan, B. , Kilic, U. , Sahin, F. , & Kucuk, O. (2012). Sensitization of cervical cancer cells to cisplatin by genistein: The role of NF B and Akt/mTOR signaling pathways. Journal of Oncology, 2012, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray, S. , Das, A. , Matzelle, D. C. , Yu, S. P. , Wei, L. , Varma, A. , Ray, S. K. , & Banik, N. L. (2016). Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. Journal of Neurochemistry, 137(4), 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavati, L. , & Uhal, B. D. (2020). ACE2, much more than just a receptor for SARS‐COV‐2. Frontiers in Cellular and Infection Microbiology, 10, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, S. M. , Varghese, E. , & Büsselberg, D. (2021). Therapeutic potential of metformin in COVID‐19: Reasoning for its protective role. Trends in Microbiology, 29, 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, M. , Uemura, K. , Sato, A. , Toba, S. , Sanaki, T. , Maenaka, K. , Hall, W. W. , Orba, Y. , & Sawa, H. (2021). SARS‐CoV‐2 variants with mutations at the S1/S2 cleavage site are generated in vitro during propagation in TMPRSS2‐deficient cells. PLoS Pathogens, 17(1), e1009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter, D. , Schwarz, S. , Wang, K. , Zhang, R. , Sun, B. , & Schwarz, W. (2014). Genistein as antiviral drug against HIV ion channel. Planta Medica, 80(8/9), 682–687. [DOI] [PubMed] [Google Scholar]
- Schoenberger, S. D. , Kim, S. J. , Sheng, J. , Rezaei, K. A. , Lalezary, M. , & Cherney, E. (2012). Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Investigative Ophthalmology & Visual Science, 53(9), 5906–5911. [DOI] [PubMed] [Google Scholar]
- Scirpo, R. , Fiorotto, R. , Villani, A. , Amenduni, M. , Spirli, C. , & Strazzabosco, M. (2015). Stimulation of nuclear receptor PPAR‐γ limits NF‐kB‐dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology (Baltimore, MD), 62(5), 1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell, K. D. , & Cassidy, A. (1999). Dietary isoflavones: Biological effects and relevance to human health. The Journal of Nutrition, 129(3), 758S–767S. [DOI] [PubMed] [Google Scholar]
- Sharma, A. , Kaur, R. , Katnoria, J. K. , Kaur, R. , & Nagpal, A. K. (2017). Family fabaceae: A boon for cancer therapy. In Malik S. (Eds.), Biotechnology and production of anti‐cancer compounds (pp. 157–175). Springer. [Google Scholar]
- Shimizu, S. , Yoshioka, K. , Aki, S. , & Takuwa, Y. (2021). Class II phosphatidylinositol 3‐kinase‐C2α is essential for Notch signaling by regulating the endocytosis of γ‐secretase in endothelial cells. Scientific Reports, 11(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, V. , Chandra, V. , Sankhwar, P. , Popli, P. , Kaushal, J. B. , Sirohi, V. K. , & Dwivedi, A. (2015). Phytoestrogen genistein inhibits EGFR/PI3K/NF‐kB activation and induces apoptosis in human endometrial hyperplasial cells. RSC Advances, 5(69), 56075–56085. [Google Scholar]
- Sirtori, C. R. (2001). Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug Safety, 24(9), 665–682. [DOI] [PubMed] [Google Scholar]
- Somanath, P. R. (2020). Is targeting Akt a viable option to treat advanced‐stage COVID‐19 patients? American Journal of Physiology‐Lung Cellular and Molecular Physiology, 319(1), L45–L47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto, M. E. , Guarner‐Lans, V. , Soria‐Castro, E. , Manzano Pech, L. , & Pérez‐Torres, I. (2020). Is antioxidant therapy a useful complementary measure for Covid‐19 treatment? An algorithm for its application. Medicina, 56(8), 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnuolo, C. , Moccia, S. , & Russo, G. L. (2018). Anti‐inflammatory effects of flavonoids in neurodegenerative disorders. European Journal of Medicinal Chemistry, 153, 105–115. [DOI] [PubMed] [Google Scholar]
- Spagnuolo, C. , Russo, G. L. , Orhan, I. E. , Habtemariam, S. , Daglia, M. , Sureda, A. , Nabavi, S. F. , Devi, K. P. , Loizzo, M. R. , Tundis, R. , & Nabavi, S. M. (2015). Genistein and cancer: Current status, challenges, and future directions. Advances in Nutrition, 6(4), 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suba, Z. (2020). Prevention and therapy of COVID‐19 via exogenous estrogen treatment for both male and female patients: Prevention and therapy of COVID‐19. Journal of Pharmacy & Pharmaceutical Sciences, 23, 75–85. [DOI] [PubMed] [Google Scholar]
- Sutrisno, S. , Aprina, H. , Simanungkalit, H. M. , Andriyani, A. , Barlianto, W. , Sujuti, H. , Santoso, S. , Dwijayasa, P. M. , Wahyuni, E. S. , & Mustofa, E. (2018). Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. Journal of Traditional and Complementary Medicine, 8(2), 278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrisno, S. , Wulandari, R. C. L. , Sulistyowati, D. W. W. , Wulandari, R. F. , Wahyuni, E. S. , Yueniwati, Y. , & Santoso, S. (2015). Effect of genistein on proinflammatory cytokines and estrogen receptor–β in mice model of endometriosis. Asian Pacific Journal of Reproduction, 4(2), 96–99. [Google Scholar]
- Suuronen, T. , Nuutinen, T. , Huuskonen, J. , Ojala, J. , Thornell, A. , & Salminen, A. (2005). Anti‐inflammatory effect of selective estrogen receptor modulators (SERMs) in microglial cells. Inflammation Research, 54(5), 194–203. [DOI] [PubMed] [Google Scholar]
- Tahmasebi, S. , El‐Esawi, M. A. , Mahmoud, Z. H. , Timoshin, A. , Valizadeh, H. , Roshangar, L. , Varshoch, M. , Vaez, A. , Aslani, S. , Navashenaq, J. G. , & Aghebati‐Maleki, L. (2021). Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID‐19 patients. Journal of Cellular Physiology, 236(7), 5325–5338. [DOI] [PubMed] [Google Scholar]
- Tan, H. K. , Moad, A. I. H. , & Tan, M. L. (2014). The mTOR signalling pathway in cancer and the potential mTOR inhibitory activities of natural phytochemicals. Asian Pacific Journal of Cancer Prevention, 15(16), 6463–6475. [DOI] [PubMed] [Google Scholar]
- Tuli, H. S. , Tuorkey, M. J. , Thakral, F. , Sak, K. , Kumar, M. , Sharma, A. K. , Sharma, U. , Jain, A. , Aggarwal, V. , & Bishayee, A. (2019). Molecular mechanisms of action of genistein in cancer: Recent advances. Frontiers in Pharmacology, 10, 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulhaq, Z. S. , & Soraya, G. V. (2020). Interleukin‐6 as a potential biomarker of COVID‐19 progression. Medecine et Maladies Infectieuses, 50(4), 382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles, S. L. , Dolz‐Gaiton, P. , Gambini, J. , Borras, C. , LLoret, A. , Pallardo, F. V. , & Viña, J. (2010). Estradiol or genistein prevent Alzheimer's disease‐associated inflammation correlating with an increase PPARγ expression in cultured astrocytes. Brain Research, 1312, 138–144. [DOI] [PubMed] [Google Scholar]
- Vaya, J. , & Tamir, S. (2004). The relation between the chemical structure of flavonoids and their estrogen‐like activities. Current Medicinal Chemistry, 11(10), 1333–1343. [DOI] [PubMed] [Google Scholar]
- Vegeto, E. , Cuzzocrea, S. , Crisafulli, C. , Mazzon, E. , Sala, A. , Krust, A. , & Maggi, A. (2010). Estrogen receptor‐α as a drug target candidate for preventing lung inflammation. Endocrinology, 151(1), 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdrengh, M. , Jonsson, I. , Holmdahl, R. , & Tarkowski, A. (2003). Genistein as an anti‐inflammatory agent. Inflammation Research, 52(8), 341–346. [DOI] [PubMed] [Google Scholar]
- Vermillion, M. S. , Ursin, R. L. , Attreed, S. E. , & Klein, S. L. (2018). Estriol reduces pulmonary immune cell recruitment and inflammation to protect female mice from severe influenza. Endocrinology, 159(9), 3306–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Shi, S. , Li, Y. , Li, H. , & Xie, M. (1956). Study on anti‐microbial activity of genistein and its mechanism. Acta Nutrimenta Sinica, 6, 4–12. [Google Scholar]
- Wang, L. , Waltenberger, B. , Pferschy‐Wenzig, E.‐M. , Blunder, M. , Liu, X. , Malainer, C. , Blazevic, T. , Schwaiger, S. , Rollinger, J. M. , Heiss, E. H. , & Schuster, D. (2014). Natural product agonists of peroxisome proliferator‐activated receptor gamma (PPARγ): A review. Biochemical Pharmacology, 92(1), 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.‐L. , Yang, J.‐W. , & Xu, J.‐F. (2021). Coronavirus (SARS‐CoV‐2) causes lung inflammation and injury. Clinical Microbiology and Infection, 28, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Lin, S. , Jiang, P. , Song, Y. , Zhao, Y. , & Zheng, Y. (2021). Focal adhesion kinase inhibitor inhibits the oxidative damage induced by central venous catheter via abolishing focal adhesion kinase‐protein kinase B pathway activation. BioMed Research International, 2021, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Węgrzyn, G. , Jakóbkiewicz‐Banecka, J. , Gabig‐Cimińska, M. , Piotrowska, E. , Narajczyk, M. , Kloska, A. , Malinowska, M. , Dziedzic, D. , Gołębiewska, I. , Moskot, M. , & Węgrzyn, A. (2010). Genistein: A natural isoflavone with a potential for treatment of genetic diseases. Biochemical Society Transactions, 38(2), 695–701. [DOI] [PubMed] [Google Scholar]
- Xiao, Y. , Ho, C.‐T. , Chen, Y. , Wang, Y. , Wei, Z. , Dong, M. , & Huang, Q. (2020). Synthesis, characterization, and evaluation of genistein‐loaded zein/carboxymethyl chitosan nanoparticles with improved water dispersibility, enhanced antioxidant activity, and controlled release property. Food, 9(11), 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, B. Y. , Tang, X. D. , Chen, J. , Wu, H. B. , Chen, W. S. , & Chen, L. (2020). Rifampicin induces clathrin‐dependent endocytosis and ubiquitin–proteasome degradation of MRP2 via oxidative stress‐activated PKC‐ERK/JNK/p38 and PI3K signaling pathways in HepG2 cells. Acta Pharmacologica Sinica, 41(1), 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y.‐Y. , Yang, C. , & Li, S.‐N. (2006). Effects of genistein on angiotensin‐converting enzyme in rats. Life Sciences, 79(9), 828–837. [DOI] [PubMed] [Google Scholar]
- Yao, W. , Luo, G. , Zhu, G. , Chi, X. , Zhang, A. , Xia, Z. , & Hei, Z. (2014). Propofol activation of the Nrf2 pathway is associated with amelioration of acute lung injury in a rat liver transplantation model. Oxidative Medicine and Cellular Longevity, 2014, 258567–258579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, S. , Chen, S. , Xiang, J. , Tan, J. , Huang, K. , Zhang, H. , Wang, Y. , & Wu, H. (2021). Genistein exerts a cell‐protective effect via Nrf2/HO‐1//PI3K signaling in Ab25‐35‐induced Alzheimer's disease models in vitro. Folia Histochemica et Cytobiologica, 59(1), 49–56. [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Maguire, T. G. , & Alwine, J. C. (2011). Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. Journal of Virology, 85(4), 1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, X. , Lin, M. , Zhang, F. , Hu, Y. , Xu, X. , Li, Y. , Liu, K. , Ma, X. , Tian, X. , & Yao, J. (2013). Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco‐2 cells. Molecular Nutrition & Food Research, 57(2), 249–259. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , & Liu, Y. (2020). Potential interventions for novel coronavirus in China: A systematic review. Journal of Medical Virology, 92(5), 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Ma, X. , Xu, H. , Wu, W. , He, X. , Wang, X. , Jiang, M. , Hou, Y. , & Bai, G. (2020). A natural AKT inhibitor swertiamarin targets AKT‐PH domain, inhibits downstream signaling, and alleviates inflammation. The FEBS Journal, 287(9), 1816–1829. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Liu, Y. , Wang, X. , Yang, L. , Li, H. , Wang, Y. , Liu, M. , Zhao, X. , Xie, Y. , Yang, Y. , & Zhang, S. (2020). SARS‐CoV‐2 binds platelet ACE2 to enhance thrombosis in COVID‐19. Journal of Hematology & Oncology, 13(1), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Qi, X. , Chen, X. , Zhang, J. , Zhang, W. , & Lin, H. (2021). Dietary selenomethionine ameliorates lipopolysaccharide‐induced renal inflammatory injury in broilers via regulating the PI3K/AKT pathway to inhibit necroptosis. Food & Function, 12(10), 4392–4401. [DOI] [PubMed] [Google Scholar]
- Zhao, H. , Song, X. , Zhang, Y. , & Sheng, X. (2019). Molecular interaction between MeOH and genistein during soy extraction. RSC Advances, 9(67), 39170–39179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Wang, Y. , Liu, J. , Wang, K. , Guo, X. , Ji, B. , Wu, W. , & Zhou, F. (2016). Protective effects of genistein and puerarin against chronic alcohol‐induced liver injury in mice via antioxidant, anti‐inflammatory, and anti‐apoptotic mechanisms. Journal of Agricultural and Food Chemistry, 64(38), 7291–7297. [DOI] [PubMed] [Google Scholar]
- Zorov, D. B. , Juhaszova, M. , & Sollott, S. J. (2014). Mitochondrial reactive oxygen species (ROS) and ROS‐induced ROS release. Physiological Reviews, 94(3), 909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


