Abstract
Background
COVID‐19 global pandemic started in late 2019 with the first wave. In this cross‐sectional and observational study, we evaluated the associations between the biomarkers, COVID‐19 pneumonia severity and 1‐year mortality.
Methods
A sample of 276 polymerase chain reaction (PCR)‐positive patients for SARS‐CoV‐2 was included. Computerized tomography severity score (CT‐SS) was used to assess the severity of COVID‐19 pneumonia in 222 cases. Multivariate analyses were performed to find the predictors of CT‐SS, severe CT‐SS (≥20) and 1‐year mortality. Biomarkers of ferritin, high‐sensitive C‐reactive protein (CRP), lactate dehydrogenase (LDH), cardiac troponin (cTn), neutrophil‐to‐lymphocyte ratio (NLR), uric acid (UA) and d‐dimer were routinely measured.
Results
Severe CT‐SS (>20) was observed in 86 (31.2%) cases. Mortality was observed in 75 (27.2%) patients at 1 year. LDH displayed the highest predictive accuracy for severe CT‐SS (AUC 0.741, sensitivity = 81% and specificity = 68%, cut‐off value: 360 mg/dl). Linear regression analysis displayed that LDH predicted CT‐SS [B = 11 (95% CI for B = 5–17, p < .001)]. Age was the most significant parameter that was associated with severe CT‐SS (OR 0.96, 95% CI 0.92–0.99, p = .015). d‐dimer was the only biomarker that predicted with 1‐year mortality (OR 1.62, 95% CI 1.08–2.42, p = .020).
Conclusion
LDH is a sensitive and specific biomarker to determine patients with severe lung injury in COVID‐19. d‐dimer is the only biomarker that predicts 1‐year mortality. Neither LDH nor CT‐SS is associated with 1‐year mortality.
Keywords: biomarkers, COVID‐19, CT severity score, first wave, mortality
1. INTRODUCTION
SARS‐CoV‐2 caused coronavirus disease 2019 (COVID‐19). COVID‐19 global pandemic started in late 2019 with the first wave. 1 Lung damage is common in COVID‐19 such as histologically‐proven pneumonitis or interstitial damage and fibrosis. Pneumonia is the leading cause of morbidity and mortality in COVID‐19. Lung involvement in patients with COVID‐19 could range from asymptomatic to overt adult respiratory distress syndrome requiring intubation. Determining the severity of lung involvement is important for the effective utilization of health care resources. Lung damage in COVID‐19 can be assessed with several noninvasive imaging methods such as chest radiography, computed tomography (CT) and lung ultrasonography. Radiological findings are largely based on CT of the chest. A suspected diagnosis of SARS‐CoV‐2 pneumonia is radiologically based upon the findings of ground‐glass opacities (GGO), crazy‐paving and consolidation in Chest CT images. 2 Several scoring systems check and score the typical findings in accordance with the standards published by the Fleischner Society. 3 The lung involvement and the radiological findings vary in relation to the phase and severity of COVID‐19. In the early phases, the GGO is frequently observed whereas in later phases as in severe and critical patients, consolidation is the predominant finding. 2 The CT findings represent the damage in the epithelium and the alveoli, which are infiltrated by inflammatory exudate. 4 Chest CT has a higher sensitivity and greater proficiency in determining the characteristic findings, particularly during the early stages of the disease. 5 Yet, CT scan cannot be used as a routine screening tool in the emergency room (ER) due to the cost and the radiation exposure. In addition to the clinical assessment in the ER, pulse oximeter (<94%) and respiratory rate (>22/min) set the decision points for admission in COVID‐19. 6 However, the assessment of pulse oximeter and respiratory rate can be difficult and subjective in the ER. Both variables can change with the position of the patient and environmental factors. Confirmatory biomarkers are needed to screen, diagnose and manage patients with severe lung damage in the ER. Similarly, the predictors of poor COVID‐19 outcomes during the first wave of the pandemic remain to be elucidated. The pathophysiology of severe and rapid lung damage, in most cases, remains uncertain. Histological and autopsy studies report that SARS‐CoV‐2 induces endotheliitis in the heart, lung, kidney, liver and other tissues. 7 Inflammation, microvascular dysfunction and thrombosis co‐exist in the pulmonary vascular system in COVID‐19. Several biomarkers are used to risk stratify the hospitalized patients with COVID‐19. 8 These biomarkers represent different biological systems such as inflammation (ferritin, white blood cell count (WBC), neutrophil‐to‐lymphocyte ratio (NLR) and high‐sensitive C‐reactive protein (CRP), tissue injury (lactate dehydrogenase (LDH) and cardiac troponins), oxidative stress (uric acid) and thrombosis (d‐dimer).
Over the last 2 years, the COVID‐19 phenotype has changed significantly. The availability of effective vaccines and resources, effective treatment algorithms and identification of novel variants with different clinical features have all added to the complexity of COVID‐19. The predictors of severe outcomes in the first wave of COVID‐19 remain to be of interest.
There is a need for easily available and cheap biomarkers for severe lung involvement COVID‐19. In the present study, we report the predictors of severe CT‐SS and 1‐year mortality in COVID‐19.
2. MATERIALS AND METHODS
The study was performed in Istanbul, Turkey. The first case of COVID‐19 in Turkey was reported on 11 March 2020 in Istanbul. Shortly after the start of the pandemic, the hospital became a designated COVID‐19 center. We reviewed the charts of patients who had been diagnosed with COVID‐19 and admitted to the hospital during the first wave of the pandemic. A cross‐sectional observational study was performed in a large tertiary care university hospital. Patients aged between 18 and 80 years were included. Patients were polymerase chain reaction (PCR) positive for SARS‐CoV‐2. Demographic, clinical and laboratory parameters were recorded with direct interviews and with using the institutional electronic medical database. All patients included in the present study had: (i) a positive nasopharyngeal swab PCR test for SARS‐CoV‐2, (ii) chest X‐ray or thoracic CT findings that were compatible with COVID‐19 pneumonia and (iii) requirement for hospital admission due to COVID‐19 according to the Ministry of Health criteria.
The study was approved by the Ethics Committee and the Ministry of Health (protocol number 2022–04‐09T11_42_37). The committee waived the requirement of informed consent due to the study design and anonymity of the database. The study conforms to the STROBE Statement for the reports of observational studies. Reporting of the study conforms to broad EQUATOR guidelines. 9
2.1. Imaging parameters
All patients were scanned in the supine position with a 16‐detector CT scanner (GE Optima CT660 GE Healthcare). CT scan parameters were as follows: X‐ray tube parameters 120 kVp, 300 mAs; rotation time 0.6 s; pitch 1.0; section thickness 5 mm; intersection space 5 mm; additional reconstruction with a slice thickness of 1.5 mm.
2.2. Grading of computerized tomography evidence of disease
Chest computed tomography severity score (CT‐SS) was used for grading CT findings. CT‐SS was developed by Yang et al 10 to assess the severity of COVID‐19 pneumonia. The lung segments were assessed for the presence and dissemination of GGO, crazy‐paving pattern and consolidation. Totally 20 lung segments and percent (%) involvement were scored on chest CT images as follows: 0%–score 0, <50%–score 1, or equal to or more than 50%–score 2, respectively. Finally, the sum of all 20 segment scores was added to a maximum score of 40 range. Univariate and multivariate logistic regression analysis was performed to predict CT‐SS. Severe COVID‐19 pneumonia is defined as a CT score ≥20 (Figure 1).
FIGURE 1.
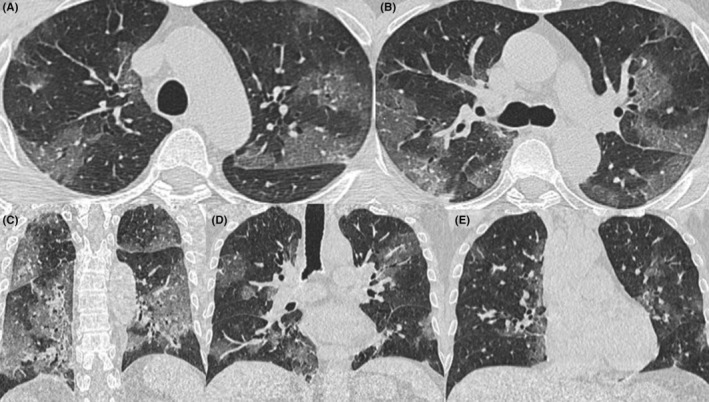
The axial (A,B) and coronal (C–E) reformatted computerized tomography (CT) images demonstrate an example of severe CT‐SS. Patchy areas of ground‐glass opacities were noted in several segments in both lungs. All 20 segments were scored from 0 to 2 based on the percent involvement. All scores were added to a total CT severity score of 31
2.3. Laboratory investigations
The nasopharyngeal swab was obtained on admission. Real‐time reverse‐transcription PCR using Coronex COVID‐19 rt‐qPCR detection kit (Gensutek) was used. The diagnosis of COVID‐19 was ascertained with a positive PCR test and thorax imaging findings that were compatible with COVID‐19 pneumonia. Biomarker concentrations of ferritin, CRP, LDH, cardiac troponin, uric acid and d‐dimer were routinely determined with electrochemiluminescence immunoassay methods, using biochemical analysis kits and Roche Cobas 6000 analysis device (Roche Diagnostics). Other laboratory analyses were done with conventional biochemical methods.
2.4. Statistical analyses
Data for continuous parameters were given as mean ± SD or median and interquartile range, depending on the distribution of the data. Categorical variables were presented as percentages. For continuous variables, patterns of distribution were analysed with a visual inspection of histograms and with the Shapiro–Wilk test. Comparisons between groups were done with the Student's t test or Mann–Whitney U test were used to compare independent samples with parametric or nonparametric distribution, respectively. For categorical variables, the χ2 test or Fisher's exact test were used to compare the groups. Correlation analyses were performed with the Pearson or Spearman test for parametric and nonparametric variables, respectively. A receiver‐operator curve was drawn to analyse the accuracy of various biomarkers to predict severe CT‐SS (≥20). Linear regression analysis was performed to find the predictors of CT‐SS. Covariates were selected from basic parameters that are assessed in the emergency room such as age, LDH, d‐dimer, haemoglobin WBC and NLR. Variables with nonparametric distribution were log‐transformed before analysis (LDH, d‐dimer, WBC). p < .05 were accepted as statistically significant. All statistical analyses were done with SPSS 25.0 (IBM Inc).
3. RESULTS
A sample of 276 PCR‐positive patients with COVID‐19 who were admitted to the hospital from April 1 to 30, 2020 to March 1 to 30, 2021 were identified. The study was performed through a detailed retrospective chart review. CT‐SS was used to assess the severity of COVID‐19 pneumonia in 222 cases, and 86 cases (39%) displayed severe CT‐SS.
Figure 1 demonstrates an example of severe CT‐SS. Patchy areas of ground‐glass opacities were noted in several segments in both lungs. All 20 segments were scored from 0 to 2 based on the percent involvement. The higher percentage of involvement (≥50%) scored 2 points for each segment. All scores were added to a total CT severity score of 31.
Demographic, clinical and laboratory results of the study groups were summarized in Table 1. Patients with severe CT‐SS displayed higher fibrinogen, troponin and hs‐CRP concentrations as compared to patients with CT‐SS < 20 (Table 2).
TABLE 1.
Demographic and laboratory characteristics of patients
| Median | IQR | Mean | SD | |
|---|---|---|---|---|
| Age (years) | 65.0 | 25.0 | 63.4 | 17.9 |
| Uric acid (mg/dl) | 5.7 | 3.3 | 6.3 | 2.8 |
| Lactate dehydgenase (units/L) | 364.0 | 261.0 | 572.5 | 1097.2 |
| Urea (mmol/L) | 39.0 | 39.5 | 59.4 | 39.5 |
| Creatinine (mg/dl) | 0.9 | 0.5 | 1.2 | 1.1 |
| Hs‐troponin (ng/L) | 18 | 90 | 879 | 5647 |
| C‐reactive protein | 11.8 | 12.4 | 16.5 | 31.1 |
| d‐dimer | 1.5 | 2.1 | 2.8 | 4.4 |
| White blood cell | 7.5 | 5.1 | 8.8 | 5.5 |
| Haemoglobin | 12.2 | 3.2 | 11.9 | 2.3 |
| Haematocrit | 37.0 | 9.0 | 36.8 | 8.1 |
| Platelet | 209 | 129 | 218 | 99 |
| Mean platelet volume | 10.1 | 1.7 | 10.1 | 1.4 |
| Platelet distribution width | 16.3 | 0.7 | 16.7 | 5.1 |
| Neutrophil‐to‐lymphocyte ratio | 4.3 | 5.7 | 9.5 | 17.9 |
| Computerized tomography of chest score | 16.0 | 17.3 | 16.1 | 10.3 |
Abbreviations: SD, standard deviation; IQR, interquartile range.
TABLE 2.
Comparison of laboratory characteristics between low and high CT score groups
| Low CT score | High CT score | p | |
|---|---|---|---|
| Hs‐troponin (ng/L) | 50.3 (313.8) | 38.0 (526.1) | .048m |
| C‐reactive protein | 14.6 (16.2) | 16.4 (17.3) | .007m |
| d‐dimer | 2.1 (2.4) | 2.5 (5.5) | <.001m |
| LDH (units/L) | 358.0 (298.5) | 516.0 (387.0) | <.001m |
| Ferritin | 340.8 (1827.0) | 1367.5 (1497.6) | <.001m |
| Uric acid (mg/dl) | 6.9 (3.2) | 7.0 (4.3) | .512m |
| White blood cell | 6.8 (5.2) | 8.4 (4.5) | .043m |
| Haemoglobin | 12.4 (3.2) | 12.1 (3.1) | .302m |
| Platelet | 201 (123) | 220 (156) | .288m |
Abbreviations: L: liter, dl: deciliter, m: Mann–Whitney U Test.
3.1. CT‐SS and biomarkers
We tested all 6 biomarkers by ROC analysis for severe CT‐SS. The sensitivities and specificities of all 6 biomarkers to detect severe CT‐SS by ROC analysis were determined (Table 3). Of all biomarkers tested, LDH [AUC 0.741 (0.672–0.809) p < .001, sensitivity = 81% and specificity = 68% for a cut‐off value 360 mg/dl] displayed the highest predictive accuracy for severe CT‐SS. AUC for other biomarkers of inflammation and organ injury remained <0.7 (Table 3). Gender differences were noted in the specificity and sensitivity of LDH in predicting severe lung damage. In both female and male patients, LDH displayed higher AUC than other biomarkers (Figure 2). Linear regression analysis was performed to find the predictors of CT‐SS. Covariates were age, LDH, d‐dimer, haemoglobin and WBC. Variables with nonparametric distribution were log‐transformed (LDH, d‐dimer and WBC). LDH was the only predictor of CT‐SS (Figure 3).
TABLE 3.
ROC statistics of biomarkers to predict high CT score
| Biomarker | AUC in men | AUC in women | AUC | % 95 CI for AUC | p | Sensitivity (%) | Specificity (%) | Cut‐off value | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| LDH | 0.702 | 0.788 | 0.741 | 0.672 | 0.809 | <.001 | 81 | 68 | 360 mg/dl |
| Hs‐CRP | 0.584 | 0.717 | 0.645 | 0.550 | 0.739 | .007 | 67 | 54 | 12 mg/dl |
| d‐dimer | 0.521 | 0.625 | 0.565 | 0.484 | 0.645 | .121 | 68 | 42 | 1.0 mg/dl |
| HsCTn | 0.574 | 0.541 | 0.561 | 0.476 | 0.646 | .166 | 52 | 48 | 10.0 ng/L |
| Ferritin | 0.679 | 0.709 | 0.696 | 0.616 | 0.775 | <.001 | 77 | 63 | 405 mg/dl |
| Uric acid | 0.522 | 0.531 | 0.528 | 0.441 | 0.614 | .512 | 53 | 57 | 6 mg/dl |
FIGURE 2.
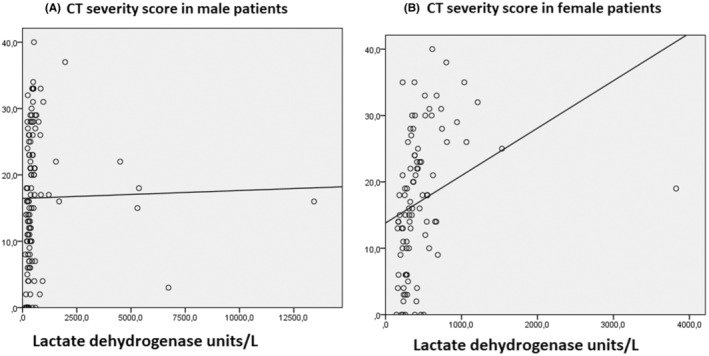
A linear regression curve estimation analysis was drawn to analyse the lactate dehydrogenase (LDH) level to predict severe COVID‐19 pneumonia CT score in males (A) and females (B)
FIGURE 3.
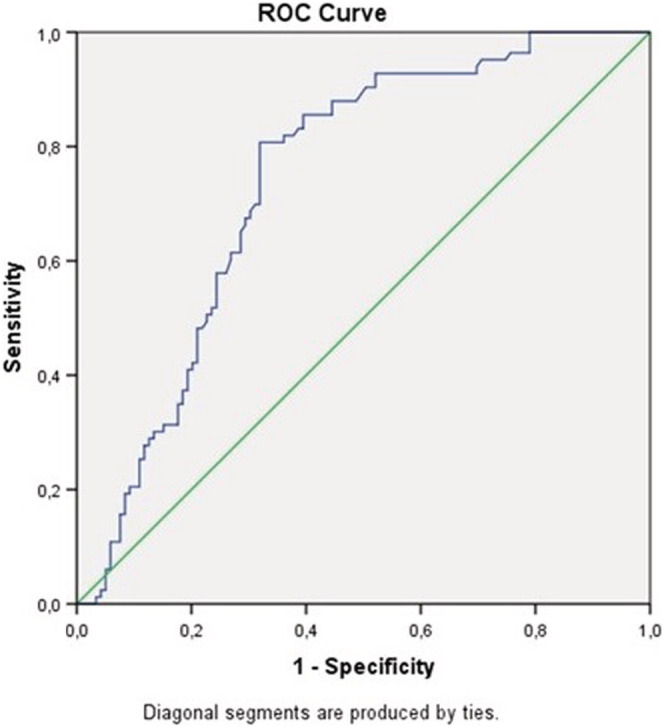
A receiver–operator curve (ROC) was drawn to analyse the accuracy of lactate dehydrogenase (LDH) to predict severe COVID‐19 pneumonia (score ≥20)
Severe CT‐SS (>20) was observed in 86 (31.2%) cases. Logistic regression analysis was performed to find the predictors of severe CT‐SS. Covariates were 7 biomarkers (Hs‐CRP, d‐dimer, LDH, troponin, ferritin, uric acid and NLR), gender and age. Age was the most significant parameter that was associated with severe CT‐SS. Age displayed negative association with CT‐SS (OR 0.96, 95% CI 0.92–0.99, p = .015, Nagelkarke R 2 .36) (Table 4).
TABLE 4.
Logistic regression analysis was performed to find the predictors of severe CT‐SS (>20)
| Sig. | Odds ratio | 95% CI for odds ratio | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age (years) | 0.015 | 0.956 | 0.922 | 0.991 |
| Gender | 0.403 | 1.669 | 0.503 | 5.540 |
| Hs‐CRP (mg/dl) | 0.956 | 0.999 | 0.968 | 1.031 |
| d‐dimer (mg/dl) | 0.028 | 1.398 | 1.037 | 1.884 |
| LDH (units/L) | 0.049 | 0.999 | 0.998 | 1.000 |
| Troponin | 0.087 | 1.000 | 1.000 | 1.000 |
| Ferritin | 0.401 | 1.000 | 1.000 | 1.000 |
| Uric acid (mg/dl) | 0.135 | 1.184 | 0.949 | 1.476 |
| NLR | 0.284 | 1.022 | 0.982 | 1.064 |
| Constant | 0.471 | 2.451 | ||
Note: Severe CT‐SS was observed in 86 (31.2%) cases. Among covariates of 7 biomarkers, gender and age, age was the most significant parameter that was associated with CT‐SS. Age displayed negative association with CT‐SS. Nagelkarke R 2 .36.
Abbreviations: CRP, high‐sensitivity C‐reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil‐to‐lymphocyte ratio.
3.2. One‐year mortality
Mortality was observed in 75 (27.2%) patients at 1 year. Multivariate binary logistic regression analysis has performed the predictors of 1‐year mortality. Covariates were age, CT‐SS, biomarker concentrations of ferritin, hs‐CRP, LDH, cardiac troponin, NLR, UA and d‐dimer. Among covariates of 7 biomarkers, CT‐SS, gender and age, d‐dimer was the only biomarker that was associated with mortality (OR 1.62 95% CI 1.08–2.42, p = .20, Nagelkarke R 2 .73) (Table 5).
TABLE 5.
The predictors of 1‐year mortality
| Sig. | Odds ratio | 95% CI for odds ratio | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age (years) | 0.530 | 1.017 | 0.966 | 1.070 |
| hs‐CRP (mg/dl) | 0.805 | 1.006 | 0.957 | 1.058 |
| d‐dimer (mg/dl) | 0.020 | 1.618 | 1.080 | 2.424 |
| LDH (units/L) | 0.264 | 1.003 | 0.998 | 1.007 |
| Troponin | 0.165 | 1.000 | 1.000 | 1.001 |
| Ferritin | 0.141 | 1.000 | 1.000 | 1.001 |
| Uric acid (mg/dl) | 0.141 | 1.261 | 0.926 | 1.719 |
| NLR | 0.147 | 1.053 | 0.982 | 1.130 |
| CT‐SS | 0.383 | 0.955 | 0.862 | 1.059 |
| Gender | 0.985 | 0.981 | 0.137 | 7.028 |
| Constant | 0.025 | 0.001 | ||
Note: Logistic regression analysis was performed to find the predictors of 1‐year mortality. Mortality was observed in 75 (27.2%) patients at 1 year. Multivariate binary logistic regression analysis was performed the predictors of 1‐year mortality. Covariates were age, CT‐SS, biomarker concentrations of ferritin, hs‐CRP, LDH, cardiac troponin, NLR, UA and d‐dimer. d‐dimer was the only biomarker that was associated with mortality (Nagelkarke R 2 .73).
Abbreviations: Hs‐CRP, high‐sensitivity C‐reactive protein; NLR, neutrophil‐to‐lymphocyte ratio; CT‐SS, computed tomography severity score.
4. DISCUSSION
Severe lung damage and 1‐year mortality are indicators of poor outcomes after COVID‐19. The study indicates that there is a discrepancy between the biomarkers of lung damage and 1‐year mortality in COVID‐19. LDH is a sensitive and specific biomarker to determine patients with severe lung damage in COVID‐19. Yet, neither LDH nor CT‐SS is associated with 1‐year mortality. d‐dimer is the only biomarker that was associated with mortality in the first wave of COVID‐19.
d‐dimer is a fibrin degradation product and a small protein fragment. d‐dimer is present in the circulation after a blood clot is degraded by fibrinolysis. 8 COVID‐19 patients display an interesting paradox. Clinical phenotype in COVID‐19 is characterized by a hypercoagulable state with decreased fibrinolytic capacity and a paradoxical increase in d‐dimer. 11
Several retrospective studies report that d‐dimer levels are associated with the disease severity and mortality in COVID‐19. 12
The hallmark of the lung damage in COVID19 is intra‐alveolar fibrin deposition. d‐dimer is a protein degradation product of fibrin that is formed by plasmin during fibrinolysis. 12 d‐dimer serves as an indirect biomarker of thrombosis. Fibrinolysis and proteolytic breakdown of the fibrin in the capillary beds of the lung segments increase the d‐dimer levels in the circulation. Observational studies report that the lungs are the potential sources of d‐dimer in COVID‐19. 11 , 12 , 13
A retrospective study from Wuhan, China reports the COVID‐19 outcome from 676 laboratory‐confirmed cases. The study comes from the first wave of the pandemic. 14 Patients are divided into 3 groups according to the extent of lung lesions: mild (0%–30%), moderate (30%–60%) and severe (>60%). Severe lung damage is more common in the mortality group compared with the survivors (65.9% vs. 16.0%). d‐dimer is the biomarker that predicts the mortality in the study. 14
A multicenter cohort study of 3418 critically ill COVID‐19 patients from the US confirms the results of the Wuhan study. d‐dimer levels are independently associated with mortality, even after adjustments for disease characteristics and severity. 15 Early autopsy and pathological studies help the clinicians to understand the pathophysiology of d‐dimer elevation in COVID‐19. The lung specimens demonstrate widespread capillary fibrinous microthrombi and fibrin at the microvascular level. 16
Global data indicate that there are differences in the risk factors, phenotype and outcome of COVID‐19 among countries. 17 The vaccination for COVID‐19 in Turkey initially started early in 2021 with the CoronaVac vaccine. Healthcare workers and old people with chronic diseases were prioritized in the order of vaccination. Pfizer‐BioNTech vaccine doses were widely available later in 2021. After the vaccination COVID‐19 disease course and phenotype changed significantly. Similarly, novel variants, effective therapies and global responses evolved in the course of pandemics. The phenotype of COVID‐19 changed significantly since the first wave of COVID‐19. The delta and omicron variants have reached global circulation after 2021. The first variants were dominant throughout the world at the time of our study. The diagnostic and prognostic effects of biomarkers differ according to the globally dominant variants.
LDH is a widely available biochemical parameter that is part of the routine laboratory panel. Estimating the extent of lung damage is challenging in the absence of imaging findings. CT cannot be offered to every patient visiting ER with a clinical suspicion of COVID‐19. The cost and radiation exposure are common concerns. The study indicates that LDH is a sensitive and specific biomarker to predict a severe CT‐SS. LDH displays better performance than hs‐CRP, d‐dimer, cTn, uric acid and ferritin in predicting severe CT‐SS. In the first wave of pandemics, respiratory rate or room air oxygen saturation are used as initial screening parameters. LDH is a routinely and easily available biomarker universally. Adding LDH level to the initial evaluation algorithms can be useful. LDH can be utilized in screening patients for severe CT‐SS.
Various scoring methods (including artificial intelligence) have been proposed to grade the CT findings in COVID‐19 10 , 18 , 19 , 20 , 21 CT‐SS is developed for assessing the severity of COVID‐19 pneumonia. 10 Chest CT findings of COVID‐19 partially overlap with other viral infections. COVID‐19 reporting and data system (CO‐RADS) is a scheme that evaluates the extent of the lung involvement. The reporting system ranges from very low to very high levels of suspicion (CO‐RADS 1–5), 20 Francone et al 4 and Pan et al 18 use semiquantitative CT severity scoring systems. Five lobes of each lung are evaluated for the extent of the involvement and all 5 lobes are scored from 0 to 5. The global CT‐SS is the sum of each lobar score, which varies from 0 to 25 4 GGOs are the main findings in the early phase of COVID‐19. Crazy‐paving and consolidation patterns are the characteristics of the late phase of the disease.
In a recent observational study, CT‐SS and age of the patients from the first and the second waves of the pandemic are compared. CT‐SS and age of the patients are higher in the second wave compared with the first wave of the pandemic. 22 In the current study, we observe a negative correlation between age and CT‐SS. The differences in the observations can be attributed to the alterations of COVID‐19 phenotype between the waveforms of the disease.
LDH is a valuable biomarker in a wide range of pathological conditions in the respiratory system. Bronchial asthma, chronic obstructive pulmonary disease, pulmonary tuberculosis, bronchopneumonia, pneumocystis jirovecii pneumonia, avian influenza and acute respiratory distress syndrome are all associated with elevated LDH levels 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 The current study findings suggest that LDH can be useful in the clinical assessment of COVID‐19 patient in the ER. Yet LDH or CT‐SS are not associated with short‐term mortality after COVID‐19. d‐dimer predicts 1‐year mortality. These observations contribute to a nascent body of literature suggesting that COVID‐19 is multisystem disease.
5. LIMITATIONS
We examine a representative sample of COVID‐19 patients from Istanbul, Turkey. One of the study's limitations is that the sample size is small and cross‐sectional. Each country has differences in treatment algorithms during the COVID‐19 pandemic. Therapies can affect biomarker levels. Further disparities exist among different regions and socioeconomical statuses within the countries. Another limitation is that the confounding effects of genetic and environmental risk factors cannot be excluded. Pertaining to the laboratory studies, preanalytical and analytical factors can affect biomarker levels. The biomarker levels were obtained on the same admission with COVID‐19. Since the study period, the COVID‐19 phenotype has changed significantly. Vaccination, novel variants, effective therapies and global response to pandemics have all modulated the phenotype of COVID‐19. Naturally, biomarkers' diagnostic and prognostic forecast values will also change according to the globally dominant variants.
6. CONCLUSIONS
COVID‐19 is a proinflammatory condition that can result in the elevation of biomarkers in several critical pathways. In this study, we investigated the biomarkers in relation to the severity of pneumonia and 1‐year mortality in hospitalized COVID‐19 patients. One‐year mortality was high in the first wave of COVID‐19. d‐dimer was the only parameter that predicted the 1‐year mortality. LDH was a sensitive and specific biomarker of severe CT‐SS. Yet, neither LDH nor CT‐SS was associated with 1‐year mortality. We need larger studies to test the efficacy of novel criteria and algorithms that can improve the outcome of COVID‐19.
CONFLICT OF INTEREST
Authors have no conflicts to disclose.
Atalay B, Cesur A, Agirbasli M. Discrepancy between biomarkers of lung injury and 1‐year mortality in COVID‐19. Eur J Clin Invest. 2022;00:e13827. doi: 10.1111/eci.13827
REFERENCES
- 1. Hu B, Guo H, Zhou P, et al. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2021;19:141‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID‐19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87‐93. [DOI] [PubMed] [Google Scholar]
- 3. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697‐722. [DOI] [PubMed] [Google Scholar]
- 4. Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID‐19 patients: correlation with disease severity and short‐term prognosis. Eur Radiol. 2020;30(12):6808‐6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pal A, Ali A, Young TR, et al. Comprehensive literature review on the radiographic findings, imaging modalities, and the role of radiology in the COVID‐19 pandemic. World J Radiol. 2021;13(9):258‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID‐19_Rehberi20200414_eng_v4_002_14.05.2020.pdf
- 7. Fox SE, Lameira FS, Rinker EB, Vander Heide RS. Cardiac endotheliitis and multisystem inflammatory syndrome after COVID‐19. Ann Intern Med. 2020;173(12):1025‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Battaglini D, Lopes‐Pacheco M, Castro‐Faria‐Neto HC, Pelosi P, Rocco PRM. Laboratory biomarkers for diagnosis and prognosis in COVID‐19. Front Immunol. 2022;27(13):857573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 10. Yang R, Li X, Liu H, et al. Chest CT severity score: an imaging tool for assessing severe COVID‐19. Radiol Cardiothorac Imaging. 2020;2(2):e200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ibañez C, Perdomo J, Calvo A, et al. High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? J Thromb Thrombolysis. 2021;51(2):308‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nasif WA, El‐Moursy Ali AS, Hasan Mukhtar M, et al. Elucidating the Correlation of D‐Dimer Levels with COVID‐19 Severity: A Scoping Review Anemia. 2022. 9104209. doi: 10.1155/2022/9104209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agirbasli M. Thrombosis and fibrosis: mutually inclusive targets to combat in COVID‐19. Future Sci OA. 2022;8(2):FSO777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang Y, Lyu X, Li D, et al. A cohort study of 676 patients indicates d‐dimer is a critical risk factor for the mortality of COVID‐19. PLoS One. 2020;15(11):e0242045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Short SAP, Gupta S, Brenner SK, et al. d‐dimer and death in critically ill patients with coronavirus disease 2019. Crit Care Med. 2021;49(5):e500‐e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carsana L, Sonzogni A, Nasr D, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐centre descriptive study. Lancet Infect Dis. 2020;20(10):1135‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarmadi M, Ahmadi‐Soleimani SM, Fararouei M, Dianatinasab M. COVID‐19, body mass index and cholesterol: an ecological study using global data. BMC Public Health. 2021;21(1):1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest ct during recovery from coronavirus disease 2019 (COVID‐19). Radiology. 2020;295(3):715‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID‐19 pneumonia. Invest Radiol. 2020;55(6):327‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prokop M, van Everdingen W, van Rees VT, et al. CO‐RADS: a categorical CT assessment scheme for patients suspected of having COVID‐19‐definition and evaluation. Radiology. 2020;296(2):E97‐E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lessmann N, Sánchez CI, Beenen L, et al. Automated assessment of COVID‐19 reporting and data system and chest CT severity scores in patients suspected of having COVID‐19 using artificial intelligence. Radiology. 2021;298(1):E18‐E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korkmaz İ, Keleş F. COVID‐19‐related lung involvement at different time intervals: evaluation of computed tomography images with semiquantitative scoring system and COVID‐19 reporting and data system scoring. Cureus. 2021;13(10):e18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142‐150. [DOI] [PubMed] [Google Scholar]
- 24. Usher DJ, Shepherd RJ, Deegan T. Serum lactate dehydrogenase isoenzyme activities in patients with asthma. Thorax. 1974;29:685‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cepelak I, Dodig S, Romic D, Ruljancic N, Popovic‐Grle S, Malic A. Enzyme catalytic activities in chronic obstructive pulmonary disease. Arch Med Res. 2006;37:624‐629. [DOI] [PubMed] [Google Scholar]
- 26. Spruit MA, Pennings HJ, Does JD, Moller GM, Janssen PP, Wouters EFM. Serum LDH and exercise capacity in COPD. Thorax. 2008;63:472. [DOI] [PubMed] [Google Scholar]
- 27. Drent M, Cobben NA, Henderson RF, et al. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9:1736‐1742. [DOI] [PubMed] [Google Scholar]
- 28. Emad A, Rezaian GR. Lactate dehydrogenase in bronchoalveolar lavage fluid of patients with active pulmonary tuberculosis. Respiration. 1999;66:41‐45. [DOI] [PubMed] [Google Scholar]
- 29. Quist J, Hill AR. Serum lactate dehydrogenase (LDH) in Pneumocystis carinii pneumonia, tuberculosis, and bacterial pneumonia. Chest. 1995;108:415‐458. [DOI] [PubMed] [Google Scholar]
- 30. Tasaka S, Hasegawa N, Kobayashi S, et al. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest. 2007;131:1173‐1180. [DOI] [PubMed] [Google Scholar]


