Abstract
Introduction
The effects of the COVID‐19 pandemic on mental health have been profound. Mental health and diabetes self‐care are inter‐related. We examined whether COVID‐19 anxiety, depressive symptoms and health anxiety were associated with domains of diabetes self‐management and investigated whether greater COVID‐19 anxiety syndrome would independently contribute to suboptimal diabetes self‐care.
Research design and methods
Surveys were sent to people attending diabetes clinics of three London hospitals. Participants completed the Diabetes Self‐Management Questionnaire (DSMQ), the COVID‐19 Anxiety Syndrome Scale (C‐19 ASS), which measures perseveration and avoidant maladaptive coping behaviour, assessed with measures of co‐existent depressive symptoms and anxiety, controlling for age, gender and social deprivation. Clinical data, including pre‐ and post‐lockdown HbA1c measures, were obtained from hospital records for 369 respondents, a response rate of 12.8%.
Results
Depressive symptom scores were high. Both pre‐existing health anxiety and depressive symptoms were independently linked to improvable measures of diabetes care, as was lower socio‐economic rank. However, avoidant COVID‐19 anxiety responses were independently associated with higher diabetes self‐care scores. HbA1c levels improved modestly over the year of UK lockdown in this cohort.
Conclusion
During the height of lockdown, avoidant coping behaviours characteristic of the COVID‐19 anxiety syndrome may in fact work to improve diabetes self‐care, at least in the short term. We recommend screening for depressive symptoms and being aware of the significant minority of people with COVID‐19 anxiety syndrome who may now find it difficult to re‐engage with face‐to‐face clinic opportunities.
Keywords: anxiety, COVID‐19, depression, diabetes, HbA1c , maladaptation
Novelty Statement.
What is already known?
Anxiety and depression are predictors for suboptimal diabetes self‐management and this may have been magnified during the COVID pandemic since people with diabetes were advised that they were at risk.
What has this study found?
Rates of COVID Anxiety Syndrome are not high amongst people with diabetes; however, rates of depressive symptoms were much higher than pre‐pandemic levels and this was significantly associated with suboptimal diabetes self‐care. Conversely, high levels of COVID‐19‐related avoidant behaviour were associated with improved measures of diabetes self‐management.
What are the implications of the study?
As we return to face‐to‐face clinical encounters, we should be aware of the high levels of mental distress amongst people with diabetes.
1. INTRODUCTION
Coronavirus disease (COVID‐19), caused by the SARS‐CoV‐2 virus, was declared a pandemic by the World Health Organisation (WHO) on 11 March 2020. By March 2021, there were 115 million confirmed cases and over 2.5 million deaths worldwide. To curb the spread of the virus and protect frontline medical services from becoming overwhelmed, many countries implemented pervasive social distancing measures. ‘Lockdown’ came into place on 26 March 2020 in the United Kingdom. By July 2021, although there have been transitions between levels of severity, the country had not yet returned to the normality of social interactions.
COVID‐19 infection carries a poorer prognosis with advancing age and also in those with co‐morbidities. With a global prevalence of 8.5%, diabetes is second only to hypertension as the most common co‐morbidity documented in patients hospitalised with COVID‐19. 1 , 2 Diabetes is an established risk factor for severe COVID‐19. The largest scale UK study of 23, 698 in‐hospital COVID‐19‐related deaths reported adjusted odds of mortality for patients with Type 1 and Type 2 diabetes of 2.86 and 1.80 respectively. 3 Accordingly, UK guidelines state that a diagnosis of diabetes confers an increased COVID‐19 risk, and during the riskiest phases of the pandemic, enhanced precautions and shielding measures were recommended for these individuals. 4
Social distancing measures were proven to prevent deaths 5 ; however, the implementation of lockdown has had a significant impact on mental health. In the UK general population, during the first phase of the pandemic, 22.1% of surveyed people had symptoms of moderate/severe depression, and 21.6% of moderate/severe anxiety, 6 proportions higher than pre‐pandemic estimates of 17.0% and 13.0% respectively. 7 Fear and anxiety related to COVID‐19 have also been reported 8 as well as a specific pattern of coping in relation to the threat of COVID‐19 infection, termed the ‘COVID‐19 anxiety syndrome’. 9 A scale, the COVID‐19 Anxiety Syndrome Scale (C‐19 ASS), enables the assessment of COVID‐19‐related avoidance, checking, worrying and threat monitoring, which has been found to predict generalised anxiety and depressive symptoms independently of age, gender, employment and risk status, personality traits and health anxiety in both US and UK community samples. 9 , 10 , 11
There is a complex bidirectional relationship between symptoms of mental health and diabetes self‐care. Amongst people with diabetes, depressive symptoms were associated with lower diabetes self‐care (as measured by the Diabetes Self‐management Questionnaire, DSMQ), and indirectly with higher HbA1c. 12 Given the possible interplay between COVID‐19 susceptibility, maladaptive responses to the fear of COVID‐19, depressive symptoms, anxiety and self‐care in people with diabetes, clinicians managing these individuals need a better understanding of their needs as lockdown restrictions ease and clinical re‐engagement occurs.
With that goal in mind, people with diabetes on outpatient clinic lists across three London hospitals were surveyed during the second major lockdown (January–March 2021) and how COVID‐19 anxiety syndrome, depressive symptoms and health anxiety linked to domains of diabetes self‐management, as measured by the DSMQ, were examined, whilst controlling for age, gender and social deprivation. Whether greater COVID‐19 anxiety syndrome prevalence would independently contribute to suboptimal diabetes self‐care was investigated.
2. METHODS
2.1. Study setting
All people on clinic lists in 2019 attending the Diabetes Service at Imperial College Healthcare NHS Trust (ICHNT), which includes three London hospitals (Charing Cross Hospital, Hammersmith Hospital and St Mary's Hospital), were invited via email (using a secure Qualtrics link) or, if no email address was on file, a postal pack to return their study surveys. Study participants had to confirm that they had read the information leaflet and signed informed consent for their responses to be included. The survey was open between 7 January and 2 March 2021. This study was approved by the Frenchay Research Ethics (REC reference 20/PR/0771).
2.2. Data collection
Participants were sent a request to fill out the following five questionnaires.
2.2.1. The diabetes self‐management questionnaire (DSMQ)
To measure diabetes self‐management, participants received the validated 16‐item DSMQ, which assesses five domains using a four‐point Likert scale (e.g., 0 for ‘does not apply to me at all’ through to 3 for ‘applies to me very much’): ‘dietary control’ (DSMQ‐DC), glucose monitoring (DSMQ‐GM), ‘medication adherence’ (DSMQ‐MA), physical activity (DSMQ‐PA) and physician contact (DSMQ‐PC). 13 Both DSMQ subscores and the cumulative score (DSMQ‐total) are calculated, with higher scores reflecting more optimal diabetes self‐care. The DSMQ has been validated for use in people with Type 1 and Type 2 diabetes. 13 , 14 Where questions are not answerable (e.g., if they relate to insulin dosing in people who do not inject), the score can be rescaled. A scaled DSMQ score was therefore calculated as the actual sum of items/maximum possible sum of items ×10. The transformed scale score can vary between 0 and 10.
2.2.2. The COVID‐19 anxiety syndrome scale (C‐19 ASS)
The C‐19 ASS is a nine‐item survey which assesses COVID‐19 anxiety‐related maladaptive coping behaviour, with two domains of perseveration (excessive symptom checking, worrying and threat monitoring) and avoidance. Participants rank their responses using a 5‐point Likert scale (0–4 points). Total scores range from 0 to 36, with higher scores indicating greater COVID‐19 anxiety syndrome severity.
2.2.3. Seven‐item Whiteley Index (WI‐7)
To account for pre‐existing health anxiety, the WI‐7 was used. Participants respond using a 5‐point Likert scale: ‘Not at all’ to ‘Great deal’ (0–4 points). Total scores range between 0 and 28, with a score > 21 suggestive of clinically relevant health anxiety. 15
2.2.4. Patient Health Questionnaire‐9 (PHQ‐9)
The PHQ‐9 has been extensively used in studies evaluating depressive symptoms in people with diabetes. Participants answer using a 4‐point Likert scale: ‘Not at all’ to ‘Nearly every day’ (0–3 points). Higher PHQ‐9 scores indicate progressively increasing depressive symptom severity, and a score ≥ 10 has 88% sensitivity and 88% specificity for depressive disorder. 16
2.2.5. Clinical data
No demographic or clinical data were requested via self‐reporting. Instead, the questionnaires had a code linking researchers to that respondent's unique hospital number and clinical data were collected from electronic health records held on the ICHNT computer system (Cerner Corporation).
For all respondents, demographics (age, sex, ethnicity and 2019 Index of Multiple Deprivation rank; IMDR—a higher number indicating greater social deprivation on a scale of 1–32, 844), anthropometrics (weight and body mass index), diabetes type and duration, treatment information (medications for diabetes but also all others e.g. antidepressants), relevant complications (microvascular: retinopathy, neuropathy, nephropathy and macrovascular: cardiovascular disease/stroke) and laboratory data (glycated haemoglobin [HbA1c], estimated glomerular filtration rate [eGFR], total cholesterol: HDL ratio and albumin: creatinine ratio [ACR]) were recorded. For each biochemical parameter, the soonest measurement before (pre‐COVID) and the latest after December 2019 was obtained to identify trends over the pandemic. Data points were more than 3 months and less than 18 months apart as a minimum and maximum.
2.3. Statistical analysis
Mean and standard deviation (SD) were used to summarise continuous variables and mean and interquartile range (IQR) used to summarise ordinal and non‐Gaussian variables. Distribution was assessed using the Shapiro–Wilk test. Where data were continuous and followed a Gaussian distribution, p‐values were calculated using either the two‐tailed t‐test (paired or unpaired) or the one‐way ANOVA (Tukey's multiple comparisons test was used for post hoc analysis). Where data were ordinal or of non‐Gaussian distribution, comparators used were the Mann–Whitney U test for unpaired data or the Wilcoxon signed‐rank test for paired data. Categorical values are summarised as a percentage and a chi‐squared test was used for comparison. To identify univariate associations, correlation matrices were calculated using two‐tailed Spearman's rank. These univariate and group comparison analyses were performed using GraphPad Prism, Version 9. An a priori power calculation, based on the requirement of 20 participants per questionnaire factor, provided the aim of a minimum of 180 responses from people with diabetes.
To uncover the independent determinants of suboptimal self‐care (low DSMQ scores), a proportional odds model was estimated in Matlab. Since the dependent variables (DSMQ factors) are ordinal scores with bounded support, DSMQ scores were divided into three categories: ‘Low’, ‘Medium’ and ‘High’. The threshold values to determine which category a given person fits into reflect the cross‐sectional distribution of the score. Hence, the bottom 30% of the sample were included in the ‘Low’ category, the mid 30% in the ‘Medium’ category and the upper 40% in the ‘High’ one.
The estimated functional form is explained in the equations:
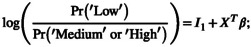 |
where X is a matrix containing the potential determinants of the score, and β is a vector of parameters to be estimated by the maximum likelihood of predicting low self‐care. Therefore, since this was modelled with ‘low’ DSMQ score as the numerator, when a variable has a positive β coefficient, it should be interpreted to mean that as the variable increases, there is a greater chance of a lower (suboptimal) DSMQ score. Conversely, a negative β coefficient means that as that variable increases, it is independently associated with a higher (better) DSMQ score. The choice of the variable to include in X was informed by previous literature but also by data quality issues. For this reason, ethnicity, which was poorly recorded in the hospital notes, was not included in the analysis.
3. RESULTS
Invitations to participate in the study were sent out to 2, 894 people, of whom 59% were women and 64% had Type 1 diabetes. Three hundred and sixty nine people with diabetes successfully completed the study, a response rate of 12.8%. Characteristics of the respondents are presented in Table 1. The average age was 50.5 ± 16.0 years (compared with a mean age of 51.6 years for all those written to). Respondents were well gender‐balanced (women: 52.9%) and most had been diagnosed with Type 1 diabetes (70.5%). Although only 7.9% of respondents for whom ethnicity data were retrievable were from minority ethnic populations, 54.2% of participants had missing ethnicity data. Socio‐economic deprivation and previous severe COVID‐19 disease necessitating hospital management were uncommon in this group.
TABLE 1.
Characteristics of patients attending a London hospital diabetes clinic that responded to a survey of mental health and diabetes self‐management questionnaires during the second UK lockdown (January‐March 2021)
| Total | Type 1 Diabetes | Type 2 Diabetes | |
|---|---|---|---|
| (n = 369) | (n = 260) | (n = 109) | |
| Demographic data | |||
| Age, mean (SD) | 50.5 (16.0) | 45.4 (16.3) | 57.8 (12.3) |
| Gender | |||
| Men % | 47.1% | 44.8% | 50.5% |
| Women % | 52.9% | 55.2% | 49.5% |
| Ethnicity, n (% total cohort) | |||
|
White Caucasian |
140 (37.9%) | 89 (34.2%) | 51 (46.8%) |
| Asian/Mixed | 22 (6.0%) | 8 (3.1%) | 14 (12.8%) |
| Black/Afro‐Caribbean | 7 (1.9%) | 2 (0.8%) | 5 (4.6%) |
| Not specified | 200 (54.2%) | 161 (61.9%) | 39 (35.8%) |
| Index of multiple deprivation rank a , n | |||
| Bottom tertile | 85 (26.2%) | 52 (24%) | 35 (32%) |
| Middle tertile | 129 (39.7%) | 86 (40%) | 37 (34%) |
| Top tertile | 111 (34.2%) | 78 (36%) | 37 (34%) |
| Not specified, n | 44 | 44 | 0 |
| Clinical data | |||
| Diabetes duration, years; mean ± SD (number of respondents data available for) | 17.0 ± 13.7 (225) | 21.8 ± 14.6 (130) | 10.3 ± 8.8 (95) |
| Most recent weight, kg; mean ± SD (number of respondents data available for) | 85.0 ± 23.9 (213) | 78.1 ± 16.5 (118) | 93.5 ± 27.0 (95) |
| ICHNT COVID admission, n (% total cohort) | 4 (1.1%) | 1 (0.4%) | 3 (2.8%) |
| ICU Admissions, n (% total cohort) | 1 (0.3%) | 1 (0.4%) | 0 (0%) |
| Treatment data | |||
| Insulin use, % total cohort | 100% | 42% | |
| Oral Hypoglycaemic medication only, n (%) | |||
| Total | 4.2% | 78.9% | |
| Metformin only | 4.2% | 30.3% | |
| Multiple oral hypoglycaemic use | 0% | 48.6% | |
| Previous bariatric surgery | 0 | 5.5% | |
| Anti‐depressant/anxiolytic use, % total cohort | 11.2% | 8.4% | |
| Complication data | |||
| Retinopathy | 46.9% | 19.2% | |
| Neuropathy | 21.3% | 21.1% | |
| Advanced nephropathy | 8.4% | 15.6% | |
| Cardiovascular disease | 9.1% | 11.0% | |
| C‐19 ASS scores | |||
| C‐19 ASS total mean score (IQR) (max score 36, scores > 18 suggestive of COVID anxiety syndrome) | 18.22 (11–25) | 18.15 (21–25) | 18.39 (11–26) |
| C‐19 ASS P perseveration subscore: mean score (IQR) | 10.52 (6–15) | 10.47 (6–15) | 10.63 (6–16) |
| C‐19 ASS A avoidance subscore: mean score (IQR) | 7.70 (4–11) | 7.68 (4–11) | 7.75 (4–12) |
| DSMQ scores (scaled): max score 10, scores >6 suggest good self‐care | |||
| DSMQ total: mean score (IQR) | 4.14 (3–4) | 4.07 (4–4) | 4.62 (4–5) |
| % with a mean DSMQ‐total score >6 | 4% | 18% | |
| DSMQ‐PC: mean score (IQR) | 4.02 (3–4) | 3.37 (3–4) | 4.43 (3–6) |
| DSMQ‐GM: mean score (IQR) | 5.40 (5–6) | 5.41 (7–9) | 5.47 (5–6) |
| DSMQ‐PA: mean score (IQR) | 2.51 (2–3) | 2.51 (3–4) | 3.55 (2–4) |
| DSMQ‐DC: mean score (IQR) | 4.21 (3–5) | 4.21 (3–5) | 4.49 (3–5) |
| Whiteley‐7 scores: max score 28, scores > 21 suggest significant health anxiety | |||
| WI‐7 total: mean score (IQR) | 9.87 (5–14) | 9.68 (5–13) | 10.31 (5–14) |
| % with a score ≥ 21 (indicating clinically significant health anxiety) | 5.5% | 5.4% | 5.5% |
| PHQ‐9 scores: max score 27, scores > 10 suggest clinically significant depressive symptoms | |||
| PHQ‐9 total: mean score (IQR) | 7.28 (2–10) | 7.03 (2–10) | 7.86 (3–12) |
| % with a score ≥ 10 (indicating clinically moderate to severe depressive symptoms) | 27.6% | 26.5% | 28.4% |
Note: Characteristics of all participants with Type 1 Diabetes (n = 260) and Type 2 Diabetes (n = 109) who usually attend hospital diabetes clinics and responded to our email/postal survey of mental health and diabetes self‐management questionnaires during the second UK lockdown (January‐March 2021). Clinical data were accessed from the CERNER patient database.
Abbreviations: C‐19 ASS, Covid‐19 Anxiety Syndrome Scale; DSMQ, Diabetes Self‐Management Questionnaire (domains—PC, Physician Contact; GM, Glucose Monitoring; PA, Physical Activity; DC, 'Dietary Control'); ICHNT, Imperial College Healthcare NHS Trust; ICU, Intensive Care Unit; IQR, Interquartile Range; kg, kilogram; PHQ‐9, Patient Health Questionnaire‐9; SD, Standard Deviation; WI‐7, 7‐item Whiteley Index.
Index of Multiple Deprivation (IMD) ranks every small area in England from 1 (most deprived area) to 32, 844 (least deprived area) based on several domains, such as employment levels, gathered by the UK Ministry of Housing each year.
The range of questionnaire scores (C‐19 ASS, DSMQ, WI‐7 and PHQ‐9) for the whole cohort, and divided by type of diabetes, is given in Table 1. Respondents with diabetes had a smooth distribution of health anxiety scores, weighted towards lower levels (Figure 1a). Similar distribution plots of C‐19 ASS results (avoidance and perseveration subscores) are presented in Figure 1c,d. These distributions point to a small group of participants with very high C‐19 ASS scores. DSMQ scaled scores, expressed as a total (DSMQ‐total) and five subscores, which reflect the different aspects of diabetes self‐care, revealed highest self‐assessed scores for glucose monitoring and lowest scores for physical activity in this cohort. However, overall, only 4% of participants with Type 1 diabetes and 18% of participants with Type 2 diabetes reported a scaled DSMQ score > 6, a commonly used cut‐off to assign ‘good’ diabetes self‐management. 12 , 13
FIGURE 1.
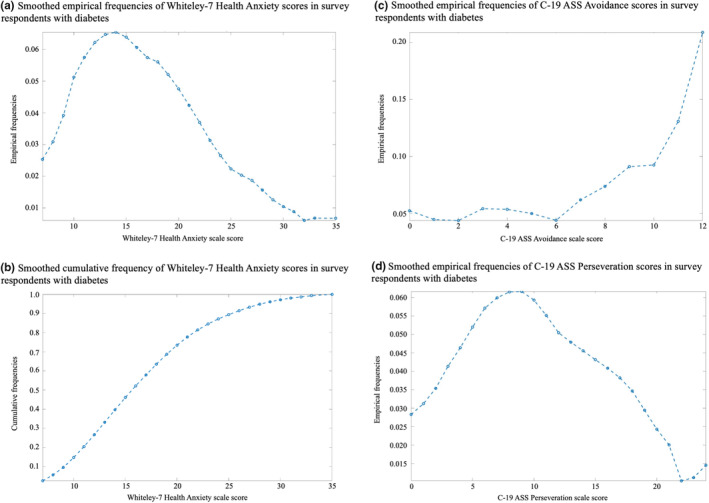
Probability distribution plots for (a) Whiteley‐7 scores (empirical frequencies), (b) Whiteley‐7 scores (cumulative frequencies), (c) C‐19 ASS avoidance and (d) C‐19 ASS perseveration scores in survey respondents with diabetes.
To examine the univariate associations between the mental health questionnaires, diabetes disease self‐management (as measured with the DSMQ) and demographic factors, a correlation matrix is presented in Figure 2. In survey respondents with diabetes of any type, C‐19 ASS scores were associated with WI‐7 (R = 0.32 each for perseveration and for avoidance, p < 0.0001) and PHQ‐9 (R = 0.22 for both perseveration and for avoidance, p < 0.0001), suggesting symptoms of health anxiety and depression are likely to occur alongside COVID‐19 anxiety syndrome in people with diabetes. WI‐7 and PHQ‐9 were both negatively associated with all aspects of DSMQ (R = −0.33 and − 0.46, p < 0.0001 for DSMQ‐total), confirming the expected association of anxiety and depressive symptoms with improvable reported measures of diabetes self‐care. Total scores on the DSMQ were not significantly associated with either the perseveration or the avoidance component of the COVID‐19 anxiety syndrome.
FIGURE 2.
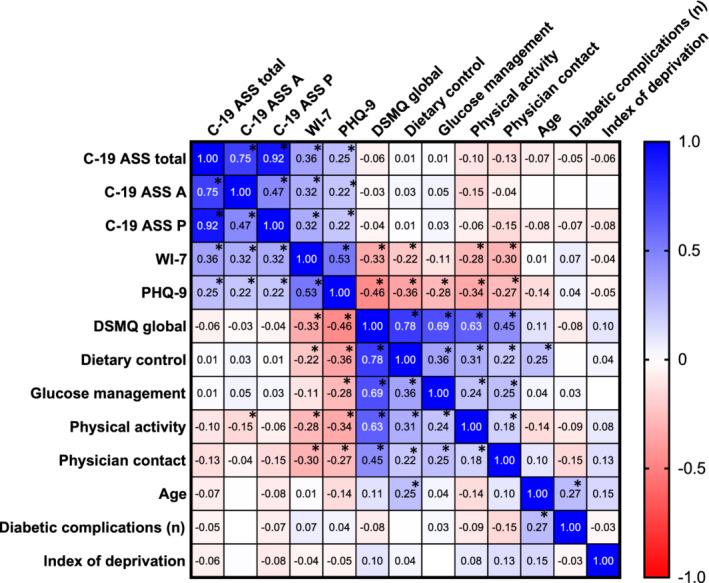
Univariate correlation table (Spearman rank coefficient) between different measured factors in participants with diabetes who responded to the email/postal survey of mental health and diabetes self‐management questionnaires during the second UK lockdown (January–March 2021; n = 369). Stars indicate correlations that reached significance when corrected for using Bonferroni's correction for multiple testing (p < 0.00385). Blank white squares indicate an R‐value of 0 to 2sf. Abbreviations: C‐19 ASS A, COVID‐19 Anxiety Syndrome Scale Avoidance subscore; C‐19 ASS P, COVID‐19 Anxiety Syndrome Scale Perseveration subscore; DSMQ, Diabetes Self‐Management Questionnaire; IMDR, Index of Multiple Deprivation Rank; PHQ‐9, Patient Health Questionnaire‐9; WI‐7, Whiteley Index‐7.
A multivariate proportional odds analysis was then performed to better understand the marginal associations with the different factors within the DSMQ (Table 2). Of note, women gender and age were not found to be independently associated with differences in DSMQ. However, greater socio‐economic deprivation was significantly related to suboptimal diabetes self‐management. The effect size was small for each division but over a scale of 32, 844 divisions, this was a major contributor; moving from the most to the least deprived score on the scale was associated with a 33% increased probability of achieving a high (vs. a medium or low) self‐management score. Surviving correction for the other measured variables, both depressive symptoms and health anxiety remained independently and significantly associated with lower DSMQ‐total and physician contact scores. Depressive symptoms were also associated with reduced ‘dietary control’ and ‘medication adherence’. Conversely, higher C‐19 ASS avoidance scores were linked to higher self‐management scores, shown by a negative association with low DSMQ global (p = 0.021) and more physician contact (p = 0.026). Higher perseveration scores of the C‐19 ASS were associated with less physician contact, similar to general health anxiety.
TABLE 2.
Factors associated with impaired diabetes self‐management
| Regressors | DSMQ global | Diet control | Physical activity | Physician contact | Glucose monitoring | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p‐value | β | p‐value | β | p‐value | β | p‐value | β | p‐value | |
| Intercept 1 | −0.482 | 0.784 | 1.694 | 0.332 | −1.592 | 0.384 | 0.482 | 0.772 | −0.123 | 0.942 |
| Intercept 2 | 0.490 | 0.781 | 2.858 | 0.103 | −0.500 | 0.784 | 1.686 | 0.311 | 0.933 | 0.577 |
| C‐19 ASS A | −0.112 | 0.021 | −0.034 | 0.482 | 0.036 | 0.443 | −0.103 | 0.026 | −0.042 | 0.352 |
| C‐19 ASS P | 0.046 | 0.098 | −0.011 | 0.693 | −0.021 | 0.441 | 0.093 | 0.001 | 0.016 | 0.558 |
| WI‐7 | 0.067 | 0.028 | 0.054 | 0.075 | 0.045 | 0.129 | 0.093 | 0.002 | −0.036 | 0.221 |
| PHQ‐9 | 0.130 | 0.001 | 0.135 | 0.001 | 0.106 | 0.007 | 0.121 | 0.001 | 0.013 | 0.720 |
| Women | 0.238 | 0.826 | −0.672 | 0.528 | 1.276 | 0.273 | −1.107 | 0.271 | 1.282 | 0.231 |
| Age | −0.007 | 0.694 | −0.018 | 0.304 | 0.036 | 0.064 | −0.025 | 0.130 | −0.010 | 0.584 |
| Index of multiple deprivation | 4.04 × 10 −5 | 0.038 | 3.05 × 10 −5 | 0.123 | 1.56 × 10 −5 | 0.419 | 1.30 × 10 −5 | 0.483 | 2.99 × 10 −5 | 0.112 |
| Type 1 diabetes | −0.623 | 0.256 | −0.096 | 0.859 | 0.488 | 0.447 | −0.251 | 0.639 | −0.507 | 0.371 |
| Type 2 diabetes | 0.207 | 0.714 | −0.797 | 0.174 | 1.543 | 0.019 | −0.276 | 0.618 | 1.424 | 0.016 |
| Psychiatric medication use | 0.685 | 0.192 | 1.402 | 0.006 | 0.364 | 0.463 | −1.432 | 0.009 | 0.324 | 0.516 |
| Cardiovascular disease presence | −0.385 | 0.481 | −0.911 | 0.120 | −0.542 | 0.296 | 0.192 | 0.709 | 0.276 | 0.593 |
| Number of microvascular complications | 0.170 | 0.289 | −0.101 | 0.549 | −0.023 | 0.881 | 0.374 | 0.017 | 0.101 | 0.498 |
Note: Multivariate regression analysis using a proportional odds model to identify independent associations of impaired diabetes self‐management in all participants with diabetes (n = 314 included for whom data set was complete). Participants were stratified based on DSMQ scores relative to the entire sample: low DSMQ (bottom 30%), middle DSMQ (mid 30%) and high DSMQ scores (upper 40%). To identify the main determinants of impaired diabetes self‐management, we used the ‘low DSMQ score group’ as the dependent variable. Therefore, a positive regression estimate indicates that the variable is associated with worse diabetes self‐care and vice versa. In the case of missing values, data in that row were not analysed (55 participants had missing data and thus were excluded). p < 0.05 was considered statistically significant.
Abbreviations: C‐19 ASS, Covid‐19 Anxiety Syndrome Scale (subscores—A, avoidance; P, perseveration); DSMQ, Diabetes Self‐Management Questionnaire; PHQ‐9, Patient Health Questionnaire‐9; WI‐7, 7‐item Whiteley Index.
To determine whether these questionnaire‐based findings aligned with clinical measures of diabetes management, paired clinical data before and during the COVID‐19 pandemic were analysed. Despite high scores on average for depressive symptoms in this cohort at the height of the UK's second lockdown, a small but significant improvement in mean HbA1c levels compared with paired pre‐pandemic values was found. Data for 86 of the respondents were available providing a paired pre‐ and post‐lockdown HbA1c comparison no more than 18 months apart. Average HbA1c in that group fell from 62.13 to 60.16 mmol/mmol (p = 0.0033). No significant differences were observed in other clinical markers.
4. DISCUSSION
This study sought to record the levels of COVID‐19‐related anxiety during the second UK lockdown, between January and March 2021, and to link these with measures of diabetes self‐management in people listed in diabetes clinics at three London hospitals. For over half of respondents, C‐19 ASS scores suggested high levels of COVID‐19 anxiety syndrome which is consistent with the UK general population. 11 The response rate was lower than other community mental health surveys at only 12.8% and of course, it is difficult to infer whether this in itself reflects a bias towards those who felt that COVID‐related anxiety was a pertinent issue for them. Whilst COVID‐19 anxiety syndrome has been found to be associated with generalised health anxiety, the high levels of COVID‐19 anxiety syndrome in this cohort were not driven by somatoform disorder, with less than 6% reporting WI‐7 scores above the cut‐off for significant health anxiety. However, over a quarter of respondents reported PHQ‐9 scores suggestive of clinically relevant depressive symptoms. Pre‐pandemic estimates of depression or depressive symptoms' prevalence in the UK and Europe are about 20%–25%. 17 , 18 Rates of depressive symptoms reported in the general population in the United Kingdom during the pandemic range from 22% to 30%. 6 , 19 Thus, whilst others have reported a disproportionate deterioration in mental health in people with diabetes, assumed to be because of additional fears about higher COVID‐19 infection risks or the pre‐existing interplay between diabetes and depression or depressive symptoms, 20 , 21 , 22 and although rates of depressive symptoms and COVID‐19 anxiety syndrome were very high due to lockdown, co‐morbid diabetes was not associated with even greater susceptibility to psychological distress in this population.
Although there is no direct comparison with pre‐pandemic scores for this cohort, DSMQ scores in this study point to self‐perception of improvable diabetes management at the height of lockdown, which is supported by other studies reporting a deterioration in self‐care in people with diabetes during the pandemic. 23 , 24 , 25 , 26 In contrast, other studies have shown that people with diabetes have been maintaining better diabetes management during this period. 27 , 28 At the outset, we hypothesised that COVID‐19 anxiety syndrome might negatively impact on diabetes self‐care. Indeed, C‐19 ASS perseveration subscores were associated with less physician contact, similar to health anxiety. It could be that people exhibiting more checking, worrying and threat monitoring in relation to COVID‐19 experienced more threat and fear during the pandemic, which in turn motivated them to seek doctors' advice and attend regular doctors' appointments. A similar pattern was observed in those with greater health anxiety. However, contrary to predictions, higher C‐19 ASS avoidance subscores were linked to better diabetes self‐management efficacy in this cohort. In other words, higher scores related to COVID‐19 avoidant (self‐protective) behaviour were independently and significantly associated with higher scores of diabetes self‐management and more physician contact. This fits with the notion that those who are more engaged with looking after their health were less likely to risk opportunities for infection, for example, using public transport. Avoidant behaviour may also lend itself to staying at home (physical activity scores were particularly low in this group) and therefore finding it easier to maintain scheduled eating times, more predictable routines or other favourable aspects of self‐care. In line with this, despite participants perceiving suboptimal diabetes self‐care scores during the pandemic and quite high levels of depressive symptoms, HbA1c levels actually improved. However, the average improvement was small and the longevity of this effect remains to be seen, casting doubt on the actual clinical significance of this observation. It is possible that during enforced lockdown, diabetes management may have been seen as an effective method to reduce the risk of COVID‐19 susceptibility. Further research will need to ascertain the relevance of C‐19 ASS avoidance, particularly as the transition back to greater face‐to‐face interaction could induce anxiety in those who were particularly avoidant.
There are some limitations in this study that should be considered when interpreting its findings. It is becoming less commonplace to perform studies on cohorts of patients with both Type 1 and Type 2 diabetes, given the differences in the management, lived experience and self‐care challenges across the conditions. Outcomes from respondents with Type 1 and Type 2 were combined here however, partly to ensure coverage of all of the clinic cohort and the exigencies of understanding the impact of the pandemic on patients' self‐care. Nevertheless, differences in the way in which patients with Type 1 and Type 2 diabetes may experience elements that are measured in the DSMQ (although validated for both conditions) should be borne in mind when interpreting the data presented. 29 Direct comparisons with pre‐pandemic figures for this particular cohort cannot be made. With a large proportion of respondents identifying as White (which is not demographically representative of the actual patient population 30 ) and with HbA1c levels in this cohort lower than previous measurements of the London average for people with diabetes, 31 our findings should not be broadly generalised. 32 It is possible that the salience of the study at a time of great challenge due to COVID may have encouraged some to reply; conversely patients with diabetes also suffering symptoms of mental illness may be less motivated to engage with a survey received by post/email, a key factor for survey non‐response. 32 , 33 For example, the Community Mental Health Survey often has one of the lowest response rates in the NHS's Patient Survey Programme, a programme that seeks patients' feedback on the healthcare they receive across England. 34 Aligned with this is the clear result on multivariate analysis of the continued independent association of socio‐economic deprivation and diabetes self‐care—another reminder of the need to address inequalities in the health service.
Finally, this study was open for the early months of 2021. During that time, the United Kingdom had only just started to roll out its vaccination programme and by the time this study had closed, only frontline healthcare workers or those aged over 80 years had at large scale received a vaccine dose. It is therefore unlikely that the security of immunity was a confounder in the responses gathered, although the survey did not ask if participants believed that they had already caught and recovered from COVID‐19.
In summary, high levels of depressive symptoms and COVID‐19 anxiety syndrome were found in participants. There was an expected significant association between low mood and suboptimal self‐management of aspects of diabetes care. During the height of lockdown, avoidant coping behaviours characteristic of the COVID‐19 anxiety syndrome may in fact work to improve diabetes self‐care, at least in the short term. We recommend screening for depressive symptoms as people return, in person, to diabetes clinics and monitoring for the potential longer term ramifications on diabetes self‐management as we discover the post‐pandemic ‘new normal’. We should also be aware of the significant minority of people with COVID‐19 anxiety syndrome who may now find it difficult to re‐engage with face‐to‐face clinic opportunities.
AUTHOR CONTRIBUTIONS
V.S., M.S., W.D., N.O., A.N. and V.M. designed the study; V.S., S.S., M.M., A.A., E.A., D.H., R.S. and K.S. contributed to the execution of the study and data collection; W.D. and V.S. prepared the data analysis; V.S. wrote the manuscript and all authors contributed to the editing of the manuscript. V.S. is the guarantor of this work, had full access to the data and accepts full responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to the participants attending Imperial College Healthcare NHS Trust diabetes clinics for their support of this study. The study was internally funded. V.S. is the recipient of a Diabetes UK Harry Keen Clinician Scientist Fellowship. The Department of Metabolism, Digestion and Reproduction is funded by grants from the MRC and Biotechnology and Biological Sciences Research Council and is supported by the NIHR Imperial BRC Funding Scheme. The views expressed are those of the authors and not necessarily those of the above‐mentioned funders, the UK National Health Service (NHS), the NIHR or the UK Department of Health.
Distaso W, Malik MMAH , Semere S, et al. Diabetes self‐management during the COVID‐19 pandemic and its associations with COVID‐19 anxiety syndrome, depression and health anxiety. Diabet Med. 2022;00:e14911. doi: 10.1111/dme.14911
REFERENCES
- 1. World Health Organization . Global report on Diabetes Online. 2016. [Available from: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf] Accessed 15th August 2021.
- 2. Guan W‐J, Liang W‐H, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID‐19‐related mortality in England: a whole‐population study. Lancet Diabetes Endocrinol. 2020;8(10):813‐822. doi: 10.1016/S2213-8587(20)30272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Department of Health and Social Care . Clinically extremely vulnerable receive updated guidance in line with new national restrictions. 2020. [Available from: https://www.gov.uk/government/news/clinically‐extremely‐vulnerable‐receive‐updated‐guidance‐in‐line‐with‐new‐national‐restrictions] Accessed 15th August 2021.
- 5. VoPham T, Weaver MD, Hart JE, et al. Effect of social distancing on COVID‐19 incidence and mortality in the US. medRxiv. 2020: 2020.06.10.20127589. doi: 10.1101/2020.06.10.20127589 [DOI] [Google Scholar]
- 6. Shevlin M, McBride O, Murphy J, et al. Anxiety, depression, traumatic stress and COVID‐19‐related anxiety in the UK general population during the COVID‐19 pandemic. BJPsych Open. 2020;6(6):e125. doi: 10.1192/bjo.2020.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giebel C, Corcoran R, Goodall M, et al. Do people living in disadvantaged circumstances receive different mental health treatments than those from less disadvantaged backgrounds? BMC Public Health. 2020;20(1):651. doi: 10.1186/s12889-020-08820-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee SA, Mathis AA, Jobe MC, Pappalardo EA. Clinically significant fear and anxiety of COVID‐19: a psychometric examination of the coronavirus anxiety scale. Psychiatry Res. 2020;290:113112. doi: 10.1016/j.psychres.2020.113112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikčević AV, Spada MM. The COVID‐19 anxiety syndrome scale: development and psychometric properties. Psychiatry Res. 2020;292:113322. doi: 10.1016/j.psychres.2020.113322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nikčević AV, Marino C, Kolubinski DC, Leach D, Spada MM. Modelling the contribution of the big five personality traits, health anxiety, and COVID‐19 psychological distress to generalised anxiety and depressive symptoms during the COVID‐19 pandemic. J Affect Disord. 2021;279:578‐584. doi: 10.1016/j.jad.2020.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albery IP, Spada MM, Nikčević AV. The COVID‐19 anxiety syndrome and selective attentional bias towards COVID‐19‐related stimuli in UKresidents during the 2020‐2021 pandemic. Clin Psychol Psychother. 2021;28:1367‐1378. doi: 10.1002/cpp.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmitt A, Bendig E, Baumeister H, et al. Associations of Depression and Diabetes Distress with Self‐Management Behavior and Glycemic Control. American Psychological Association; 2021:113‐124. [DOI] [PubMed] [Google Scholar]
- 13. Schmitt A, Gahr A, Hermanns N, Kulzer B, Huber J, Haak T. The diabetes self‐management questionnaire (DSMQ): development and evaluation of an instrument to assess diabetes self‐care activities associated with glycaemic control. Health Qual Life Outcomes. 2013;11(1):138. doi: 10.1186/1477-7525-11-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Downie GA, Mullan BA, Boyes ME, McEvoy PM. The effect of psychological distress on self‐care intention and behaviour in young adults with type 1 diabetes. J Health Psychol. 2019;26(4):543‐555. doi: 10.1177/1359105318824795 [DOI] [PubMed] [Google Scholar]
- 15. Fink P, Ewald H, Jensen J, et al. Screening for somatization and hypochondriasis in primary care and neurological in‐patients: a seven‐item scale for hypochondriasis and somatization. J Psychosom Res. 1999;46(3):261‐273. doi: 10.1016/S0022-3999(98)00092-0 [DOI] [PubMed] [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606‐613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salinero‐Fort MA, Gómez‐Campelo P, San‐Andrés‐Rebollo FJ, et al. Prevalence of depression in patients with type 2 diabetes mellitus in Spain (the DIADEMA study): results from the MADIABETES cohort. BMJ Open. 2018;8(9):1‐11. doi: 10.1136/bmjopen-2017-020768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diabetes UK . Chapter 6 – Depression. [Available from: https://www.diabetes.org.uk/professionals/resources/shared‐practice/psychological‐care/emotional‐health‐professionals‐guide/chapter‐6‐depression#:~:text=Depression%20in%20people%20with%20diabetes&text=People%20with%20depression%20are%20more,recurrent%20in%20people%20with%20diabetes] Accessed 17th March 2022.
- 19. Iob E, Frank P, Steptoe A, Fancourt D. Levels of severity of depressive symptoms among at‐risk groups in the UK during the COVID‐19 pandemic. JAMA Netw Open. 2020;3(10):e2026064. doi: 10.1001/jamanetworkopen.2020.26064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan AF, Sun X, Zheng J, et al. Perceived risk, behavior changes and health‐related outcomes during COVID‐19 pandemic: findings among adults with and without diabetes in China. Diabetes Res Clin Pract. 2020;167:108350. doi: 10.1016/j.diabres.2020.108350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142:S8‐S21. doi: 10.1016/S0165-0327(12)70004-6 [DOI] [PubMed] [Google Scholar]
- 22. Sayeed A, Kundu S, Al Banna MH, et al. Mental health outcomes of adults with comorbidity and chronic diseases during the COVID‐19 pandemic: a matched case‐control study. Psychiatr Danub. 2020;32(3–4):491‐498. doi: 10.24869/psyd.2020.491 [DOI] [PubMed] [Google Scholar]
- 23. Utli H, Vural DB. The effect of the COVID‐19 pandemic on self‐management in patients with type 2 diabetics. Prim Care Diabetes. 2021;S1751‐9918(21):e00126‐e00121. doi: 10.1016/j.pcd.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fragala MS, Kaufman HW, Meigs JB, Niles JK, McPhaul MJ. Consequences of the COVID‐19 pandemic: reduced hemoglobin A1c diabetes monitoring. Popul Health Manag. 2021;24(1):8‐9. doi: 10.1089/pop.2020.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hosomi Y, Munekawa C, Hashimoto Y, et al. The effect of COVID‐19 pandemic on the lifestyle and glycemic control in patients with type 1 diabetes: a retrospective cohort study. Diabetol Int. 2021;13:1‐6. doi: 10.1007/s13340-021-00507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merzon E, Green I, Shpigelman M, et al. Haemoglobin A1c is a predictor of COVID‐19 severity in patients with diabetes. Diabetes Metab Res Rev. 2021;37(5):e3398. doi: 10.1002/dmrr.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grabowski D, Overgaard M, Meldgaard J, Johansen LB, Willaing I. Disrupted self‐management and adaption to new diabetes routines: a qualitative study of how people with diabetes managed their illness during the COVID‐19 lockdown. Diabetol. 2021;2(1):1‐15. doi: 10.3390/diabetology2010001 [DOI] [Google Scholar]
- 28. Nachimuthu S, Vijayalakshmi R, Sudha M, Viswanathan V. Coping with diabetes during the COVID ‐ 19 lockdown in India: results of an online pilot survey. Diabetes Metab Syndr. 2020;14(4):579‐582. doi: 10.1016/j.dsx.2020.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adu MD, Malabu UH, Malau‐Aduli AEO, Malau‐Aduli BS. Enablers and barriers to effective diabetes self‐management: a multi‐national investigation. PLoS One. 2019;14(6):1‐22. doi: 10.1371/journal.pone.0217771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sekhon Inderjit Singh HK, Lal N, Majeed A, Pawa N. Ethnic disparities in the uptake of colorectal cancer screening: an analysis of the West London population. Colorectal Dis. 2021;23(7):1804‐1813. doi: 10.1111/codi.15682 [DOI] [PubMed] [Google Scholar]
- 31. James GD, Baker P, Badrick E, et al. Ethnic and social disparity in glycaemic control in type 2 diabetes; cohort study in general practice 2004‐9. J R Soc Med. 2012;105(7):300‐308. doi: 10.1258/jrsm.2012.110289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health. 2005;27(3):281‐291. doi: 10.1093/pubmed/fdi031 [DOI] [PubMed] [Google Scholar]
- 33. Paddock LE, Veloski J, Chatterton ML. Development and validation of a questionnaire to evaluate patient satisfaction with diabetes disease management. Diabetes Care. 2000;23(7):951‐956. doi: 10.2337/diacare.23.7.951 [DOI] [PubMed] [Google Scholar]
- 34. Gooden T, Wright A, Swinn E, Sizmur S. Optimising response rates in a national postal survey evaluating community mental health care: four interventions trialled. J Ment Health. 2021;1‐7. doi: 10.1080/09638237.2021.1922646. Online ahead of print. [DOI] [PubMed] [Google Scholar]


