Abstract
Objective
To investigate the association between demographic, clinical, psychological, cognitive, and health‐related variables and the Central Sensitization Inventory (CSI) in previously hospitalized COVID‐19 survivors exhibiting “de novo” post‐COVID pain.
Methods
Seventy‐seven (n = 77) COVID‐19 survivors with “de novo” post‐COVID pain completed demographic (age, height, and weight), clinical (duration and intensity of the pain), psychological (depressive/anxiety levels and sleep quality), cognitive (catastrophizing and kinesiophobia levels), and health‐related quality of life variables as well as the CSI. A multivariable correlation analysis was conducted to determine the association between variables, and a stepwise multiple linear regression model was performed to identify CSI predictors.
Results
Patients were assessed a mean of 6.0 (SD 0.8) months after hospital discharge. Twenty‐six (33.7%) individuals showed indications of sensitization‐associated symptoms (CSI score ≥40 points). The CSI score was positively associated with pain intensity (r: 0.371), anxiety (r: 0.784), depressive (r: 0.709), catastrophizing (r: 0.620), and kinesiophobia (r: 0.359) levels (all, p < 0.001). The stepwise regression analysis revealed that 60.2% of CSI was explained by anxiety levels and pain intensity.
Conclusion
This study found that psychological and cognitive variables were associated with the CSI score in previously hospitalized COVID‐19 survivors with “de novo” post‐COVID pain. Anxiety levels and the intensity of pain symptoms were independently associated with CSI score suggesting a significant overlap with psychological construct. The “de novo” post‐COVID pain association with CSI may indicate changes in the pain processing important for managing the pain.
Keywords: anxiety, COVID‐19, pain, post‐COVID, sensitization
INTRODUCTION
Among the multiple symptoms experienced by people affected by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), musculoskeletal pain (myalgia) is common during the acute phase of the infection. 1 , 2 In addition, evidence supports that up to 60% of COVID‐19 survivors may develop different post‐COVID symptoms (ie, long COVID) after the infection. 3 A recent meta‐analysis reported a prevalence from 4.6% to 18.1% for post‐COVID pain at different follow‐ups during the first‐year post‐infection. 4
Characterization of post‐COVID pain is crucial for better understanding of potential mechanisms and for orientating personalized treatments. A recent study observed that the most common type of post‐COVID pain described on social media was musculoskeletal (nociceptive) pain. 5 In fact, a large cohort study has reported a prevalence up to 45% of musculoskeletal post‐COVID pain in previously hospitalized COVID‐19 survivors 8 months after hospitalization. 6 Musculoskeletal chronic pain can be associated with central sensitization, 7 which is the underlying concept defining nociplastic pain. Nociplastic pain is defined as “pain that arises from altered nociception without clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing pain.” 8 In fact, nociplastic pain is not just associated with exaggerated pain symptomatology but also with central nervous system‐associated symptoms including fatigue, sleep problems, memory loss, and mood disturbances. 9
Preliminary evidence suggests the presence of sensitization in people exhibiting post‐COVID pain. In fact, a Delphi study tried to identify the sensitization phenotypes of individuals with post‐COVID pain. 10 This study proposed that symptoms were associated with sensitization included pain, fatigue, dyspnea, orthostatic intolerance, and gastrointestinal problems. 10 Oguz‐Akarsu et al. 11 found that almost 60% of COVID‐19 survivors reported multiple pain sites and more than two types of pain after hospitalization. Ursini et al. 12 observed, through a web‐based survey, that 30% of patients with post‐COVID pain self‐reported common features similar to fibromyalgia, indicating nociplastic pain. None of these studies included either objective (eg, quantitative sensory testing) or self‐reported (eg, sensitization inventory) variables of sensitization.
The Central Sensitization Inventory (CSI) consists of a self‐reported questionnaire used to assist within the identification of sensitization‐associated symptoms. 13 Goudman et al. 14 used the CSI in people with post‐COVID pain and reported that 70% of individuals showed a score of >40/100 points suggestive of altered pain processing pointing toward sensitization. However, this study did not collect other potential cofounder variables, for example, psychological or cognitive variables, which all may influence the pain processing. The exclusive use of the CSI for inferring sensitization in individuals with chronic pain is not recommended since it generally overlaps with psychological construct 15 and because just a self‐reported tool cannot capture the complexity of central sensitization. 16 The current study investigated the association of sensitization‐associated symptoms, as evaluated with the CSI, with psychological variables (concurrent validity) and the presence of de novo post‐COVID. Our aims were as follows: (1) to analyze the associations between symptoms of central sensitization and pain‐related, psychological, cognitive, and quality of life variables, and; (2) to identify the potential risk factors explaining the variance of the CSI score in a sample of previously hospitalized COVID‐19 survivors exhibiting “de novo” post‐COVID pain.
METHODS
Study design
An observational cross‐sectional cohort study following the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines 17 was conducted. This study was approved by the Local Institutional Ethics Committee of INDIVAL Cantabria (code 2020.416). Participants were informed of the study, and all provided their written informed consent prior to their inclusion in the study.
Participants
This cohort study included patients hospitalized at one urban hospital in Santander (Spain) who had recovered from acute SARS‐CoV‐2 infection. Patients requiring internal care unit (ICU) admission were excluded. All were diagnosed with reverse transcription‐polymerase chain reaction (PCR) assay and the presence of clinical and radiological findings at hospital admission. They were included if presented: (1) “de novo” pain symptoms starting after hospitalization compatible with a diagnosis of chronic primary musculoskeletal pain 18 ; (2) symptoms experienced for at least three consecutive months, and (3) absence of any potential medical condition which could best explain pain, for example, arthritis. Participants were excluded if reported a previous history of pain symptoms and or any pre‐existing medical comorbidity explaining symptoms to reduce confounding variables associated with sensitization prior to hospitalization.
Collection data procedure
Participants were recruited from those attending to a specific post‐COVID unit at an urban hospital (Hospital Universitario Marqués de Valdecilla) in Santander (Spain) from June 1, 2021, to October 31, 2021. Patients reporting that their main post‐COVID symptom consisted of pain were invited to participate and evaluated for the inclusion and exclusion criteria. A structured questionnaire including clinical data of their pain and self‐reported questionnaires was used for data collection. Age, weight, height, and intensity (numerical pain rating scale, NPRS, 0–10) and location of pain symptoms were collected. Questionnaires included sensitization‐associated symptoms, psychological variables (eg, anxiety levels, depressive levels, or sleep quality), cognitive variables (kinesiophobia and catastrophism), and health‐related quality of life.
Central Sensitization Inventory
The CSI is a self‐reported questionnaire assessing a total of 25 sensitization‐associated symptoms, on a 5‐point Likert scale rating. 19 The total score ranges from 0 to 100, where >40/100 points suggest the presence of sensitization‐associated symptoms. 20 The CSI has proven psychometric strength for evaluating sensitization‐associated symptom in patients with persistent chronic pain. 21
Psychological variables
Anxiety and depressive symptoms were assessed with the anxiety (HADS‐A, 7 items, 0–21 points) and depressive (HADS‐D, 7 items, 0–21 points) scales of the Hospital Anxiety and Depression Scale (HADS). Higher scores suggest higher anxiety/depressive levels. 22 The following cut‐off scores were considered as indicative of anxiety (HADS‐A ≥12 points) and depressive (HADS‐D ≥10 points) symptoms. 23
The Pittsburgh Sleep Quality Index (PSQI, 0–21 points) evaluates sleep quality by including 19 self‐rated questions assessing different aspects of sleep during the previous month. 24 Higher scores indicate worse sleep quality, and a score ≥8.0 points is indicative of poor sleeper. 24
Cognitive variables
The Pain Catastrophizing Scale (PCS) was used to assess pain catastrophizing. 25 It includes 13 items evaluating rumination, magnification, and despair aspects in relation to the pain experience. Items are answered in a 5‐point Likert scale ranging from 0 (“never”) to 4 (“always”), providing a total score from 0 to 52 points. 25
The 11‐item short form of the Tampa Scale for Kinesiophobia (TSK‐11, 0–44 points) was used to quantify the fear of movement perceived by the patient. 26 It consists of 11 items where the patients choose from a 4‐point Likert scale (1: “complete disagreement”; 4 “complete agreement”) how much they agree with each item. 26
Health‐related quality of life
The paper‐based five‐level version of EuroQol‐5D is a generic questionnaire used for assessing health‐related quality of life. 27 It includes five health dimensions (mobility, self‐care, daily activities, pain, and depression/anxiety) into a 5‐item Likert scale (0: no problems; 4: severe problems). Responses were converted into a single index number by applying crosswalk index values for Spain life (0: equivalent to death; 1: optimal health status). 28
Sample size determination
An adequate sample size for prediction models was based on a range of 10–15 subjects per predictor variable, with no more than five predictors within the model as suggested by Jenkins and Quintana‐Ascencio. 29 Accordingly, for five potential predictor variables, a minimum of 75 participants would be required.
Statistical analysis
Descriptive analyses (means and standard deviations ‐SD‐) were used to describe the sample. The Kolmogorov–Smirnov test revealed that all quantitative data exhibited a normal distribution. Between‐group differences depending on the CSI score (<40 or ≥40 points) were assessed with the independent Student t‐tests. A multiple linear regression analysis was used to determine which variables could explain the variance of CSI. First, Pearson correlation coefficients (r) were used to determine the correlation between predictors and the dependent variable (CSI). The correlation coefficients were also used to identify multicollinearity between the variables (defined when r > 0.8). All statistically significant variables associated with the CSI score were included in a stepwise multiple linear regression model (hierarchical regression analysis) to assess those independent variables contributing significantly to the variance of the dependent variable (CSI), except variables showing multicollinearity. The significance criterion of the critical F value for entry into the regression equation was set at p < 0.05. Changes in adjusted R 2 were reported after each step of the regression model to determine the contribution of the additional variables.
RESULTS
From 150 individuals attending the post‐COVID unit from June 1, 2021, to October 31, 2021, 73 (48%) were excluded because their main post‐COVID symptom was respiratory (n = 45) or the presence of previous pain symptoms (n = 28). Finally, 77 patients (37.6% women, age: 60, SD: 11.5 years) satisfied all inclusion criteria, were included, and assessed a mean of 6.0 (SD 0.8) months after hospital discharge. They were hospitalized a mean of 12.4 (SD 11.1) days at a hospital ward. Table 1 details clinical, psychological, cognitive, and health‐related features of the sample. All participants reported pains as their primary post‐COVID symptom. In fact, 46 (59.7%) only experienced post‐COVID pain. In fact, 20.8% of patients reported generalized post‐COVID pain as visualized in Figure 1. The remaining 31 (40.3%) also reported other less bothersome symptomatology: anosmia (n = 10, 13%), gastrointestinal problems (n = 8, 10.4%), brain bog (n = 7, 9%), or ageusia (n = 6, 7.8%).
TABLE 1.
Baseline outcomes (mean ± SD) of the sample
| Variable | Total sample (n = 77) | CSI ≥40 points (n = 26) | CSI <40 points (n = 51) |
|---|---|---|---|
| Demographic variables | |||
| Age (years) | 60.0 ± 11.5 | 59.9 ± 11.2 | 60.0 ± 11.7 |
| Height (m) | 1.69 ± 0.09 | 1.66 ± 0.09 | 1.68 ± 0.08 |
| Weight (kg) | 75.8 ± 15.4 | 72.7 ± 12.7 | 74.9 ± 12.4 |
| Previous medical co‐morbidities | |||
| Hypertension, n (%) | 23 (29.9%) | 8 (30.7%) | 15 (29.4%) |
| Obesity, n (%) | 15 (19.5%) | 6 (23.1%) | 9 (17.6%) |
| Diabetes, n (%) | 11 (14.3%) | 4 (15.4%) | 7 (13.7%) |
| Asthma, n (%) | 10 (13.0%) | 3 (11.5%) | 7 (13.7%) |
| Chronic obstructive pulmonary disease, n (%) | 5 (6.5%) | 2 (7.7%) | 3 (5.9%) |
| Pain and sensitization‐related variables | |||
| Time with symptoms (months) | 6.0 ± 0.8 | 5.8 ± 0.9 | 6.0 ± 0.8 |
| Pain intensity (0–10)* | 5.4 ± 1.8 | 6.2 ± 1.3 | 4.9 ± 2.0 |
| CSI (0–100)* | 30.0 ± 17.3 | 51.3 ± 7.5 | 19.9 ± 9.8 |
| Psychological variables | |||
| HADS‐A (0–21)* | 5.8 ± 4.4 | 10.7 ± 3.5 | 3.5 ± 2.6 |
| HADS‐D (0–21)* | 5.7 ± 4.7 | 10.5 ± 3.9 | 3.4 ± 3.1 |
| PSQI (0–21) | 8.9 ± 4.3 | 9.2 ± 4.1 | 8.9 ± 4.6 |
| Cognitive and health‐related variables | |||
| PCS (0–52)* | 16.1 ± 13.1 | 27.3 ± 12.7 | 11.5 ± 10.0 |
| TSK‐11 (0–44)* | 24.2 ± 9.1 | 29.3 ± 8.7 | 22.3 ± 7.8 |
| EuroQol‐5D (0–1) | 0.75 ± 0.25 | 0.8 ± 0.2 | 0.7 ± 0.25 |
Abbreviations: CSI, Central Sensitization Inventory; HADS, Hospital Anxiety and Depression Scale; PCS, Pain Catastrophizing Scale; PSQI, Pittsburgh Sleep Quality Index; TSK‐11, Tampa Scale for Kinesiophobia.
Significant differences between patients according to the CSI score (Student t‐test, p < 0.01).
FIGURE 1.
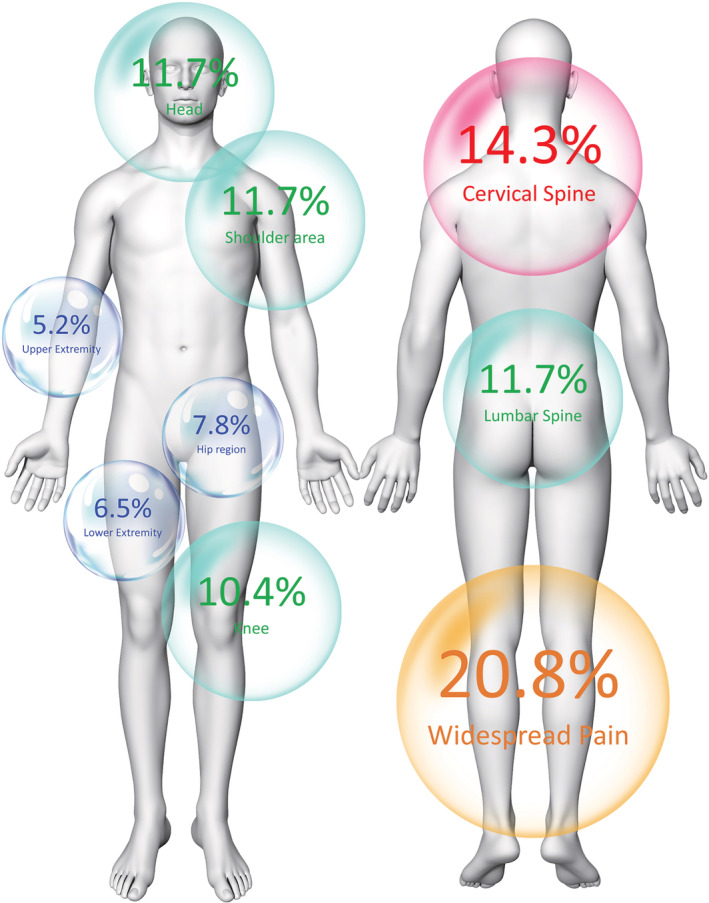
Location of pain symptomatology in previously hospitalized COVID‐19 survivors exhibiting “de novo” post‐COVID pain (n = 77)
The mean CSI score was 30.0 (SD: 17.3), where 26 (33.7%) patients had a CSI score ≥40/100 and 51 (66.3%) a score <40/100. Individuals with sensitization‐associated features (CSI ≥40 points) exhibited higher pain intensity (p = 0.01), more anxiety/depressive (p < 0.001), and more catastrophizing and kinesiophobia (p < 0.001) levels than those without sensitization‐associated symptoms (CSI score <40 points).
Bivariate correlation analysis
Bivariate correlation analyses are reported in Table 2. The CSI score was positively associated with pain intensity, anxiety/depressive levels, catastrophizing, and kinesiophobia levels (all, p < 0.001). Significant positive associations were also found among pain‐related, psychological, and cognitive variables (r from 0.321 to 0.639).
TABLE 2.
Pearson‐product moment correlation matrix between sociodemographic, psychological, neuro‐physiological, and clinical characteristics
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | |||||||||||
| 2. Weight | n.s. | ||||||||||
| 3. Height | n.s. | 0.521** | |||||||||
| 4. Time with symptoms | n.s. | n.s. | n.s. | ||||||||
| 5. Mean pain intensity | n.s. | n.s. | n.s. | n.s. | |||||||
| 6. HADS‐A | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||
| 7. HADS‐D | n.s. | n.s. | n.s. | n.s. | 0.301* | 0.867** | |||||
| 8. PSQI | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||
| 9. PCS | n.s. | n.s. | n.s. | n.s. | 0.321* | 0.672** | 0.619** | n.s. | |||
| 10. TKS‐11 | n.s. | n.s. | n.s. | n.s. | 0.429** | 0.409** | 0.365** | n.s. | 0.639** | ||
| 11. EuroQol‐5D | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| 12. CSI | n.s. | n.s. | n.s. | n.s. | 0.371** | 0.784** | 0.709** | n.s. | 0.620** | 0.359** | n.s. |
Abbreviations: CSI, Central Sensitization Inventory; HADS, Hospital Anxiety and Depression Scale; PCS, Pain Catastrophizing Scale; PSQI, Pittsburgh Sleep Quality Index; TSK‐11, Tampa Scale for Kinesiophobia.
*p < 0.05; **p < 0.01.
Since multicollinearity was identified between HADS‐D and HADS‐A (r: 0.867, p < 0.001), HADS‐D was “a priori” excluded from the regression analysis. Its inclusion in the logistic regression did not alter the results.
Multiple regression analysis
The hierarchical regression analysis explaining the variance of the CSI score is shown in Table 3. Stepwise regression analyses revealed that anxiety (contributing 57.3%) and pain intensity (2.9%) were significant predictors of CSI, and, when combined, they explained 60.2% of the variance of the outcome (r 2 adjusted: 0.602, Figure 2).
TABLE 3.
Summary of the stepwise regression analyses to determine predictors of sensitization‐associated symptoms
| Predictor outcome | Β | SE B | 95% CI | B | t | p | |
|---|---|---|---|---|---|---|---|
| CSI | Step 1 | ||||||
| HADS‐A | 2.667 | 0.340 | 1.981, 3.352 | 0.763 | 7.840 | <0.001 | |
| Step 2 | |||||||
| HADS‐A | 2.505 | 0.338 | 1.824, 3.187 | 0.717 | 7.415 | <0.001 | |
| Mean pain intensity | 1.631 | 0.798 | 0.022, 3.329 | 0.198 | 2.044 | 0.045 | |
Note: = 0.573 for step 1, = 0.602 for step 2.
Abbreviations: CSI, Central Sensitization Inventory; HADS, Hospital Anxiety and Depression Scale.
FIGURE 2.
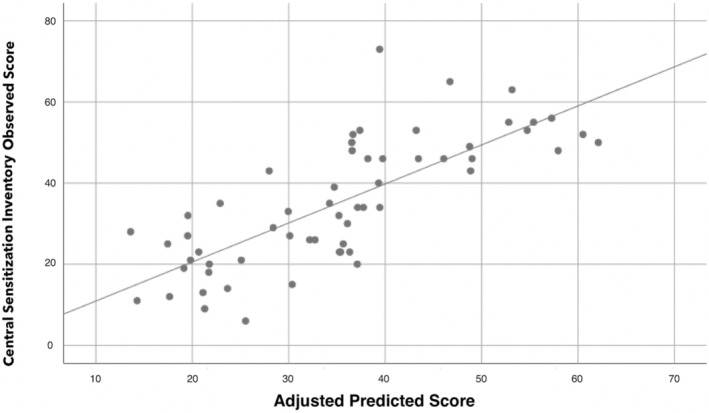
Scatter plot of the adjusted predicted score (r 2 adjusted: 0.602) explaining Central Sensitization Inventory (CSI) score in COVID‐19 survivors exhibiting “de novo” post‐COVID pain symptoms (n = 77). Note that some points can be overlapping
DISCUSSION
This is the first study conducting regression analyses to study which factors may contribute to the variance of sensitization‐associated symptoms in previously hospitalized COVID‐19 survivors exhibiting “de novo” post‐COVID pain. Sensitization‐associated characteristics were correlated with psychological and cognitive variables. Anxiety levels and intensity of pain symptoms were independently associated with CSI explaining up to 65% of its variance.
Sensitization‐associated symptoms and emotional‐cognitive variables
Serrano‐Ibañez et al. 30 found that individuals suffering from pre‐existing chronic pain sensitization syndromes were at a higher risk of developing psychological distress during the worldwide COVID‐19 outbreak. The current study reported an association between pain intensity, emotional distress, and sensitization‐associated features. In fact, current evidence supports that stress and psychological factors had a significant impact on the pain processing and our study showed that psychological disorders, particularly anxiety levels, were associated with CSI. A possible explanation may be serotonergic and noradrenergic neurons dysfunction affecting psychological and somatic pain pathways. 31 The fact that anxiety levels were positively associated with the CSI score agrees with previous studies in people with chronic pain, 32 and the finding that high‐trait anxiety‐related personality predicts the extent of symptoms of sensitization in patients with chronic low back pain. 33 Anxiety was the major contributor to CSI score (up to 57%) of all the variables assessed in our study. Our results support the assumption that the CSI questionnaire can exhibit a significant overlap with psychological construct as previously suggested. 34
Current data also identified that cognitive variables such as catastrophizing and kinesiophobia levels were linearly associated with CSI score, although these variables were not maintained in the logistic regression analysis. Hruschak et al. 35 found that chronic pain patients with worse disability and higher catastrophizing levels were at a higher risk of social isolation during the first COVID‐19 outbreak. Precision pain medicine implies that patient education, management, and treatments should be adapted to pain phenotypes. For instance, implementation of telemedicine for the management of the identified factors associated with sensitization‐associated symptoms such as anxiety or kinesiophobia level can be applied. 36 Supporting this assumption, preliminary evidence suggests that social technology use is associated with a decrease in depressive levels and loneliness in patients with chronic pain. 37 As sensitization is related to cognitive or emotional factors including catastrophizing, anxiety or depressive levels, kinesiophobia, stress, or maladaptive illness perception, clinicians should consider individual‐tailored multimodal treatments combining pain neuroscience education with physical therapy and stress management.
Sensitization‐associated symptoms and post‐COVID pain
We also found the intensity of post‐COVID pain to be independently positively associated with CSI after adjusting by all variables supporting that the magnitude of the nociceptive input as a relevant factor for sensitization. 38 This topic is not only relevant for COVID‐19 survivors but also for people with chronic pain, but not infected. Current literature supports that individuals with chronic pain (but not infected) had exhibited an increase in their pain during the first COVID‐19 lockdown. 39 , 40 Proper monitoring of pain symptoms and stress levels in people with chronic pain would help in the early identification of these potentially modifiable factors in people at a risk for developing changes in pain processing networks.
We found that widespread pain was the most common feature of “de novo” post‐COVID pain. In line with our results, subjects who had survived to the acute severe acute respiratory syndrome (SARS) also exhibit widespread pain as a sequela 1 year after the infection. 41 Generalized pain symptomatology, combined with the presence of high CSI score, resembles the features of fibromyalgia. Our results are slightly inferior to those previously reported by Ursini et al. 12 who report that 30% of patients with post‐COVID pain self‐reported clinical features of fibromyalgia. In fact, spreading pain is also considered a clinical feature of sensitization 7 and is included as mandatory criteria for determining the presence of nociplastic pain. 8
Current theories hypothesize that SARS‐CoV‐2 cytokine and interleukin‐induced storms may lead to sensitization of pain pathways. 42 , 43 In such a scenario, SARS‐CoV‐2 infection could trigger nociplastic pain responses by altering the balance between those neuromodulation systems of nociception. 44 Additionally, widespread symptomatology has been suggested to be related to deficient immune regulatory mechanisms 45 and could indicate a prolonged immune system impact in post‐COVID pain sufferers which, in fact, will promote more sensitization.
Limitations
First, current data can be only applicable to previously hospitalized COVID‐19 survivors with mild‐to‐moderate severity, since none required ICU admission. In fact, critically ill COVID‐19 survivors requiring ICU admission also develop “de novo” post‐COVID pain. 46 Second, the presence of pre‐existing symptoms before the infection is a risk factor for developing post‐COVID pain. 6 In the current study, we included patients without previous history of pain; accordingly, we do not currently know if the presence of symptoms before the infection would lead to a facilitation of sensitization. Third, we collected a patient‐reported outcome measure, for example, CSI score, for assessing the presence of sensitization‐associated symptoms. It has been found that scores <40/100 points in the CSI do not exclude the presence of sensitization, since CSI scores may be confounded by emotional factors with features that tend to underreport themselves in other objective measures such as quantitative sensory tests. 33 In fact, the current study supports that CSI has a similar construct with psychological variables, particularly anxiety levels. Fourth, although our results suggest that post‐COVID pain resembles a nociplastic pain condition, differentiating between nociceptive, neuropathic, or nociplastic pain conditions remains challenging. 47 In fact, it has been also seen that neuropathic pain can be also present in COVID‐19 survivors. 48 Studies examining the clinimetric and psychometric properties of the proposed criteria for defining a nociplastic condition are also needed. 49 Finally, data were collected from just one hospital, questioning the external validity of the study findings.
CONCLUSIONS
Self‐reported symptoms of sensitization were associated with the intensity of pain, anxiety and depressive levels and catastrophizing and kinesiophobia levels in previously hospitalized COVID‐19 survivors exhibiting “de novo” post‐COVID pain. The regression analysis reported that 60.2% of the variance of the CSI score was explained by anxiety levels and pain intensity. These results suggest that post‐COVID pain resembles features of a nociplastic condition.
AUTHOR CONTRIBUTIONS
All authors contributed to the study concept and design. MHM and CFdlP conducted the literature review and did the statistical analysis. All authors recruited participants and collected data. PPB supervised the study. All authors contributed to interpretation of data. All authors contributed to drafting the paper. All authors revised the text for intellectual content and have read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declared that there is no conflict.
ACKNOWLEDGEMENTS
The Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121) and Novo Nordisk Foundation (NNF21OC0067235).
Fernández‐de‐las‐Peñas C, Parás‐Bravo P, Ferrer‐Pargada D, Cancela‐Cilleruelo I, Rodríguez‐Jiménez J, Nijs J, et al. Sensitization symptoms are associated with psychological and cognitive variables in COVID‐19 survivors exhibiting post‐COVID pain. Pain Pract. 2022;00:1–9. 10.1111/papr.13146
Funding information
Proyecto financiado por la convocatoria Next‐Val 2021 de la Fundación Instituto de Investigación Marqués de Valdecilla (IDIVAL) and by a grant from the Novo Nordisk Foundation 0067235. The sponsors had no role in the design, collection, management, analysis, or interpretation of the data, draft, review, or approval of the manuscript or its content. The authors were responsible for the decision to submit the manuscript for publication, and the sponsor did not participate in this decision
DATA AVAILABILITY STATEMENT
All data derived from this study are included in the paper.
REFERENCES
- 1. Abdullahi A, Candan SA, Abba MA, Bello AH, Alshehri MA, Afamefuna Victor E, et al. Neurological and muscuslokeletal features of COVID‐19: a systematic review and meta‐analysis. Front Neurol. 2020;11:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciaffi J, Meliconi R, Ruscitti P, Berardicurti O, Giacomelli R, Ursini F. Rheumatic manifestations of COVID‐19: a systematic review and meta‐analysis. BMC Rheumatol. 2020;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. Sci Rep. 2021;11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernández‐de‐las‐Peñas C, Navarro‐Santana M, Plaza‐Manzano G, Palacios‐Ceña D, Arendt‐Nielsen L. Time course prevalence of post‐COVID pain symptoms of musculoskeletal origin in patients who had survived to SARS‐CoV‐2 infection: a systematic review and meta‐analysis. Pain. 2022;163:1220–1231. 10.1097/j.pain.0000000000002496 [DOI] [PubMed] [Google Scholar]
- 5. D'Souza RS, Kilgore AE, D'Souza S. Manifestations of pain during the COVID‐19 pandemic portrayed on social media: a cross‐sectional study. Pain Med. 2022;23(2):229–33. 10.1093/pm/pnab305 [DOI] [PubMed] [Google Scholar]
- 6. Fernández‐de‐las‐Peñas C, de‐la‐Llave‐Rincón AI, Ortega‐Santiago R, Ambite‐Quesada S, Gómez‐Mayordomo V, Cuadrado ML, et al. Prevalence and risk factors of musculoskeletal pain symptoms as long‐term post‐COVID sequelae in hospitalized COVID‐19 survivors: a multicenter study. Pain. 2021. 10.1097/j.pain.0000000000002564 [DOI] [PubMed] [Google Scholar]
- 7. Nijs J, George SZ, Clauw DJ, Fernández‐de‐las‐Peñas C, Kosek E, Ickmans K, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3:e383–92. [DOI] [PubMed] [Google Scholar]
- 8. Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. 2021;162:2629–34. [DOI] [PubMed] [Google Scholar]
- 9. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. 2021;397:2098–110. [DOI] [PubMed] [Google Scholar]
- 10. Bierle DM, Aakre CA, Grach SL, Salonen BR, Croghan IT, Hurt RT, et al. Central sensitization phenotypes in post‐acute sequelae of SARS‐CoV‐2 infection (PASC): defining the post COVID syndrome. J Prim Care Community Health. 2021;12:21501327211030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oguz‐Akarsu E, Gullu G, Kilic E, Dinç Y, Ursavas A, Yilmaz E, et al. Insight into pain syndromes in acute phase of mild‐to‐moderate COVID‐19: frequency, clinical characteristics, and associated factors. Eur J Pain. 2021;26:492–504. 10.1002/ejp.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ursini F, Ciaffi J, Mancarella L, Lisi L, Brusi V, Cavallari C, et al. Fibromyalgia: a new facet of the post‐COVID‐19 syndrome spectrum? Results from a web‐based survey. RMD Open. 2021;7:e001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scerbo T, Colasurdo J, Dunn S, Unger J, Nijs J, Cook C. Measurement properties of the central sensitization inventory: a systematic review. Pain Pract. 2018;18:544–54. [DOI] [PubMed] [Google Scholar]
- 14. Goudman L, De Smedt A, Noppen M, Moens M. Is central sensitisation the missing link of persisting symptoms after COVID‐19 infection? J Clin Med. 2021;10:5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hendriks E, Voogt L, Lenoir D, Coppieters I, Ickmans K. Convergent validity of the Central Sensitization Inventory in chronic whiplash‐associated disorders: association with quantitative sensory testing, pain intensity, fatigue, and psychosocial factors. Pain Med. 2020;21:3401–12. [DOI] [PubMed] [Google Scholar]
- 16. Nijs J, Huysmans E. Clinimetrics: the Central Sensitisation Inventory: a useful screening tool for clinicians, but not the gold standard. J Physiother. 2021;S1836‐9553(21)00119‐3. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 18. Perrot S, Cohen M, Barke A, Korwisi B, Rief W, Treede RD, et al. The IASP classification of chronic pain for ICD‐11: chronic secondary musculoskeletal pain. Pain. 2019;160:77–82. [DOI] [PubMed] [Google Scholar]
- 19. Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, et al. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain. 2013;14:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuesta‐Vargas AI, Neblett R, Chiarotto A, Kregel J, Nijs J, van Wilgen CP, et al. Dimensionality and reliability of the Central Sensitization Inventory in a pooled multi‐country sample. J Pain. 2018;19:317–29. [DOI] [PubMed] [Google Scholar]
- 22. Herrmann‐Lingen C, Buss U, Snaith RP. Hospital Anxiety and Depression Scale – Deutsche Version (HADS‐D). Bern: Verlag Hans Huber; 2011. [Google Scholar]
- 23. Grupo de Trabajo de la Guía de Práctica Clínica para el Manejo de Pacientes con Trastornos de Ansiedad en Atención Primaria 2008 . Guías de Práctica Clínica en el SNS – UETS N° 2006/10. Madrid: Plan Nacional para el SNS del MSC, Unidad de Evaluación de Tecnologías Sanitarias, Agencia Laín Entralgo, Comunidad de Madrid; 2008. [Google Scholar]
- 24. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 25. García Campayo J, Rodero B, Alda M, Sobradiel N, Montero J, Moreno S. Validation of the Spanish version of the Pain Catastrophizing Scale in fibromyalgia. Med Clin. 2008;131:487–92. [DOI] [PubMed] [Google Scholar]
- 26. Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK‐11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117:137–44. [DOI] [PubMed] [Google Scholar]
- 27. Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Hout B, Janssen MF, Feng YJ, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health. 2012;15:708–15. [DOI] [PubMed] [Google Scholar]
- 29. Jenkins DG, Quintana‐Ascencio PF. A solution to minimum sample size for regressions. PLoS One. 2020;15:e0229345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serrano‐Ibáñez ER, Esteve R, Ramírez‐Maestre C, Ruiz‐Párraga GT, López‐Martínez AE. Chronic pain in the time of COVID‐19: Stress aftermath and central sensitization. Br J Health Psychol. 2021;26:544–52. [DOI] [PubMed] [Google Scholar]
- 31. Shigetoh H, Tanaka Y, Koga M, Osumi M, Morioka S. The mediating effect of central sensitization on the relation between pain intensity and psychological factors: a cross‐sectional study with mediation analysis. Pain Res Manag. 2019;2019:3916135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Wilgen CP, Vuijk PJ, Kregel J, Voogt L, Meeus M, Descheemaeker F, et al. Psychological distress and widespread pain contribute to the variance of the Central Sensitization Inventory: a cross‐sectional study in patients with chronic pain. Pain Pract. 2018;18:239–46. [DOI] [PubMed] [Google Scholar]
- 33. Clark JR, Nijs J, Yeowell G, Holmes P, Goodwin PC. Trait sensitivity, anxiety, and personality are predictive of central sensitization symptoms in patients with chronic low back pain. Pain Pract. 2019;19:800–10. [DOI] [PubMed] [Google Scholar]
- 34. Adams GR, Gandhi W, Harrison R, van Reekum CM, Gilron I, Salomons TV. Do "central sensitization" questionnaires reflect measures of nociceptive sensitization or psychological constructs? Protocol for a systematic review. Pain Rep. 2021;6:e962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hruschak V, Flowers KM, Azizoddin DR, Jamison RN, Edwards RR, Schreiber KL. Cross‐sectional study of psychosocial and pain‐related variables among patients with chronic pain during a time of social distancing imposed by the coronavirus disease 2019 pandemic. Pain. 2021;162:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Emerick T, Alter B, Jarquin S, Brancolini S, Bernstein C, Luong K, et al. Telemedicine for chronic pain in the COVID‐19 era and beyond. Pain Med. 2020;21:1743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Y, Grol‐Prokopczyk H, Reid MC, Pillemer K. The relationship between pain and psychological distress during the COVID‐19 pandemic: is social technology use protective? Pain Med. 2022;23(2):280–7. 10.1093/pm/pnab262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Staud R. Peripheral pain mechanisms in chronic widespread pain. Best Pract Res Clin Rheumatol. 2011;25:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meulders A, Vlaeyen JWS, Evers AW, Köke AJ, Smeets RJ, Van Zundert JH, et al. Chronic primary pain in the COVID‐19 pandemic: how uncertainty and stress impact on functioning and suffering. Pain. 2021;163:604–9. 10.1097/j.pain.0000000000002428 [DOI] [PubMed] [Google Scholar]
- 40. Karos K, McParland JL, Bunzli S, Devan H, Hirsh A, Kapos FP, et al. The social threats of COVID‐19 for people with chronic pain. Pain. 2020;161:2229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post‐SARS syndrome; a case‐controlled study. BMC Neurol. 2011;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID‐19 cytokine storm: systematic review and meta‐analysis. Eur J Clin Invest. 2021;51:e13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coomes EA, Haghbayan H. Interleukin‐6 in COVID‐19: a systematic review and meta‐analysis. Rev Med Virol. 2020;30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cascella M, Del Gaudio A, Vittori A, Bimonte S, Del Prete P, Forte CA, et al. COVID‐pain: acute and late‐onset painful clinical manifestations in COVID‐19: molecular mechanisms and research perspectives. J Pain Res. 2021;14:2403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ryabkova VA, Churilov LP, Shoenfeld Y. Neuroimmunology: what role for autoimmunity, neuroinflammation, and small fiber neuropathy in fibromyalgia, chronic fatigue syndrome, and adverse events after human papillomavirus vaccination? Int J Mol Sci. 2019;20:5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ojeda A, Calvo A, Cuñat T, Mellado‐Artigas R, Comino‐Trinidad O, Aliaga J, et al. Characteristics and influence on quality of life of new‐onset pain in critical COVID‐19 survivors. Eur J Pain. 2022;26:680–694. 10.1002/ejp.1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shraim MA, Massé‐Alarie H, Hodges PW. Methods to discriminate between mechanism‐based categories of pain experienced in the musculoskeletal system: a systematic review. Pain. 2021;162:1007–37. [DOI] [PubMed] [Google Scholar]
- 48. Magdy R, Eid RA, Fathy W, Abdel‐Aziz MM, Ibrahim RE, Yehia A, et al. Characteristics and risk factors of persistent neuropathic pain in recovered COVID‐19 patients. Pain Med. 2022;23(4):774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nijs J, Lahousse A, Kapreli E, Bilika P, Saraçoğlu İ, Malfliet A, et al. Nociplastic pain criteria or recognition of central sensitization? Pain phenotyping in the past, present and future. J Clin Med. 2021;10:3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data derived from this study are included in the paper.


