Abstract
Our aim was to analyse, on a population level, the year‐long decline in cancer diagnoses in the region of Lombardy (Italy), and to characterise the tumours with the greatest reduction in diagnosis by patient age, sex and tumour stage at diagnosis. We used the health care utilisation databases of the Lombardy region to identify cancer patients' characteristics (eg, sex, age) and cancer‐related information (eg, cancer site, stage at diagnosis). The frequency of new cancer diagnoses in 2019 and 2020 were compared in terms of percentage differences in undiagnosed cases. We observed two peaks in the decline in cancer diagnoses: March to May 2020 (−37%) and October to December 2020 (−19%). The decline persisted over the course of 2020 and was higher in males and patients aged 74+. Diagnoses of all four common cancers analysed (female breast, lung, colorectal and prostate) remained below pre‐pandemic levels. For breast and colorectal cancers, the decline in diagnoses was high in the age groups targeted by population‐based screening programmes. We observed a reduction in localised stage cancer diagnoses for all four cancers. Our data confirm that timely monitoring of cancer diagnoses and interventions to prevent disruption of routine diagnostic services are needed to mitigate the impact of emergencies on cancer patients.
Keywords: cancer diagnoses, COVID‐19, population‐based data, screening programmes
What's new?
The impact of COVID‐19 restrictions on cancer diagnosis and treatment has become a widespread source of concern. This population‐based study reports a persisting decline in new cancer diagnoses during 2020 compared to pre‐pandemic levels and a reduction in localised‐stage cancer diagnoses for female breast, lung, prostate and colorectal cancer. Moreover, our study reveals inequalities across cancer patient groups, with males and patients over 74 being more negatively impacted by the COVID‐19 pandemic. Timely monitoring of cancer diagnoses and interventions to prevent disruption of routine diagnostic services are needed to mitigate the impact of public health emergencies on cancer patients.
Abbreviations
- ATC
Anatomical Therapeutic Chemical classification system
- COVID‐19
coronavirus diseases 2019
- ICD‐9CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NHS
National Health System
- PSA
prostate‐specific antigen
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
1. INTRODUCTION
Following its outbreak in China in December 2019, the COVID‐19 pandemic spread rapidly, with Italy being the first Western country to experience a massive outbreak of the virus. The Italian government imposed strict measures to counteract the spread of SARS‐CoV‐2. 1 , 2 The Healthcare System reorganised or reduced many activities in order to adapt to the emergency, and routine diagnostic procedures, including cancer screening, were either halted or postponed. 3 , 4 , 5 Italian oncological societies recommended limiting follow‐up appointments and second opinions and established a priority scale designed to optimise available resources while continuing to offer the best possible care to cancer patients. 6 However, the impact of these measures on cancer diagnoses and treatment has become a widespread source of concern.
Disruption of activities also affected surveillance systems, such as population‐based cancer registration. 7 As a result, population‐based data on the decline in cancer diagnoses are limited. 8 , 9 , 10 , 11 , 12 Dutch, Danish and USA studies reported a notable decrease in cancer diagnoses during the early months of the COVID‐19 pandemic (February to April 2020). The decline was observed in males and females, and at nearly all cancer sites. 9 , 10 , 11 In Northern Ireland, a 23% reduction was observed in diagnosed cancer cases during the 6‐month peak of the first wave of the pandemic. 12 In Belgium, Peacock 8 and colleagues observed the whole of 2020 and reported a steep decline in cancer diagnoses in the first wave of the pandemic (−44% in April 2020 compared to April 2019) and a small 2% dip in diagnoses during the second wave, in November 2020.
Data on the impact of the COVID‐19 pandemic on cancer detection in Italy come mainly from mono‐ or multicentre studies. 13 , 14 , 15 Our goal is to analyse, at a population level, the year‐long decline in cancer diagnoses in Lombardy, that is, the epicentre of the Italian COVID‐19 pandemic. 16 Specifically, we aim to characterise the tumours with the greatest overall reduction in diagnoses, according to patients' age and sex, and tumour stage at diagnosis. The latter is particularly important since early diagnosis is known to improve cancer patient prognosis, but currently available population‐based evidence does not report any data disaggregated by stage. To our knowledge, this is the first time such a study has been conducted in Italy at a population level.
2. METHODS
2.1. Data sources
2.1.1. Health care utilisation databases
The data used for the present study were retrieved from the health care utilisation databases of Lombardy, the second Italian region by resident population, with around 10 million inhabitants. 17 All Italian citizens have equal access to health care services as part of the NHS. The NHS guarantees partly or entirely free of charge access to a number of health care services to all Italian citizens. In Lombardy, this has been associated since late 1990s with an automated system of health care utilisation databases including: hospital discharge (inpatient and day‐hospital) database containing information about primary diagnosis, coexisting conditions, provided procedures (coded according to the ICD‐9CM) 18 and clinical condensed stage (1: tumour confined to organ of origin; 2: tumour beyond organ of origin; 3: metastases to regional lymph node; 4: tumour beyond organ of origin + metastases to regional lymph nodes; 5: distant metastases; 6: distant lymph nodes; 7: not confined to organ of origin but unknown if 2, 3, 4, 5 or 6; 8: no distant organs invasions but unknown if 1, 2, 3 or 4; 9: unknown); drug prescription database providing information on all the drugs reimbursed by the NHS (coded according to the ATC classification system); outpatient database, including visits in specialist ambulatories and diagnostic laboratories accredited from the NHS (coded according to the regional outpatients services coding); and copayment exception database, including exception for chronic disease (coded according to the national exceptions coding). Pathological reports are not included in the healthcare utilisation databases. These various types of data are interconnected, since a single individual identification code is used by all databases for each citizen enrolled. Further details of the databases of health care use in Lombardy have been described in previous studies. 19
2.1.2. Case definition
To identify cancer patients, we used the hospital discharge records database. The hospital discharge records are fed from the medical records for all patients discharged from public and private healthcare institutions, as defined in art. 1 of the decree of the Minister of Health October 27, 2000, n. 380. In details, we selected cases with at least one hospital stay for a diagnosis of a solid cancer (ICD‐9‐CM codes 140.*‐199.*) between January 31, 2019 and December 31, 2020 from among all residents in Lombardy aged over 18 years. We defined the first admission for a cancer diagnosis as the ‘index hospitalisation’. To select only incident cases, we excluded patients admitted for the same cancer diagnosis as the index hospitalisation in the 10 years prior to the index admission. Patients reporting more than one cancer at the index date were excluded because hospital discharge database provide staging information for one cancer only. Haematological neoplasms (ICD‐9‐CM codes: 200.*‐208.*), melanoma of skin and other malignant skin cancers (ICD‐9‐CM codes: 172.* and 173.*) were not included because these cancers are mainly treated with radiotherapy and chemotherapy administered on an outpatient basis. By working only with hospital discharge database we will have greatly underestimated their incidence.
2.2. Statistical analyses
We compared the frequency of new diagnoses between 2019 and 2020 in terms of percentage differences in undiagnosed cases. We estimated this as the difference between the number of cancer cases diagnosed between February and December in 2020 and those diagnosed between February and December in 2019, divided by the number of cancer cases diagnosed in 2019. We present percentage differences together with 95% confidence intervals for proportions calculated assuming a normal distribution. We report percentage differences for all malignant solid tumours combined and stratified by three main age groups (<50‐young; 50‐74; >74 years old/elderly), based on the age groups screened in Lombardy. In Lombardy the recommended screening programmes include those for breast, colorectal and cervical cancer and are aimed, respectively, at women between 50 and 74 years, women and men between 50 and 74 years and women between 25 and 64 years old.
We stratified percentage differences by age, sex and stage for the four most common cancers in Italy: female breast (ICD‐9‐CM code = 174.*), colorectal (ICD‐9‐CM codes = 153.*‐154.*), lung (ICD‐9‐CM codes = 162.1* − 162.9*) and prostate (ICD‐9‐CM codes = 185.*). The clinical condensed stage reported in the clinical discharge was classified as localised (tumour confined to organ of origin), locally advanced (tumour beyond organ of origin; metastases to regional lymph node; tumour beyond organ of origin + metastases to regional lymph nodes), metastatic (distant metastases; distant lymph nodes) or unknown (not confined to organ of origin but unknown if locally advanced or metastatic; no distant organs invasions but unknown if localised or locally advanced; unknown). When staging information was missing at the index date, we searched for it in patients' hospital discharge records for up to 6 months after cancer diagnosis.
All analyses were performed using SAS Studio software.
3. RESULTS
3.1. Overall changes over 2020
We observed a significant reduction of approximately −20% in the number of new cancers diagnosed between February 1 and December 31, 2020 compared to those diagnosed in the same period in 2019 (39 036 vs 49 012 new cancer cases; Figure 1A,B). The percentage difference varied widely over the course of the year (Figure 1A,B). The largest difference between 2020 and 2019 was observed in March and April with percentage reductions of −40% (2786 vs 4622 cancer cases) and −42% (2629 vs 4514 cancer cases), respectively. From June to September, the differences gradually narrowed, reaching −8% (4128 vs 4511 cancer cases) in September. The differences increased again in October and November (3952 vs 4811 cancer cases, −18% and 3321 vs 4352 cancer cases, −24%, respectively), but were stably lower than those of the first wave of the pandemic. We observed two peaks of decrease, the first in the first wave (March‐May 2020; 8778 vs 13 988 cancer cases, −37%) and the second in the second wave (October‐December 2020; 10 797 vs 13 288 cancer cases, −19%).
FIGURE 1.
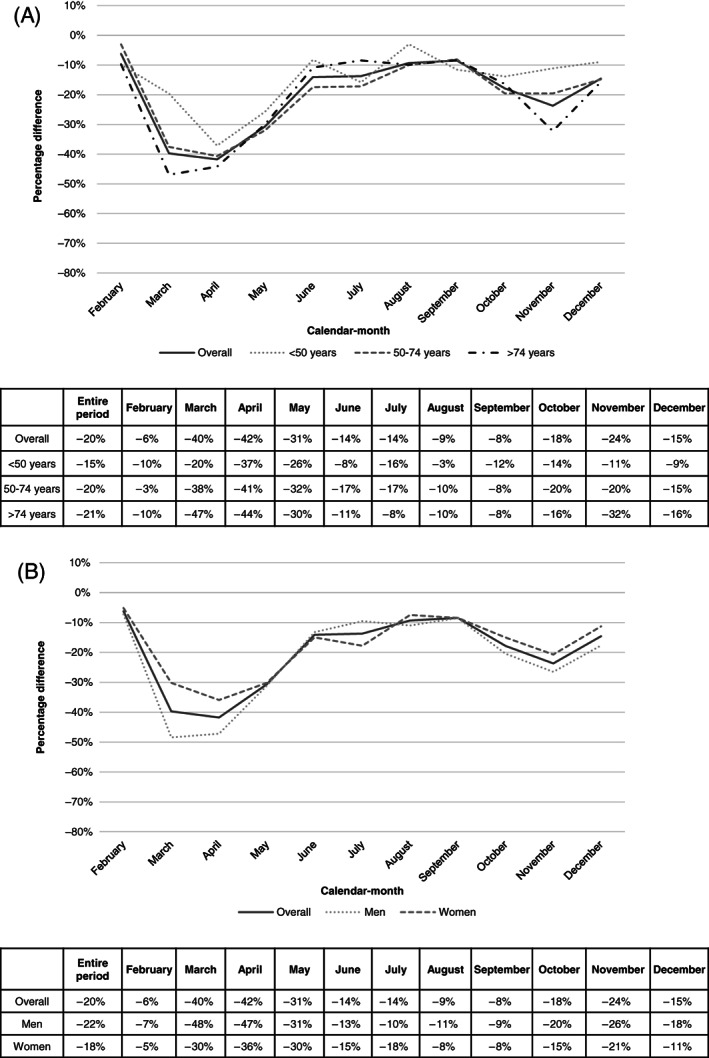
Percentage difference between all cancer cases diagnosed in 2020 and in 2019 by age group (A) or sex (B) by calendar‐month
3.2. Changes over 2020 by age groups
The percentage difference also varied by age group. Older people (>74 years old) showed the biggest difference between 2019 and 2020, with a fall of approximately −46% in new cancers diagnosed in March and April and of −32% in November (Figure 1A). Young people (<50 years old), on the other hand, had the smallest reduction in newly diagnosed cancers. However, a reduction of −37% was observed for them too in April 2020. The summer months showed the lowest reduction in newly diagnosed cancers. Notably, the greatest decline in these months was observed for younger patients (both <50 and 50‐74 age groups).
3.3. Changes over 2020 by sex
We observed a sex‐based difference in the reduction in new cancer diagnoses: −22% in men and −18% in women (Figure 1B). The sex gap was wider during the first wave (March‐May 2020; −42% and −32% for men and women, respectively), while percentage differences were similar for the rest of the year.
3.4. Changes over 2020 by major cancer site
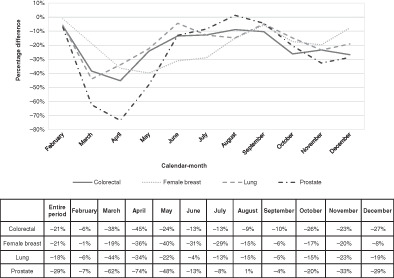
Figure 2 shows the percentage differences in cancer diagnoses over the year for colorectal, breast, lung and prostate cancers. The biggest differences between the new cancer diagnoses in 2020 and 2019 were seen for prostate cancer (3462 vs 5138 new cancer cases, −29%), followed by female breast (6896 vs 8686 cancer cases, −21%) and colorectal cancer (4525 vs 5762 cancer cases, −21%). The smallest decline was observed for lung cancer (4450 vs 5457 cancer cases, −18%).
FIGURE 2.
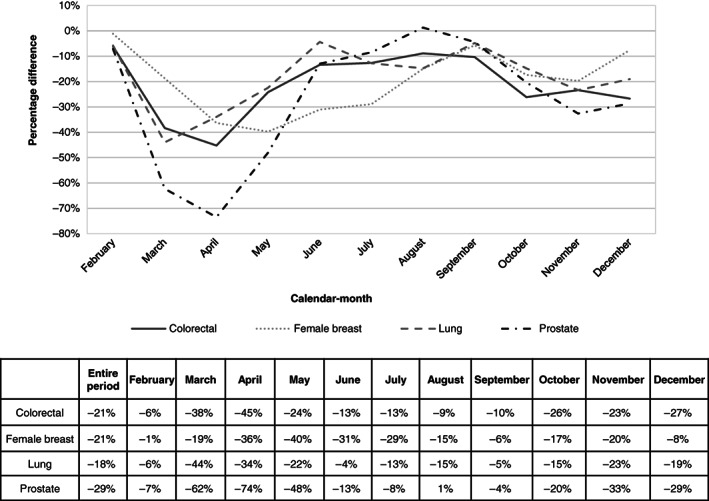
Percentage difference between cancer cases diagnosed in 2020 and in 2019, by main cancer site and calendar month
3.5. Changes over 2020 by cancer site and age groups
During the first wave of the pandemic (March‐May 2020), the percentage difference in cancer diagnoses was greater in elderly (aged >74 years) than in younger (aged <50 years) patients for both colorectal and female breast cancer: −37% vs −6% in colorectal and −42% vs −15% in female breast cancer (Figure 3). After the first wave, the largest reduction in new cancer diagnoses for colorectal and female breast cancer was found in the screening‐target age group (−21%; 50‐74 years).
FIGURE 3.
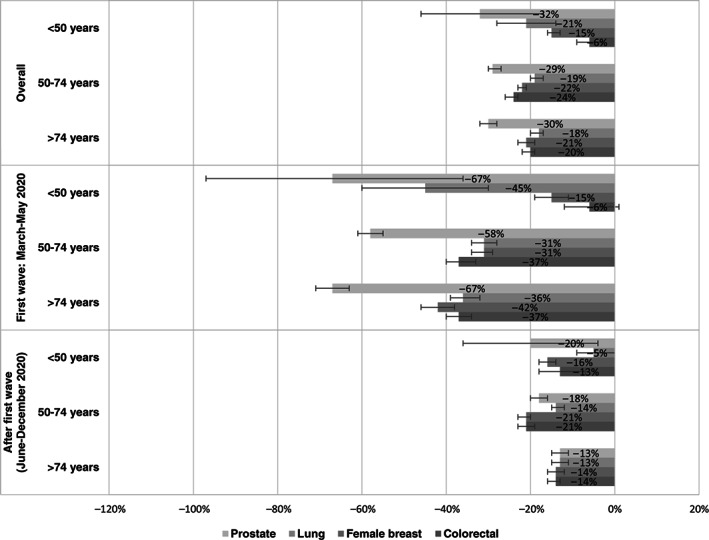
Overall and period‐specific percentage differences (together with 95% confidence intervals) between cancer cases diagnosed in 2020 and in 2019, by main cancer site and age group
For lung and prostate cancer, the overall decrease was similar across the age groups. However, after the first wave, the reduction was higher at older ages for lung cancer (−14% and −13% at age 50‐74 and >74 years, respectively, vs −5% at <50 years) and highest in young patients for prostate cancer (−20% in those aged <50 years; Figure 3).
3.6. Changes over 2020 by cancer site and sex
In colorectal cancer, we observed a lower decline in new cancer diagnoses in women compared to men, in both the younger (<50 years old) and older age groups (>74 years old). In terms of lost new cancer diagnoses, there were no major differences between women and men either overall or in the screening‐target age group (50‐74 years old) (Table 1).
TABLE 1.
Percentage difference (together with 95% confidence intervals) between cancer cases diagnosed in 2020 and in 2019, by main cancer site, sex and age group
| Cancer site | Age group | Females | Males |
|---|---|---|---|
| Colorectal | |||
| <50 years | 8% [3%; 13%] | −16% [−23%; −10%] | |
| 50‐74 years | −24% [−26%; −22%] | −25% [−27%; −23%] | |
| >74 years | −16% [−18%; −14%] | −24% [−26%; −22%] | |
| Lung | |||
| <50 years | −30% [−40%; −19%] | −12% [−19%; −4%] | |
| 50‐74 years | −16% [−19%; −14%] | −20% [−22%; −18%] | |
| >74 years | −21% [−23%; −18%] | −17% [−19%; −15%] | |
For lung cancer, we observed a higher decline in new cancer diagnosis in young women (<50 years old) compared to young men (Table 1). No substantial differences were observed between males and females for the other age groups.
3.7. Changes over 2020 by cancer site and stage
In all four cancers considered, localised stage diagnoses presented the largest reduction: −22%, −27%, −27% and −31% in female breast, colorectal, lung and prostate cancers, respectively (Figure 4). The largest reduction in localised stage diagnoses was observed during the first wave of the pandemic: −34%, −41%, −46% and −64% in female breast, colorectal, lung and prostate cancers, respectively. The smallest reduction for the whole period was observed for metastatic breast cancer patients.
FIGURE 4.
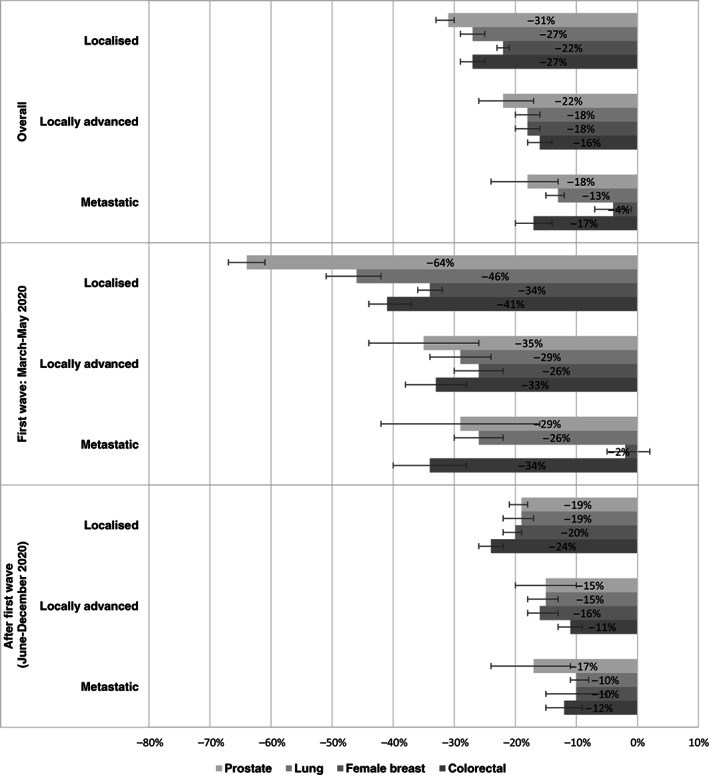
Overall and period‐specific percentage differences (together with 95% confidence intervals) between cancer cases diagnosed in 2020 and in 2019, by main cancer site and stage at diagnosis (unknown stage not reported)
Stage distribution did not change substantially between 2019 and 2020 for all cancers considered (Table S1).
4. DISCUSSION
Two key findings of our study were the reduction in cancer diagnoses, which persisted over the course of 2020—although some recovery did occur—and the emergence of inequalities across patient groups. Male and middle‐aged/older adults, particularly 74+ years old, lagged behind in terms of a return to expected numbers of cancer diagnoses, based on 2019 data.
Another major study finding was that diagnoses of all four analysed common solid cancers (female breast, lung, colorectal and prostate) remained below pre‐pandemic levels. The decline in breast and colorectal cancer diagnoses was high in the age groups targeted by population‐based screening programmes. For all four cancers we also report a large decline in diagnoses of localised stage cancer.
The explanation for the fall in cancer diagnoses is multifactorial and involves patients and primary and secondary care organisations. Fear of contracting COVID‐19 restricted patients' access to the healthcare system and their likelihood of reporting symptoms. 20 Recommendations to limit primary and secondary care access to urgent conditions may have resulted in missed detection of important symptoms or signs, or delays in appropriate diagnostic work‐ups (eg, radiology or endoscopy). 6 , 14 , 21 , 22 , 23 Furthermore, capacity for diagnostic investigations was likewise subject to disruption or re‐allocation of resources to the COVID‐19 emergency.
During 2020 as a whole, we observed a decrease of approximately 20% in diagnoses. However, the reduction stood at 37% and 19% in the first and second waves of the COVID‐19 pandemic, respectively (Figure 1). The impact of the first wave was similar to the situation reported in Belgium, Denmark, the Netherlands and the United States. 8 , 9 , 10 , 11 The impact of the second wave was instead reported in Belgium only and was lower than in Lombardy. This difference may be due to the data sources used. We used cancer admissions, which were largely influenced by the number of COVID‐19 hospitalisations, whereas pathology laboratories were used to detect cancer in Belgium. From September to December 2020, the maximum number of COVID‐19 hospitalisations in Belgium was 7500 compared to 38 000 in Italy, of which roughly 9000 were in Lombardy. 24 , 25 However, estimates of ‘missing cancer diagnoses’ due to the second wave could be underestimated in both Italy and Belgium because observation ended on December 31, 2020 whereas the second wave of the pandemic lasted until the early months of 2021. Despite the similar age distribution of the population residing in Lombardy between 2019 and 2021, 17 we observed the highest reduction in cancer diagnosis in the elderly (−46% in March and April and −32% in November), particularly men, (−48% reduction in cancers diagnosis in March and April and −26% in November). Severe forms of COVID‐19 were more common among the elderly and patients with comorbidities (eg, diabetes mellitus, hypertension and cardiovascular disease). 26 Elderly men were also at higher risk of complications and death from COVID‐19, 27 so it is conceivable that older men are the least likely to see a doctor. Additionally, as deaths were higher among the elderly, 27 it is also likely that some cancer diagnoses in this age group were missed by dying from COVID‐19 before being diagnosed with cancer. Although elderly were particularly impacted by the COVID‐19, there was still a 15% drop in cancer diagnoses in young patients (<50 years old).
Our results confirmed a high reduction in diagnoses of breast, colorectal and prostate cancers, and a lower, but relevant decline in diagnoses for lung cancer. 8 , 9 , 12 Belgian data previously showed that poor prognosis cancers were less impacted. In the case of lung cancer, symptoms mimicking those of COVID‐19 may have contributed to keeping the diagnosis rate higher compared to other cancers. 8 A slight reduction in newly diagnosed lung cancer cases was observed in 2020 compared to 2019 in several other studies. 28 , 29 , 30 , 31 However, some studies have observed relevant decrease in lung cancer diagnoses 32 , 33 likely due to the disruption of lung cancer screening 34 , 35 , 36 or to the different setting of the study (hospital vs population‐based). Lung cancer screening is not available in Italy.
We observed a major decline in cancer diagnoses for colon and breast cancers in the age groups targeted by population‐based screening programmes. Italian national data showed a significant decline in the number of screening tests performed in 2020 compared to 2019. For breast cancer, there was a −54% reduction in the number of screening tests performed between January and May 2020 and a −24% fall between September and December 2020. In Lombardy, the reduction was −62% and −25% in the first and last months of 2020, respectively. 4 For colorectal cancer, the decrease in the number of screening tests performed was −58% between January and May 2020 and −24% between September and December 2020. In Lombardy, the reduction was −69% and −55% in the first and last months of 2020, respectively. This can again be explained by patients' fear of being infected during hospital access and the recommendation to limit primary and secondary care access to more fragile patients, including the elderly. Our study demonstrates the substantial effect that the COVID‐19 pandemic had on the number of men diagnosed with prostate cancer: nearly 70% of the expected diagnoses were not made in the first lockdown period in Lombardy most likely because prostate biopsies were restricted to patients who were suspected to have high‐risk disease. Prostate cancer is the most common urological malignancy in men in Italy. Thus, the significant reduction in prostate cancer diagnosis observed during the first wave of the pandemic may help explain the greater impact of COVID‐19 on cancer diagnosis in men compared to women. Finally, the 20% reduction in prostate cancer diagnoses in people under the age of 50 after the first wave of the pandemic can be explained by the decrease in the number of total PSA tests performed 22 , 37 which are not recommended for people over the age of 70.
We also report a large decline in localised stage cancer diagnoses across the four analysed cancers. In Italy, several steps of the integrated patient pathway for lung cancer were impacted by COVID‐19, including a decrease in endoscopic diagnostic procedures and major resections for early‐stage lung cancers. Personnel shortages and different tumour sample processing methods slightly lengthened the time for diagnostic pathway completion. Personnel protection strategies led to the remodelling of multidisciplinary team discussions on a web basis and the need to reduce the number of cases discussed. 23 For colorectal cancers, the screening programme was discontinued, endoscopic procedures were reduced and high‐risk patients were more likely to undergo colonoscopy during the lockdown period. 14 In the case of prostate cancer, the number of clinical visits, prostate biopsies and men enrolled in active surveillance were significantly lower during the pandemic. Conversely, the number of cases with advanced and metastatic prostate cancer increased. 38 Furthermore, lockdown resulted in a dramatic decrease in the total number of PSA tests performed, even though local restrictions did not include a ban on access to routine diagnostic testing. 22 While the increased volume of testing recorded post lockdown may have filled the gap observed during confinement in terms of total number of annual tests, it cannot instead void delays that may have occurred in diagnosing some prostate cancers. 22 The breast cancer screening programme was disrupted, leading to a significant decrease in in situ breast cancer diagnoses and an increase in node‐positive and stage III tumours. 39 Furthermore, waiting times on list and times between biopsy/cytological examination and surgery were significantly longer during lockdown and were associated with an increase in pathological node involvement. 40 In any event, despite observing a differential relative decrease in localised stage cancer diagnoses, stage distribution did not change substantially between 2019 and 2020 for any of the cancers considered (Table S1). This could be due to the limited follow‐up (December 2020), which did not allow us to observe the long‐term impact of the two COVID‐19 waves, and to the cancer stage grouping, which prevented us from observing detailed changes such as tumour size. The large reduction in prostate cancer diagnoses with missing stage information may be explained by the decline in PSA testing during the first wave of the pandemic.
Our study has several limitations which need to be acknowledged. We used hospital discharge database, which are claims data collected for administrative, not epidemiological purposes, so they are not completely precise, especially in terms of diagnostic codes, and only include procedures performed in hospital. This could also explain some observed differences between our data and those reported by CRs which are based on different sources of information, including pathological reports. To address these issues, we focused on solid tumours that can be adequately retrieved via the ICD‐9CM codes used in the hospital discharge database, limiting more in‐depth analyses to the four tumours with a clear hospital pathway, which can be identified without losing cases via the hospital discharge database. However, we were unable to report on haematological cancers and skin melanomas, which were heavily impacted by the emergency. Another limitation is that our follow‐up ended in December 2020, preventing us from assessing the full impact of the second wave of the pandemic and full retrieval of cancer diagnoses. However, we did have access to the latest updated data as of December 31, 2020. Although the number of diagnoses increased in several countries 12 , 41 in 2021, the pandemic and COVID‐19 restrictions differed substantially across EU countries, hence their impact on cancer detection across countries could likewise differ. 42
Despite the weaknesses mentioned above, our study has the merit of supporting the use of health care utilisation database to study the impact of emergency situations on cancer detection (and beyond) when population cancer registries cannot promptly contribute. This is the first population‐based study performed in Lombardy, the Italian region most hit by the COVID‐19 pandemic.
To conclude, our data confirm a strong, not yet fully recovered reduction in cancer diagnoses in Lombardy, Italy. Although the distribution of stages did not change substantially between 2019 and 2020, the large decline observed in localised stage diagnoses could have an impact on stage at diagnosis in the coming years. Substantial increases in the number of avoidable cancer deaths are expected as a result of COVID‐19‐related diagnostic delays. 43 Hence, our data confirm that timely monitoring of cancer diagnoses and interventions to prevent the disruption of routine diagnostic services are needed to mitigate the impact of emergencies on cancer patients.
AUTHOR CONTRIBUTIONS
Annalisa Trama, Alice Bernasconi, Paolo Lasalvia and Laura Botta contributed to writing‐original draft, writing‐review and editing. Alice Bernasconi, Paolo Lasalvia and Laura Botta contributed also to formal analysis, investigation, software and methodology. Annalisa Trama, Serena Di Cosimo, Rosalba Miceli, Melanie Claps contributed also to conceptualization, project administration and methodology. All authors contributed to writing‐review and editing the article, read and approved the final article. The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
This work was supported by “5 per 1000” 2016 funds (Italian Ministry of Health, financial support for healthcare research) ‐ project tile “CAncRo E covid (CARE)”, Principal Investigator Annalisa Trama.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The project was approved by the Institutional Review Board and Ethics committee of the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, protocol number 0257/20.
Supporting information
Table S1 Changes in stage distribution between 2019 and 2020 by major cancer site.
ACKNOWLEDGEMENT
We thank Giulia Del Monego for helping us with the article submission.
Trama A, Bernasconi A, Botta L, et al. COVID‐19 outbreak in Lombardy: Impact on reducing solid cancer diagnoses in 2020. Int J Cancer. 2022;151(9):1502‐1511. doi: 10.1002/ijc.34168
Annalisa Trama and Alice Bernasconi have contributed equally to this work and share first authorship.
Funding information Italian Ministry of Health “5 per 1000” 2016 funds
DATA AVAILABILITY STATEMENT
Please contact Regione Lombardia (website: https://www.en.regione.lombardia.it/wps/portal/site/en-regione-lombardia) to get access to the data. Further information is available from the corresponding author upon reasonable request.
REFERENCES
- 1. EpiCentro . COVID‐19 integrated surveillance data in Italy. Retrieved from https://www.epicentro.iss.it/en/coronavirus/sars‐cov‐2‐dashboard
- 2. Ministry of Health . Covid‐19 – Situazione in Italia. Retrieved from https://opendatadpc.maps.arcgis.com/apps/dashboards/b0c68bce2cce478eaac82fe38d4138b1
- 3. Maida M, Sferrazza S, Savarino E, et al. Impact of the COVID‐19 pandemic on gastroenterology services in Italy: a national survey. Dig Liver Dis. 2020;52(8):808‐815. doi: 10.1016/j.dld.2020.05.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rapporto sui ritardi accumulati dai programmi di screening Italiani in seguito alla pandemia da Covid 19. Terzo Rapporto aggiornato al 31 Dicembre 2020. Retrieved from https://www.osservatorionazionalescreening.it/content/rapporto‐sulla‐ripartenza‐degli‐screening‐dicembre‐2020
- 5. Scioscia M, Noventa M, Palomba S, Laganà AS. Effect of the COVID‐19 pandemic on oncology screenings: it is time to change course. BJOG. 2021;128(13):2213‐2214. doi: 10.1111/1471-0528.16857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. AIOM . The treatment of cancer patients during COVID19 pandemia. Retrieved from https://www.aiom.it/wp-content/uploads/2020/03/20200325_COVID19_SICO-AIOM-AIRO.pdf
- 7. Neamtiu L, Martos C, Giusti F, et al. ENCR steering committee. Impact of the first wave of the COVID‐19 pandemic on cancer registration and cancer care: a European survey. Eur J Public Health. 2021;32:311‐315. doi: 10.1093/eurpub/ckab214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peacock HM, Tambuyzer T, Verdoodt F, et al. Decline and incomplete recovery in cancer diagnoses during the COVID‐19 pandemic in Belgium: a year‐long, population‐level analysis. ESMO Open. 2021;6(4):100197. doi: 10.1016/j.esmoop.2021.100197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skovlund CW, Friis S, Dehlendorff C, Nilbert MC, Mørch LS. Hidden morbidities: drop in cancer diagnoses during the COVID‐19 pandemic in Denmark. Acta Oncol. 2021;60(1):20‐23. doi: 10.1080/0284186X.2020.1858235 [DOI] [PubMed] [Google Scholar]
- 10. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID‐19) pandemic. JAMA Netw Open. 2020;3(8):e2017267. doi: 10.1001/jamanetworkopen.2020.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID‐19 epidemic in The Netherlands. Lancet Oncol. 2020;21(6):750‐751. doi: 10.1016/S1470-2045(20)30265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamilton AC, Donnelly DW, Loughrey MB, et al. Inequalities in the decline and recovery of pathological cancer diagnoses during the first six months of the COVID‐19 pandemic: a population‐based study. Br J Cancer. 2021;125(6):798‐805. doi: 10.1038/s41416-021-01472-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Vincentiis L, Carr RA, Mariani MP, Ferrara G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID‐19 pandemic, compared with the 2018‐2019: an audit study from cellular pathology. J Clin Pathol. 2021;74(3):187‐189. doi: 10.1136/jclinpath-2020-206833 [DOI] [PubMed] [Google Scholar]
- 14. Buscarini E, Benedetti A, Monica F, et al. Changes in digestive cancer diagnosis during the SARS‐CoV‐2 pandemic in Italy: a nationwide survey. Dig Liver Dis. 2021;53(6):682‐688. doi: 10.1016/j.dld.2021.02.021 [DOI] [PubMed] [Google Scholar]
- 15. Ferrara G, De Vincentiis L, Ambrosini‐Spaltro A, et al. Cancer diagnostic delay in northern and Central Italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic. Am J Clin Pathol. 2021;155(1):64‐68. doi: 10.1093/ajcp/aqaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. COVID‐19 ITALIA – Desktop. Retrieved from https://www.arcgis.com/apps/dashboards/b0c68bce2cce478eaac82fe38d4138b1
- 17. Istat, Popolazione residente al 1° gennaio. Retrieved from http://dati.istat.it/Index.aspx?DataSetCode=DCIS_POPRES1
- 18. International Classification of Diseases,Ninth Revision, Clinical Modification (ICD‐9‐CM). Retrieved from https://www.cdc.gov/nchs/icd/icd9cm.htm [PubMed]
- 19. Corrao G, Mancia G. Generating evidence from computerized healthcare utilization databases. Hypertension. 2015;65(3):490‐498. doi: 10.1161/HYPERTENSIONAHA.114.04858 [DOI] [PubMed] [Google Scholar]
- 20. Vanni G, Materazzo M, Pellicciaro M, et al. Breast cancer and COVID‐19: the effect of fear on patients' decision‐making process. In Vivo. 2020;34(3 Suppl):1651‐1659. doi: 10.21873/invivo.11957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armellini E, Repici A, Alvisi C, et al. Analysis of patients attitude to undergo urgent endoscopic procedures during COVID‐19 outbreak in Italy. Dig Liver Dis. 2020;52(7):695‐699. doi: 10.1016/j.dld.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrari A, Sanchis‐Gomar F, Mattiuzzi C, Henry BM, Lippi G. Is COVID‐19 impacting prostate cancer screening? A survey of prostate‐specific antigen test requests during a local outbreak. EJIFCC. 2021;32(1):69‐77. [PMC free article] [PubMed] [Google Scholar]
- 23. Pasello G, Menis J, Pilotto S, et al. How the COVID‐19 pandemic impacted on integrated care pathways for lung cancer: the parallel experience of a COVID‐spared and a COVID‐dedicated center. Front Oncol. 2021;11:669786. doi: 10.3389/fonc.2021.669786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Number of COVID‐19 patients in hospital in Belgium and Italy. Retrieved from https://ourworldindata.org/grapher/current-covid-patients-hospital?country=BEL~ITA
- 25. Dashboard Covid‐19. Retrieved from https://www.regione.lombardia.it/wps/portal/istituzionale/HP/servizi‐e‐informazioni/cittadini/salute‐e‐prevenzione/coronavirus/dashboard‐covid19
- 26. Characteristics of COVID‐19 patients dying in Italy. Retrieved from https://www.epicentro.iss.it/en/coronavirus/sars‐cov‐2‐analysis‐of‐deaths
- 27. Impatto dell'epidemia covid‐19 sulla mortalità totale della popolazione residente periodo gennaio‐maggio 2020. Retrieved from https://www.epicentro.iss.it/coronavirus/pdf/Rapp_Istat_Iss_9luglio.pdf
- 28. Cantini L, Mentrasti G, Russo GL, et al. Evaluation of COVID‐19 impact on DELAYing diagnostic‐therapeutic pathways of lung cancer patients in Italy (COVID‐DELAY study): fewer cases and higher stages from a real‐world scenario. ESMO Open. 2022;7(2):100406. doi: 10.1016/j.esmoop.2022.100406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greene G, Griffiths R, Han J, et al. Impact of the SARS‐CoV‐2 pandemic on female breast, colorectal and non‐small cell lung cancer incidence, stage and healthcare pathway to diagnosis during 2020 in Wales, UK, using a national cancer clinical record system. Br J Cancer. 2022;1‐11. doi: 10.1038/s41416-022-01830-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coma E, Guiriguet C, Mora N, et al. Impact of the COVID‐19 pandemic and related control measures on cancer diagnosis in Catalonia: a time‐series analysis of primary care electronic health records covering about five million people. BMJ Open. 2021;11(5):e047567. doi: 10.1136/bmjopen-2020-047567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morais S, Antunes L, Rodrigues J, Fontes F, Bento MJ, Lunet N. The impact of the coronavirus disease 2019 pandemic on the diagnosis and treatment of cancer in northern Portugal. Eur J Cancer Prev. 2022;31(2):204‐214. doi: 10.1097/CEJ.0000000000000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kasymjanova G, Anwar A, Cohen V, et al. The impact of COVID‐19 on the diagnosis and treatment of lung cancer at a Canadian academic center: a retrospective chart review. Curr Oncol. 2021;28(6):4247‐4255. doi: 10.3390/curroncol28060360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. London JW, Fazio‐Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID‐19 pandemic on cancer‐related patient encounters. JCO Clin Cancer Inform. 2020;4:657‐665. doi: 10.1200/CCI.20.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Haren RM, Delman AM, Turner KM, et al. Impact of the COVID‐19 pandemic on lung cancer screening program and subsequent lung cancer. J Am Coll Surg. 2021;232(4):600‐605. doi: 10.1016/j.jamcollsurg.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henderson LM, Benefield T, Bosemani T, Long JM, Rivera MP. Impact of the COVID‐19 pandemic on volumes and disparities in lung cancer screening. Chest. 2021;160(1):379‐382. doi: 10.1016/j.chest.2020.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Englum BR, Prasad NK, Lake RE, et al. Impact of the COVID‐19 pandemic on diagnosis of new cancers: a national multicenter study of the veterans affairs healthcare system. Cancer. 2022;128(5):1048‐1056. doi: 10.1002/cncr.34011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaufman HW, Chen Z, Niles JK, Radcliff J, Fesko Y. Patterns of prostate‐specific antigen testing and prostate biopsies during the COVID‐19 pandemic. JCO Clin Cancer Inform. 2021;5:1028‐1033. doi: 10.1200/CCI.21.00074 [DOI] [PubMed] [Google Scholar]
- 38. Pepe P, Pepe L, Pennisi M, Fraggetta F. Prostate cancer diagnosis and management during one year of the COVID‐19 pandemic. Anticancer Res. 2021;41(6):3127‐3130. doi: 10.21873/anticanres.15097 [DOI] [PubMed] [Google Scholar]
- 39. Toss A, Isca C, Venturelli M, et al. Two‐month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6(2):100055. doi: 10.1016/j.esmoop.2021.100055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanni G, Tazzioli G, Pellicciaro M, et al. Delay in breast cancer treatments during the first COVID‐19 lockdown. A multicentric analysis of 432 patients. Anticancer Res. 2020;40(12):7119‐7125. doi: 10.21873/anticanres.14741 [DOI] [PubMed] [Google Scholar]
- 41. Covid‐19 en kanker. Retrieved from https://iknl.nl/covid-19
- 42. Daily new confirmed cases in Belgium, Italy and Netherlands. Retrieved from https://ourworldindata.org/explorers/coronavirus‐data‐explorer?zoomToSelection=true&facet=none&hideControls=true&Metric=Confirmed+cases&Interval=New+per+day&Relative+to+Population=false&Color+by+test+positivity=false&country=BEL~ITA~NLD
- 43. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21(8):1023‐1034. doi: 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Changes in stage distribution between 2019 and 2020 by major cancer site.
Data Availability Statement
Please contact Regione Lombardia (website: https://www.en.regione.lombardia.it/wps/portal/site/en-regione-lombardia) to get access to the data. Further information is available from the corresponding author upon reasonable request.


