Abbreviations
- CAR‐T
chimaeric antigen receptor T‐cell therapy
- CLL
chronic lymphocytic leukaemia
- COVID‐19
SARS‐CoV‐2 disease 2019
- CRP
C‐reactive protein
- DLBCL
diffuse large B‐cell lymphoma
- FL
follicular lymphoma
- HM
haematological malignancies
- HSCT
haematopoietic stem cell transplantation
- ICU
intensive‐care unit
- IQR
interquartile range
- LOS
length of stay
- mAB
monoclonal antibodies
- NHL
non‐Hodgkin lymphoma
- PCR
polymerase chain reaction
- PET
positron emission tomography
- PFS
progression‐free survival
- RCHOP
rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride (doxorubicin hydrochloride), vincristine (oncovin), and prednisone
- SMC
Soroka University Medical Center
- T‐C
tixagevimab–cilgavimab
- VOC
variant of concern
Patients with haematological malignancies (HM) who fall prey to COVID‐19, especially those receiving anti‐CD20 monoclonal antibodies (mAB), are at a higher risk for complications, mortality, protracted disease, and relapse. 1 , 2
Anti‐CD‐20 mAB became widely used for HM, especially for B‐cell non‐Hodgkin lymphoma (NHL). These antibodies neutralise B cells and suppress the humoral response to viral replication, 3 resulting in a higher risk for severe COVID‐19. 2 Obinutuzumab is a novel type 2 anti‐CD20 mAB that enhances antibody‐dependent cell‐mediated cytotoxicity, thus inducing higher levels of direct cell death than rituximab. 4 Current use of obinutuzumab includes combination with chemotherapy for previously untreated or relapsed follicular lymphoma or chronic lymphocytic leukaemia (CLL). Obinutuzumab‐based immunochemotherapy and maintenance resulted in more prolonged progression‐free survival for patients with advanced‐stage follicular lymphoma, at the cost of an increased rate of adverse events, including infections. 5
During the study, Omicron (B.1.1.529) was the dominant variant of concern (VOC), 6 generally characterised by a lower case fatality rate and a lower propensity for complications but substantially higher transmissibility. 7
We performed a single‐centre population‐based cohort study to determine whether patients treated with obinutuzumab were at a higher risk for adverse COVID‐19 outcomes than those of rituximab‐treated patients. We included all HM patients treated with either obinutuzumab or rituximab between June 2021 and April 2022 at Soroka University Medical Center, a 1110‐bed referral hospital. After obtaining a waiver of informed consent (SOR‐20‐156), we collected data from the patients' electronic medical records. For those who tested positive for SARS‐CoV‐2, the authors performed an in‐depth review of the chart. During the study period, SARS‐CoV‐2 PCR testing was available by self‐referral to all HM patients whenever deemed necessary regardless of treatment and as part of universal screening upon hospital admission. 8 We identified the Omicron variant by either sequencing or when compatible mutations were found in the S gene by Allplex™ SARS‐CoV‐2 Master Assay (Seegene, Seoul, South Korea). Follow‐up continued until 1 April 1 2022 or death.
We defined aggressive haematologic disease as B‐cell NHL, including DLBCL, mantle cell lymphoma, and high‐grade lymphoma, treated with RCHOP or CHOP‐like treatment. Indolent B‐cell lymphomas included follicular lymphoma, marginal zone lymphoma and CLL. Our primary outcome was COVID‐19‐related hospitalisation. Secondary outcomes were COVID‐19 disease severity, admission to an intensive‐care unit, and all‐cause mortality.
We utilised the t‐test to compare continuous variables, the χ2 or Fisher's exact tests for categorical data, and a Mann–Whitney test for comparing variables with non‐normal distribution. Multivariate analysis for hospitalisation was performed using a logistic regression model. We used SPSS version 25 (IBM Corp, Armonk, NY, USA); a two‐tailed p value of ⩽0.05 was considered significant.
During the study period, 147 HM patients received at least one dose of anti‐CD20 mAB. We excluded four who became ill before the Omicron wave. In the remaining 143, 105 (73%) received rituximab, and 38 (27%) received obinutuzumab.
A third of our cohort (47/143) contracted SARS‐CoV‐2 during the follow‐up period, 27 in the rituximab group and 20 in the obinutuzumab group. The demographic, clinical characteristics, and comorbidities of these participants are shown in Table 1.
TABLE 1.
Characteristics of the positive COVID‐19 population according to anti‐CD‐20 type (n = 47)
| Variable | Total (n = 47) | Rituximab (n = 27) | Obinutuzumab (n = 20) | p value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (mean ± SD) | 65.3 ± 12.2 | 65.7 ± 14.9 | 64.8 ± 7.8 | 0.769 |
| Gender, female n (%) | 23 (48.9) | 18 (66.7) | 5 (25.0) | 0.005 |
| Charlson (median, IQR) | 6.0, 4.0–8.0 | 6, 4.0–8.0 | 6, 4.0–7.7 | 0.712 |
| Charlson ≥ 4 n (%) | 39 (83.0) | 22 (81.5) | 17 (85.0) | 1.00 |
| Hypertension n (%) | 21 (44.7) | 13 (48.1) | 8 (40.0) | 0.579 |
| Diabetes mellitus n (%) | 13 (27.7) | 5 (18.5) | 8 (40.0) | 0.104 |
| Haematological malignancy status | ||||
| Haematologic diagnosis n (%) | ||||
| Aggressive lymphoma | 16 (34.0) | 16 (59.3) | 0 (0) | <0.001 |
| Indolent lymphoma/CLL | 31 (66.0) | 11 (40.7) | 20 (100.0) | |
| s/p CAR‐T n (%) | 3 (6.4) | 3 (11.1) | 0 (0) | 0.251 |
| s/p HSCT n (%) | 3 (6.4) | 3 (11.1) | 0 (0) | 0.251 |
| Anti‐CD20 indication n (%) | ||||
| Induction | 28 (59.6) | 21 (77.8) | 7 (35.0) | 0.003 |
| Maintenance | 19 (40.4) | 6 (22.2) | 13 (65.0) | |
| Remission n (%) | 36 (76.6) | 21 (77.8) | 15 (75.0) | 0.824 |
| Relapse or refractory disease n (%) | 13 (27.7) | 8 (29.6) | 5 (27.7) | 0.726 |
| Vaccination status n (%) | ||||
| 0–2 doses | 12 (25.5) | 7 (25.9) | 5 (25.0) | 0.943 |
| 3–4 doses | 35 (74.5) | 20 (74.1) | 15 (75.0) | |
| Covid‐19 disease | ||||
| Covid severity n (%) | ||||
| Asymptomatic‐mild–moderate | 38 (80.9) | 25 (92.6) | 13 (65.0) | 0.017 |
| Severe–critical | 9 (19.1) | 2 (7.4) | 7 (35.0) | |
| Time from last anti‐CD‐20 to COVID‐19 infection, days (median, IQR) | 49.0, 16.0–171.0 | 62.0, 18.0–217.0 | 47.5, 14.5–66.0 | 0.186 |
| Hospitalisations n (%) | 19 (40.4) | 7 (25.9) | 12 (60.0) | 0.019 |
| Hospital LOS (median, IQR) (n = 19) | 5.0, 3.0–13.0 | 4.0, 2.0–8.0 | 5.5, 3.2–14.5 | 0.523 |
| Elevated CRP (≥0.5 mg/dl) n (%) (n = 21) | 21 (100) | 8 (100) | 13 (100) | 1.00 |
| Lymphopenia (<900 a 103/μl) n (%) (n = 28) | 19 (67.9) | 8 (61.5) | 11 (73.3) | 0.689 |
| Neutropenia (<1000 a 103/μl) n (%) (n = 28) | 5 (17.9) | 2 (15.4) | 3 (20.0) | 1.00 |
| Treatment n (%) | ||||
| Glucocorticoids | 10 (21.3) | 3 (11.1) | 7 (35.0) | 0.048 |
| Remdesivir | 11 (23.4) | 4 (14.8) | 7 (35.0) | 0.106 |
| Nirmatrelvir/ritonavir | 12 (25.5) | 8 (29.6) | 4 (20.0) | 0.454 |
| Molupiravir | 6 (12.8) | 4 (14.8) | 2 (10.0) | 1.00 |
| Any anti‐viral a | 25 (53.2) | 12 (44.4) | 13 (65.0) | 0.163 |
| Baricitinib | 2 (4.3) | 1 (3.7) | 1 (5.0) | 1.00 |
| Tixagevimab–cilgavimab | ||||
| Pre‐ exposure | 3 (6.4) | 1 (3.7) | 2 (10.0) | 0.549 |
| Post‐exposure | 10 (21.3) | 5 (18.5) | 5 (25.0) | |
| Outcomes | ||||
| Mortality n (%) | 3 (6.4) | 0 (0) | 3 (15.0) | 0.038 |
| ICU hospitalisation n (%) | 4 (8.5) | 1 (3.7) | 3 (15.0) | 0.298 |
| Cumulative follow‐up weeks | 753.4 | 430.4 | 323.0 | — |
Abbreviations: CAR‐T, chimaeric antigen receptor (CAR) T‐cell therapy; CLL, chronic lymphocytic leukaemia; CRP, C‐reactive protein, normal range 0.02–0.5 mg/dl; HSCT, haematopoietic stem cell transplantation; ICU, intensive‐care unit; IQR, interquartile range; LOS, length of stay; SD standard deviation.
Any antiviral medication—remdesivir, ritonavir‐boosted nirmatrelvir or molnupiravir.
All patients receiving obinutuzumab in our cohort were treated for indolent lymphomas, most of whom (13/20, 65%) were in the maintenance phase of treatment (6/27, 22% in the rituximab group, p = 0.003). Remission rates and the proportions of anti‐CD20 mAB as second‐line therapy were similar.
Almost 75% of patients (35/47) in both groups were fully vaccinated (3–4 doses of BNT162b2 vaccine). Only three patients received tixagevimab–cilgavimab as pre‐exposure prophylaxis.
More patients in the obinutuzumab group were diagnosed with severe to critical COVID‐19 than in the rituximab group (35% vs. 7.4%, p = 0.017). In addition, COVID‐related hospitalisation occurred in 26/47 patients, of which 12/20 (60%) were treated with obinutuzumab, and 7/27 (25.9%) were treated with rituximab (p = 0.019). All three patients who died were treated with obinutuzumab (Figure 1).
FIGURE 1.
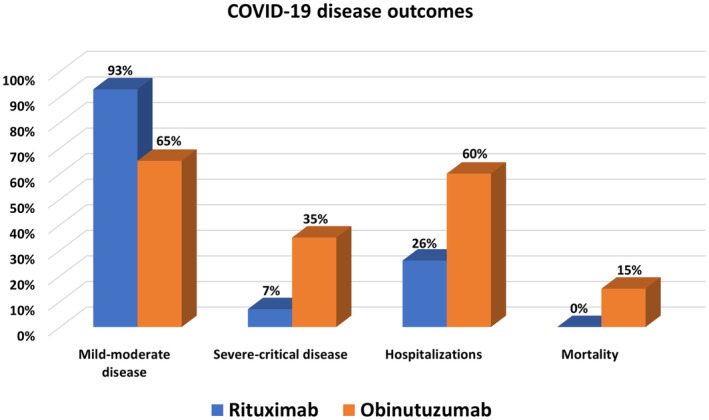
COVID‐19 clinical outcomes of the study population, comparing rituximab and obinutuzumab groups (n = 47).
In the multivariable analysis for hospitalisation, obinutuzumab treatment was associated with a 4.3‐fold (95% confidence interval [CI] 1.22–15.06, p = 0.023) increase in hospital admissions compared to rituximab after adjustment to age older than 60 and a Charlson comorbidity index of 4 or more (Tables S1, S2).
The key finding in our study is that in HM patients who contracted the Omicron VOC, prior treatment with obinutuzumab rather than rituximab was associated with worse clinical outcomes, higher hospitalisation rates, and severe–critical illness. Furthermore, even though the rituximab group included patients with more aggressive lymphoma [16/27 (59%) vs none, p < 0.0001] and more instances of anti‐CD20 as part of the induction phase [21/27 (78%) vs. 7/20 (35%), p = 0.003], their outcomes were better. This observation substantiates that the treatment with obinutuzumab rather than the haematological condition is the leading risk factor.
Current evidence shows that maintenance treatment with anti‐CD20 mAB for follicular lymphoma (FL) increases progression‐free survival, but does not significantly impact overall survival rates. 5 , 9 , 10 In light of our findings, we raise the question whether clinicians should withhold maintenance treatment with obinutuzumab during the current pandemic.
Another critical issue that should be addressed is the underutilisation of tixagevimab–cilgavimab and antiviral medications, as seen by meagre uptake rates in our cohort (Table 1).
Limitations to our study include its single‐centre, retrospective design with no routine serial SARS‐CoV‐2 testing, raising the possibility that some infections may have been missed. However, since we recommend all HM patients to be tested when symptomatic and a mandatory screen is performed upon admission to the hospital, we assume the number of missed diagnoses was not substantial. Additionally, the limited number of observed outcomes did not allow for multivariable analysis to examine the association between treatment with obinutuzumab or rituximab and mortality.
In conclusion, we observed worse clinical outcomes during Omicron VOC infection in HM patients treated with obinutuzumab. As this treatment has not been shown to increase overall survival when given as maintenance, it may be prudent to defer treatment with obinutuzumab or replace it with a less potent anti‐CD20 for the duration of the COVID‐19 pandemic.
AUTHOR CONTRIBUTIONS
Study conceptualisation: Tali Shafat, Lior Nesher, Daniel Grupel and Yael Yagel. Data curation, methodology: Tali Shafat, Daniel Grupel and Tzvika Porges. Formal analysis: Tali Shafat. Writing – original draft: Tali Shafat, Daniel Grupel and Tzvika Porges; Writing – review and editing: Yael Yagel, Itai Levi and Lior Nesher.
FUNDING INFORMATION
No funding has been sourced for the performance of the current study.
CONFLICT OF INTEREST
All the authors confirm that they have no financial conflict of interest with regard to publication of this manuscript.
Supporting information
Table S1
Table S2
Table S3
Tali Shafat and Daniel Grupel contributed equally to the study.
DATA AVAILABILITY STATEMENT
The data collected for this study will be made available by the corresponding author upon reasonable request.
REFERENCES
- 1. Pagano L, Salmanton‐García J, Marchesi F, Busca A, Corradini P, Hoenigl M, et al. COVID‐19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):1–15. 10.1186/s13045-021-01177-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calderón‐Parra J, Múñez‐Rubio E, Fernández‐Cruz A, García‐Sánchez MC, Maderuelo‐González E, López‐Dosil M, et al. Incidence, clinical presentation, relapses and outcome of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) infection in patients treated with Anti‐CD20 monoclonal antibodies. Clin Infect Dis. 2021;74:1786–94. [DOI] [PubMed] [Google Scholar]
- 3. Kow CS, Hasan SS. Use of rituximab and the risk of adverse clinical outcomes in COVID‐19 patients with systemic rheumatic disease. Rheumatol Int. 2020;40(12):2117–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freeman CL, Sehn LH. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182(1):29–45. [DOI] [PubMed] [Google Scholar]
- 5. Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the first‐line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331–44. [DOI] [PubMed] [Google Scholar]
- 6. WHO Weekly epidemiological update on COVID‐19 ‐ 18 January 2022 Edition 75. [cited 2022 May 9]. Available from: https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐‐‐18‐january‐2022
- 7. European Centre for Disease Prevention and Control . Assessment of the further spread and potential impact of the SARS‐CoV‐2 Omicron variant of concern in the EU/EEA, 19th update ‐ 27 January 2022. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/en/publications‐data/covid‐19‐omicron‐risk‐assessment‐further‐emergence‐and‐potential‐impact [Google Scholar]
- 8. Saidel‐Odes L, Shafat T, Nativ R, Borer A, Nesher L. SARS‐CoV‐2 universal screening upon adult hospital admission in Southern Israel. J Hosp Infect. 2021;114:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salles G, Seymour JF, Offner F, López‐Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet (London, England). 2011;377(9759):42–51. [DOI] [PubMed] [Google Scholar]
- 10. Hill BT, Nastoupil L, Winter AM, Becnel MR, Cerhan JR, Habermann TM, et al. Maintenance rituximab or observation after frontline treatment with bendamustine‐rituximab for follicular lymphoma. Br J Haematol. 2019;184(4):524–35. 10.1111/bjh.15720 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
The data collected for this study will be made available by the corresponding author upon reasonable request.


