Abstract
Purpose
The COVID‐19 pandemic had an impact on health care, with disruption to routine clinical care. Our aim was to describe changes in prescription drugs dispensing in the primary and outpatient sectors during the first year of the pandemic across Europe.
Methods
We used routine administrative data on dispensed medicines in eight European countries (five whole countries, three represented by one region each) from January 2017 to March 2021 to compare the first year of the COVID‐19 pandemic with the preceding 3 years.
Results
In the 10 therapeutic subgroups with the highest dispensed volumes across all countries/regions the relative changes between the COVID‐19 period and the year before were mostly of a magnitude similar to changes between previous periods. However, for drugs for obstructive airway diseases the changes in the COVID‐19 period were stronger in several countries/regions. In all countries/regions a decrease in dispensed DDDs of antibiotics for systemic use (from −39.4% in Romagna to −14.2% in Scotland) and nasal preparations (from −34.4% in Lithuania to −5.7% in Sweden) was observed. We observed a stockpiling effect in the total market in March 2020 in six countries/regions. In Czechia the observed increase was not significant and in Slovenia volumes increased only after the end of the first lockdown. We found an increase in average therapeutic quantity per pack dispensed, which, however, exceeded 5% only in Slovenia, Germany, and Czechia.
Conclusions
The findings from this first European cross‐national comparison show a substantial decrease in dispensed volumes of antibiotics for systemic use in all countries/regions. The results also indicate that the provision of medicines for common chronic conditions was mostly resilient to challenges faced during the pandemic. However, there were notable differences between the countries/regions for some therapeutic areas.
Keywords: COVID‐19, cross‐national comparison, DDD volume, drug utilization, pandemic, pharmacoepidemiology, stockpiling
Key Points
Dispensed volumes of systemic antibiotics and nasal preparations decreased in all countries/regions.
Provision of medicines for common chronic conditions was mostly resilient to challenges faced during the COVID‐19 pandemic.
Stockpiling of pharmaceuticals was observed typically in March 2020, except for Slovenia, where increases of DDD volume were observed only 2 months later.
Plain Language Summary
The COVID‐19 pandemic had an impact on many aspects of life including health care. We assumed that also drug prescribing would be affected. Our aim was to describe changes in prescription drug dispensing in the outpatient sector during the first year of the pandemic across Europe. We used large data sets on dispensed volumes of medicines and their pack sizes in eight European countries (five whole countries and three represented by one region each), comparing the first year of the COVID‐19 pandemic and the preceding 3 years. In all countries/regions we observed a substantial decrease in dispensed volumes of antibiotics for systemic use (i.e., usually oral antibiotics) and nasal preparations. In March 2020 we observed an increase in dispensed volume of medicines in all countries/regions but Slovenia, where this effect occurred only after the end of the lockdown connected to the first wave of the pandemic. Increases in average pack size dispensed were rather mild and exceeded 5% only in three of the countries/regions. Although the first year of the pandemic brought substantial changes in dispensed volumes of medicines, it is reassuring that medicines for treating common chronic conditions were usually little affected.
1. INTRODUCTION
The first cases of COVID‐19 were confirmed in Europe at the end of January 2020. In March 2020, the World Health Organization (WHO) declared a COVID‐19 pandemic and countries began to take measures to mitigate the spread of the pandemic. Many European countries introduced strict measures during March 2020, including physical distancing, wearing face masks, and limiting possibilities of social contacts, however, the nature of these measures varied substantially between and within countries. 1
The COVID‐19 pandemic has affected peoples' lives and behavior in many aspects and has also had a significant impact on health care. 2 , 3 Many planned health care examinations and procedures have been cancelled or postponed, as clinical staff were re‐allocated to health care services related to COVID‐19 and patients tried to limit their visits to physicians to avoid the risk of contracting the infection in healthcare facilities. In December 2020, the EU‐funded European Network to Advance Best practices & technoLogy on medication adherencE (ENABLE) COST Action conducted a survey in 39 European countries to assess barriers and facilitators for patients accessing their chronic medication during the pandemic. 4 , 5 The survey indicated significant disruption of chronic disease services, especially in countries with a greater number of COVID‐19 cases per 100 000 inhabitants, and a large variation between countries in measures taken to ensure adequate drug management for these patients.
We hypothesized that, like other areas of health care, the pandemic would affect also the patterns of drug prescribing and dispensing. Given that there were substantial differences between countries in measures to maintain medicines management during the pandemic, cross‐national comparisons of drug utilization patterns may add value as a tool to identify areas for improvement. The aim of this paper was to characterize and compare changes that took place in prescription drugs dispensing in primary care and the outpatient sector in several European countries or regions during the first year of the pandemic.
2. METHODS
2.1. Study design and data
We conducted a retrospective observational cross‐national comparative study. Participating countries and regions were Czechia, Germany, Romagna (Italy), Lithuania, Slovenia, Catalonia (Spain), Sweden, and Scotland (United Kingdom). For each country/region, we used dispensing data 6 from January 2017 to March 2021. Data covered all dispensed prescription medicines of the countries/regions and included the codes of the Anatomical‐Therapeutic‐Chemical classification (ATC) and corresponding numbers of dispensed defined daily doses (DDDs) and packs, both aggregated by month. We used the ATC classification of 2021, as published by the World Health Organization (WHO), 7 for the whole study period. The details on data sources and populations covered are in Table S1.
2.2. Data analysis
To describe changes in dispensed volumes of pharmaceuticals we used percentages. For the analysis of changes in pack sizes we employed index decomposition methods from economics. 8 , 9
To compare the drug consumption before the COVID‐19 pandemic and during the COVID‐19 pandemic, we used only data for four successive 12‐months periods from March 2017 to February 2021. We compared the drug consumption in the first 12‐month period with COVID‐19 with the three preceding 12‐month periods. To assess total market development on a monthly scale, we used monthly data for the whole period from January 2017 to March 2021. Data analysis was performed using R 4.1.0. 10
2.2.1. Definitions used
DDD/TID—number of dispensed DDDs 7 per thousand inhabitants per day.
Therapeutic quantity per pack—number of defined daily doses (DDDs) per pack; also referred to as pack size.
Period 1—From March 2017 to February 2018.
Period 2—From March 2018 to February 2019.
Period 3—From March 2019 to February 2020 (pre‐COVID period).
Period 4—From March 2020 to February 2021 (COVID period).
2.2.2. Choice of therapeutic drug subgroups for analysis
The first step was an analysis of therapeutic subgroups (ATC level 2). 7 We adopted two complementary approaches: firstly, we analyzed the therapeutic subgroups with the highest DDD volume; secondly, we analyzed the therapeutic subgroups with the most marked changes in DDD volume when comparing the COVID period with preceding periods.
For the purpose of comparing periods and countries/regions, we expressed the volume of the dispensed DDDs in each period as DDD/TID. We used the following equation:
v p : dispensed volume in period p [DDD/TID]. u m,p : dispensed volume in month m of period p [DDD]. d p : number of days in period p. n p : size of population covered in period p (see Table S1 for details).
To calculate relative changes in dispensed DDD/TID between consecutive 12‐month periods we used the following equation:
Δ p : relative change between periods p and p − 1.
2.2.3. Changes in dispensed DDDs—ATC groups with the highest dispensed volume
In order to identify changes in the COVID period compared to pre‐COVID times, we first focused on the 10 therapeutic subgroups with the highest dispensed DDD/TID volume (for details of the selection procedure see Figure S1). For these 10 groups and for each country/region separately, we then investigated changes in dispensed DDDs for the COVID period compared to the previous period. To guard against mistaking longer‐term trends for COVID‐related changes, we also checked changes in earlier periods.
2.2.4. Changes in dispensed DDDs—ATC groups with the most marked changes
Additionally, for each country, we determined the 10 therapeutic subgroups with the most marked changes. We excluded all therapeutic subgroups where dispensed DDD/TID in the pre‐COVID period were less than 0.1, because infrequently dispensed pharmaceuticals are more prone to high fluctuations which could be unrelated to COVID. (For further details of the selection procedure see Figure S2.) For each country/region and its 10 selected therapeutic subgroups, we identified the main volume contributors on ATC level 4 (chemical subgroups) and described their changes in volume.
2.2.5. Changes in dispensed DDDs—total market development on a monthly scale
To see the development of the total market during the COVID period in finer temporal resolution and to compare it to previous years, we used for each country the monthly volumes (in DDD/TID) of all pharmaceuticals. Because of seasonality and autocorrelation in the data we used ARIMA models to assess the impact of the pandemic. The first step was to estimate the noise in the time series using only data from January 2017 to February 2020. In step two we modelled the whole time series (January 2017 to March 2021), adding two events: one with immediate onset and permanent duration to model lasting influence of the pandemic, the other with immediate onset and short duration to capture the large fluctuations in the first 3 months of the pandemic. We used the Ljung‐Box test on the residuals to test for the presence of remaining autocorrelation. Stationarity and seasonal stationarity were tested with the Kwiatkowski‐Phillips‐Schmidt‐Shin test and the Hylleberg‐Engle‐Granger‐Yoo test respectively. Statistical significance of coefficients in the models was tested using the z‐test. Tests were performed at the 0.05 significance level.
2.2.6. Changes in dispensed therapeutic quantity per pack
The average therapeutic quantity per pack is calculated as the ratio of number of dispensed DDDs and number of dispensed packs. Changes in this average pack size are influenced both by changes in the number of units or the dosage strengths of the units dispensed and by changes in the market mix, because some therapeutic areas typically use bigger pack sizes than others. For instance, the pack size of pharmaceuticals typically prescribed for antidiabetic treatment is greater than in antibiotic therapy. Thus any reduction in antibiotic dispensing would lead to an increase of average pack size reported for the total market even if average pack size dispensed within antibiotic therapy and also average pack size within diabetic therapy remained unchanged. In order to account for this fact we employed a concept of index theory from economics. 8 , 9 We explain the relative change in average pack size Δu as a combined effect of shifts in average dispensed pack sizes Δv within the therapeutic subgroups and of structural changes in the market Δs, i.e., shifts in the market shares of those subgroups, where Δu = Δv · Δs. (See Supplement, section Changes in therapeutic quantity per pack, for more details.)
In order to concentrate on longer‐lasting effects, we compared pack sizes dispensed during the second half of the COVID period (September 2020–February 2021) with the values from the corresponding period one year before (September 2019–February 2020), just before the start of the pandemic. To assess possible longer‐term trends, we also obtained the corresponding values for the same months in 2017/18 and 2018/19. The analysis was performed on ATC level 2. In order to check stability with respect to the choice of ATC level, we also performed the calculations on ATC level 4.
3. RESULTS
3.1. Changes in dispensed DDDs
3.1.1. Changes in dispensed DDDs between the pre‐COVID period and the COVID period—ATC groups with the highest dispensed volume in the pre‐COVID period
The 10 therapeutic groups with the highest dispensed volumes in the pre‐COVID period (measured by DDD/TID) across all countries/regions were drugs for acid related disorders (A02), antidiabetics (A10), antithrombotics (B01), antianemic preparations (B03), beta‐blockers (C07), calcium channel blockers (C08), ACE inhibitors/ARBs (C09), lipid modifying agents (C10), psychoanaleptics (N06) and drugs for asthma/COPD (R03). The relative changes between the pre‐COVID period and the COVID period in all these therapeutic subgroups (apart from antiasthmatics) were mostly of a magnitude similar to the changes between previous periods (Figure 1). For antiasthmatics/COPD‐drugs, more fluctuating patterns were observed. In Catalonia, Romagna, and Slovenia the dispensed DDD volume decreased, whereas in Scotland and Sweden we observed a slight increase and in the rest of the countries/regions the volumes stayed approximately on the same level (Figure 1). For more detail see Table S3. For a comparison of first and second half year of the pandemic see Figures S3 and S4.
FIGURE 1.
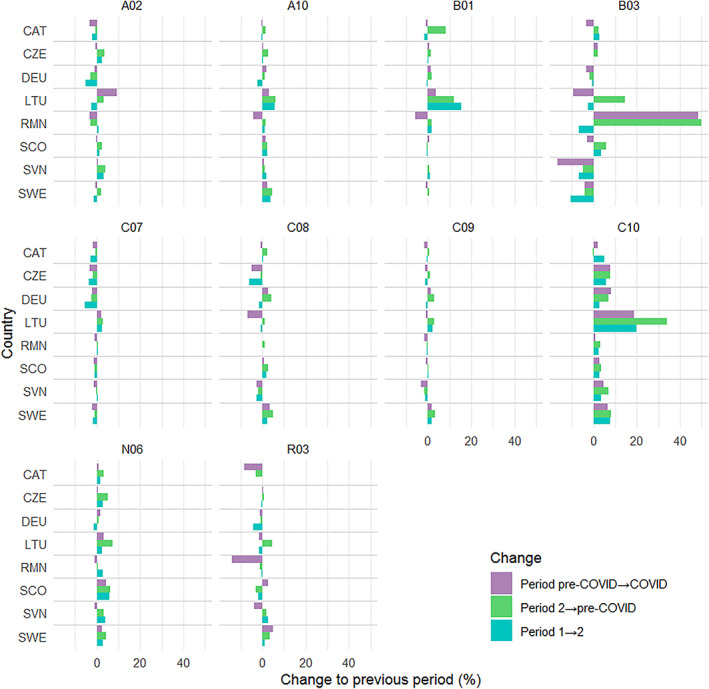
Top 10 therapeutic subgroups with highest dispensed volume (in the pre‐COVID period) across eight European countries/regions: relative change of defined daily doses per 1000 inhabitants per day (DDD/TID) between four 12‐month periods March 2017 to February 2021. Periods: Period 1, March 2017—February 2018; Period 2, March 2018—February 2019; Pre‐COVID period, March 2019—February 2020; COVID period, March 2020—February 2021. Country codes: CAT, Catalonia; CZE, Czechia; DEU, Germany; LTU, Lithuania; RMN; Romagna; SCO, Scotland; SVN, Slovenia; SWE, Sweden. Therapeutic subgroups: A02, drugs for acid related disorders; A10, drugs used in diabetes; B01, antithrombotic agents; B03, antianemic preparations; C07, beta blocking agents; C08, calcium channel blockers; C09, agents acting on the renin‐angiotensin system; C10, lipid modifying agents; N06, psychoanaleptics; R03, drugs for obstructive airway diseases
3.1.2. Changes in dispensed DDDs between the pre‐COVID period and the COVID period—ATC groups with the most marked changes compared to the pre‐COVID period
The 10 therapeutic subgroups with the greatest relative changes (i.e., | Δ p |) between the pre‐COVID period and the COVID period for each participating country/region are shown in Figure 2. The main contributors on chemical (ATC 4th level) subgroups which together cover at least 2/3 of the DDD volume for the therapeutic (ATC 2nd level) subgroups are shown in Tables S4–S11.
FIGURE 2.
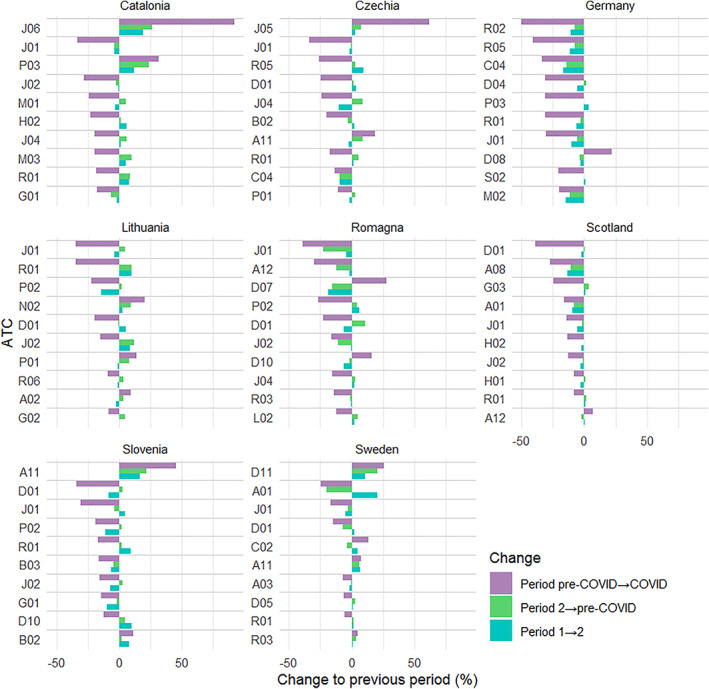
Top 10 therapeutic subgroups with most marked relative change in defined daily doses per 1000 inhabitants per day (DDD/TID) volume (in the COVID period relative to the pre‐COVID period) for eight European countries/regions between four 12‐month periods within March 2017 to February 2021. (Only changes that were larger for the COVID period than for any period before the onset of COVID‐19 are shown. Therapeutic subgroups with DDD/TID ≤0.1 were excluded.) Periods: Period 1, March 2017—February 2018; Period 2, March 2018—February 2019; Pre‐COVID period, March 2019—February 2020; COVID period, March 2020—February 2021. Country codes: CAT, Catalonia; CZE, Czechia; DEU, Germany; LTU, Lithuania; RMN, Romagna; SCO, Scotland; SVN, Slovenia; SWE, Sweden. Therapeutic subgroups: A01, stomatological preparations; A02, drugs for acid related disorders; A03, drugs for functional gastrointestinal disorders; A08, antiobesity preparations, excl. diet products; A11, vitamins; A12, mineral supplements; B02, antihemorrhagics; B03, antianemic preparations; C02, antihypertensives; C04, peripheral vasodilators; D01, antifungals for dermatological use; D04, antipruritics, incl. antihistamines, anesthetics, etc.; D05, antipsoriatics; D07, corticosteroids, dermatological preparations; D08, antiseptics and disinfectants; D10, anti‐acne preparations; D11, other dermatological preparations; G01, gynecological antiinfectives and antiseptics; G02, other gynecologicals; G03, sex hormones and modulators of the genital system; H01, pituitary and hypothalamic hormones and analogues; H02, corticosteroids for systemic use; J01, antibacterials for systemic use; J02, antimycotics for systemic use; J04, antimycobacterials; J05, antivirals for systemic use; J06, immune sera and immunoglobulins; L02, endocrine therapy; M01, antiinflammatory and antirheumatic products; M02, topical products for joint and muscular pain; M03, muscle relaxants; N02, analgesics; P01, antiprotozoals; P02, anthelmintics; P03, ectoparasiticides, incl. scabicides, insecticides and repellents; R01, nasal preparations; R02, throat preparations; R03, drugs for obstructive airway diseases; R05, cough and cold preparations; R06, antihistamines for systemic use; S02, otologicals.
In all countries/regions, systemic antibiotics (J01) were among the top 10 therapeutic subgroups, and in all countries/regions their volume decreased. All subgroups of systemic antibiotics decreased in the COVID period compared to the pre‐COVID period, except for tetracyclines (J01AA) in Lithuania with 0.9% increase of DDD/TID and third generation cephalosporins (J01DD) in Czechia, where DDD/TID increased by 52%, however it accounted for only 0.7% of the J01 DDD volume in the pre‐COVID period.
Also nasal preparations (R01) belonged to the top 10 therapeutic subgroups with the greatest changes in volume in almost all countries/regions. The only exception was Romagna. In all countries/regions including Romagna the volumes decreased.
The third most frequently appearing group in the list of groups with most marked changes were the antimycotics for systemic use (J02). It reached the top 10 in five countries/regions (Catalonia, Romagna, Lithuania, Scotland, and Slovenia). In all cases the volumes decreased during the COVID period. Volume also decreased in Sweden, Germany and Czechia, although in Germany and Czechia the decrease was only approximately 2% and did not exceed changes seen in previous periods. Overall, there was a high variability in the top 10 therapeutic subgroups with most marked changes in volume among countries/regions.
For a comparison of first and second half year of the pandemic see Figures S5 and S6.
3.1.3. Changes in dispensed DDDs—total market development on a monthly scale
In Germany, Lithuania, Scotland, and Sweden, we observed a marked increase in March 2020 followed by a decrease which in April and/or May 2020 went below the levels in previous years. In Romagna and Catalonia this pattern was weaker. In Czechia there were hints of a similar pattern too, however in this country a strong quarterly rhythm could be observed in previous years. In Slovenia, unlike in other countries/regions, there was a decrease in March and April 2020, followed by a peak in May 2020, followed by a return to the level of 2019 in July (Figure 3). In all countries/regions but in Czechia taking into account the events with immediate effect and short duration (see Methods) significantly improved the fit of the model, whereas the durable impact of the pandemic was significant only in Catalonia and Romagna. For more detail see Table S12 and Figures S7 and S8.
FIGURE 3.
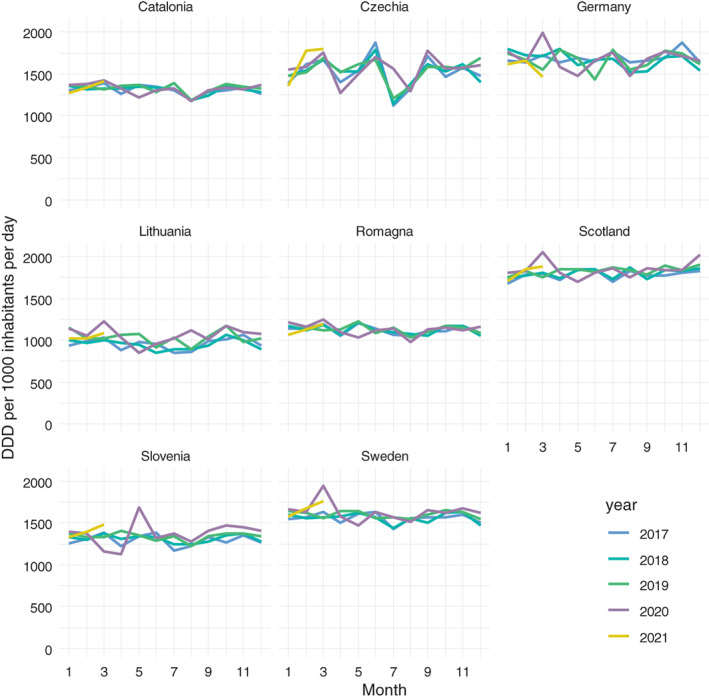
Total market development in defined daily doses per 1000 inhabitants per day (DDD/TID) on a monthly scale for eight European countries/regions, January 2017 to March 2021.
3.2. Changes in average number of DDDs per pack
3.2.1. Analysis for therapeutic (ATC 2nd level) subgroups
In the 6 months immediately preceding the pandemic, the increases in the average therapeutic quantity per pack dispensed were between +0.5 and +3.1% in all the countries/regions, with the exception of Romagna at +4.3% (Table 1). During the pandemic, the increases were below +2.5% in four countries/regions. The lowest value of these, 0.0% (no increase), was recorded for Lithuania, where a shift towards smaller pack sizes (−1.6%) was offset by a positive structural shift of the same magnitude.
TABLE 1.
Relative change (%) in average therapeutic quantity per pack (Δu) broken down to changes of average therapeutic quantity per pauck within therapeutic subgroups (Δv) and change of market shares of the therapeutic subgroups (Δs) for eight European countries/regions, September to February in the years 2017 to 2021; therapeutic subgroups at ATC level 2.
| Base period | 9/2017–2/2018 | 9/2018–2/2019 | 9/2019–2/2020 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Period under review | 9/2018–2/2019 | 9/2019–2/2020 | 9/2020–2/2021 | ||||||
| Country | Δv | Δs | Δu | Δv | Δs | Δu | Δv | Δs | Δu |
| Catalonia | 1.013 | 1.001 | 1.014 | 1.006 | 0.998 | 1.004 | 1.014 | 1.010 | 1.024 |
| Czechia | 1.015 | 0.996 | 1.011 | 1.021 | 1.005 | 1.027 | 1.043 | 1.014 | 1.058 |
| Germany | 1.004 | 1.012 | 1.016 | 1.006 | 1.008 | 1.014 | 1.019 | 1.041 | 1.060 |
| Lithuania | 1.001 | 1.009 | 1.010 | 1.012 | 0.999 | 1.011 | 0.984 | 1.016 | 1.000 |
| Romagna | 1.003 | 1.001 | 1.005 | 1.028 | 1.014 | 1.043 | 1.031 | 1.016 | 1.047 |
| Scotland | 1.005 | 1.008 | 1.013 | 0.997 | 1.008 | 1.004 | 1.009 | 1.013 | 1.022 |
| Slovenia | 1.019 | 0.999 | 1.018 | 1.025 | 1.006 | 1.031 | 1.060 | 1.016 | 1.077 |
| Sweden | 0.998 | 1.008 | 1.006 | 1.010 | 1.003 | 1.012 | 1.008 | 1.009 | 1.017 |
Notes: Δv, relative change in average therapeutic quantity per pack within therapeutic subgroups; Δs, structural changes in the market; Δu, combined total effect.
The other four countries/regions showed increases of between +4.7 and +7.7%. Most of these increases were due to shifts to higher numbers of DDDs per pack within the therapeutic subgroups (Czechia +4.3%, Romagna +3.1%, Slovenia +6.0%), whereas in Germany such shifts accounted for only +1.9%. Here the structural changes in the market contributed +4.1% to the total increase in average pack size.
3.2.2. Analysis for chemical (ATC 4th level) subgroups
Results were similar to those observed for therapeutic subgroups. In the majority of countries, shifts between the chemical subgroups were slightly stronger than between therapeutic subgroups, except for Slovenia, where the shifts towards bigger packs within subgroups were even stronger than at a higher ATC‐level. (See Table S13)
4. DISCUSSION
In this cross‐national comparative study including eight European countries/regions we assessed utilization patterns of all prescription drugs before and during the first year of the COVID‐19 pandemic. We found limited impact of the pandemic on the most commonly used prescription drugs in all countries/regions, but there were some differences between countries/regions in trends of certain pharmaceuticals.
The small changes observed in the 10 groups with the highest dispensed volume suggest that drug usage for the most common chronic diseases was little affected by the pandemic. With few exceptions, the change in volume of these drugs followed trends seen in previous years. This holds particularly for the cardiovascular diseases and diabetes drugs, which is compatible with the findings of Carr et al. 11 It is positive that we found no substantial decrease in utilization of these drugs in any country. However, it is important to acknowledge that there may still be problems of underuse of these agents. Diabetes and hypertension have both been found to be strong independent risk factors for severe COVID‐19, 12 and there is an increasing number of studies showing the beneficial effects of treatment for diabetes and cardiovascular diseases in the prognosis and outcome of COVID‐19. 13 , 14 , 15 , 16
A partial exception to these trends are drugs used in the treatment of COPD and asthma. Several countries/regions experienced a decrease in the use of these medicines, which might be connected to less exposure to exacerbating factors due to lockdowns and similar measures. Notably, Sweden as the only country without any formal lockdown during the reporting period showed a small increase in the dispensed volume of these drugs.
There have been discussions about the impact of the pandemic on mental health. Although there is some evidence for increased loneliness, anxiety, stress, and depression, 17 , 18 , 19 , 20 we found no strong reflection of this on the dispensed volumes of either psycholeptic or psychoanaleptic drugs (see Figure S9).
In all participating countries/regions, we noted a decrease in dispensed volume of antibiotics and nasal preparations. The observed decrease in dispensed volume of antibiotics was in line with other studies. 21 , 22 , 23 , 24 We assume that lower need of these pharmaceuticals in the COVID period might be a consequence of lockdown and other forms of social distancing, resulting in fewer occasions for transmitting infections.
In general, the largest decreases were observed in therapeutic subgroups used predominantly for non‐life‐threatening conditions. This suggests a rational approach by patients and/or physicians to the minimisation of contacts during the pandemic: postponing treatment of less serious conditions while maintaining vital treatment regimes.
A number of countries/regions showed a marked increase of the overall dispensed volume right at the beginning of the pandemic, followed by a notable decrease. We surmise that this is a case of stockpiling, 25 as was also seen for a number of other (non‐pharmaceutical) goods. 26 , 27 In times of uncertainty over the imminent future, drugs for which the need was known or possible to plan were prescribed and redeemed in bigger quantities than usual, and were consequently used over subsequent periods. Again, this is compatible with rational behaviour in two respects: firstly, this safeguarded the individual against shortages, and secondly, it obviated the need for visits to physicians' offices with the risk of catching a COVID infection.
Interestingly, Slovenia showed the opposite development. There was a substantial decrease during March and April 2020, followed by a peak in May. Slovenian patients apparently also avoided visits to physicians for receiving renewal prescriptions, but trusted the system to guarantee continued supply. After the lockdown terminated, depleted stocks were refilled.
For the beginning of the second wave of the COVID‐19 pandemic at the break of summer and autumn 2020 we did not observe stockpiling patterns, which suggests a calmer approach by patients based on the experience gained during the first wave.
4.1. Strengths and limitations
To the best of our knowledge, this is the first cross‐national study of the trends of dispensed prescriptions through the pandemic. It uses large administrative databases with complete data on all dispensed prescription medicines in the participating countries resp. regions. Thus, it allows identification of both common trends and differences between countries/regions.
The major limitation is that we investigated only medicines dispensed in ambulatory care. The data included both dispensed prescriptions from general practice and secondary care specialists, but there were no inpatient utilization data assessed, and this may have varied between countries/regions. Secondly, we had to restrict the cross‐national comparison to rates of change. A comparison of absolute values of dispensed volumes was not possible due to different national rules for reimbursement in some therapeutic areas. Thirdly, we used aggregated data and could, consequently, not assess if any changes in prevalence, incidence or discontinuation had taken place for the specific drug groups. For this, further research and more detailed analysis on the patient level would be needed. Fourthly, we cannot make causal attribution of changes in the COVID period to the pandemic because we cannot exclude concomitant factors. Finally, it is important to acknowledge that it was not possible to assess the impact of lock‐down and other measures given that these varied substantially in nature and across time, both between and within countries/regions.
5. CONCLUSIONS
In this study we assessed utilization patterns of all prescription medicines before and during the first year of the COVID‐19 pandemic in eight European countries/regions. We have identified a number of patterns common to all the countries/regions. In particular, we observed a substantial decrease in dispensed volumes of antibiotics for systemic use. It is reassuring that medicines from therapeutic subgroups used for treating common chronic conditions were usually little affected, which suggests that there was no under‐supply of these medicines. However, there were also notable differences between the countries/regions for some therapeutic areas, which may reflect different approaches by physicians and/or patients to the pandemic situation.
AUTHOR CONTRIBUTIONS
Iva Selke Krulichová and Gisbert W. Selke conceptualized the study with support from Björn Wettermark and Marion Bennie. Gisbert W. Selke and Iva Selke Krulichová analysed the data. Iva Selke Krulichová drafted the manuscript with support from Gisbert W. Selke, Björn Wettermark, Marion Bennie, Mohammadhossein Hajiebrahimi, and Fredrik Nyberg. Corinne Zara Yahni, Caridad Pontes and Marta Turu Pedrola provided data on Catalonia. Juraj Slabý and Václav Kočí provided data on Czechia. Gisbert W. Selke provided data on Germany. Kristina Garuolienė provided data on Lithuania. Elisabetta Poluzzi, Chiara Reno, Simona Rosa, and Mattia Altini provided data on Romagna. Marion Bennie and Stuart McTaggart provided data on Scotland. Jurij Fürst and Mitja Udovič provided data on Slovenia. Björn Wettermark provided data on Sweden. All authors participated in critical revision of the manuscript, contributed comments, and approved the final version.
FUNDING INFORMATION
This study was supported in part by COOPERATIO Program (Charles University), Research Area Medical Diagnostics and Basic Medical Sciences. Mohammadhossein Hajiebrahimi received funding from NordForsk.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENT
The authors thank Maria Juhasz Haverinen from Stockholm County Council for her help with providing the Swedish data.
Selke Krulichová I, Selke GW, Bennie M, et al. Comparison of drug prescribing before and during the COVID‐19 pandemic: A cross‐national European study. Pharmacoepidemiol Drug Saf. 2022;31(10):1046‐1055. doi: 10.1002/pds.5509
Funding information NordForsk; Univerzita Karlova v Praze; Stockholm County Council
DATA AVAILABILITY STATEMENT
The data and code that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.5912080.
REFERENCES
- 1. European Centre for Disease Prevention and Control . Data on country response measures to COVID‐19. https://www.ecdc.europa.eu/en/publications-data/download-data-response-measures-covid-19. Accessed 11 December, 2021.
- 2. Chang AY, Cullen MR, Harrington RA, Barry M. The impact of novel coronavirus COVID‐19 on noncommunicable disease patients and health systems: a review. J Intern Med. 2021;289:450‐462. doi: 10.1111/joim.13184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong SYS, Zhang D, Sit RWS, et al. Impact of COVID‐19 on loneliness, mental health, and health service utilisation: a prospective cohort study of older adults with multimorbidity in primary care. Br J Gen Pract. 2020;70:e817‐e824. doi: 10.3399/bjgp20X713021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ENABLE Collaborators , Kardas P, van Boven JFM, et al. Disparities in European healthcare system approaches to maintaining continuity of medication for non‐communicable diseases during the COVID‐19 outbreak. Lancet Regional Health. 2021;4:4. doi: 10.1016/j.lanepe.2021.100099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ágh T, van Boven JF, Wettermark B, et al. A cross‐sectional survey on medication management practices for noncommunicable diseases in Europe during the second wave of the COVID‐19 pandemic. Front Pharmacol. 2021;12. doi: 10.3389/fphar.2021.685696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selke Krulichová I, Selke GW, Bennie M, et al. Comparison of drug prescribing before and during the COVID‐19 pandemic: A cross‐national European study. 2022. doi: 10.5281/zenodo.5912080 [DOI] [PMC free article] [PubMed]
- 7. WHOCC ‐ ATC/DDD Index . https://www.whocc.no/atc_ddd_index/. Accessed 12 December, 2021.
- 8. Andersson O. Indexzahlen. In: Albers W, ed. Handwörterbuch der Wirtschaftswissenschaft. Vol 4. Gustav Fischer; 1978:98‐108. [Google Scholar]
- 9. Reichelt H. Eine Methode der statistischen Komponentenzerlegung. Wissenschaftliches Institut der Ortskrankenkassen; 1988. [Google Scholar]
- 10. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 11. Carr MJ, Wright AK, Leelarathna L, et al. Impact of COVID‐19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: a UK‐wide cohort study involving 618 161 people in primary care. BMJ Qual Saf. 2021;31:503‐514. doi: 10.1136/bmjqs-2021-013613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fishkin T, Goldberg MD, Frishman WH. Review of the metabolic risk factors for increased severity of coronavirus disease‐2019. Cardiol Rev. 2021;29:292‐295. doi: 10.1097/crd.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chow R, Im J, Chiu N, et al. The protective association between statins use and adverse outcomes among COVID‐19 patients: a systematic review and meta‐analysis. PLoS One. 2021;16:e0253576. doi: 10.1371/journal.pone.0253576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baral R, Tsampasian V, Debski M, et al. Association between renin‐angiotensin‐aldosterone system inhibitors and clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. JAMA Netw Open. 2021;4:e213594. doi: 10.1001/jamanetworkopen.2021.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luk AOY, Yip TCF, Zhang X, et al. Glucose‐lowering drugs and outcome from COVID‐19 among patients with type 2 diabetes mellitus: a population‐wide analysis in Hong Kong. BMJ Open. 2021;11:e052310. doi: 10.1136/bmjopen-2021-052310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berlie HD, Kale‐Pradhan PB, Orzechowski T, Jaber LA. Mechanisms and potential roles of glucose‐lowering agents in COVID‐19: a review. Ann Pharmacother. 2021;55:1386‐1396. doi: 10.1177/1060028021999473 [DOI] [PubMed] [Google Scholar]
- 17. McQuaid RJ, Cox SML, Ogunlana A, Jaworska N. The burden of loneliness: implications of the social determinants of health during COVID‐19. Psychiatry Res. 2021;296:113648. doi: 10.1016/j.psychres.2020.113648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palgi Y, Shrira A, Ring L, et al. The loneliness pandemic: loneliness and other concomitants of depression, anxiety and their comorbidity during the COVID‐19 outbreak. J Affect Disord. 2020;275:109‐111. doi: 10.1016/j.jad.2020.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okruszek Ł, Aniszewska‐Stańczuk A, Piejka A, Wiśniewska M, Żurek K. Safe but lonely? Loneliness, anxiety, and depression symptoms and COVID‐19. Front Psychol. 2020;11:3222. doi: 10.3389/fpsyg.2020.579181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID‐19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55‐64. doi: 10.1016/j.jad.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Pol AC, Boeijen JA, Venekamp RP, et al. Impact of the COVID‐19 pandemic on antibiotic prescribing for common infections in The Netherlands: a primary care‐based observational cohort study. Antibiotics. 2021;10:196. doi: 10.3390/antibiotics10020196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussain AZ, Paudyal V, Hadi MA. Impact of the COVID‐19 pandemic on the prescribing patterns of first‐line antibiotics in English primary care: a longitudinal analysis of National Prescribing Dataset. Antibiotics. 2021;10:591. doi: 10.3390/antibiotics10050591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. King LM, Lovegrove MC, Shehab N, et al. Trends in US outpatient antibiotic prescriptions during the coronavirus disease 2019 pandemic. Clin Infect Dis. 2021;73:e652‐e660. doi: 10.1093/cid/ciaa1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Högberg LD, Vlahović‐Palčevski V, Pereira C, Weist K, Monnet DL, ESAC‐Net Study Group . Decrease in community antibiotic consumption during the COVID‐19 pandemic, EU/EEA, 2020. Eurosurveillance. 2021;26(46):26, 2101020. doi: 10.2807/1560-7917.ES.2021.26.46.2101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karlsson P, Nakitanda AO, Löfling L, Cesta CE. Patterns of prescription dispensation and over‐the‐counter medication sales in Sweden during the COVID‐19 pandemic. PLoS One. 2021;16:e0253944. doi: 10.1371/journal.pone.0253944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lehberger M, Kleih A‐K, Sparke K. Panic buying in times of coronavirus (COVID‐19): extending the theory of planned behavior to understand the stockpiling of nonperishable food in Germany. Appetite. 2021;161:105118. doi: 10.1016/j.appet.2021.105118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Micalizzi L, Zambrotta NS, Bernstein MH. Stockpiling in the time of COVID‐19. Br J Health Psychol. 2021;26:535‐543. doi: 10.1111/bjhp.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data and code that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.5912080.


