Abstract
COVID‐19 is arguably the biggest health crisis the world has faced in the 21st century. Therefore, two of the polyherbal formulations, Infuza and Kulzam were assessed for the prevention of COVID‐19 infection as a repurposed medication. Four hundred seven high‐risk subjects were recruited in the present open‐label randomized controlled clinical trial for eligibility. After assessment for eligibility, remaining 251 subjects were randomized to the test and control groups. Further, 52 high‐risk subjects in Infuza, 51 in Kulzam, 51 in Infuza & Kulzam and 53 in control group completed the 14 days of intervention/assessment. The phenotyping of lymphocytes at baseline (0 day) and after 14 days of treatment was carried out by flow cytometry assays. A total of 15.09% high‐risk subjects in control group turned positive as compared to only 7.69% in Infuza, 3.92% in Kulzam and 1.96% in Infuza & Kulzam groups. The rate of conversion to COVID‐19 infection in Infuza & Kulzam group was minimal and statistically significant as compared to control group (p0.017). No significant changes in phenotype of lymphocytes (T, B, NK cells), absolute lymphocyte count and cytokine levels were found in study groups. However, there was a decreasing trend of hs‐CRP level in high‐risk subjects after intervention of polyherbal formulations for 14 days. The combination of Infuza and Kulzam may synergistically prevent COVID‐19 infection in high‐risk subjects of COVID‐19.
Keywords: 2019 novel coronavirus disease, clinical trial, inflammation, Infuza, Kulzam, phytochemicals, phytotherapy
Abbreviations
- CD
cluster of differentiation
- COVID‐19
Coronavirus disease
- GC
gas chromatography
- hs‐CRP
high sensitivity‐c reactive protein
- TLC
thin layer chromatography
1. INTRODUCTION
Amid October 2021, World reported above 236 million novel coronavirus disease (COVID‐19) cases and the total official count of deaths was over 4 million (World Health Organization., 2021). Multiple variants of SARS‐CoV 2 are circulating worldwide including India. The Variant of Concern (VOC) B.1.17 (United Kingdom), Brazilian P.1 and South African B.1.351 strains have been reported from different parts of the country. The variants differ in terms of transmissibility and disease severity. The B.1.617 variant, also called a double mutant, has been attributed to the second wave that ravaged India since March 2021 (World Health Organization, 2021a).
The cornerstone in the fight against COVID‐19 is the prevention of its infection. Along with social distancing, masks, other protective gears, surface disinfection and mass vaccination, many drugs have also been tried as preventive medication. Few formulations have been suggested and repurposed as prophylactic options for COVID‐19 (Salvi & Patankar, 2020). Anti‐parasitic drugs like hydroxychloroquine and ivermectin were used for this purpose extensively. Later multiple clinical trials found no or very limited role of hydroxychloroquine in prevention and cure of COVID‐19 (Cohen, 2020; Jorge, 2020). Similarly, efficacy reports of ivermectin are still inconclusive (World Health Organization, 2021b). Known antivirals like acyclovir, amprenavir, baloxavir marboxil, darunavir, entecavir, Tipranavir, Umifenovir, Zanamivir, and so forth and anti‐parasitic like amodiaquine are under drug repurposing clinical trials (Andrade et al., 2020). Vaccination, the best solution to control this pandemic has its limitation and challenges (Richman, 2021). Production and administration of vaccine dosages for a hugely populated country like India will take long time. Duration of protection provided by vaccine and escape potential of rapidly emerging variants are important question marks and matters of concern (Forman, Shah, Jeurissen, Jit, & Mossialos, 2021).
Traditional medicine has proven its utility over the centuries in the treatment of wide range of diseases including infectious ones (Ma et al., 2019). Polyherbal formulations are the potential candidates for the prevention of COVID‐19 infection due to their immunomodulatory and other pharmacological effects (Saboo, 2021). Glycyrrhiza glabra (Mulethi), Mentha arvensis (Pudina), Trachyspermum ammi (Ajwain), Cinnamumum camphor (Kafoor), Syzygium aromaticum (Laung), Nigella sativa (Kalaunji), Tinospora cordifolia (Gilo), Cinnamomum zeylanicu (Dalchini), Eucalyptus globulus are listed in Indian traditional system of medicines and have established for antiviral, anti‐inflammatory, strong decongestant, anti‐tussive, bronchodilatory, analgesic and immunomodulatory activities (GOI, 2016; Government of India, 2009; Hakeem Najmul Gani Rampuri, 2009; Hasan, Ara, Mondal, & Kabir, 2021; Ministry of AYUSH, 2009, n.d.; Unani Pharmacopeia committee. Government of India, 2007). Infuza and Kulzam are the proprietary polyherbal formulations of M/S Hamdard Laboratories (Medicine Division) (Licence number U‐212/78 and U‐312/78, respectively) that include many ingredients from these herbal plants and therefore, have a potential for prevention of COVID‐19 infection. In absence of any proven and efficient preventive medication for COVID‐19 and limitations of vaccine effectiveness, the present study attempted to elucidate the role of Infuza and Kulzam polyherbal formulations for prevention of COVID‐19 infection in high‐risk subjects as a repurposed medication.
2. MATERIAL AND METHODS
Polyherbal formulations, that is, Infuza and Kulzam of M/S Hamdard Laboratories, India, which are already in use and prescribed for the management of common cold, headache and epidemic flu for decades were selected for the present study to repurpose their use in prevention of COVID‐19 infection. The compositions of the polyherbal formulations (Infuza and Kulzam) are given in Table 1.
TABLE 1.
Composition of investigational polyherbal formulations, that is, Infuza and Kulzam
| S.no. | Common name | Scientific name | Part used | Quantity |
|---|---|---|---|---|
| Infuza polyherbal formulation (each 2.5 ml contains): | ||||
| 1 | Rubb‐e‐Mulethi | Glycerrhiza glabra | Rhizome extract | 1.550 g |
| 2 | Sat‐e‐Gilo | Tinospora cordifolia | Stem decoction | 0.200 g |
| 3 | Naushadar | Ammonium chloride | 0.390 g | |
| 4 | Sat‐e‐Ajwain | Trachyspermum ammi | Crystal | 0.500 mg |
| 5 | Roghan Laung | Eugenia caryophyllata | Oil | 0.075 ml |
| 6 | Roghan Kalaunji | Nigella sativa | Oil | 0.100 ml |
| 7 | Roghan Dalchini | Cinnamomum zeylanicum | Oil | 0.075 ml |
| Purified water | q.s. | |||
| Kulzam polyherbal formulation (each 1 ml contains): | ||||
| 1 | Sat‐e‐Pudina | Mentha gravis | Crystal | 80 mg |
| 2 | Sat‐e‐Ajwain | Trachyspermum ammi | Crystal | 150 mg |
| 3 | Kafoor | Camphor | Crystal | 300 mg |
| 4 | Roghan Asfidar | Eucalyptus globulus | Oil | 0.18 ml |
| 5 | Roghan Dalchini | Cinnamomum zeylanicum | Oil | 0.18 ml |
| 6 | Roghan Zaitun | Olea europaea (olive oil) | Oil | 0.03 ml |
| 7 | Roghan Laung | Syzygium aromaticum | Oil | 0.01 ml |
| Colour red | q.s. | |||
2.1. Standardization of polyherbal formulations
The quality assessment of herbal formulations has vital importance to justify their adequacy in the modern system of medicine. The High Performance‐Thin Layer Chromatography (HP‐TLC) fingerprinting of Infuza was performed on Merck TLC plates; silica gel 60F254 (stationary phase) using the mobile phase solution of ethyl acetate: formic acid: acetic acid: water (15:1:1:2 v/v/v/v). Moreover, the quality of Kulzam was established by Gas Chromatography analysis using ZB‐624 plus; 30 m × 0.32 mm ID × 1.80 μm film thickness column and other standard conditions (Chandra et al., 2020; Chandra, Khan, Jetley, Ahmad, & Jain, 2018). The quality assessment of formulations was done at NABL accredited third party laboratories (Anchrome enterprises [Mumbai] and Vimta Labs [Hyderabad], India).
2.2. Research design
A randomized, open‐label controlled clinical study was conducted to assess the efficacy of Infuza and Kulzam in high‐risk subjects of COVID‐19 at the Department of Respiratory Medicine/flu clinic of the Hakeem Abdul Hameed Centenary Hospital, New Delhi, India. The study was ethically approved by the Institutional Ethics Committee (IEC) of Hamdard Institute of Medical Sciences and Research, New Delhi, and the guidelines of the declaration of Helsinki and Tokyo for humans were followed. The study was technically approved by the Project Approval Committee of the Central Council for Research in Unani Medicine, Ministry of AYUSH, Government of India and registered with the clinical trials registry of India (CTRI/2020/08/027222, registered on: August 18, 2020) (www.ctri.nic.in). All the study subjects had known exposure to a laboratory‐confirmed COVID‐19 patient(s), whether as a household contact, a health care worker, or a passenger in close proximity of symptomatic patient in a conveyance. All the eligible subjects were screened for COVID‐19 Real Time‐Polymerase Chain Reaction (RT‐PCR)/ antibody tests and only subjects with negative report were recruited for drug allocation. Written informed consent was taken from all the participants in the study. The CONSORT checklist of information for reporting a randomized trial is enclosed in Table S1.
2.3. Selection of study subjects
For this interventional clinical study, 6,961 subjects were screened from September 18, 2020, to May 21, 2021, and out of this, 407 subjects were found fit for preliminary eligibility following stringent inclusion and exclusion criteria. Further, RT‐PCR and antibody tests of COVID‐19 were performed for eligibility and a total of 251 subjects, who had tested negative for COVID‐19 RT‐PCR and antibody test were further randomized in Infuza, Kulzam, Infuza & Kulzam and control groups using excel generated random numbers. The difference in time for RT‐PCR test of COVID‐19 for eligibility and starting the treatment of test drug(s) was a maximum of 36 hr in each group of the study. Considering the power of 80% and α level of 0.05, a total of 42 subjects were required in each group for the generation of statistically significant data. Hence, a total of 207 subjects (minimum 50 subjects in each group) were administered the test drug(s) in the study. The high‐risk subjects (National Centre for Disease Control et al., 2020), who had direct or indirect contact with COVID‐19 positive patients within last 7 days were the main inclusion criteria of study. The subjects with following conditions were excluded from study: (a) COVID‐19 positive patients, (b) previously diagnosed with COVID‐19 and had taken vaccination for COVID‐19, (c) had comorbidities, (d) were taking other prophylactic and herbal medicine(s), (e) subjects below 18 and above 65 years and (f) were pregnant and lactating.
2.4. Study assessments
The eligible study subjects were randomized 1:1 to receive Infuza, Kulzam, Infuza & Kulzam, for 14 days. The study subjects were given 2.5 ml of Infuza dissolved in 100 ml of lukewarm water, twice a day, orally and 5 drops (0.5 ml) of Kulzam in 500 ml of hot water for steam inhalation till all the fragrance of polyherbal formulation disappeared, every 12 hourly were allocated as per their test group. The infuza & kulzam group of subjects were given both the drugs to see synergistic effect, if any. The control group of subjects were not given any medicine and advised to home isolate for 14 days. The drug compliance for study subjects was assured by telephonic confirmation and/or cross‐check of empty bottles of infuza and Kulzam. Nasal and oropharyngeal swabs in Viral Transport Media (VTM) and blood samples in EDTA, Serum Separator Tube (SST) and sodium heparin vacutainers (VACU‐ ETTE®, Greiner Bio‐One) were collected from the study subjects at the time of enrolment (baseline) and after 14th day of treatment or if the patient developed the symptoms of COVID‐19 after enrolment. Samples for lymphocyte subsets analysis were tested up to 6 hr after sampling. For the analysis of cytokines (IL‐4, IL‐6, INF‐γ and IL‐12), samples were stored at ‐80°C till the analysis. The rate of conversion to COVID‐19 positive after administration of test drug (s) or successful completion of 14 days of observation, whichever earlier was considered the primary endpoint of the study. Besides, the immune‐modulatory markers, that is, phenotyping of lymphocytes, cytokines, inflammatory markers and need of hospitalization/intensive care unit admission for COVID‐19 infection after giving test drugs were used as the secondary endpoints.
The RTPCR test for COVID‐19 was performed by RT‐PCR COVIWOK, SNP technologies, RTPCR machine (MX3005P, AGILENT) using the RNA extraction kit of Qiagen, supplied by Himedia, India. The antibody test of COVID‐19 in study subjects was performed at the time of enrolment using Meril COVID‐19 Ig G/Ig M test kit. The biochemical parameters for liver and kidney toxicities such as alanine transaminase, aspartate transaminase, alkaline phosphatase, creatinine, urea, and so forth were analysed by Beckman AU480 automated biochemistry analyser using the kits supplied by Beckman Coulter Diagnostic, USA. High sensitive C‐reactive protein (hs‐CRP) was analysed using Roche Cobas® e411 CLIA analyser with the kits purchased from Roche, India. Plasma interleukin 4, 6, 12 and interferon‐γ were analysed by ELISA method using commercially available kits (Bioron, Roemerberg, Germany) following the manufacturer's protocol.
2.5. Detection and quantification of cluster of differentiation/classification determinant; CD3, CD4, CD8, CD16, CD19 and CD56 expressed immune cells in human peripheral blood
The phenotyping of lymphocytes and absolute lymphocyte counts were analysed by BD FACSCanto II Flow cytometry using BD FACSDiva™ software. BD FACS canto‐ll has three lasers (Blue‐405 nm, Red‐488 nm, Violet‐633 nm) with a capacity for up to eight colour detection. Lymphocytes were identified and quantified based on forward and side scatter characteristics. The samples were processed using the Lyse No Wash Staining (LNW) procedure. Human T, B and NK cells were identified using allophycocyanine labelled antihuman CD3, BD HorizonTM V450 antihuman CD4, fluorescein isothiocyanate antihuman CD8, peridinin chlorophyll protein‐ cyanine 5.5 antihuman CD16, PE‐cyanine 7 antihuman CD19, allophycocyanine‐H7 antihuman CD45 and phycoerythrin antihuman CD56 (BD catalogue number 330440, 651849, 347313, 338426, 341043, 641399 and 340363, respectively). The processed sample was acquired on a precalibrated flow cytometer. Absolute counts (cells/μl) were calculated as follows; (Events in cell population/Events in absolute count bead region) *(Beads per test/Test volume). The quality control analysis was carried out before the analysis of the study samples and the % CV of quality control samples was <5%.
2.6. Safety monitoring
The test drugs (Infuza and Kulzam) are using in Traditional Unani System of Medicine for decades and are well tolerated and not associated with any safety issue. All adverse events/reactions were monitored in the study subjects and recorded on the individual case reports of the subjects. The detailed safety procedures were also submitted to the ethics committee when the proposal was approved by Institutional Ethics Committee.
2.7. Statistical analysis
All the laboratory tests were performed using prior quality control analysis. The data were analysed through SPSS 26 version. For categorical data, we used Z test of two proportions to find statistical significance levels between control and test groups. Descriptive statistics were applied in continuous data. The normality test was performed for all continuous variables using “Kolmogorov–smirnov” test. The continuous variables were analysed by unpaired/paired “t” test, analysis of variance and “Tukey test” for multiple comparison. If the p value was <.05, it was considered to be a statistically significant difference.
3. RESULTS
3.1. Quality analysis of polyherbal formulations; Infuza and Kulzam
The polyherbal formulation; Infuza contains Glycyrrhiza glabra as the major ingredient and glycyrrhizic acid is the key phytochemical present. Other ingredients are Tinospora cordifolia, Trachyspermum ammi, Eugenia caryophyllata, Nigella sativa and Cinnamomum zeylanicum. Quality analysis of Infuza was performed by HP‐TLC and the plate was derivatized with 10% methanolic sulfuric acid. Glycyrrhizic acid was detected at Rf 0.15 in standard and Infuza samples (Figure 1a). Mentha Arvensis, Trachyspermum ammi, Camphor and essential oils from Eucalyptus globulus, Cinnamomum zeylanicum, Olea europaea and Syzygium aromaticum are the part of Kulzam polyherbal formulation. Further, camphor, menthol and thymol are the major phytochemicals present in Kulzam. These phytochemicals in Kulzam polyherbal formulations were detected at Rt 17.7, 18.7, 20.6 in gas chromatography analysis and the peak is matched with a standard solution of camphor, menthol and thymol (Figure 1b–e). The analysis revealed that Infuza and Kulzam polyherbal formulations are in conformity with the quality standards as per the previous report in literature (Khanna et al., 2021) and pharmacopeia (GOI, 2016) for their therapeutic use.
FIGURE 1.
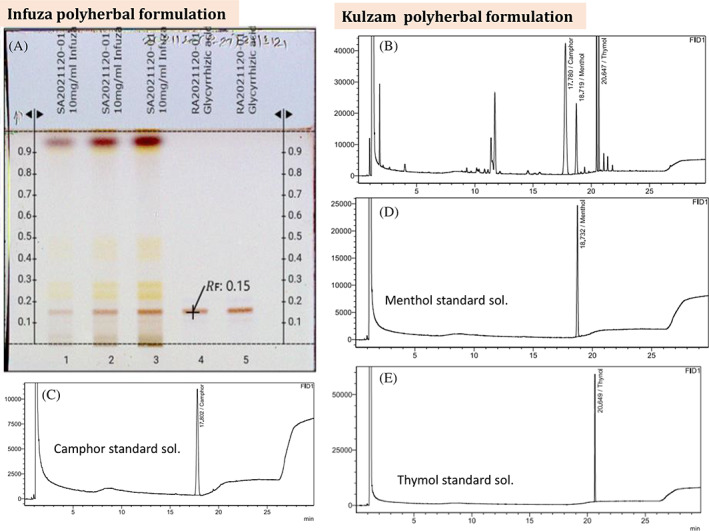
(A) HP‐TLC analysis of Infuza. The plate was derivatized with 10% methanolic sulfuric acid. Brown colour band of Glycyrrhizic acid was detected in sample and standard at Rf value of 0.15. (B) Gas Chromatogram of Kulzam polyherbal formulation. The peak of camphor, menthol and thymol is present in gas chromatogram of Kulzam polyherbal formulation at Rt 17.11, 18.79, 20.65, respectively and further it is matched with the chromatogram of standard solutions; Camphor (C), Menthol (D) and Thymol (D)
3.2. Therapeutic efficacy of polyherbal formulations (Infuza and Kulzam) for the prevention of COVID‐19 infection
Four hundred seven high‐risk subjects were enrolled for eligibility after preliminary screening of 6,961 participants in this study (Figure 2). Out of which, one hundred fifty‐six subjects were excluded further from the study as they were positive for COVID‐19 RT‐PCR and/or antibody. The remaining 251 subjects were randomized between the Infuza (n = 65), Kulzam (n = 66), Infuza & Kulzam (n = 58) and control (n = 62) groups. However, a total of 207 subjects including 52 in Infuza, 51 in Kulzam, 51 in Infuza & Kulzam and 53 in control groups completed the study as 43 subjects were further excluded due to their protocol violation and loss of follow‐up. The demographic and clinical characteristics of the subjects in all the groups at baseline are shown in Table 2. The mean age of the study subjects in Infuza, Kulzam, Infuza & Kulzam and control groups was 29.8 ± 0.98, 31.5 ± 1.4, 29.7 ± 0.10 and 31.6 ± 1.5, respectively. The age and gender distribution of the study subjects in test groups were comparable to the subjects of control group (p > .05). The average body mass index (BMI) was 23.2 ± 0.61 kg/m2 in Infuza group, 23.7 ± 0.51 kg/m2 in Kulzam group, 24.2 ± 0.57 kg/m2 in Infuza & Kulzam group and 22.9 ± 0.46 kg/m2 in control group. The difference of BMI in all the study groups was insignificant (p > .05) and comparable. The study subjects in all the groups had no co‐morbidities and their blood pressure, pulse rate and oxygen saturation were normal at the time of enrolment. Further, 76.9% in Infuza, 86.3% in Kulzam, 78.4% in Infuza & Kulzam and 77.4% in control group had direct contact with COVID‐19 positive patients. The average time (mean ± SEM) from exposure to enrolment of the study subjects was 3.6 ± 0.23 days, 3.6 ± 0.20 days, 3.1 ± 0.19 days and 3.5 ± 0.23 days in Infuza, Kulzam, Infuza & Kulzam and control groups, respectively. The type/days of exposure in study subjects with COVID‐19 patients in test groups were comparable to the control group (p > .05). Further, RT‐PCR test of COVID‐19 was negative for all the study subjects at the time of enrolment in study.
FIGURE 2.
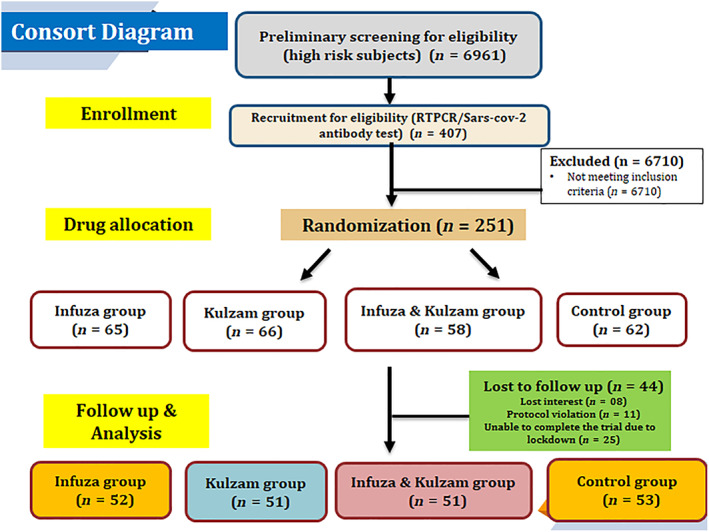
CONSORT diagram of the study
TABLE 2.
Demographic and clinical characteristics of study subjects
| Test groups | |||||
|---|---|---|---|---|---|
| Characteristics | Infuza (n = 52) | Kulzam (n = 51) | Infuza and Kulzam (n = 51) | Control (n = 53) | p‐value |
| Average age; years (mean ± SEM) (range) | 29.8 ± 0.98 (21–50) | 31.5 ± 1.4 (20–53) | 29.7 ± 0.10 (18–52) | 31.6 ± 1.5 (18–62) | >.05 |
| Male (%) | 61.5 | 58.8 | 58.8 | 54.7 | — |
| Female (%) | 38.5 | 41.2 | 41.2 | 45.3 | — |
| BMI; kg/m2 (mean ± SEM) | 23.2 ± 0.61 | 23.7 ± 0.51 | 24.2 ± 0.57 | 22.9 ± 0.46 | >.05 |
| Systolic blood pressure (mean ± SEM [mmHg]) | 112.9 ± 0.78 | 113.2 ± 0.75 | 113.6 ± 0.67 | 114.2 ± 0.71 | >.05 |
| Diastolic blood pressure (mean ± SEM [mmHg]) | 81.0 ± 0.91 | 81.7 ± 0.78 | 81.8 ± 0.64 | 81.7 ± 0.78 | >.05 |
| Pulse rate (mean ± SEM [BPM]) | 76.6 ± 0.67 | 78.0 ± 0.73 | 77.5 ± 0.62 | 78.2 ± 0.72 | >.05 |
| Oxygen saturation (%) (mean ± SEM) | 97.3 ± 0.22 | 97.2 ± 0.21 | 98.2 ± 0.10 | 97.2 ± 0.23 | >.05 |
| Any co‐morbidities | Nil | Nil | Nil | Nil | — |
| Direct contact with COVID‐19 positive patients (n [%]) | 40 (76.9) | 44 (86.3) | 40 (78.4) | 41 (77.4) | >.05 |
| Healthcare workers/others | 12 (23.1) | 07 (13.7) | 11 (21.6) | 12 (22.6) | >.05 |
| Average time from exposure to enrolment (days) (mean ± SEM) | 3.6 ± 0.31 | 3.6 ± 0.20 | 3.1 ± 0.19 | 3.5 ± 0.23 | >.05 |
| Subject(s) turned positive after enrollment in study (% [n]) | 7.69 (4)* | 3.92 (2)** | 1.96 (1)*** | 15.09 (8) | .233* |
| .533** | |||||
| .017*** | |||||
Note: Data are expressed as number, percentage and mean ± SEM. The continuous and categorical variables are analysed by unpaired “t” test and “Z” test of two proportion, respectively as compared to control group. *, **, *** Statistical comparison of Infuza, Kulzam and in combination of Infuza and Kulzam groups, respectively with control group. (p < .05: significant difference, p > .05: not significant).
Abbreviation: BPM; beats per minute.
3.3. Safety analysis
To assess the toxicity if any, after intervention of test drugs (Infuza and Kulzam alone and in combination) for 14 days, safety parameters such as liver enzymes and biomarkers of kidney toxicity were measured. The differences in these parameters between 0 day and 14 days in test and control groups were not statistically significant (p > .05), suggesting that Infuza and Kulzam are not producing any toxic ailment in the study subjects (Table 3). Further, no serious event or reactions were reported in any of the test groups during the 14 days of interventional study.
TABLE 3.
Safety parameters in test groups after 14 days of treatment
| Infuza group Kulzam group Infuza and Kulzam Control group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | O day | 14 days | O day | 14 days | O day | 14 days | O day | 14 days |
| Hb (g/dl) | 14.9 ± 0.16 | 14.5 ± 0.19 | 13.7 ± 0.30 | 13.7 ± 0.31 | 14.9 ± 1.7 | 14.1 ± 0.23 | 13.8 ± 0.33 | 13.8 ± 0.32 |
| AST (U/L) | 29.0 ± 1.9 | 30.1 ± 2.6 | 29.3 ± 1.5 | 31.9 ± 2.1 | 31.5 ± 2.1 | 31.5 ± 2.3 | 30.5 ± 2.5 | 30.9 ± 2.9 |
| ALT (U/L) | 34.2 ± 3.5 | 36.6 ± 4.1 | 31.5 ± 2.9 | 34.2 ± 3.1 | 38.3 ± 4.1 | 36.2 ± 3.5 | 34.9 ± 5.6 | 37.0 ± 4.8 |
| ALP (U/L) | 95.6 ± 6.6 | 97.9 ± 6.9 | 84.9 ± 4.7 | 90.0 ± 4.2 | 94.0 ± 3.6 | 101.3 ± 3.8 | 91.4 ± 3.7 | 96.6 ± 4.4 |
| Urea (mg/dl) | 26.0 ± 1.1 | 26.2 ± 2.1 | 25.2 ± 0.95 | 23.2 ± 0.81 | 24.7 ± 0.74 | 24.4 ± 0.95 | 22.6 ± 1.0 | 21.4 ± 0.99 |
| Cret. (mg/dl) | 0.78 ± 0.03 | 0.78 ± 0.03 | 0.74 ± 0.02 | 0.74 ± 0.03 | 0.76 ± 0.23 | 0.75 ± 0.02 | 0.75 ± 0.02 | 0.75 ± 0.02 |
| TP (g/dl) | 7.7 ± 1.3 | 7.4 ± 0.6 | 7.4 ± 0.08 | 7.4 ± 0.06 | 7.5 ± 0.51 | 7.5 ± 0.05 | 7.4 ± 0.07 | 7.3 ± 0.07 |
| Alb (g/dl) | 4.5 ± 0.76 | 4.4 ± 0.06 | 4.4 ± 0.36 | 4.4 ± 0.04 | 4.5 ± 0.07 | 4.4 ± 0.05 | 4.5 ± 0.12 | 4.4 ± 0.04 |
| UA (mg/dl) | 5.5 ± 0.23 | 5.6 ± 0.24 | 5.4 ± 0.20 | 5.5 ± 0.22 | 5.5 ± 0.19 | 5.6 ± 0.18 | 5.2 ± 0.23 | 5.4 ± 0.22 |
Note: Data are expressed as mean ± SEM. 0 day data; collected at the time of recruitment without intervention (baseline), 14 days of data collected after giving intervention/observation; treatment data compared with 0 week data and without intervention; p > .05, not significant). Further, 14 days data of each group were compared each other using post hoc “Tukey” multiple comparison test and difference is not statistically significant (p > .05: not significant).
Abbreviations: Alb, albumin; ALP, alanine transaminase; ALT, alanine transaminase; AST, aspartate transaminase; Cret, creatinine; Hb, hemoglobin; TP, total protein; UA, uric acid.
3.4. Outcome of the study
3.4.1. Primary outcome
Rate of conversion to COVID‐19 positive after intervention of polyherbal formulations (Infuza and Kulzam) in high‐risk subjects
The study subjects in the clinical trial are at high‐risk for COVID‐19 infection. The subjects in control group were under home isolation and did not take any medication. The rate of conversion to COVID‐19 infection in Infuza & Kulzam group was minimal and the difference was statistically significant as compared to control group (p .017). Interestingly, a total of 15.09% (n = 8) high‐risk subjects in control group turned positive as compared to only 7.69% (n = 4) in Infuza, 3.92% (n = 2) in Kulzam and only 1.96% (n = 1) in Infuza & Kulzam groups during 14 days of treatment/observation. The average cycle threshold (CT) value in the subjects, who turned to COVID‐19 positive after drug allocation was 26.14 ± 4.3 (n = 4) in Infuza, 23.5 ± 0.57 (n = 2) in Kulzam, 28.47 (n = 1) in Infuza & Kulzam and 24.69 ± 2.69 (n = 8) in control groups. The viral load in positive subjects of Infuza & Kulzam group was found lowest as compared to control group, however, the difference was not statistically significant (p > .05). Additionally, most of the subjects, who later turned COVID‐19 positive in test groups during drug trial were either asymptomatic or showed mild symptoms as compared to positives in control group. However, none of COVID‐19 positive subjects in study groups needed hospitalization and took only symptomatic treatment.
3.4.2. Secondary outcomes
Effect of polyherbal formulations on inflammation, cytokine levels, phenotyping of lymphocytes and lymphocyte counts
The treatment outcome of polyherbal formulations; Infuza and Kulzam on inflammation, cytokine levels and lymphocyte counts are shown in Table 4. The hs‐CRP is the marker of acute‐phase reaction and it showed the early stage of inflammation. The mean hs‐CRP level in control group was increased by 11.5% (from 2.3 ± 0.35 mg/L to 2.6 ± 0.67 mg/L) (p .70 [ns]) after 14 days of assessment. Interestingly, mean serum hs‐CRP level in Infuza, Kulzam and Infuza & Kulzam groups was reduced by 11.8% (from 1.7 ± 0.27 mg/L to 1.5 ± 0.20 mg/L), 12.5% (from 2.4 ± 0.37 mg/L to 2.1 ± 0.33 mg/L) and 3.8% (from 2.6 ± 0.44 mg/L to 2.5 ± 0.36 mg/L), respectively after 14 days of intervention. However, this reduction was not statistically significant in paired “t” test (p > .05). Further, the level of cytokines; interleukin 4 (IL‐4), IL‐6, Interferon γ and IL‐12 were not changed statistically after 14 days of treatment (p > .05). The results are comparable to the control group.
TABLE 4.
Treatment outcome of polyherbal formulations; Infuza and Kulzam on inflammation, cytokine levels and lymphocyte counts
| test groups a (mean ± SEM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Infuza (n = 48) | Kulzam (n = 49) | Infuza & Kulzam (n = 50) | Control (n = 45) | ||||||||
| 0 day | 14 days | p‐value | 0 day | 14 days | p‐value | 0 day | 14 days | p‐value | 0 day | 14 days | p‐value | |
| hs‐CRP (mg/L) | 1.7 ± 0.27 | 1.5 ± 0.20 | .25 | 2.4 ± 0.37 | 2.1 ± 0.33 | .17 | 2.6 ± 0.44 | 2.5 ± 0.36 | .75 | 2.3 ± 0.35 | 2.6 ± 0.67 | .72 |
| Interleukin‐4 (pg/dl) | 39.0 ± 0.10 | 40.0 ± 0.70 | .40 | 40.0 ± 0.60 | 39.0 ± 0.02 | .32 | 38.6 ± 0.30 | 39.0 ± 0.20 | .30 | 40.0 ± 1.1 | 41.1 ± 1.85 | .68 |
| Interleukin‐6 (pg/dl) | 32.0 ± 8.50 | 19.0 ± 0.2 | .15 | 26.2 ± 6.9 | 19.0 ± 0.02 | .30 | 22.7 ± 3.5 | 19.0 ± 0.02 | .25 | 25.7 ± 5.7 | 19.0 ± 0.2 | .25 |
| Interferon‐ γ (pg/dl) | 180.0 ± 1.0 | 181.0 ± 1.0 | .48 | 180.0 ± 1.0 | 180.0 ± 0.1 | .99 | 180.0 ± 0.1 | 180.0 ± 0.1 | .99 | 180.0 ± 0.1 | 181.0 ± 0.1 | .32 |
| Interleukin‐12 (pg/ml) | 71.5 ± 8.8 | 85.2 ± 8.3 | .08 | 91.4 ± 8.6 | 93.1 ± 8.1 | .99 | 95.4 ± 12.7 | 97.5 ± 12.3 | .81 | 100.7 ± 7.4 | 111.6 ± 6.9 | .15 |
| Absolute lymphocyte count/μl | 2,115.7 ± 74.0 | 2,102.3 ± 103.3 | .12 | 2,287.0 ± 121.5 | 2,161.5 ± 135.1 | .49 | 2,327.6 ± 89.8 | 2,306.9 ± 88.8 | .81 | 2,372.1 ± 134.6 | 2,210.0 ± 108.5 | .265 |
Note: Data are expressed as mean ± SEM; 0 day data; collected at the time of recruitment without intervention (baseline). 14 days of data collected after giving intervention; treatment data compared with 0 day data (without intervention); p‐value is calculated using paired “t” test. Further, 14 days data of each group were compared each other using post hoc “Tukey” multiple comparison test and the difference is not statistically significant (p > .05: not significant).
4 in Infuza, 2 in Kulzam, 1 in Infuza & Kulzam and 8 subjects in control groups were positive after enrolment in the study. The data of these subjects were not included in the analysis.
The level (mean ± SEM/μl) of blood T‐cells (CD3+), T‐helper (CD3+, CD4+) and T‐cytotoxic cells (CD3+, CD8+) in high‐risk subjects was 1,603.5 ± 75.9, 873.2 ± 58.9, 625.1 ± 32.7, respectively. Moreover, the level (mean ± SEM/μl) of B cells (CD19+) and Natural Killer (NK) cells (CD16+, CD56+) in high‐risk subjects was 322.0 ± 20.0 and 230.9 ± 18.0. The effect of polyherbal formulations on immune cells (T, B and NK cells) is shown in Figures 3a–d and 4a–d. There was no significant change in T, B and NK cells after intervention of polyherbal formulations (Infuza and Kulzam) for 14 days (p > .05). Furthermore, absolute lymphocyte counts in control and Infuza & Kulzam groups were decreased by 6.8% and 0.63%, respectively at 14 days of analysis from baseline (0 day) (Table 4).
FIGURE 3.
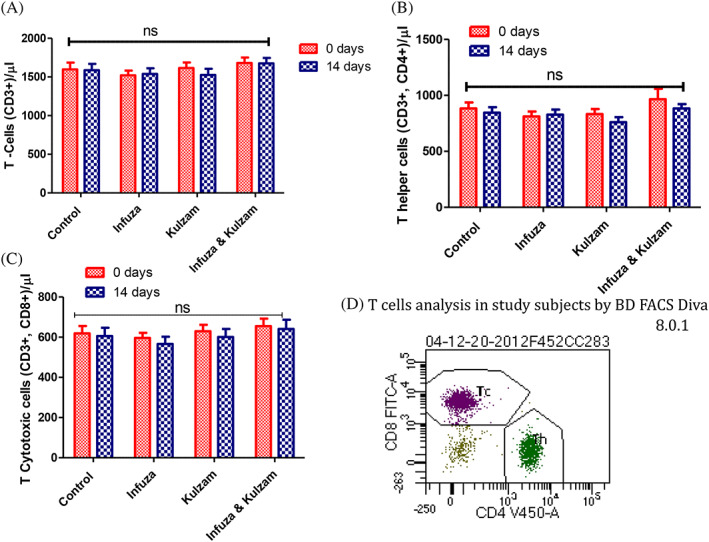
Level of T‐cells (CD3+), T‐helper (CD3+, CD4+) and T‐cytotoxic cells (CD3+, CD8+) of high‐risk subjects in study groups and alteration after administration of test drugs. (A): mean level of T‐cells (CD3+), (B): T‐helper cells (CD3+, CD4+), (C) T‐cytotoxic cells (CD3+, CD8+) at baseline (0 day) and after intervention of test drugs for 14 days. (D); Flow cytogram (BD FACS Diva 8.0.1) of T‐helper and cytotoxic cells in study subject. Data are expressed as mean ± SEM; 0 day data; collected at the time of recruitment without intervention (baseline). 14 days of data collected after giving intervention; p‐value is calculated using paired “t” test between 0 day and 14 days data. Further, 14 days data of each group were compared each other using post hoc “Tukey” multiple comparison test. The difference among all these groups is not statistically significant (p > .05: not significant)
FIGURE 4.
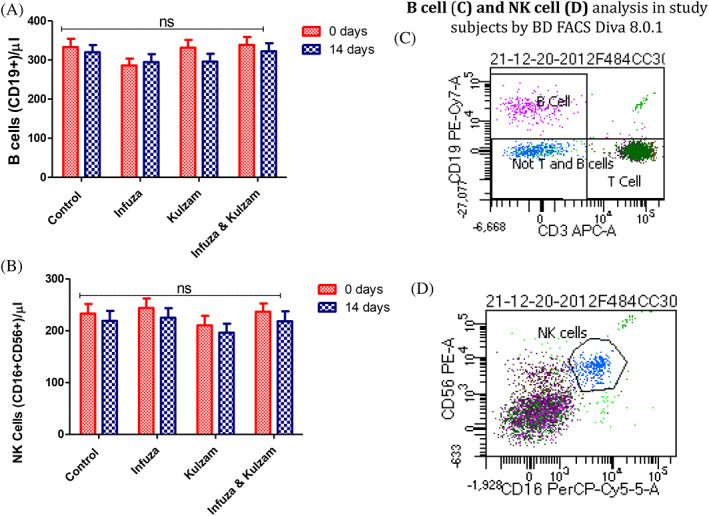
Levels of B cells (CD19+) and Natural Killer (NK) cells (CD16+, CD56+) in high‐risk subjects and alteration after administration of test drugs. (A): mean level of B cells (CD19+); (B) NK cells (CD16+, CD56+) at baseline (0 day) and after intervention of test drugs for 14 days. (C) & (D); Flow cytogram (BD FACS Diva 8.0.1) of B and NK cells in study subject. Data are expressed as mean ± SEM; 0 day data; collected at the time of recruitment without intervention (baseline). 14 days of data collected after giving intervention; p‐value is calculated using paired “t” test between 0 day and 14 days data. Further, 14 days data of each group were compared each other using post hoc “Tukey” multiple comparison test. The difference among all these groups is not statistically significant (p > .05: not significant)
4. DISCUSSION
The present study was undertaken to assess the efficacy of polyherbal formulations; Infuza and Kulzam for post‐exposure prophylaxis against COVID‐19 infection. The interaction between Sars‐Cov 2 spike protein and ACE‐2 inhibitor is the key event to COVID‐19 infection. Medicinal plants and their phytochemicals are known for their antiviral activities (Shah et al., 2021). The antiviral property is due to activity of phytochemicals for their efficacy in the inhibition of spike‐protein‐ACE 2 complex, entry of virus into host cells, viral RNA synthesis and replication, RNA dependent‐RNA polymerase, viral proteases and assembly of virion particles (Shah et al., 2021). The present clinical study was conducted in high‐risk subjects of COVID‐19 to assess the efficacy of Infuza and Kulzam for prevention of COVID‐19 infection. The relative risk (RR) score in Infuza, Kulzam and Infuza & Kulzam groups was 0.51, 0.26 and 0.13, respectively. Hence, the efficacy of Infuza, Kulzam and Infuza & Kulzam intervention was 49%, 74% and 87%, respectively for prevention of COVID‐19 infection (E = 1 − RR*100). The analysis showed that combination of Infuza and Kulzam provides significant protection against COVID‐19 infection. The protective action against COVID‐19 infection of polyherbal formulations tested in the present study may be due to their anti‐inflammatory, antioxidative, antiviral and anti‐tussive activities that eventually lead to a balanced immune system. Glycyrrhiza glabra, Trachyspermum ammi, Tinospora cordifolia and essential oils from Nigella sativa, Cinnamomum zeylanicum and Eugenia caryophyllata are the part of Infuza polyherbal formulation. Further, Kulzam polyherbal formulation contains Mentha arvensis, Trachyspermum ammi, Cinnamumum camphor, Syzygium aromaticum, Cinnamomum zeylanicu, Eucalyptus globulus and Olea europaea. Infuza and Kulzam polyherbal formulations are the rich source of polyphenols, flavonoids, β‐sitosterol and hydroxyl coumarins (Khanna et al., 2021). Phytochemicals present in these herbs have anti‐inflammatory and antioxidant potential that augment the formation of interferons in human body (Ramos‐Tovar & Muriel, 2019). However, in the present study, we did not observe any change in cytokines and interferon levels after 14 days of intervention in test as well as control groups. Glycyrrhizic acid, kaemferol, emodin, chrysin are reported for their inhibitory action against spike‐ACE2 complex (Basu, Sarkar, & Maulik, 2020). Glycyrrhizin a major chemical part of Glycyrrhiza glabra reduces the ability of Sar‐CoV virus to attach to the cell at the early stages of viral invasion (Feng Yeh et al., 2013). Recently, Glycyrrhizin has also shown a similar action against COVID‐19 virus under in silico experimentation (Mohammadi & Shaghaghi, 2020). Glycyrrhiza species are well known for their anti‐viral activity. It reduces the viral transport to the host membrane, modification of hepatitis B‐virus surface antigen, limits the viral membrane fluidity, increases the synthesis of gamma interferon, and reduction of viral latency (Akram et al., 2018; Fiore et al., 2008). Interestingly, Glycyrrhiza glabra is the key ingredient of Infuza polyherbal formulation. Essential oils such as cinnamyl acetate, cinnamaldehyde, carvacrol are the inhibitor of Sars‐Cov2 spike protein (Sharanya, Sabu, & Haridas, 2021). Phytochemicals such as curcumin, rosmarinic acid, nigelledine, eugenol, piperine, jansenone, magnoflorine and piperamide are present in Tinospora cordifolia, Eugenia caryophyllata, Nigella sativa, Syzygium aromaticum and Eucalyptus globulus have a binding affinity with COVID‐19 virus/protease and spike proteins leads to boost immunity and decrease the chance of COVID‐19 infection (Alabboud & Javadmanesh, 2020; Dev Sharma & Kaur, 2020; Koshak & Koshak, 2020; Maurya & Sharma, 2020; Mohammadi & Shaghaghi, 2020). Polyherbal formulations (Infuza and Kulzam) tested in the present study contain most of these phytochemicals and might have played a possible role in the prevention of COVID‐19 infection.
C‐reactive protein (CRP) is a marker of inflammation and has been positively correlated with the early stage of COVID‐19 disease and its severity (Wang, 2020). No study is available for hs‐CRP level in high‐risk contact of COVID‐19 till date. Our observation in the present study regarding Infuza, Kulzam and combination of Infuza & Kulzam administration in high‐risk subjects showed a decreasing trend of hs‐CRP level, though it was not statistically significant (p > .05). The decreasing trend of hs‐CRP levels shows the possible role of polyherbal formulations in reducing inflammation. The beneficial effect of polyherbal formulations was further evaluated for their probable mechanism of action, therefore complete profiling of immune cells and cytokines was studied in study subjects at baseline (0 day) and after 14 days of treatment. Immune system plays a crucial role to protect against any type of viral infection. Ministry of AYUSH (Ayurvedic, Yoga and Naturopathy, Unani, Siddha and Homeopathy) was also recently advocated to drink “Kadha” (decoction of many herbs) for boosting the immunity to prevent COVID‐19 infection (AYUSH advisories, 2020). Calder, Carr, Gombart, and Eggersdorfer (2020) postulated that immune system mediators such as NK cells, helper T Cells, lymphocytes, interleukin 4 and other cytokines are impaired which leads to elevated chances of infection along with the reactivation of latent viruses (Calder et al., 2020). Further, mild to moderate infection of COVID‐19 showed an augmented level of T‐helper, T‐cytotoxic and B‐cells. However, in severe cases of COVID‐19, there is marked lymphocytopaenia and reduced level of T, B and NK cells (Khanna et al., 2021). In present study, the level of T, B and NK cells was not significantly changed in the subjects, who were spared for COVID‐19 infection in the study after 14 days of treatment/assessment in test and control groups. Further, it has been observed that lymphocyte counts remain constant in asymptomatic as compared to symptomatic COVID‐19 patients (Han et al., 2020). The control group in the present study showed higher percentage of reduction in absolute lymphocyte count (6.8%) in contrast to Infuza & Kulzam group (0.8%). Herbal plants have the potential to prevent/treat a variety of infectious diseases (Mukherjee & Mao, 2021). The protective role of herbal plants is substantiated in the present study as 15.09% of high‐risk subjects in control group turned positive during the assessment of 14 days. Interestingly, only 7.69% of high‐risk subjects in Infuza, 3.92% in Kulzam and 1.96% in Infuza & Kulzam groups turned positive after 14 days of treatment in spite of being in a high‐risk environment. However, sample size and open‐label study design are the limitations of the present study. Results of this trial cannot be generalized to the older age group and subjects with co‐morbidities.
5. CONCLUSION
To summarize, polyherbal formulations Infuza and Kulzam reduce the inflammation individually and synergistically in high‐risk subjects. The combination of Infuza and Kulzam showed a significant beneficial effect on the prevention of COVID‐19 infection. However, their mechanism of action is not established in the present study. This study demonstrated the potential efficacy of traditional Unani medicine in the prevention of serious infections like COVID‐19.
AUTHOR CONTRIBUTIONS
Conceptualization: Mridu Dudeja, Kailash Chandra, Ayan Kumar Das, Naushad Ali Rana, Santosh Joshi, Asad Mueed; Data curation: Sumeera Banday, Mohini Arora, Shashank Agarwal, Santosh Joshi, Kailash Chandra; Formal analysis: Kailash Chandra, Varun Kashyap, Ayan Kumar Das, Sumeera Banday; Investigation: Mohini Arora, Santosh Joshi, Kailash Chandra, Ayan Kumar Das; Methodology: Ayan Kumar Das, Kailash Chandra, Varun Kashyap, Sonal Jain, Shashank Agarwal; Project administration: Mridu Dudeja, Kailash Chandra, Ayan Kumar Das; Resources: Asad Mueed, Santosh Joshi, Mridu Dudeja; Supervision: Mridu Dudeja, Farzana Islam; Validation: Kailash Chandra, Ayan Kumar Das, Sonal Jain; Visualization: Writing ‐ original draft: Kailash Chandra, Ayan Kumar Das; Writing ‐ review & editing: Mridu Dudeja, Sumeera Banday, Naushad Ali Rana, Asad Mueed, Sonal Jain, Shashank Agarwal, Santosh Joshi, Farzana Islam, Varun Kashyap. The corresponding author confirms that all listed authors meet authorship criteria and no one others meeting the criteria have been omitted. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
CONFLICT OF INTEREST
We confirm that there is no conflict of interest associated with this publication. The medications were provided by M/S Hamdard Laboratories (Medicine Division), India. Clinical trial was conducted at Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital, New Delhi. M/S Hamdard Laboratories is not involved in any aspect of clinical trial reported in the study.
Supporting information
Table S1. CONSORT 2010 checklist of information to include when reporting a randomised trial*
ACKNOWLEDGEMENT
We are very thankful to Hon'ble CEO, Dr. G.N.Qazi, Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital for his relentless support and valuable guidance throughout the study. We acknowledge the dedication and commitment of Hakeem Abdur Rashid, Mr. Khursheed Alam, Ms. Heena (phlebotomist), Dr. Neha Dhyani and Mr. Kanhaiya, who contributed sincerely to this study. We express our thanks to Dr. Arjun Dang and Dr. Leena Chatterjee from M/S Dang Labs for helping in the flow cytometry analysis in study. We sincerely thanks M/S Hamdard Laboratories (Medicine Division) for providing the medicines for conducting this trial. We extend our heartfelt gratitude to the study subjects, who gave consent to participate in this study. The present study was funded by M/S Hamdard Laboratories (Medicine Division), India. No grant from other funding agency was received. The study was technically approved by Special Project Approval Committee of Ministry of AYUSH, Govt. of India.
Chandra, K. , Das, A. K. , Banday, S. , Rana, N. A. , Arora, M. , Jain, S. , Islam, F. , Agarwal, S. , Kashyap, V. , Joshi, S. , Mueed, A. , & Dudeja, M. (2022). Efficacy of polyherbal formulations for prevention of COVID‐19 infection in high‐risk subjects: A randomized open‐label controlled clinical trial. Phytotherapy Research, 36(9), 3632–3643. 10.1002/ptr.7531
Funding information Hamdard Laboratories (Medicine Division), Grant/Award Number: 01/2020
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the authors upon reasonable request.
REFERENCES
- Akram, M. , Tahir, I. , Shah, S. M. A. , Mahmood, Z. , Altaf, A. , Ahmad, K. , … Mehboob, H. (2018). Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytotherapy Research, 32(5), 811–822. 10.1002/PTR.6024 [DOI] [PubMed] [Google Scholar]
- Alabboud, M. , & Javadmanesh, A. (2020). In silico study of various antiviral drugs, vitamins, and natural substances as potential binding compounds with SARS‐CoV‐2 main protease. DYSONA ‐ Life Science, 1(2), 44–63. 10.30493/DLS.2020.225404 [DOI] [Google Scholar]
- Andrade, B. S. , de Rangel, F. S. , Santos, N. O. , dos Freitas, A. S. , de Soares, W. R. A. , Siqueira, S. , … de Azevedo, V. A. C. (2020). Repurposing approved drugs for guiding COVID‐19 prophylaxis: A systematic review. Frontiers in Pharmacology, 11, 1–7. 10.3389/fphar.2020.590598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu, A. , Sarkar, A. , & Maulik, U. (2020). Molecular docking study of potential phytochemicals and their effects on the complex of SARS‐CoV2 spike protein and human ACE2. Scientific Reports, 10(1), 1–15. 10.1038/s41598-020-74715-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder, P. C. , Carr, A. C. , Gombart, A. F. , & Eggersdorfer, M. (2020). Optimal nutritional status for a well‐functioning immune system is an important factor to protect against viral infections. Nutrients, 12(4), 1181. 10.3390/nu12041181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, K. , Jain, V. , Azhar, M. , Khan, W. , Alam, O. , Ahmad, S. , & Jain, S. K. (2020). Effect of augmented glycation in mobilization of plasma free fatty acids in type 2 diabetes mellitus. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 14(5), 1385–1389. 10.1016/j.dsx.2020.07.028 [DOI] [PubMed] [Google Scholar]
- Chandra, K. , Khan, W. , Jetley, S. , Ahmad, S. , & Jain, S. (2018). Antidiabetic, toxicological, and metabolomic profiling of aqueous extract of Cichorium intybus seeds. Pharmacognosy Magazine, 14(57), 377. 10.4103/pm.pm_583_17 [DOI] [Google Scholar]
- Cohen, M. S. (2020). Hydroxychloroquine for the prevention of Covid‐19 — Searching for evidence. New England Journal of Medicine, 383(6), 585–586. 10.1056/nejme2020388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev Sharma, A. , & Kaur, I. (2020). Bioactive molecules from eucalyptus essential oil as potential inhibitors of COVID‐19 corona virus infection by molecular docking studies. Kragujevac Journal of Science, 42, 29–43. [Google Scholar]
- Feng Yeh, C. , Chih Wang, K. , Chai Chiang, L. , Shieh, D. E. , Hong Yen, M. , & San Chang, J. (2013). Water extract of licorice had anti‐viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Journal of Ethnopharmacology, 148(2), 466–473. 10.1016/j.jep.2013.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore, C. , Eisenhut, M. , Krausse, R. , Ragazzi, E. , Pellati, D. , Armanini, D. , & Bielenberg, J. (2008). Antiviral effects of Glycyrrhiza species. Phytotherapy Research, 22(2), 141–148. 10.1002/PTR.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman, R. , Shah, S. , Jeurissen, P. , Jit, M. , & Mossialos, E. (2021). COVID‐19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy, 125(5), 553–567. 10.1016/j.healthpol.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of India, M. of A . (2009). Unani Pharmacopoeial of India ‐(government of India) (Vol. 6). [Google Scholar]
- Government of India, M. of A . (2016). The Unani pharmacopoeia of India part ‐II volume ‐III (formulations) (1st ed.). Gaziabad: Pharmacopoeia Commission for Indian Medicine & Homeopathy. [Google Scholar]
- Hakeem Najmul Gani Rampuri . (2009). Khazain ul Advia (Vol. 2). [Google Scholar]
- Han, H. , Xu, Z. , Cheng, X. , Zhong, Y. , Yuan, L. , Wang, F. , … Xia, Y. (2020). Descriptive, retrospective study of the clinical characteristics of asymptomatic COVID‐19 patients. MSphere, 5(5), e0092–20. 10.1128/msphere.00922-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, M. K. , Ara, I. , Mondal, M. S. A. , & Kabir, Y. (2021). Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra . Heliyon, 7(6), e07240. 10.1016/J.HELIYON.2021.E07240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge, A. (2020). Hydroxychloroquine in the prevention of COVID‐19 mortality. The Lancet Rheumatology, 3, e2–e3. 10.1016/S2665-9913(20)30390-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, K. , Kohli, S. K. , Kaur, R. , Bhardwaj, A. , Bhardwaj, V. , Ohri, P. , … Ahmad, P. (2021). Herbal immune‐boosters: Substantial warriors of pandemic Covid‐19 battle. Phytomedicine, 85, 1–21. 10.1016/j.phymed.2020.153361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshak, D. A. E. , & Koshak, P. E. A. (2020). Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: A mini review of in silico studies. Current Therapeutic Research, Clinical and Experimental, 93, 100602. 10.1016/j.curtheres.2020.100602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Chen, M. , Guo, Y. , Liu, J. , Chen, W. , Guan, M. , … Wang, Y. (2019). Prevention and treatment of infectious diseases by traditional Chinese medicine: A commentary. APMIS, 127(5), 372–384. 10.1111/apm.12928 [DOI] [PubMed] [Google Scholar]
- Maurya, D. K. , & Sharma, D. (2020). Evaluation of traditional ayurvedic Kadha for prevention and management of the novel coronavirus (SARS‐CoV‐2) using in silico approach. Journal of Biomolecular Structure and Dynamics, 40, 3949–3964. 10.1080/07391102.2020.1852119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of AYUSH . (2009). Unani Pharmacopoeia of India (Government of India) (Vol. 6).
- Ministry of AYUSH . (n.d.). The Unani Pharmacopoeia of India ‐ Part 1, Volume 1 (Vol. V1).
- Mohammadi, N. , & Shaghaghi, N. (2020). Inhibitory effect of eight secondary metabolites from conventional medicinal plants on COVID‐19 virus protease by molecular docking analysis. ChemRxiv, Preprint. 10.26434/CHEMRXIV.11987475.V1 [DOI]
- Mukherjee, P. K. , & Mao, A. A. (2021). Compendium of antiviral medicinal plants of north East India.
- National Centre for Disease Control, Directorate General of Health Services, & MoHFW, G . (2020). The updated case definitions and contact‐categorisation. Retrieved from https://ncdc.gov.in/WriteReadData/l892s/89568514191583491940.pdf
- Ramos‐Tovar, E. , & Muriel, P. (2019). Phytotherapy for the liver. In Dietary interventions in liver disease: Foods, nutrients, and dietary supplements (pp. 101–121). Mexico: Academic Press. 10.1016/B978-0-12-814466-4.00009-4 [DOI] [Google Scholar]
- Richman, D. D. (2021). COVID‐19 vaccines: Implementation, limitations and opportunities. Global Health & Medicine, 3(1), 1–5. 10.35772/ghm.2021.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboo, S. (2021). Immunomodulator in traditional healthcare system. In Intech Open (Vol. 2021). IntechOpen (online). 10.5772/INTECHOPEN.94965 [DOI] [Google Scholar]
- Salvi, R. , & Patankar, P. (2020). Emerging pharmacotherapies for COVID‐19. Biomedicine & Pharmacotherapy, 128, 110267. 10.1016/J.BIOPHA.2020.110267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, M. A. , Rasul, A. , Yousaf, R. , Haris, M. , Faheem, H. I. , Hamid, A. , … Batiha, G. E. S. (2021). Combination of natural antivirals and potent immune invigorators: A natural remedy to combat COVID‐19. Phytotherapy Research, 35(12), 6531–6551. 10.1002/PTR.7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharanya, C. S. , Sabu, A. , & Haridas, M. (2021). Potent phytochemicals against COVID‐19 infection from phyto‐materials used as antivirals in complementary medicines: A review. Future Journal of Pharmaceutical Sciences, 7(1), 1–20. 10.1186/S43094-021-00259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unani Pharmacopeia committee. Government of India . (2007). National Formulary of Unani Medicine (N.F.U.M.) (Vol. 1).
- Wang, L. (2020). C‐reactive protein levels in the early stage of COVID‐19. Médecine et Maladies Infectieuses, 50(4), 332–334. 10.1016/j.medmal.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2021). India: WHO Coronavirus Disease (COVID‐19) Dashboard With Vaccination Data. Retrieved from https://covid19.who.int/region/searo/country/in
- World Health Organization . (2021a). Weekly epidemiological update on COVID‐19 ‐ May 11, 2021.
- World Health Organization . (2021b). WHO advises that ivermectin only be used to treat COVID‐19 within clinical trials. Retrieved from https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CONSORT 2010 checklist of information to include when reporting a randomised trial*
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.


