Dear Editor,
The vaccination against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) continues to expand across the globe. Concurrently, a large number of dermatological adverse effects related to vaccination are vigorously reported. A study conducted by Rerknimitr et al encompassing over 35 000 vaccine receiving healthcare workers stated the incidence of cutaneous adverse effects to be <1%. India launched its vaccination drive on 16 January 2021 and has vaccinated over 320 million individuals (23.4% of the total population) by July 2021. 1 , 2 Amongst various vaccine‐related adverse effects, leprosy reactions have sparse mention in the literature limited to mere case reports and case series. We report a series of four cases of Hansen's disease with leprosy reactions spanning across the entire spectrum after being administered the vaccine from a leprosy care centre in India.
Of the four cases included in the series, three were borderline tuberculoid (BT) leprosy while one was of borderline lepromatous (BL) pole of the Ridley–Jopling classification. Of the three BT leprosy cases, two were released from treatment (RFT) after completing 6 months and one year of MDT, respectively. Two kinds of vaccines were administered, covishield (Recombinant ChAdOx1‐S) vaccine manufactured by the serum institute of India and covaxin (the whole virion inactivated vaccine NIV‐2020‐770 strain) by Bharat biotech, India. The BL leprosy case was administered covaxin while the other three cases were given covishield. The duration between the administration of the vaccine and the onset of leprosy reactions ranged from 5 to 11 days. The clinical profile of the cases was varied, and precipitation of the entire spectrum of leprosy reactions was noted which included cutaneous type I reaction, ulnar nerve neuritis, median nerve neuritis with nerve abscess and type II reaction in the form of necrotic erythema nodosum leprosum (ENL). The diagnosis was established with corroborating radiological and histopathological findings in a background of characteristic clinical presentation. The demographic, clinical profile and details of vaccination are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of cases.
| Patient # | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age (in years) | 35 | 28 | 25 | 45 |
| Gender | Male | Male | Male | Male |
| Spectrum of Hansen's disease | Borderline tuberculoid (BT) | Borderline tuberculoid (BT) | Borderline tuberculoid (BT) | Borderline lepromatous (BL) |
| Status of Multidrug therapy (MDT) | On multibacillary MDT since the past 05 months | Release from therapy 06 months back after 12 months of MDT | Release from therapy one year back after 12 months of MDT | On multibacillary MDT since the past 04 months |
| Type of lepra reaction | Type I reaction (cutaneous) | Type I reaction (right ulnar nerve neuritis) | Type I reaction (left median nerve abscess) | Type II reaction (necrotic erythema nodosum leprosum) |
| Clinical characteristics |
Presented with few light‐coloured numb patches over trunk and back. Slit skin smear (SSS) for acid‐fast bacilli (AFB‐L) ‐ 1 + . |
Sudden onset sharp shooting pain down right elbow | Sudden onset pain and swelling over volar aspect of left forearm around wrist joint | Crops of multiple erythematous tender nodules with subsequent ulceration distributed symmetrically over trunk and extremities (Fig. 1) |
| Acute onset erythema and oedema over these pre‐existing patches (Fig. 1) | Right ulnar nerve grade III thickened and grade II tender | Left median nerve grade III thickened and grade II tender |
Associated fever and joint pains. No features of organ involvement. SSS for AFB‐ 4+ |
|
| Histopathology revealed multiple ill‐defined epithelioid granulomas centred around nerve twigs and appendages with neutrophilic infiltrate and dermal oedema | USG of right upper limb suggestive of ulnar nerve thickening with surrounding edematous changes suggesting acute neuritis (Fig. 1) | Contrast‐enhanced MRI suggestive of median nerve neuritis with focal granuloma/abscess (Fig. 1) |
Histopathology revealed infiltration of neutrophils and sparse lymphocytes extending to subcutaneous tissue with vasculitis (Fig. 1). Special stains for AFB (L): Globi teeming with lepra bacilli (Fig. 1) |
|
| Treatment | Tapering doses of oral steroids that was started at 0.75 mg/kg/day of prednisolone | Tapering doses of oral steroids that was started at 1 mg/kg/day of prednisolone | Tapering doses of oral steroids that was started at 1 mg/kg/day of prednisolone | Managed with thalidomide 100 mg four times a day tapered monthly |
| Details of vaccine and dose | First dose of covishield (recombinant ChAdOx1‐S vaccine) | Second dose of covishield (recombinant ChAdOx1‐S vaccine) | Second dose of covishield (recombinant ChAdOx1‐S vaccine) | Second dose of covaxin (whole virion inactivated vaccine NIV‐2020‐770 strain) |
| Duration between onset of reaction and Covid‐19 vaccine administration | 11 days | 5 days | 8 days | 7 days |
Figure 1.
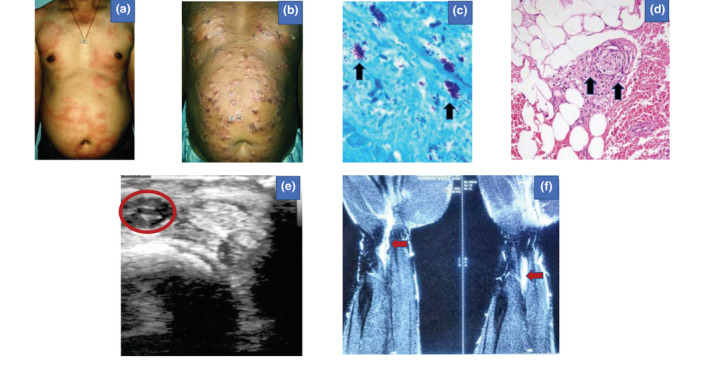
(a) Erythema and oedema over pre‐existing patches distributed over trunk and back (Patient 1). (b) Crops of multiple erythematous tender nodules with subsequent ulceration distributed symmetrically over trunk and extremities (Patient 4). (c) Multiple acid‐fast lepra bacilli in globi suggestive of multibacillary leprosy indicated by black arrows (Patient 4) (Fite Faraco stain of tissue for AFB [L], 40×). (d) Histopathology revealed infiltration of neutrophils and sparse lymphocytes of the subcutaneous tissue suggestive of lobular panniculitis with vasculitis indicated by black arrows (Patient 4) (H & E, 40×). (e) Ultrasonography of right upper limb suggestive of ulnar nerve thickening with surrounding edematous changes suggesting acute neuritis indicated by the red circle and red arrows (Patient 2). (f) Contrast‐enhanced magnetic resonance imaging (MRI) of the left forearm and wrist region suggestive of median nerve neuritis with focal granuloma/abscess indicated by red arrows (Patient 3).
Leprosy reactions are immunologically mediated episodes of acute or subacute inflammation interrupting a relatively uneventful disease process and are classified as type 1 and type 2 reactions. Initiation of multidrug therapy (MDT) is the primary trigger for these reactions, but they are known to be influenced by pregnancy, infections, stress and vaccinations. Barring vaccination, there were no identifiable triggers of reactions in the four cases reported. Amongst vaccines, influenza, pneumococcal, Bacillus Calmette and Guerin (BCG), and hepatitis‐B vaccines have been implicated with leprosy reactions. Covid‐19 vaccines after administration are known to increase TNF‐α, IFN‐γ and IL‐8 levels stimulated by glycoprotein‐S. 2 Panda et al. have proposed a hypothesis of increased neutrophil–lymphocyte ratio postcovid infection and vaccination. A conglomeration of neutrophilic inflammation with increased TNF‐α, IFN‐γ and IL‐8 could trigger leprosy reactions. An average interval of 5.1 to 11.5 days is mentioned for onset of reactions postvaccination which in our study was 7.5 days. 3 Moreover, neurological complications of Covid‐19 vaccination have been reported such as Guillian‐Barre syndrome (GBS), ischaemic stroke, facial nerve palsy and acute transverse myelitis.. Enhanced IL‐6 and IL‐12B gene expressions, inherent to Covid‐19 infection, are key mediators of neuritis in lepra reactions. The vaccines possess enhanced immunostimulatory effects, and it could be contemplated that IL‐6 and IL‐12B expressions result in subsequent leprous neuritis in our cases. 4
Covid‐19 vaccine‐associated ENL and foot drop in Hansen's disease have been reported previously, but type I reaction with cutaneous flare and acute neuritis has probably not been reported so far. 3 , 5 The four cases reported are from a leprosy care centre in India where the disease is endemic. CXCL10 and IL‐6 along with cortisone–cortisol shuttle enzymes are potential laboratory markers of type 1 reactions, and IL‐17 and platelet‐derived growth factor BB (PDGF‐BB) are for type 2 reactions. The sheer versatility of clinical presentation of leprosy reactions following Covid‐19 vaccination warrants further large‐scale molecular studies including assays of potential laboratory inflammatory markers to establish the exact pathogenesis of vaccine‐related adverse effects.
Conflicts of Interests
None declared.
Author contribution
The manuscript has been read and approved by all the authors. The requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Acknowledgement
The patient in the manuscript has given written informed consent to the publication of case details and photographs.
Data availability statement
The authors declare that data supporting the findings of this case are available within the article and its supplementary information file.
References
- 1. Choudhary OP, Choudhary P, Singh I. India's COVID‐19 vaccination drive: key challenges and resolutions. Lancet Infect Dis 2021; 21(11): 1483–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rerknimitr P, Puaratanaarunkon T, Wongtada C et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID‐19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol 2022; 36: e158–e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rebello PF, Pennini SN. Erythema nodosum leprosum and active leprosy after ChAdOx1‐S/nCoV‐19 recombinant vaccine. A report of two cases. Lepr Rev 2021; 92: 421–426. [Google Scholar]
- 4. Panda AK, Begum F, Panda M, Jena AK. Trigger of type 2 lepra reaction with acute foot drop following Covid‐19 vaccination. J Eur Acad Dermatol Venereol 2022; 36: e334‐335. [DOI] [PubMed] [Google Scholar]
- 5. Santos Morais Junior G, Shu Kurizky P, Penha Silva Cerqueira SR et al. Enhanced IL‐6 and IL‐12B gene expression after SARS‐CoV‐2 infection in leprosy patients may increase the risk of neural damage. Am J Trop Med Hyg 2021; 104: 2190–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this case are available within the article and its supplementary information file.


