Abstract
Background
COVID‐19 convalescent plasma (CCP) was approved under emergency authorization to treat critically ill patients with COVID‐19 in the United States in 2020. We explored the demographics of donors contributing plasma for a hyperimmune, plasma‐derived therapy to evaluate factors that may be associated with anti‐SARS‐CoV‐2 antibody response variability and, subsequently, antibody titers.
Study Design
An electronic search of CCP donors was performed across 282 US plasma donation centers. Donations were screened for nucleocapsid protein‐binding‐IgG using the Abbott SARS‐CoV‐2 IgG assay.
Results
Overall, 52 240 donors donated 418 046 units of CCP. Donors were of various ethnicities: 43% Caucasian, 34% Hispanic, 17% African American, 2% Native American, 1% Asian, and 3% other. Females had higher initial mean anti‐SARS‐CoV‐2 antibody titers but an overall faster rate of decline (P < .0001). Initial antibody titers increased with age: individuals aged 55 to 66 years had elevated anti‐SARS‐CoV‐2 titers for longer periods compared with other ages (P = .0004). African American donors had the lowest initial antibody titers but a slower rate of decline (P < .0001), while Caucasian (P = .0088) and Hispanic (P = .0193) groups had the fastest rates of decline. Most donor antibody levels decreased below the inclusion criteria (≥1.50) within 30 to 100 days of first donation, but donation frequency did not appear to be associated with rate of decline.
Conclusion
Several factors may be associated with anti‐SARS‐CoV‐2 antibody response including donor age and sex. Evaluating these factors during development of future hyperimmune globulin products may help generation of therapies with optimal efficacy.
Keywords: convalescent COVID‐19 plasma, hyperimmune globulins, plasma donor demographics, SARS‐CoV‐2
1. INTRODUCTION
Convalescent plasma (CP) has been used to treat various infectious diseases including Ebola, middle eastern respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and, more recently, COVID‐19, caused by SARS coronavirus 2 (SARS‐CoV‐2). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 CP can be collected from non‐remunerated donors at blood centers or at source plasma centers, where donors receive payment for donations, and can be used for both direct patient infusion and to make hyperimmune globulin products. On March 24, 2020, under an emergency access program, the Food and Drug Administration (FDA) issued an emergency use authorization for COVID‐19 convalescent plasma (CCP) in critically ill patients with COVID‐19 or those at high risk of severe disease. 12 Studies evaluating CCP have yielded heterogenous findings: some demonstrate an improvement in COVID‐19 disease course, with a potential benefit being particularly evidenced when CCP is transfused early in disease course, 13 , 14 , 15 , 16 while others suggest little benefit of CCP in treatment of COVID‐19. 3 , 17 , 18 , 19 , 20 , 21 , 22 , 23
Early in the COVID‐19 pandemic, CSL Plasma and several other companies joined efforts as the CoVIg‐19 Plasma Alliance to collect CCP for development of a COVID‐19 hyperimmune globulin product (CoVIg‐19). Two other companies (Grifols and Emergent BioSolutions) worked separately with the Biomedical Advanced Research Development Authority (BARDA) to develop two additional COVID‐19 hyperimmune globulin products. These COVID‐19 hyperimmune globulin products were subsequently investigated in a Phase 3 clinical trial (NCT04546581) that sought to study the safety, efficacy, and tolerability of a combination of remdesivir (and other standard of care) and hyperimmune globulins to treat patients with COVID‐19. Approximately, 600 adult patients with COVID‐19 were enrolled in the United States and 10 other countries across 5 continents. 24 , 25 Patients were enrolled if they had been hospitalized and symptomatic with COVID‐19 for 12 or fewer days without life‐threatening pulmonary dysfunction or organ failure. 24 , 25 Though the study demonstrated that there were no safety concerns with CoVIg‐19, there was no evidence of improved clinical outcomes at day 7 with CoVIg‐19 compared with placebo. 24 , 25 While further analysis is pending, it is important to establish why the hyperimmune COVID‐19 immunoglobulin product did not provide significant clinical benefit and what can be done in the future to improve study outcomes with hyperimmune globulins.
In this study, we sought to assess the demographics of the donors whose CCP was used to develop the CoVIg‐19 product manufactured by CSL Behring for the Plasma Alliance to better understand factors that correlate with the anti‐SARS‐CoV‐2 antibody response and the rate of antibody dissipation. Through an improved understanding of these factors, we hope to gain further insight into the immune response to COVID‐19 and how we might better respond to the need for a hyperimmune product in the future.
2. MATERIALS AND METHODS
2.1. Donor recruitment
Between April 28, 2020, and April 21, 2021, CSL Plasma solicited convalescent donors from the community and required proof of previous COVID‐19 infection (either testing positive for anti‐SARS‐CoV‐2 antibodies or having had a positive COVID‐19 PCR test), provided by the donor. Donors who were already giving plasma in the United States were offered anti‐SARS‐CoV‐2 antibody testing at plasma collection centers. All CCP donors were required to be at least 14 days post‐recovery from COVID‐19 symptoms or at least 14 days post‐positive COVID‐19 test. CCP volumes collected were similar to volumes collected during non‐COVID‐19 plasmapheresis in source plasma centers. Donors who had been vaccinated were not eligible for inclusion in the study. Each plasma donation was tested for anti‐SARS‐CoV‐2 antibody levels to ensure inclusion of high titer donations only.
2.2. Donor demographics data collection
An electronic search of all CCP donors was performed across 282 plasma donation centers in the United States operated by CSL Plasma. All donors were applicants or qualified by the Plasma Protein Therapeutics Association's (PPTA) International Quality Plasma Program (IQPP) process. Donor demographics assessed included age, sex, ethnicity, location, and number and frequency of donations. Donor sex and ethnicity were self‐reported.
2.3. Anti‐SARS‐CoV‐2 antibody testing
All plasma donations were screened for nucleocapsid (NC) protein‐binding‐IgG using the Abbott SARS‐CoV‐2 IgG diagnostic method (Cat. #06R8620, Abbott Laboratories, Abbott Park, Illinois) run on an Abbott ARCHITECT Analyzer, according to the manufacturer instructions. This assay qualitatively detects IgG antibodies against the SARS‐CoV‐2 NC protein, with an assay specificity of 99.6% to 99.9% and sensitivity of 100%. 26 The signal to cut‐off ratio in each sample was determined automatically through comparison of the measured chemiluminescent relative light unit (RLU) to the calibrator RLU, which is reported as index sample/calibrator (S/C). Results ≥1.40 index S/C are considered positive and donors whose initial anti‐SARS‐CoV‐2 antibody levels fell below this level were either not entered into, or excluded from, the CCP donor program. For practical reasons, a ≥1.50 cut‐off was utilized for longitudinal analysis of donor titers in this study.
2.4. Evaluation of rate of decline
From the total cohort, only donors who provided ≥5 donations were considered for the rate of decline studies to achieve a robust estimation. Based on the linear relationship between titer and time (in days) observed for most donors, we utilized the following linear model:
where T(d) = titer d days after first donation; T 0 = estimated initial titer; d = days since first donation; and r = rate of decline (or increase) of the titer per day. T 0 and r were estimated via linear regression, but separately for each donor. Donor titers were assessed up to 120 days post‐first donation, as this was the longest period in which there was a large enough cohort of donors.
2.5. Statistical analysis
Univariate analysis was performed using standard, two‐sided t‐tests with the null hypothesis assuming no difference between two groups. Tested variables were donor sex, age, and ethnicity. A cut off of P < .05 indicated significance. Multivariate analysis was performed to investigate the impact of sex and titer of first donation on the rate of decline and results are displayed via boxplots showing the rate of decline distribution, stratified by sex and ranges of the titer of first donation. All statistical analyses were performed using R. 27
3. RESULTS
3.1. Donor demographics
A total of 52 240 donors donated 418 046 units of convalescent COVID‐19 plasma between April 2020 and April 2021. There were 8399 (16%) donors who donated once and 43 841 (84%) donors who donated more than once. The majority of CCP donors donated every 4 days. There was a slight male donor predominance (n = 28 210, 54%) compared with females (n = 24 030, 46%; Table 1). Donor age ranged from 18 to 66 years old, with a mean (SD) age of 36.42 ± 12.39 years old. Donors were of various ethnicities, with the most common being Caucasian (n = 22 463, 43%) and Hispanic (n = 17 762, 34%; Table 1). The most common donor region was the Southwest (n = 21 418, 41%).
TABLE 1.
Donor demographics
| Characteristic | N = 52 240 |
|---|---|
| Sex | |
| Male | 28 210 (54%) |
| Female | 24 030 (46%) |
| Age (years) | |
| 18‐25 | 9403 (18%) |
| 26‐40 | 21 418 (41%) |
| 41‐55 | 16 195 (31%) |
| 56‐66 | 5224 (10%) |
| Ethnicity | |
| African American | 8881 (17%) |
| Asian | 522 (1%) |
| Caucasian | 22 463 (43%) |
| Hispanic | 17 762 (34%) |
| Native American | 1045 (2%) |
| Other | 1567 (3%) |
| Region | |
| Midwest | 12 538 (24%) |
| Northeast | 2612 (5%) |
| Rocky Mountains | 3134 (6%) |
| Southeast | 11 493 (22%) |
| Southwest | 21 418 (41%) |
| West | 1045 (2%) |
Note: All values are n (%).
3.2. Initial anti‐SARS‐CoV‐2 antibody titers
The mean (SD) initial anti‐SARS‐CoV‐2 antibody level for females was 4.28 ± 1.94 compared with 4.08 ± 1.92 for males (P < .0001; Figure 1A, Table 2). Mean initial anti‐SARS‐CoV‐2 antibody levels varied by both age and ethnicities: antibody levels tended to rise with increasing age, with significant differences observed between all age groups when compared with each other (P < .0001), while both the Asian and Native American subgroups demonstrated statistically significant higher initial anti‐SARS‐CoV‐2 antibody levels compared with all other groups (P < .0001). In contrast, the initial anti‐SARS‐CoV‐2 antibody levels were significantly lower in the African American subgroup compared with all other ethnicities (P < .0001; Table 2).
FIGURE 1.

Anti‐SARS‐CoV‐2 antibody levels and sex. (A) Distribution of mean initial anti‐SARS‐CoV‐2 titers by sex. Anti‐SARS‐CoV‐2 antibody levels (S/C ratio) were determined by the Abbott assay (x‐axis), with an S/C of <1.40 indicating negativity. The y‐axis represents the relative frequency (density) of donors. (B) Change in anti‐SARS‐CoV‐2 antibody titers per day by sex. The x‐axis shows the change in anti‐SARS‐CoV‐2 antibody levels and the y‐axis represents donor density. (C) Change in anti‐SARS‐CoV‐2 titer per day by initial titer range and sex
TABLE 2.
Initial mean anti‐SARS‐CoV‐2 antibody level and rate of decline by sex, age, and ethnicity
| Initial mean anti‐SARS‐CoV‐2 antibody level | Rate of decline a | |||
|---|---|---|---|---|
| Mean ± SD | P value b | Mean ± SD | P value b | |
| Sex | ||||
| Male | 4.08 ± 1.92 | <.0001 | −1.57 ± 1.00 | <.0001 |
| Female | 4.28 ± 1.94 | <.0001 | −1.92 ± 1.09 | <.0001 |
| Age | ||||
| 18‐25 | 3.66 ± 1.71 | <.0001 | −1.81 ± 1.04 | <.0001 |
| 26‐40 | 4.09 ± 1.93 | <.0001 | −1.74 ± 1.07 | .2807 |
| 41‐55 | 4.55 ± 1.97 | <.0001 | −1.71 ± 1.05 | .0886 |
| 55‐66 | 4.98 ± 1.95 | <.0001 | −1.66 ± 1.08 | .0004 |
| Ethnicity | ||||
| African American | 4.06 ± 1.98 | <.0001 | −1.61 ± 1.04 | <.0001 |
| Asian | 4.81 ± 2.02 | <.0001 | ND | ND |
| Caucasian | 4.16 ± 1.91 | .0961 | −1.76 ± 1.10 | .0088 |
| Hispanic | 4.25 ± 1.94 | .0110 | −1.76 ± 1.02 | .0193 |
| Native American | 4.54 ± 1.94 | <.0001 | −1.72 ± 1.07 | .4002 |
| Other | 4.17 ± 1.89 | .3886 | −1.71 ± 1.01 | .2165 |
Abbreviations: CCP, COVID‐19 convalescent plasma; ND, not determined; SD, standard deviation.
Rate of decline was determined from donors who donated at least five times.
P value determined for each subgroup vs all others. ND values could not be calculated due to limited sample size.
3.3. Rate of antibody decline by sex
The mean rate of anti‐SARS‐CoV‐2 antibody decline was significantly different between the sexes, with a rate of decline of −1.92 ± 1.09 for females and −1.57 ± 1.0 for males, respectively (P < .0001; Table 2). The change in anti‐SARS‐CoV‐2 titer per day was also greater in females than males (Figure 1B). Multivariate analysis evaluating the relationship between rate of decline, sex, and the titer of first donation further confirmed these findings and also suggested that, regardless of sex, higher initial antibody titers, up to a certain level, may give rise to a stronger rate of decline over time (Figure 1C).
3.4. Rate of antibody decline by age
A trend of increasing mean initial levels of anti‐SARS‐CoV‐2 antibody was observed with increasing donor age, which was paralleled by a slower rate of decline with increasing age (Figure 2A,B, Table 2). The mean rate of antibody decline was significantly faster for the 18 to 25‐year‐old group (P < .0001) and significantly slower for the 55 to 66‐year‐old group (P = .0004) compared with the other age groups. Thus, older donors appeared to have anti‐SARS‐CoV‐2 antibody levels that were initially higher and persisted for extended periods of time compared with younger donors.
FIGURE 2.
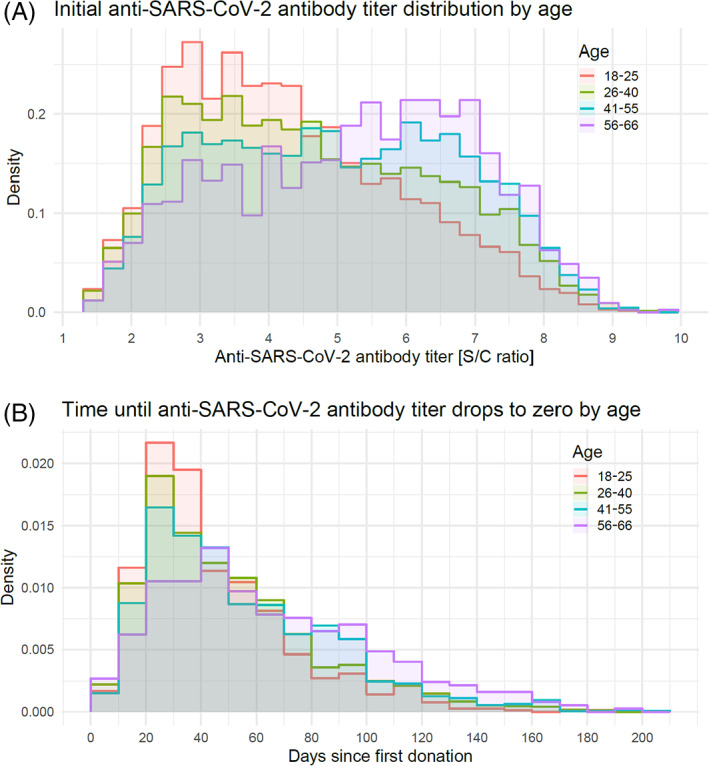
Anti‐SARS‐CoV‐2 antibody levels and age. (A) Distribution of initial anti‐SARS‐CoV‐2 titers by age. Anti‐SARS‐CoV‐2 antibody levels (S/C ratio) were determined by the Abbott assay (x‐axis), with an S/C of <1.40 indicating negative titers. The y‐axis represents the relative frequency (density) of donors. (B) Time until anti‐SARS‐CoV‐2 antibody titer drops to <1.50 by age
3.5. Rate of antibody decline by ethnicity
The rate of decline demonstrated significant variability between ethnicities, most notably being significantly slower in the African American group compared with all other groups (−1.61 ± 1.04, P < .0001; Table 2). Of note, African American donor initial antibody levels were also the lowest of any ethnic group analyzed. Intergroup comparisons also revealed various significant differences in the rate of decline between ethnicities, with the Caucasian and Hispanic groups demonstrating the fastest rates of decline (−1.76 ± 1.10, P = .0088 and −1.76 ± 1.02, P = .0193, respectively) (Table 2).
3.6. Rate of antibody decline by frequency of donation
The time for anti‐SARS‐CoV‐2 antibody levels to decrease to a level of <1.50 was right skewed with the majority of donors decreasing to <1.50 between 30 and 100 days after their first donation. By approximately 120 days, almost all donors had antibody levels that were essentially undetectable; however, approximately 5% of the donor population appeared to have at least a neutral titer evolution up until day 120 (Figure 3). The rate of antibody decline was unchanged when analyzed using frequency of donation as an additional explanatory variable. Additionally, the rate of antibody decline was not faster in the donors who donated twice weekly compared with those who donated less frequently.
FIGURE 3.
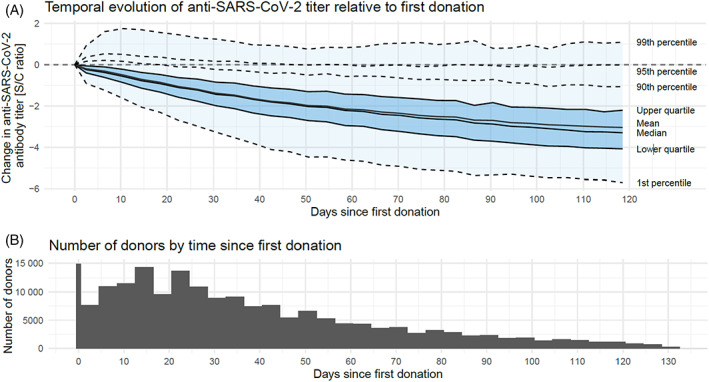
Temporal evolution of anti‐SARS‐CoV‐2 antibody levels. (A) Change of anti‐SARS‐CoV‐2 titer over time relative to the first donation and out to 120 days after the first donation. Assessing titers beyond 120 days resulted in a significant reduction in donor numbers and thus too small a cohort for meaningful analyses. The distribution of the change in anti‐SARS‐CoV‐2 titer across the donor population is displayed by quantiles, with the inner 98% of the distribution shaded in light blue and the central 50% in darker blue. (B) The number of donors with donations after X days since the first donation
4. DISCUSSION
This study assessed anti‐SARS‐CoV‐2 antibody titers and their rate of decline in a large CCP cohort to identify correlations between plasma donor characteristics and antibody response in the United States.
In this study, female CCP donors had higher mean initial anti‐SARS‐CoV‐2 antibody levels compared with males; however, the rate of decline was more rapid in females, perhaps suggesting that lower initial titers in males might be offset by a slower rate of decline. Indeed, multivariate analysis confirmed that, regardless of sex, higher initial antibody titers appeared to be associated with a faster rate of antibody decline. In contrast to our findings, several studies have found that males tend to have higher levels of convalescent anti‐SARS‐CoV‐2 antibodies. 28 , 29 , 30 , 31 , 32 , 33 , 34 Jain et al demonstrated that higher levels of anti‐nucleocapsid IgG S/C ratios were associated with males, older age, Hispanic ethnicity, and fewer days between symptom onset and first donation. 35 Another study found that higher neutralizing antibody levels were observed in males, older donors, and previously hospitalized donors, 33 while a study using the same antibody assay as in this study (Abbott SARS‐CoV‐2 IgG assay) did not find any association between donor sex and anti‐SARS‐CoV‐2 antibody level. 36 The discrepancies in the association of CCP donor sex with antibody response could be a result of several factors. Of note, several of the studies that found higher convalescent anti‐SARS‐CoV‐2 antibody levels in males evaluated neutralizing antibody levels, while in our study, we examined anti‐NC IgG levels using the Abbott assay. Although the Abbott assay has been confirmed to correlate well with neutralizing antibody assays (Figure S1), methodological differences may play a role in these discrepancies. This is partially supported by Karbiener et al who employed the Abbott assay and did not find a difference in antibody levels between the sexes. 36 Additionally, there is likely an impact of temporal heterogeneity between studies: females had faster rate of anti‐SARS‐CoV‐2 antibody decline thus, depending upon when titers were assessed, their antibody levels could have fallen below those of their male counterparts. Biologically, females tend to have stronger immune responses to infection which is thought to be, in part, due to genetic and hormonal influences on the immune system. 37 , 38 Additionally, testosterone has been found to have a suppressive effect on immune function. 39 The higher titers observed in females in this study could, therefore, also be a reflection of these biological factors. The differences in anti‐SARS‐CoV‐2 titers between males and females in our study are numerically small but statistically significant, and though potentially not imperative in choosing CCP units for direct infusion to patients, could be important in the manufacture of hyperimmune globulin products where 3000 to 5000 units are pooled and fractionated. Thus, further studies are needed to evaluate sex discrepancies in the SARS‐CoV‐2 antibody response and rate of decline to determine the potential impact for future hyperimmune globulins.
Like several of the previously described studies, we found that older age was associated with higher anti‐SARS‐CoV‐2 antibody levels in CCP. Individuals in the highest age bracket (55‐66 years old) had elevated antibody levels that persisted for extended periods of time (>100 days) compared with donors of all other ages, while donors aged 18 to 30 years old tended to have lower initial anti‐SARS‐CoV‐2 antibody levels that diminished more quickly. This aligns with the study by Karbiener et al, who evaluated anti‐SARS‐CoV‐2 antibody levels in 8749 CCP units in a longitudinal study and saw a decrease in mean antibody titer over time which was attributed to an increase in CCP donation by younger donors. 36 They found that younger donors had lower levels of anti‐SARS‐CoV‐2 antibody levels and that CCP units with the highest anti‐SARS‐CoV‐2 antibody levels were obtained soon after convalescence from COVID‐19 patients who required hospitalization, from advanced age donors, and from Black/African/Hispanic American vs White/Caucasian ethnicities. Similarly, several other studies have also found that older CCP donors had higher neutralizing antibody levels. 33 , 34 , 40 It may be surprising to some that older individuals tended to demonstrate higher anti‐SARS‐CoV‐2 antibody levels that persisted for longer than younger donors, but this has been a consistent finding in CCP studies. This phenomenon may be secondary to older individuals being more prone to being sicker and more symptomatic with COVID‐19 compared with younger people. 41 , 42 , 43 Typically, individuals who are more symptomatic with a particular illness develop a stronger immune response to fight the illness and recover. 38 , 44 This is well established and these individuals have long been targeted as a source of CP. 45 , 46 Thus, older donors who had perhaps experienced enhanced immune responses (both in initial titers and in longevity of response) to SARS‐CoV‐2 than their younger counterparts may have had significantly higher levels anti‐SARS‐CoV‐2. Additionally, immune response is known to wane over time; however, the anti‐SARS‐CoV‐2 antibody response is particularly fast to wane. 47 Of note, in the last few years, CSL Plasma has expanded the donor age limits to include individuals up to age 75. Older donors currently comprise a small proportion of source donors (as suggested by the oldest age bracket in our study, 55‐66, being the smallest proportion of donors [10% total]). This may be in part caused by the regulatory changes to donor age being relatively new. Overall, the source plasma donor population tends to be younger in comparison to whole blood donors. 48 The data in this study validates CSL Plasma's donor age group expansion and the low proportions of older donors could perhaps suggest that older donors should be actively recruited when developing hyperimmune globulin products in the future.
Hispanic, African American, and Native American ethnic groups comprised a considerable proportion of the CCP donors in this study compared with others. 33 , 49 , 50 , 51 We found that donors of Asian ethnicity had the highest mean initial anti‐SARS‐CoV‐2 antibody levels of all ethnicities, while African Americans had the lowest mean initial antibody levels. In contrast, Karbiener et al found that Black/African American CCP donors had higher anti‐SARS‐CoV‐2 antibody levels compared with White/Caucasian ethnicities, though they also reported higher titers in donors of Hispanic ethnicity compared with White/Caucasian Americans. 36 Jain et al also saw higher levels of anti‐SARS‐CoV‐2 antibodies in Hispanic convalescent donors. 35 Interestingly, African American CCP donors had the slowest rate of antibody decline as compared with other groups. Vassallo et al studied anti‐SARS‐CoV‐2 antibody seropositivity and demographics of blood donors and found that female sex, non‐Hispanic White or Asian ethnicity, and age ≥65 were associated with lower odds of anti‐SARS‐CoV‐2 antibody reactivity. 51 They observed that donors that were male, ethnicity other than non‐Hispanic White, age 16 to 17 years, and geographic location were associated with positive anti‐SARS‐CoV‐2 antibody results. 51 These findings are not in opposition to ours as they were investigating all blood donors and which groups are more likely to test positive for anti‐SARS‐CoV‐2 antibodies, while in our study all donors were known to be positive as it was a prerequisite. Our study contains one of the largest CCP donor cohorts to date and may, therefore, present the best reflection of ethnic differences in anti‐SARS‐CoV‐2 antibody response in the United States between the dates studied. The results of this study are generalizable to the US population because of the diverse population that was studied, which is distinct from the whole blood donor population. 48 Of note, regional demographic data indicated that the most common location for donors was the Southwest. This is likely to align with the locations of numerous plasma centers in the Southwest, particularly Texas.
In our study, anti‐SARS‐CoV‐2 antibody levels decreased to below positive levels as defined by the Abbott assay between 30 and 100 days after first donation. Multiple other studies have previously suggested a similar period for decline of the anti‐SARS‐CoV‐2 antibody response, including Schlickeiser et al, who found that at 58 days after symptom onset, only 12.6% of potential plasma donors showed high levels of neutralizing antibodies. 52 Likewise, a study by Miller et al found that donors who were ≥60 days from COVID‐19 symptom onset were less likely to have qualifying (titer ≥1:80) neutralizing antibody levels. 40 To obtain the maximum CCP product, donors with high anti‐SARS‐CoV‐2 antibody levels should be encouraged to donate frequently within this window. Under current regulations, when collecting source plasma, donors can donate twice within 7 days, meaning a substantial number of donations from a single donor could theoretically be collected within the denoted window. Of note, while there was a relatively narrow decay band for a large proportion of the donor population, approximately 5% demonstrated at least a neutral titer evolution until around 120 days post‐first donation. Identification of these 5% could be very meaningful in terms of both evaluation of the SARS‐CoV‐2 immune response and development of future hyperimmune globulins.
Our temporal data also indicated that frequency of donation was not related to decreased anti‐SARS‐CoV‐2 antibody titers, suggesting that maximizing donation frequency within the first few weeks of initial donation should offer the optimal immunoglobulin yield for hyperimmune globulin products. The rate of anti‐SARS‐CoV‐2 antibody decline was calculated only for those donating at least five times. While the frequency of donations was not associated with the rate of antibody decline, this may have been influenced by the absolute number of donations as more donations occur over time and antibody level declines with time. Of note, multivariate analysis suggested that higher initial anti SARS‐CoV‐2 titers may be associated with a faster rate of decline. This could suggest that more careful selection of donors in early vs late windows post‐first donation may be warranted and that those with lower antibody titers at first donation should not be disregarded for later donations, as they may maintain the immune response for a longer period than those with higher initial titers.
This study has several limitations. First and foremost, minimal information was available regarding donor COVID‐19 disease course or severity, symptoms, hospitalization, or date of illness. Moreover, timing from infection to donation was unknown. This data would have been useful knowledge since proximity to and severity of infection can result in heightened anti‐SARS‐CoV‐2 immune responses and therefore could have explained some of the differences in antibody titers we observed. Second, donors were only required to bring proof of a positive COVID‐19 test when they came to donate and were required to be at least 14 days out from either the positive test or last symptom, while later in the pandemic, anti‐SARS‐CoV‐2 antibody testing was offered to known, qualified donors. This may have resulted in recruiting asymptomatic COVID‐19 donors. Third, different SARS‐CoV‐2 virus variants were not taken into account. Finally, only one assay was used to assess antibody levels. While use of an additional assay would be an excellent method to validate antibody titers, it would have been difficult to implement in this study given the substantial number of plasma donations and the manner in which they were assessed. The ARCH SARS‐CoV‐2 IgG assay is, nevertheless, widely used and has been shown to positively correlate with neutralizing antibody assays therefore should provide robust assessment of antibody titers. 53
5. CONCLUSIONS
CCP was in high demand during the recent surges of the more aggressive and highly transmissible COVID‐19 variants. By better understanding the demographics of donors associated with higher anti‐SARS‐CoV‐2 antibody levels and the rates of decline following infection, donors with higher antibody titers could be selectively recruited in the future. In this study, which is one of the largest in looking at convalescent donor demographics to date, we found that donors of older age had higher anti‐SARS‐CoV‐2 antibody titers, suggesting that older donors could be targeted for collection of high‐titer CCP. This aligns with recent changes to donor recruitment policies, with donors aged 65 to 75 no longer requiring physician approval to continue donating. We also found some variability in antibody titers between ethnicities: those of Asian ethnicity had higher initial antibody titers while those of African American ethnicity had lower initial titers but a slower rate of decline. Overall, these findings support a source plasma donor recruitment approach that aims to encompass all ethnicities to reflect the diverse population of the United States, though the clinical significance of the variations in antibody responses according to ethnicity remains to be fully understood. The geographical findings of plasma donor distribution largely reflect the locations of the CSL Plasma donor centers, including some donors from Mexico in centers near the border in Texas. Finally, the frequency of donations did not impact the rate of antibody decline in donors, suggesting that those with high initial titers could perhaps be targeted for quick repeat donations, according to FDA guideline to maximize collection of their highest antibody titers for future products. Overall, these data contribute to the growing literature on how CCP donor demographic might influence the antibody response and indicate areas to explore to identify how we might better respond to the need for a hyperimmune product in the future.
CONFLICT OF INTEREST
All of the authors are employees of CSL Plasma or CSL Behring.
Supporting information
Figure S1 Correlation of the Abbott assay S/C ratio vs neutralization titer. Neutralizing titers were assessed using the methodology described in Barnes et al. 53 Each data point represents one donation. A linear regression was fitted to each graph.
ACKNOWLEDGMENTS
Editorial assistance was provided by Meridian Healthcomms Ltd, funded by CSL Plasma.
Schmidt AE, Vogel P, Chastain CA, Barnes T, Roth NJ, Simon TL. Analysis of 52 240 source plasma donors of convalescent COVID‐19 plasma: Sex, ethnicity, and age association with initial antibody levels and rate of dissipation. J Clin Apher. 2022;37(5):449‐459. doi: 10.1002/jca.21998
Funding information CSL Plasma
DATA AVAILABILITY STATEMENT
Author elects to not share data.
REFERENCES
- 1. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. JAMA. 2020;324(5):460‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeng F, Chen X, Deng G. Convalescent plasma for patients with COVID‐19. Proc Natl Acad Sci U S A. 2020;117(23):12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130(4):1545‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rockman S, Maher D, Middleton D. The use of hyperimmune serum for severe influenza infections. Crit Care Med. 2012;40(3):973‐975. [DOI] [PubMed] [Google Scholar]
- 9. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450‐1451. [DOI] [PubMed] [Google Scholar]
- 10. Arabi Y, Balkhy H, Hajeer AH, et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springerplus. 2015;4:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanne JH. Covid‐19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. [DOI] [PubMed] [Google Scholar]
- 13. Kanj S, Al‐Omari B. Convalescent plasma transfusion for the treatment of COVID‐19 in adults: a global perspective. Viruses. 2021;13(5):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klassen SA, Senefeld JW, Johnson PW, et al. The effect of convalescent plasma therapy on mortality among patients with COVID‐19: systematic review and meta‐analysis. Mayo Clin Proc. 2021;96(5):1262‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Psaltopoulou T, Sergentanis TN, Pappa V, et al. The emerging role of convalescent plasma in the treatment of COVID‐19. Hemasphere. 2020;4(3):e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanz C, Nomdedeu M, Pereira A, et al. Efficacy of early transfusion of convalescent plasma with high‐titer SARS‐CoV‐2 neutralizing antibodies in hospitalized patients with COVID‐19. Transfusion. 2022;62:974‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Libster R, Perez Marc G, Wappner D, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384(7):610‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korley FK, Durkalski‐Mauldin V, Yeatts SD, et al. Early convalescent plasma for high‐risk outpatients with Covid‐19. N Engl J Med. 2021;385(21):1951‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Begin P, Callum J, Jamula E, et al. Convalescent plasma for hospitalized patients with COVID‐19: an open‐label, randomized controlled trial. Nat Med. 2021;27(11):2012‐2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abani O, Abbas A, Abbas F, et al. Convalescent plasma in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised controlled, open‐label, platform trial. Lancet. 2021;397(10289):2049‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. CoVIg‐19 Plasma Alliance . CoVIg‐19 Plasma Alliance announces topline results from NIH‐sponsored clinical trial of investigational COVID‐19 hyperimmune globulin medicine. Press release, April 2nd, 2021. https://www.takeda.com/newsroom/newsreleases/2021/covig-19-plasma-alliance-announces-topline-results-from-nih-sponsored-clinical-trial-of-investigational-covid-19-hyperimmune-globulin-medicine/. Accessed July 7, 2022.
- 25. Polizzotto MN, Nordwall J, Babiker AG, et al. Hyperimmune immunoglobulin for hospitalised patients with COVID‐19 (ITAC): a double‐blind, placebo‐controlled, phase 3, randomised trial. Lancet. 2022;399(10324):530‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS‐CoV‐2 IgG assay and Seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- 28. Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71(10):2688‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID‐19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141‐6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Focosi D, Franchini M. Clinical predictors of SARS‐CoV‐2 neutralizing antibody titers in COVID‐19 convalescents: implications for convalescent plasma donor recruitment. Eur J Haematol. 2021;107(1):24‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Markmann AJ, Giallourou N, Bhowmik DR, et al. Sex disparities and neutralizing‐antibody durability to SARS‐CoV‐2 infection in convalescent individuals. mSphere. 2021;6(4):e0027521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mehew J, Johnson R, Roberts D, Harvala H. Convalescent plasma for COVID‐19: male gender, older age and hospitalisation associated with high neutralising antibody levels, England, 22 April to 12 May 2020. Euro Surveill. 2020;25(45):2001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Del Fante C, Franchini M, Baldanti F, et al. A retrospective study assessing the characteristics of COVID‐19 convalescent plasma donors and donations. Transfusion. 2021;61(3):830‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jain S, Garg K, Tran SM, et al. Characteristics of coronavirus disease 19 convalescent plasma donors and donations in the New York metropolitan area. Transfusion. 2021;61(8):2374‐2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karbiener M, Farcet MR, Ilk R, et al. Longitudinal analysis of SARS‐CoV‐2 antibodies in 8000 U.S. first‐time convalescent plasma donations. Transfusion. 2021;61(4):1141‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. 2004;10(11):2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arnold CG, Libby A, Vest A, Hopkinson A, Monte AA. Immune mechanisms associated with sex‐based differences in severe COVID‐19 clinical outcomes. Biol Sex Differ. 2022;13(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta‐analysis. Biol Rev Camb Philos Soc. 2017;92(1):551‐571. [DOI] [PubMed] [Google Scholar]
- 40. Miller MJ, Skrzekut A, Kracalik I, et al. How do I … facilitate a rapid response to a public health emergency requiring plasma collection with a public‐private partnership? Transfusion. 2021;61(10):2814‐2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim L, Garg S, O'Halloran A, et al. Risk factors for intensive care unit admission and in‐hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID‐19)‐associated hospitalization surveillance network (COVID‐NET). Clin Infect Dis. 2021;72(9):e206‐e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldberg EM, Southerland LT, Meltzer AC, et al. Age‐related differences in symptoms in older emergency department patients with COVID‐19: prevalence and outcomes in a multicenter cohort. J Am Geriatr Soc. 2022;70(7)1918‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao L, Lu L, Ma W. Immunopathological changes, complications, sequelae and immunological memory in COVID‐19 patients. Heliyon. 2022;8(4):e09302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bozzo J, Jorquera JI. Use of human immunoglobulins as an anti‐infective treatment: the experience so far and their possible re‐emerging role. Expert Rev Anti Infect Ther. 2017;15(6):585‐604. [DOI] [PubMed] [Google Scholar]
- 46. Burnouf T, Seghatchian J. Ebola virus convalescent blood products: where we are now and where we may need to go. Transfus Apher Sci. 2014;51(2):120‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumar S, Saxena SK, Maurya VK, Tripathi AK. Progress and challenges toward generation and maintenance of long‐lived memory T lymphocyte responses during COVID‐19. Front Immunol. 2021;12:804808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmidt AE, MacKercher J, Youngling B, Simon TL. Source plasma deferral trends: a 3‐year analysis of 255 centers in the United States. J Clin Apher. 2022;37(1):31‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vinkenoog M, Steenhuis M, Brinke AT, et al. Associations between symptoms, donor characteristics and IgG antibody response in 2082 COVID‐19 convalescent plasma donors. Front Immunol. 2022;13:821721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fazeli A, Sharifi S, Mohammadi S, et al. The demographic and serological characteristics of COVID‐19 convalescent plasma donors: identification of basic criteria for optimal donor selection. Transfus Apher Sci. 2021;61:103302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vassallo RR, Dumont LJ, Bravo MD, Hazegh K, Kamel H. Progression and predictors of SARS‐CoV‐2 antibody seroreactivity in US blood donors. Transfus Med Rev. 2021;35(3):8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schlickeiser S, Schwarz T, Steiner S, et al. Disease severity, fever, age, and sex correlate with SARS‐CoV‐2 neutralizing antibody responses. Front Immunol. 2020;11:628971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barnes TW, Schulte‐Pelkum J, Steller L, et al. Determination of neutralising anti‐SARS‐CoV‐2 antibody half‐life in COVID‐19 convalescent donors. Clin Immunol. 2021;232:108871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Correlation of the Abbott assay S/C ratio vs neutralization titer. Neutralizing titers were assessed using the methodology described in Barnes et al. 53 Each data point represents one donation. A linear regression was fitted to each graph.
Data Availability Statement
Author elects to not share data.


