Abstract
Since the declaration of the novel SARS‐CoV‐2 virus pandemic, health systems/ health‐care‐workers globally have been overwhelmed by a vast number of COVID‐19 related hospitalizations and intensive care unit (ICU) admissions. During the early stages of the pandemic, the lack of formalized evidence‐based guidelines in all aspects of patient management was a significant challenge. Coupled with a lack of effective pharmacotherapies resulted in unsatisfactory outcomes in ICU patients. The anticipated increment in ICU surge capacity was staggering, with almost every ICU worldwide being advised to increase their capacity to allow adequate care provision in response to multiple waves of the pandemic. This increase in surge capacity required advanced planning and reassessments at every stage, taking advantage of experienced gained in combination with emerging evidence. In University Hospital Southampton General Intensive Care Unit (GICU), despite the initial lack of national and international guidance, we enhanced our ICU capacity and developed local guidance on all aspects of care to address the rapid demand from the increasing COVID‐19 admissions. The main element of this success was a multidisciplinary team approach intertwined with equipment and infrastructural reorganization. This narrative review provides an insight into the approach adopted by our center to manage patients with COVID‐19 critical illness, exploring the initial planning process, including contingency preparations to accommodate (360% capacity increment) and adaptation of our management pathways as more evidence emerged throughout the pandemic to provide the most appropriate levels of care to our patients. We hope our experience will benefit other intensive care units worldwide.
This article is categorized under:
Infectious Diseases > Genetics/Genomics/Epigenetics
Keywords: COVID‐19, critical care, planning, surge capacity, training
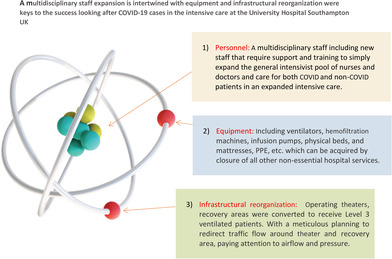
A multidisciplinary staff expansion is intertwined with equipment and infrastructural reorganisation were keys to the success looking after covid‐19 cases in the intensive care at the university hospital Southampton UK
1. INTRODUCTION
COVID‐19 caused by the SARS‐CoV‐2 beta‐coronavirus infection has inflicted devastating health and economic consequences worldwide (Cutler & Summers, 2020; WHO, 2022). It was first recognized in Wuhan, China (Huang et al., 2020), and the United Kingdom; the index case was identified in late January 2021. By March 2021, there was a rapid rise in cases across Europe, particularly in Lombardy, Italy, which resulted in significant anxiety and uncertainty over approaching such a rapidly growing number of cases in the United Kingdom. The lack of effective medical therapies or national/international guidelines coupled with a potentially overwhelming number of hospitalized patients resulted in a state of alarm among the medical community. Consequently, there was a rapid development of several internal committees and guideline development groups in our local hospital to address the concerns surrounding capacity, infection control, rapid testing, personnel protective equipment, training, and education in managing critically ill COVID‐19 patients and organization and participation into clinical research. The first wave was primarily of the original “Wuhan” variant and the subsequent second wave was due to the alpha variant. Our General Intensive Care Unit (GICU) admitted 340 patients with COVID‐19 during these two distinct pandemic waves (Figure 1). Despite the initial lack of national and international guidance on managing such patients, several management strategies were proposed and adopted. In this narrative review, we aim to provide an insight into the approach adopted by our center to manage patients with COVID‐19 critical illness, exploring the planning process, including contingency preparations to accommodate (360% GICU capacity increment), preparation of common pathways from existing evidence from China, and overall management strategies and outcomes. Our critical care outcome for the first two waves was better than the national average (Doidge et al., 2021; ICNARC, 2022). As more evidence emerged, we continued to adapt with our management pathways to provide appropriate levels of care. All clinical data presented here are collected as part of a larger observational study (Research evaluation alongside clinical treatment in COVID‐19 [REACT‐COVID‐19]) and the study was approved by Northwest Research Ethics Committee (REC 17/NW/0632) (Burke et al., 2020).
FIGURE 1.
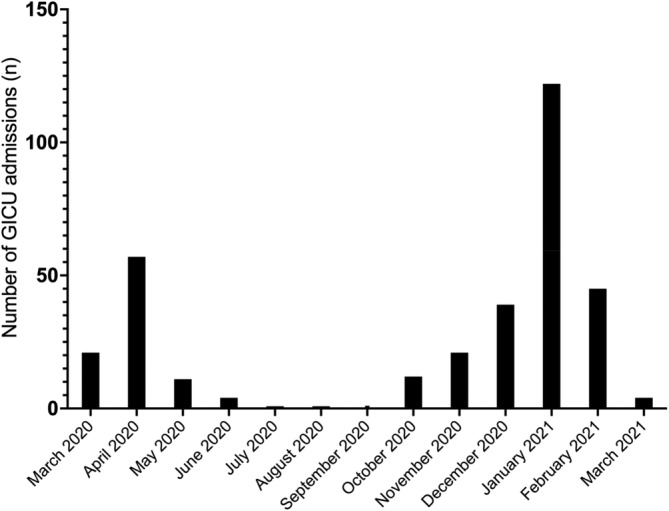
COVID‐19 related intensive care unit admissions over the first and second wave in Southampton University Hospital, United Kingdom
2. PREPARATION
2.1. Preparation for surge capacity
2.1.1. Rapid strategic planning and structural, personnel, equipment reorganization
The fundamental principle of responding to any major incident requires three main components: infrastructure, equipment, and personnel. With a novel infection and minimal data on the likely illness severity and management strategies, following a video conference call with an ICU team in Lombardy, Italy, from their recent experience, initially it was decided that our GICU should expand to provide invasive mechanical ventilation to potentially 165 patients at any one time. Ordinarily, our hospital has 54 ICU beds (including specialist ICU areas such as cardiac, neuro, and GICU); therefore, this threefold increase represented a significant increment from our standard practice. To address this anticipated increment following actions were implemented around three main areas personnel, equipment, and infrastructure (Table 1).
TABLE 1.
Initial infrastructural, equipment and personnel reorganization and expansion; key factors to success
| Personnel |
Care was done through a multidisciplinary staff composed of existing GICU staff and new non‐ICU supporting staff. A rapid development of a training program to induct non‐ICU medical and nursing staff to work with critically ill patients was inaugurated. The new staffs were distributed in teams to work alongside experienced existing GICU personnel to allow a very diluted patient to trained staff ratio. Through this we were able to expand the general intensivist pool of nurses and doctors and care for both COVID and non‐COVID patients in an expanded level 3 footprint. Senior medical cover The expansion of our capacity required multiple consultants to be present during the working day and at least one consultant to be resident overnight. The general ratio for consultant to patients was kept below 13 most of the time by the redeployment of ex‐ICU trained physicians. The Allied Health Professionals (AHP) including physiotherapists were supported by staff who were now not able to deliver their routine services and included radiologists and orthopedists among other colleagues. The practice required increased pharmacovigilance which was provided by the pharmacy department which was crucial in this multidisciplinary environment. The consultant intensivists relinquished all non‐ICU clinical activity and continued only those non‐clinical duties required to maintain safe running of GICU. The ICU consultant team was bolstered by “augmentee” intensivists drawn from our hospital's Neurosciences ICU and Pediatric ICU groups, who willingly joined in a full share of the COVID 19 workload. Rotas were reviewed and rewritten regularly to adapt and flex to demand and changing patient numbers. From the early stages of the pandemic we instituted resident night shifts, to ensure on‐site consultant presence around the clock. At the peak of activity, we had six consultants working during the daytime across the clinical footprint: five of these were responsible for their own clinical area, and one acted as consultant coordinator for the whole Unit. One of these would be scheduled to work until mid‐evening (2130), supported by a “Twilight” consultant (1600‐0000) and a Night consultant (2000‐0900). These consultants were supported by a single GICU clinical microbiologist (infection specialist) during working hours, and out of hours by an on‐call infection specialist. The role of the microbiologist was to provide updated microbiology advice for each patient, infection diagnostic stewardship, guiding antimicrobial therapy in cases of secondary bacterial infection, providing infection control advice to staff and decisions around isolation, de‐isolation of these patients during their GICU stay. The dedicated GICU microbiologist allowed for continuity of care among the rotating and everchanging GICU staff. Junior medical cover Medical Human Resources department in the hospital helped in organizing redeployment of junior medical staff from various departments across the hospital to supplement our baseline junior GICU medical staff. These doctors showed great adaptability and willingness to respond to the demands of COVID 19, as well as a highly professional and flexible attitude to the inevitable changes in shift allocations and increased proportion of out of hours. They were supported and trained to maintain a maximum patient ratio 1:6. Training A training package was introduced and delivered by the consultant intensivist team in the run‐up to the first pandemic wave, consisting of a series of bespoke lectures covering the basics of Critical Care and specific approaches to managing COVID 19‐associated critical illness, as well as hands‐on simulation scenarios to help introduce redeployed doctors and other clinical personnel to ICU equipment and how to approach everyday emergencies. These sessions were well‐received and gained positive feedback. In addition, a series of online resources were made available via the MicroGuide app (an application used to guide antimicrobial prescribing) and on Microsoft Teams covering the initial management, standard care “bundles” and other “SOPs” for management of prone ventilation, use of pulmonary vasodilators, agitation and delirium, renal replacement therapy etc. to guide all levels of staff in their care of our patients (references). In parallel, the GICU senior nursing team provided multiple hands‐on practical teaching sessions for level 1 and level 2 nursing teams to improve their ventilator management competency, setting up intravenous infusions, monitoring pressure areas, prone positioning, and standard provision of ICU care bundles. All level 1 and level 2 medical and nursing team members were always supervised by dedicated ICU staff. |
| Equipment |
Equipment was either re‐purposed or acquired from national suppliers which included ventilators, hemofiltration machines, infusion pumps, physical beds, and mattresses. This was only possible with the closure of all non‐essential hospital services. There were ongoing challenges with the personal protective equipment (PPE) supply including the FFP3 masks, from national sources as they rapidly ran short of the three types of masks (Valmy duckbill, Easimask duckbill, and 3M 7500) that the majority of UHS staff were fit tested on and therefore, we had to continue to fit test staff on various mask brands several times over until we introduced the Personal Respirator Southampton (PeRSo) as backup RPE. For specific infection control training please refer to other sections of the manuscript. |
| Infrastructure |
All our operating theaters, recovery areas (other than five used for emergencies) were converted to receive level 3 ventilated patients. A meticulous planning was done to redirect traffic flow around theater and recovery area, paying attention to airflow and pressure. Safe areas for donning and doffing personal protective equipment (PPE) were allocated with guidance from microbiologists and infection control team. The surge capacity which was initially estimated at 165 ventilated patients was quickly revised depending on the national picture and our clinical management. Our institution was part of local and regional network which worked in concert to re‐distribute patients from units which were under considerable pressure. The anticipated capacity increment was as follows:
Hospital COVID‐19 guidelines committee COVID‐19 guidelines committee was rapidly established to review, process, and implement the emerging evidence. This committee included a multitude of multidisciplinary individuals including respiratory physicians, infectious diseases and virology specialists, pharmacists, and intensive care physicians. The rapid development and weekly review of guidelines in the hospital in addition for ICU specifically indicated how quickly our understanding of the disease was evolving. We were able to upload these guidelines on the MicroGuide app, accessible via smart phones and devices; and on the hospital intranet service. The clinical information systems and electronic‐prescribing tools required modification to allow novel therapeutics to be prescribed in a safe and swift manner. |
2.1.2. Admission surge planning
Even from the start, the consensus decision in our department was to provide non‐invasive ventilation (NIV) as the primary oxygenation strategy for those requiring >60% of oxygen and only invasively ventilate those who failed NIV (Sivaloganathan et al., 2020). Patients were admitted from two fronts: (1) emergency department and (2) respiratory high dependency unit/ward. The admission process from the emergency department created a segregated respiratory assessment area. Patients were triaged to either suspected or confirmed COVID‐19 groups. Patients who were assessed to be suitable and likely to benefit from invasive mechanical ventilation were admitted to GICU either directly from the emergency department or the respiratory high respiratory dependency unit. Cross infection and delays in interdepartmental transfers were minimized with the rapid development and implementation of point‐of‐care testing for the SARS‐CoV‐2 virus (Brendish et al., 2020). The use of this rapid point of care testing enabled us to promptly escalate care to the critical care units while mitigating the risk of hospital‐onset COVID‐19 infection (HOCI) in the non‐COVID critically ill patients. Rapid testing also allowed us to safely escalate patients to level 3 care without compromising other non‐COVID‐19 patients' care. The respiratory teams outside GICU managed patients with level 2 as a ceiling of care (Table 1).
In our institution, we diverted the care of non‐COVID patients who require level 3 care (during the first wave between March 2020 and May 2020) to the Cardiac Intensive Care (CICU) and Neurology Intensive Care (NICU) departments based on the expectation that the whole of the GICU team would be required to manage the huge influx of COVID‐19 patients.
2.2. Preparation for medical and nursing personnel
The need to rapidly increase the specialized ICU staffing levels to cope with precipitous increase numbers of critically ill COVID‐19 patients was a significant challenge. Patient numbers soon significantly outstripped our usual funded and staffed GICU bed numbers. We ensured that there was always a fully trained intensive care consultant on duty to supervise and coordinate care. The senior clinical team was supported by our existing ICU junior medical and nursing team, further augmented by many redeployed personnel from other clinical areas in the hospital (Table 1).
2.3. Infection control and PPE training and education
2.3.1. Mask fit testing
ICU staffs are generally well‐rehearsed in wearing FFP3 masks during aerosol‐generating procedures (AGPs) for respiratory viruses such as influenza, and therefore there was a high percentage of GICU staff already‐fit tested to wear FFP3 masks. However, as the number of positive cases continued to increase, more staffs were required to provide clinical care, and as a result, the requirement for fit testing was also rose rapidly. In addition to redeployed personnel, agency nurses and health care support workers were also recruited, with an escalating requirement for regular fit testing programs with individualized assessments for suitable FFP3 mask interfaces. The hospital infection control team was responsible for ensuring a successful fit‐testing program inclusive to all hospital staff involved in managing COVID‐19 patients. We predominantly used the qualitative method (taste test), which takes around 30 min depending on the first‐time “pass” rate. Despite multiple testing of various masks, some staff continued to fail their fit‐tests, causing significant concern about potential increased infection risk. The advent of the locally designed Personal Respirator Southampton (PeRSo) hood provided a rapid alternative and reassurance to those who consistently failed mask fit‐testing (Elkington et al., 2021; Munro et al., 2021). As a department within a large hospital, we have fit tested more than 420 members of staff over 12 months, several staff members more than once.
2.3.2. Personal respirator Southampton
The personal respirator Southampton (PeRSo) hood was introduced to all staff who were predominantly caring for COVID‐19 positive patients across the hospital. Following successful local design and development (Elkington et al., 2021), a PeRSo deployment team was assembled, including members from microbiology, PPE operations, health & safety, specialty divisional leads and the site manager. Training across the hospital was implemented with the help of training videos. In ICU, all staff were trained by a dedicated infection prevention link sister. This included doff, don, and decontamination procedures. We trained over 780 staff to use the PeRSo hoods over pandemic. In critical care, many staff were beginning to suffer from skin breakdown on the bridge of their nose, ears, and back of neck because of wearing either the disposal or reusable FFP3 masks for prolonged periods. The PeRSo gave much relief from this aspect. The PeRSo was also introduced to protect those members of staff who could not fit tested with the available FFP3 masks, which was an essential part of the PPE that was/is required to be worn for contact with COVID positive or suspected patients and those undergoing aerosolized generating procedures.
2.3.3. Additional training and infection prevention and control
Following the introduction of national guidance on personal protective equipment (PPE), hospital wide infection prevention and control (IPC), and our local infection prevention team convened and cascaded the relevant information via email and Staffnet (electronic web‐based hospital specific communication system) to the wider personnel within the hospital. This was reviewed and modified regularly depending on emerging local data in the GICU. For example, during the second wave of the pandemic, we noticed an increase in the incidence of central line‐associated bloodstream infections. Although this could have been due to multiple factors, we identified a possible association between infections and the process of proning. Many of the sick COVID‐19 positive mechanically ventilated patients were being proned regularly by a dedicated prone team. The team members moved between patients with the same base layer PPE (long‐sleeved gown and two pairs of gloves) and the only item of PPE that was changed between patients was the outer pair of gloves, and little or no hand/ glove hygiene took place.
Following this finding, new measures were introduced where all staff in close contact with the patient (i.e., involved in maneuvering a patient) should wear an additional long‐sleeved gown on top, which should be changed between patients to prevent onward transmission. This was particularly advocated for the head/airway and endotracheal tube holder who had their hands and arms around the head and neck and quite often is exposed to oral and nasal secretions. However, wearing an additional gown in combination with the rest of the PPE was very uncomfortable. Although the UK government guidance advocated using long‐sleeved gowns for all staff exposed to COVID‐19 patients (GOV.UK, 2020), including in cohort areas, we modified this practice following a multidisciplinary risk assessment review process. In collaboration with the hospital microbiology team, local infection prevention team and ICU staff, we modified our PPE gowns to short sleeves to minimize the infection risk to both patient and staff. We recommended thorough hand and arm hygiene between patients and during decontamination. Moreover, the use of double gloves was eliminated. Although there was much resistance at first, continuous education with reassurance helped successfully implement these changes in practice.
3. DIAGNOSTICS
A summary of diagnostic investigations performed for COVID‐19 patients in GICU are summarized in Table 2.
TABLE 2.
Summary of diagnostics for COVID patients admitted to The General Intensive Care Unit, at University Hospital Southampton, United Kingdom
| Diagnostics | Comments |
|---|---|
| In‐laboratory COVID‐19 diagnosis |
Patients were tested for SARS‐CoV‐2 RNA at our Specialist Virology Centre. Using combined mid‐turbinate and throat swabs were placed in to VIROCULT virus transport medium (Sigma). Swabs were extracted using MicrosensDx RapiPREP nucleic acid extraction reagents and purified using magnetic particle extraction on the Thermo Scientific KingFisher Flex using the current standard operational procedures. All lower respiratory tract samples were extracted using the QIAsymphony SP and the QIAsymphony DSP Virus/Pathogen Mini Kit (both from Qiagen, Germantown, MD) according to the manufacturer's recommendations. Amplification took place on Applied Biosystems (ABI) 7500s using the Viasure NCO2 SAR‐CoV‐2 RT‐PCR kit (Prolabs, ORF1ab, and N genes). Additionally, each sample was tested using an in‐house World Health Organization E sarbecco gene assay (including an internal amplification control from extraction), to enhance sensitivity and prevent false negative results from being reported. The difference in method reflects the move from QIAsymphony to Kingfisher for respiratory swabs; however, saliva and lower respiratory samples were still processed using the QiaSymphonySP. PCR was performed using the ABI 7500 no matter which extraction technology was used. Patients provided an upper respiratory tract (combined mid‐turbinate and throat) swab prior to GICU admission in ICU most of these samples being endotracheal aspirates, sputum specimens and BALs. Average turnaround time was around 8 h for in lab PCR and at the time ~2500–3000 test per day during the peak period from both waves. |
| Point of care COVID‐19 testing | Within the hospital a number of point of care (POCT) and rapid diagnostics were adopted, including the Biofire/bioMérieux assay with panels detecting around 20 respiratory pathogens, including SARS‐CoV‐2, allowing clinicians to quickly rule in or rule out COVID‐19 and other common causes of respiratory illness in about 45 min. This was mostly used at the point of admission‐by‐admission unit staff allowing rapid diagnosis and patient triaging including escalation and admission to GICU when clinically. Later the laboratory adopted a similar platform as well as Cepheid's Xpert Xpress CoV‐1/Flu/RSV plus test which also delivered results in <1 h. |
| Other microbiology/virology testing |
In addition to routine clinical investigations, e.g., blood cultures if a patient was behaving in septic way. The following were performed: Weekly sputum surveillance for culture and sensitivities; this was very valuable not only in establishing microbiology that guided our subsequent choices of antibiotics when necessary, but also it allowed us in early identification of potential cross infection and outbreaks. Serum HIV testing, hepatitis B, C testing on admission. Admission CMV, EBV serology, weekly CMV/EBV PCR, weekly serum Beta D glucan check a After initial positive results: weekly SARS‐CoV‐2 PCR and SARS‐CoV‐2 antibody testing: these were used by the microbiologist in assessment for de‐isolation. Once de‐isolated these were not performed unless clinically indicated. |
| COVID‐19 blood panel bundle |
From the outset, a COVID‐19 laboratory panel bundle was developed for admission screening and follow‐up surveillance profiles. These included daily routine hematological and biochemical panel with additional specialist investigations including: Bundle for new COVID‐19 patients: · 25‐OH‐Vitamin D · HBA1C · Aspartate aminotransferase (AST) · Creatinine kinase (CK) · C‐Reactive protein · Ferritin · HS troponin · Inorganic phosphate · Lactate dehydrogenase (LDH) · Liver profile · Renal profile · Triglycerides · Coagulation screen · D‐Dimer assay · Full blood count · Blood cultures · Sputum/BAL for microscopy, culture, and sensitivity · SARS‐CoV‐2 anti‐S IgG · HIV antibody/antigen screen · SARS‐CoV‐2 PCR · X‐ray chest Bundle for monitoring COVID‐19: · AST · Creatinine kinase (CK) · C‐Reactive protein · Ferritin · HS troponin I · Lactate dehydrogenase · Liver profile · Renal profile · D‐Dimer assay · Full blood count |
| Other investigations |
All patients had a chest X‐ray and arterial blood gases in the event of oxygen requirement. We performed a cardiac echocardiogram (transthoracic echocardiogram) on all patients to assess their cardiac function and to exclude any overt incidental valvular abnormalities. We also had the facility to perform focused intensive care echocardiography (FICU) during deteriorations and to optimize fluid and inotropic management. Deteriorating patients had regular assessment of their right heart and pulmonary pressures and in the event of raised pulmonary pressures, additional therapies with pulmonary vasodilators were offered. We did not perform CT scans of thorax routinely. However, these were done in the format of CT pulmonary angiogram in the event of continued deterioration to assess pulmonary vasculature as well to quantify parenchymal abnormalities. |
For those who required additional steroid.
4. ADMISSION AND MANAGEMENT PATHWAYS
All COVID‐19 patients were admitted to level 1 dedicated cohorted COVID‐19 areas. If the patient required additional organ support measures, particularly non‐invasive ventilation support, they were admitted to a 14‐bed respiratory high dependency unit (level 2 area). This unit was able to provide non‐invasive respiratory support including continuous positive airway pressure (CPAP), non‐invasive ventilation (NIV), and high flow nasal oxygen (HFNO), outside ICU. Rapidly deteriorating patients were either admitted directly from the emergency department or level 2 respiratory area to the GICU for subsequent management. Patients deemed clinically unsuitable for intubation, and mechanical ventilation were continued to be managed in the ward or level 2 areas. Nearly all patients admitted to GICU had acute hypoxic respiratory failure and consequently required non‐invasive or invasive mechanical ventilation. The primary reason for admission was escalating oxygen requirement or respiratory distress. (Figure 2). All emergency referrals were made via a dedicated critical care bleep system, carried by a nominated ICU referral/outreach consultant for that day. Daily multidisciplinary discussions between the respiratory consultant managing respiratory wards and the level 2 area and the GICU outreach consultant ensured prompt detection of patients at risk of deterioration and facilitated patient flow between departments in a timely manner.
FIGURE 2.
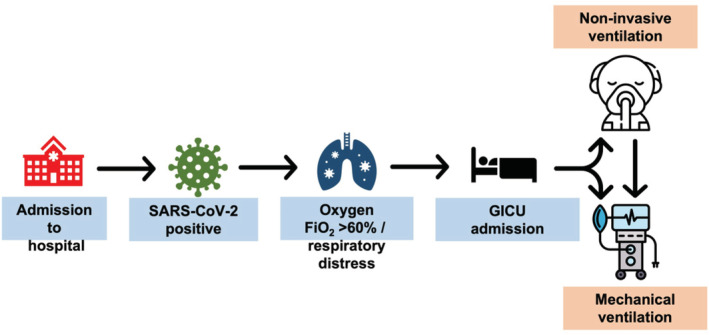
Admission and management pathways to the general intensive care unit
4.1. Primary oxygenation strategies
All patients with hypoxic respiratory failure with a requirement of inspired oxygen (FiO2 > 0.6) to maintain arterial oxygen (PaO2) of >8.0 kPa were trialed on bi‐level non‐invasive ventilation (NIV)/continuous positive airway pressure (CPAP) as the primary oxygenation strategy. The type of ventilation initiated was at the discretion of the treating clinician. All patients had an arterial line sited to facilitate blood gas sampling and a nasogastric tube (NGT) to enhance nutritional intake. All awake NIV patients were encouraged to self‐prone at maximum duration where possible (Burton‐Papp et al., 2020). Patients in respiratory distress or unable to tolerate non‐invasive ventilation masks were intubated and mechanically ventilated. Moreover, those who persistently required FiO2 of more than 0.8, with a significantly increased work of breathing, were subsequently intubated and placed on a mechanical ventilator. The NIV failure rate was around 50% (Sivaloganathan et al., 2020). Endotracheal intubation of such critically ill patients required very close attention and was only performed in the presence of a senior clinician. Patients were intubated with a video laryngoscope to ensure maximal success at the first attempt. NIV was also used for extubation in many patients to prevent immediate decruitment following extubation. High flow nasal oxygen (HFNO) was not used as the primary oxygenation strategy in our unit. However, patients were treated with HFNO in the other level 2 areas. Moreover, HFNO was consistently used to give breaks during NIV and after extubation. NIV and HFNO were provided only in dedicated areas, and all care personnel were protected with full PPE measures. We have identified no persistent environmental contamination with regular cleaning and no increase in COVID‐19 infection among health personnel due to the use of non‐invasive ventilation (Shahi et al., 2021), where appropriate PPE were used.
4.2. Specific medical therapies and interventions
At the start of the pandemic, despite consideration of various specific therapies, the evidence for these were limited by lack of randomized controlled trials. Consequently, most of the targeted specific therapies were only given in the context of a clinical trial. We participated in several clinical trials utilizing antiviral medications, immune modulators (anti‐IL‐6, JAK‐2, and corticosteroids), convalescent plasma, angiotensin converting enzyme inhibitors (ACE‐I), and exogenous pulmonary surfactant.
4.2.1. Antibiotics
Most patients who were admitted to the GICU in the initial couple of weeks of the first wave of the pandemic were commenced on standard antibiotics (co‐amoxiclav and azithromycin combination), this was based on anecdotal reports from Italy and China, as well as generic guidance from the UK's National Institute for Health and Care Excellence (NICE) published a COVID‐19 (NICE, 2020). Additionally, the same rapid guideline suggested that there is insufficient evidence to recommend routine procalcitonin (PCT) testing to guide decisions about antibiotics and encouraged centers already using PCT to participate in research and data collection.
We used PCT as a stewardship tool in these patients aiming to reduce unnecessary exposure to antibiotics given the increasing level of antimicrobial resistance globally. PCT was measured from serum samples using sequential two‐step immunoenzymatic (“sandwich”) assay (Beckman Coulter) as part of its access range for the DxI immunoassay instruments (800 version). Proposed antibiotic decisions based on incorporating PCT cut‐off levels with clinical judgment and experience based on previously published guidance for non‐COVID‐19 (Schuetz et al., 2019). A level of 0.5 μg/L was used as the upper limit to help determine the probability of bacterial infection, along with clinical judgment of a bacterial infection. Our published data (Heesom et al., 2020) suggested that for patients with low PCT of <0.5 ng/ml antibiotics can be safely stopped, minimizing the total duration of antibiotic exposure and without negative impact on outcomes including, length of stay in and outside ICU and mortality (Heesom et al., 2020). The unit continued to use PCT to guide antibiotic decision making throughout further waves.
4.2.2. Augmented anticoagulation
The emerging early laboratory, pathological and clinical data suggested an increased tendency to develop venous and arterial thromboembolic complications, particularly in critically ill patients. There was clear evidence of thrombotic phenomenon contributing to adverse clinical outcomes (Dushianthan, Abdul, et al., 2021). In combination with critical care physicians, the local thrombosis committee developed anticoagulation guidance for chemical thromboprophylaxis using unfractionated heparin or low molecular weight heparin (enoxaparin) targeting anti‐factor Xa target levels of 0.50–1.00 (Lowe et al., 2021).
4.2.3. Prone positioning
Prone positioning was a vital part of our management strategy in all COVID‐19 patients with severe hypoxic respiratory failure. Proning of a mechanically ventilated patient is a maneuver that involves sequentially alternating a patient's position from supine to prone. This primarily improves gas exchange by improving ventilation/perfusion mismatch. The prone position is typically maintained for at least 16 h, with position changes every 4–6 h to limit the development of pressure injuries while prone.
Proning is a well‐established therapy in acute respiratory distress syndrome (ARDS) with proven mortality benefits (Guérin et al., 2013). A decision was made locally that early prone ventilation would form an integral part of our GICU treatment strategy for all COVID‐19 patients requiring mechanical ventilation. We proned all patients with FiO2 > 0.6 and PaO2/FiO2 ratio <13.3 kPa for a minimum of at least three prone cycles. Due to the extreme severity of the respiratory failure, almost all mechanically ventilated patients received prone ventilation.
During the start of the pandemic, we were able to call upon expertise within the hospital to rapidly establish a dedicated, cross‐disciplinary proning team that we feel helped deliver optimal care to our patients, which is reflected in our relative better survival rates. Setting up a dedicated proning team involved significant planning. Simulation‐based training delivered in spare operating theaters to replicate an ICU bed area was provided to more than 550 staff, including anesthetists, intensivists, nursing staff, and allied health care professionals. In the first wave, a smaller group of 78 staff were eventually deployed to work as part of the proning team. We used local firefighters during the second wave to complement the existing prone team members. A dedicated standard operating procedure (SOP) checklist based on the Intensive Care Society (ICS), United Kingdom and Faculty of Intensive Care Medicine (FICM) (ICS and FICM, 2019) was used for training and to maintain consistency to minimize procedure‐related adverse events. Online website provided instructional videos and a logbook used to document proning episodes to share patient safety incidents (Stubbs, 2020). During the first wave, 1208 proning maneuvers were completed on 42 patients. Most of this work was out of hours (20:00–08:00). Analysis of incident logs identified that the most common safety incident was circuit disconnection (mainly relating to suction catheter mounts). Other events were related to the disconnection invasive lines and sub‐optimal or cardiovascular changes from the use of sedation/muscle relaxants prior to proning. The development of a dedicated prone team was a crucial part of our patient management and a real, local success. Crucial to this project's success was clear communication, a comprehensive training program and a process of collecting continual feedback, which allowed us to cascade learning points readily and safety incidents as well as improve proning team operations for subsequent waves.
4.2.4. Rescue strategies
Some patients demonstrated a relentless form of severe hypoxic respiratory failure despite maximal support with or without multiorgan failure. In this situation, multiple strategies were adopted. Our standard mandatory ventilation mode was adaptive pressure ventilation‐controlled mechanical ventilation (APVcmv). However, we increasingly used airway pressure release ventilation (APRV) at later stages of the first wave of the pandemic as a rescue mode of ventilation. We found anecdotal evidence suggesting APRV ventilation improved oxygenation during desperate times. This was supported by a survey in the United Kingdom where there was an increase in the use of APRV ventilation during COVID‐9 pandemic (Dushianthan, Cumpstey, et al., 2021). Moreover, we used prone positioning for all patients with a PaO2/FiO2 ratio of <13.3 kPa as a rescue measure even after several days of ICU admission. We also used pulmonary vasodilators (nitric oxide and aerosolized prostaglandin analogues) as a rescue measure guided by the PaO2/FiO2 ratio improvement. Augmented corticosteroids in the form of methylprednisolone at a dose of 1 mg/kg twice a day was also used for 5–7 days in failing patients to address continued hyperinflammatory state. We performed weekly microbiology surveillance for those who had high doses of corticosteroids (Table 2). In the eventual scenario of all measures failing despite maximal support, suitable patients were referred to the local tertiary extracorporeal membrane oxygenation (ECMO) center for additional support.
4.2.5. Acute kidney injury and hemofiltration
Acute kidney injury (AKI) associated with critical illness carries a high mortality. The pathophysiology of AKI associated with COVID‐19 is multi‐factorial and likely to involve a combination of acute intrinsic renal insults and pre‐renal factors (Legrand et al., 2021). Severe AKI in critically ill patients is managed by attempting to rectify the underlying cause and then, if necessary, providing support with continuous renal replacement therapy (CRRT). This involves extracorporeal hemofiltration or a combination of filtration and dialysis (hemodiafiltration) to aid solute clearance (e.g., urea) and manage fluid balance. The provision of CRRT requires large‐bore central vascular access, appropriately trained staff, hemofilter machines, disposable hemofilters, and replacement fluids. Early reports from the United States suggested that around 20% of critically ill COVID patients required renal replacement therapy (Gupta et al., 2021). At our hospital, initial predictions suggested 100–200 critically ill COVID patients, which would completely overwhelm our ability to deliver CRRT. It is probably fair to suggest that initial planning focused initially on the provision of ventilatory support with CRRT was somewhat ignored.
Early in the pandemic, correspondence from NHS England (April 2020) made several recommendations, including intermittent utilization of CRRT machines—ensuring three patients received CRRT over 48 h from each machine. This has the effect of maximizing the use of the CRRT machines but at the cost of significantly higher turnover of disposables—namely hemofilters and replacement fluids. Later NHS England recommendations in May 2020 recognized that the scarcity of RRT fluids and consumables was the limiting step in the provision of CRRT. This led to the development of a National Standard Operating Procedure for the allocation of CRRT consumables whereby all consumables were held centrally within each region, and hospitals were only able to order products a few days at a time. Allocation of consumables was then being based on the previous 4 days rolling average of hemofiltration activity.
During the first wave one (March–May 2020), UHS admitted 81 critically ill COVID patients. Thirty‐six (44%) had an acute kidney injury, and 16 (19%) required CRRT. Those patients with an AKI had an increased length of ICU stay and higher mortality (25% vs. 6.7%). These figures are in keeping with current evidence that AKI is an independent risk factor for increased mortality in all critical illnesses (Lowe et al., 2021). As well as the supply of consumables, there were COVID specific challenges around managing CRRT in these patients. Managing hemofilter life was paramount given the restricted supply of consumables. Failure of the hemofilter circuit is often due to clotting within the filter. To undergo CRRT most patients require anticoagulation. This can be done by anticoagulating the hemofilter circuit using citrate or systemic anticoagulation of the patient. To mitigate the risk of circuit failure, we developed a protocol to deliver regional anticoagulation using citrate and systemic heparinization. Also, the standard advice is that the hemofilter should be disconnected and discarded after 72 h of use. In view of the shortage of hemofilters, we used a hemofilter for significantly longer than the recommended 72 h and used a modified fluid replacement program to preserve fluid stocks.
4.2.6. Physiotherapy
In addition to the dedicated GICU physiotherapists, additional physiotherapists were deployed from other areas to address the ongoing significant demand. Input from the physiotherapists was vital from the start. They were involved in daily airway clearance to prevent secondary respiratory infections. Early mobilization and respiratory weaning for those requiring prolonged mechanical ventilation were a crucial part of rehabilitation, which was managed daily by the dedicated GICU physiotherapy team.
4.2.7. COVID‐19 research participation and data collection
Despite the overwhelming challenges of clinical care, some of our specialist research nurses retained research duties and enrolled patients into clinical research. We participated in observational studies such as International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and GenOMICC COVID‐19 study. We also contributed to the nationally prioritized multiplatform study (A Randomized, Embedded, Multifactorial, Adaptive Platform Trial for Community‐Acquired Pneumonia‐CAP [REMAP‐CAP]), enrolling a staggering number of COVID‐19 patients into this complex interventional study. We also developed locally designed studies such as nebulized surfactant therapy for patients with severe COVID‐19 (COVSurf) and participated in other interventional and national studies such as Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (mesenchymal stem cells) (REALIST) and Seamless Phase I/IIa Platform for the Rapid Evaluation of Candidates for COVID‐19 treatment (ACCORD). We also established a real‐time data collection and analysis platform to assess patients' characteristics, interventions, and outcomes, which enabled modifications of management pathways more rapidly (Burke et al., 2020).
5. OBSERVED COMPLICATIONS
The observed complications for the combined data from waves 1 and 2 are documented in (Table 3). The most common complications were related to glycemic control, occurring in 20.9% of all admissions. Cardiac complications such as myocarditis and myocardial infarctions occurred in 15% of patients. Chronic respiratory complications occurred in 13.5% of the population. Pulmonary fibrosis was the most common occurrence in 6.2% of patients. Pneumothoraxes and pulmonary hypertension were recorded in 3.8% and 3.5% of cases, respectively. Exactly 11.2% of patients experienced either venous thromboembolism (DVT or PE). Conversely, 4.7% experienced issues with bleeding. Surgical complications occurred in 7.1% of patients. 0.6% of patients experienced pancreatitis. In total, 3.8% of patients experienced a neurological complication.
TABLE 3.
Summary of observed COVID‐19 complications among the 340 patients admitted to the General Intensive Care Unit, University Hospital Southampton, United Kingdom
| Complication class | N | % |
|---|---|---|
| Respiratory complications | 46 | 13.5 |
| Pneumothorax | 13 | 3.8 |
| Pulmonary hypertension | 12 | 3.5 |
| Pulmonary fibrosis | 21 | 6.2 |
| Cardiac complications | 51 | 15.0 |
| Glycemic complications | 71 | 20.9 |
| Pancreatitis | 2 | 0.6 |
| Neurological complications | 13 | 3.8 |
| Surgical complications | 24 | 7.1 |
| Bleeding complications | 16 | 4.7 |
| DVT/PE | 38 | 11.2 |
As mentioned earlier, we noticed an increased incidence of central venous catheter‐related bloodstream infections (CRBSI) throughout both pandemic waves. We identified several potential risk factors. These include the use of immunosuppression agents (corticosteroids and anti‐IL6), proning of patients with high BMI and sessional PPE use for prolonged periods. Moreover, during the peak, we used supporting ICU staff who are not entirely familiar with ICU interventions and infection control in the ICU setting. We also decreased the trained staff to patient ratio and doubled the bed spaces. These measures could have also contributed to the increase in CRBSI events. Most of these infections were Gram‐negatives (Klebsiella spp., Escherichia spp., and Citrobacter spp.) and the organisms were different from patient to patient, suggesting an endogenous nature rather than a common source. These infections were reviewed regularly in infection control meetings, and steps were taken to provide continuous training, education, and auditing measures to assess compliance with aseptic techniques to minimize the incidence of these complications.
We did not observe an increase in COVID‐19 associated pulmonary aspergillosis (CAPA); however, we had environmental contamination of Aspergillus within part of our GICU due to leaking pipes in the ceiling above. The weekly surveillance sputum samples assisted in identifying three patients with COVID‐19 colonization without subsequent progression to CAPA. The problem was identified and managed promptly, and following this, we did not observe any further colonization with Aspergillus.
6. TRACHEOSTOMY AND POST TRACHEOSTOMY SYSTEMIC INFLAMMATORY RESPONSE
Among those mechanically ventilated (N = 184), 45 patients (24.5%) had a tracheostomy for prolonged respiratory weaning. Except for one patient, all were decannulated at hospital discharge. Our standard practice was not to provide prophylactic antibiotics during the percutaneous/surgical tracheostomy insertion. However, it was noted that some patients following the insertion of a tracheostomy developed signs of a systemic inflammatory response (SIRS)/infection with fever and raised inflammatory markers. Some patients had worsened their respiratory function, which was sustained for the following few days after the tracheostomy. The local protocol was subsequently modified to commence a stat dose of a broad‐spectrum antibiotic prior to the procedure. Based on the routine and surveillance samples, Gram‐negative organisms such as Klebsiella spp., Escherichia coli, Enterobacter spp., and Pseudomonas spp. were most isolated. From these surveillance results, most patients received a stat dose of gentamicin 3 mg/kg prior to tracheostomies if they were known to be colonized with these organisms; alternatively, a targeted antibiotic was chosen based on the patient's microbiology results. It was clinically challenging to determine whether the SIRS was due to the tracheostomy or any other clinical reasons, and more detailed studies are required to determine this.
7. FAMILY LIAISON ROLE IN CRITICAL CARE
During the first wave of the pandemic in March 2020, many members of the critical care nursing team were unable to work in patient‐facing clinical practice due to their own increased health risk. At the same time, all visitations from family members were ceased. In response to this, a team of nursing staff were relocated to a vacated office, phone lines established, and the “family liaison team” (FLT) were set up. It was necessary to train some staff on how to undertake difficult conversations and how to break bad news. In anticipation of this role being highly emotive and stressful, weekly psychological support was established with a clinical psychologist.
Each morning, the team would assemble to obtain a handover from the duty consultants. Each team member on duty would then start to provide a daily telephone update to every patient appointed next of kin (NOK)/care home manager—who were all hyperanxious and struggling to comprehend the complexity and “unpredictability” of this disease. Incoming telephone calls were diverted to the FLT as staff in the clinical areas were finding themselves too busy to answer the phone and would struggle to hear or be heard using the FFP3 masks. If a patient deteriorated, the FLT would call the dedicated NOK again to update and support them. If this was expected to be an end‐of‐life situation, a COVID‐19‐negative family member was supported in visiting their relative. The staff member from the FLT would meet and greet at reception and take them to the unit and, at times, sit in on discussions with the duty consultant. Connections with colleagues working in London facilitated a prompt sign up with “Lifelines.” This project enabled us to obtain android devices for use on each unit and was enabled with 4G data. An application for virtual visiting was pre‐installed on each device, and the FLT would obtain the email addresses of each NOK to enable a video link with the patient and staff attending to them. Relatives described these video calls as a “lifeline,” which helped bridge the gap as the no visiting policy continued.
A template was established to document all conversations in the clinical notes. Requests for medical updates or video calls were also noted here. The team frequently liaised with the research nurses as many relatives had ongoing questions on the studies that the patient had been enrolled into. Knitted hearts, donated by members of the community, were sent to the appointed NOK along with a card. One half of the pair was retained with the patient. A small symbol of bringing them closer together at such a dreadful time. As we discovered that many self‐isolating relatives were deteriorating at home, the FLT had to request urgent medical assistance on at least three occasions. Concern for relatives at home also involved signposting them to community volunteers for food and medicine supplies or the Patient Support Hub at the hospital. They would also be directed to the peer support group on Facebook. This practice was continued in the subsequent waves.
8. GICU OUTCOMES
Our ICU outcomes were comparably better to the nationally published outcomes in the United Kingdom (Doidge et al., 2021; ICNARC, 2022). We feel this may be due to several factors detailed in the previous sections. The detailed outcome of our patients is presented in (Table 4). All data are complete as of June 30, 2021.
TABLE 4.
Outcomes of COVID‐19 patients in the General Intensive Care Unit at University Hospital, United Kingdom
| Outcomes (N = 340 patients) | Details |
|---|---|
| Mechanical ventilation, n (%) | 184 (54.1%) |
| Non‐invasive mechanical ventilation, n (%) | 137 (40.1%) |
| Renal replacement therapy, n (%) | 54 (15.9%) |
| Length of mechanical ventilation (days), (N = 184) a | 11.5 (5–20) |
| Length of ICU stay (days), (N = 340) b | 10 (4–21) |
| Length of hospital stay (days), (N = 430) b | 20 (12–37) |
| ICU mortality, n (%) | 78 (22.9%) |
| Overall hospital mortality, n (%) | 85 (25%) |
Length of mechanical ventilation is only presented for patients who had invasive mechanical ventilation and does not include patients with non‐invasive respiratory support.
Includes all patients admitted to ICU.
9. PATIENT'S FOLLOW‐UP
Post GICU discharge, we conducted multi‐professional visits to recovering COVID‐19 patients on the medical wards and reviewed their progress both physically and psychologically. We worked closely with the rehabilitation team to facilitate timely discharge of patients to the appropriate rehab facility. We continued to provide telephone clinics after hospital discharge. Non‐clinical staff contacted all mechanically ventilated COVID‐19 patients post discharge, to assess their function and signpost them to appropriate, early support where necessary. We had the assistance from local rehabilitation facilities to assess and manage all patients' who had NIV at the community level. Many of these awake patients experienced disturbing visual memories of proned patients, dying patients, and fear for their own survival.
In June 2020, we re‐established the follow up clinic on a virtual platform using “Attend Anywhere.” To date we have assessed over 80 COVID patients via this video link. This format is now our established means of follow up clinic and has continued to date. This virtual platform has been very beneficial as it provides greater accessibility to this vulnerable patient population and helps overcome the limited access to hospital premises. Pre‐COVID‐19, paper‐based screening questionnaires were posted to patients for completion prior to face‐to‐face clinic. As this was no longer feasible, the Patient Support Hub kindly facilitated telephone completion of these screening tools. For Mutual aid patients there was close liaison with the respective GICU follow up teams to ensure continuity of care. Many of the patients seen in clinic experienced the common symptoms of Post‐Intensive Care Syndrome (PICS). Appropriate patients were also followed up in the respiratory department for assessment of their respiratory function and subsequent management of ongoing symptoms and requirements for additional oxygen and ventilatory support at home.
10. CONCLUSIONS
COVID‐19 remains a significant challenge to all health care systems, particularly in the intensive care unit setting. Despite many unknowns, especially at the start of the pandemic, we rapidly enhanced our surge capacity and modified the usual standard pathways to manage all critically ill admissions to achieve the anticipated outcomes. The success was cent red around reorganizing our personnel, equipment and infrastructure that included many hours of planning, interdisciplinary work, training, and support. As a result of this multidisciplinary response, we were able to respond to the exceptional demand which was thrust upon us by the multiple pandemic waves. Although it is impossible to elaborate on all the effort that went on during these unprecedented situations in a narrative review, we aim to provide a glimpse of our experience, which may be helpful to other intensive care units worldwide.
AUTHOR CONTRIBUTIONS
Ahilanadan Dushianthan: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Melanie Griffiths: Writing – original draft (equal); writing – review and editing (equal). Fiona Hall: Writing – original draft (equal); writing – review and editing (equal). Kathleen Nolan: Writing – original draft (equal); writing – review and editing (equal). Dominic Richardson: Writing – review and editing (supporting). Benjamin Skinner: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Lewis Matthews: Data curation (equal); writing – review and editing (supporting). David Charles: Writing – original draft (equal); writing – review and editing (equal). Razaz Elsheikh: Writing – original draft (equal); writing – review and editing (equal). Renato Pignatari: Writing – original draft (equal); writing – review and editing (equal). Rezaur Rahman: Writing – original draft (equal); writing – review and editing (equal). Shenthiuiyan Theivendrampillai: Writing – original draft (equal); writing – review and editing (equal). Rebecca Egglestone: Conceptualization (equal); methodology (equal); project administration (equal); writing – original draft (equal); writing – review and editing (equal). Aaron Stokes: Writing – original draft (equal); writing – review and editing (equal). Giovani Danibenvenutti: Writing – original draft (equal); writing – review and editing (equal). Michael Stewart: Writing – original draft (equal); writing – review and editing (equal). Michael Celinski: Writing – original draft (equal); writing – review and editing (equal). Rebecca Cusack: Writing – original draft (equal); writing – review and editing (equal). Sanjay Gupta: Data curation (equal); formal analysis (equal); funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal). Kordo Saeed: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); software (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead).
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
RELATED WIREs ARTICLE
Explainable deep learning in healthcare: A methodological survey from an attribution view
ACKNOWLEDGMENTS
Authors would like to thank the following groups and staff in non‐COVID intensive care units including cardiac, neuro and pediatric intensive cares, staff in emergency medicine and admission units including point of care team. We also would like to thank all laboratory staff, physiotherapy, radiology, orthopedics, general medicine, anesthetics, palliative care teams, pharmacy colleagues, IT department, human resources, REACT‐COVID‐19 study team, Research and Development, infection prevention teams, managers, patients, relatives, and everyone who supported us through those challenging times.
Dushianthan, A. , Griffiths, M. , Hall, F. , Nolan, K. , Richardson, D. , Skinner, B. , Matthews, L. , Charles, D. , Elsheikh, R. , Pignatari, R. , Rahman, R. , Theivendrampillai, S. , Egglestone, R. , Stokes, A. , Danibenvenutti, G. , Stewart, M. , Celinski, M. , Cusack, R. , Gupta, S. , & Saeed, K. (2022). Caring for COVID‐19 patients through a pandemic in the intensive care setting: A narrative review. WIREs Mechanisms of Disease, e1577. 10.1002/wsbm.1577
Edited by: Jessica Lawler, Executive Editor
DATA AVAILABILITY STATEMENT
Data available on request.
REFERENCES
- Brendish, N. J. , Poole, S. , Naidu, V. , Mansbridge, C. T. , Norton, N. J. , Wheeler, H. , Presland, L. , Kidd, S. , Cortes, N. J. , Borca, F. , Phan, H. , Babbage, G. , Visseaux, B. , Ewings, S. , & Clark, T. W. (2020). Clinical impact of molecular point‐of‐care testing for suspected COVID‐19 in hospital (COV‐19POC): A prospective, interventional, non‐randomised, controlled study. The Lancet Respiratory Medicine, 8(12), 1192–1200. 10.1016/S2213-2600(20)30454-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, H. , Freeman, A. , Cellura, D. C. , Stuart, B. L. , Brendish, N. J. , Poole, S. , Borca, F. , Phan, H. T. T. , Sheard, N. , Williams, S. , Spalluto, C. M. , Staples, K. J. , Clark, T. W. , Wilkinson, T. M. A. , Wilkinson, T. , Freeman, A. , Burke, H. , Dushianthan, A. , Celinski, M. , … Kipps, C. (2020). Inflammatory phenotyping predicts clinical outcome in COVID‐19. Respiratory Research, 21(1), 245. 10.1186/s12931-020-01511-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton‐Papp, H. C. , Jackson, A. I. R. , Beecham, R. , Ferrari, M. , Nasim‐Mohi, M. , Grocott, M. P. W. , Chambers, R. , & Dushianthan, A. (2020). Conscious prone positioning during non‐invasive ventilation in COVID‐19 patients: Experience from a single Centre. F1000Research, 9: 859. 10.12688/f1000research.25384.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, D. M. , & Summers, L. H. (2020). The COVID‐19 pandemic and the $16 trillion virus. JAMA, 324(15), 1495–1496. 10.1001/jama.2020.19759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doidge, J. C. , Gould, D. W. , Ferrando‐Vivas, P. , Mouncey, P. R. , Thomas, K. , Shankar‐Hari, M. , Harrison, D. A. , & Rowan, K. M. (2021). Trends in intensive care for patients with covid‐19 in England, Wales, and Northern Ireland. American Journal of Respiratory and Critical Care Medicine, 203(5), 565–574. 10.1164/rccm.202008-3212OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushianthan, A. , Abdul, N. , Dmochowski, J. , James, I. , Heesom, L. , Westwood, J. , Effney, J. , Bruty, S. , Saeed, K. , Rangarajan, S. , & Kazmi, R. (2021). Predictive role of haematological determinants on outcomes of critically ill COVID‐19 patients admitted to intensive care unit. Cureus, 13, e16764. 10.7759/cureus.16764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushianthan, A. , Cumpstey, A. F. , Ferrari, M. , Thomas, W. , Moonesinghe, S. R. , Summers, C. , Montgomery, H. , & Grocott, M. P. W. (2021). Intensive care physicians' perceptions of the diagnosis & management of patients with acute hypoxic respiratory failure associated with COVID‐19: A UK based survey. Journal of the Intensive Care Society. [online first] 10.1177/17511437211002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington, P. T. , Dickinson, A. S. , Mavrogordato, M. N. , Spencer, D. C. , Gillams, R. J. , de Grazia, A. , Rosini, S. , Garay‐Baquero, D. J. , Diment, L. E. , Mahobia, N. , Mant, A. , Baynham, T. , & Morgan, H. (2021). A personal respirator to improve protection for healthcare workers treating COVID‐19 (PeRSo). Frontiers in Medical Technology, 3. [online first] 10.3389/fmedt.2021.664259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOV.UK . (2020). https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control
- Guérin, C. , Reignier, J. , Richard, J.‐C. , Beuret, P. , Gacouin, A. , Boulain, T. , Mercier, E. , Badet, M. , Mercat, A. , Baudin, O. , Clavel, M. , Chatellier, D. , Jaber, S. , Rosselli, S. , Mancebo, J. , Sirodot, M. , Hilbert, G. , Bengler, C. , Richecoeur, J. , … Ayzac, L. (2013). Prone positioning in severe acute respiratory distress syndrome. New England Journal of Medicine, 368(23), 2159–2168. 10.1056/nejmoa1214103 [DOI] [PubMed] [Google Scholar]
- Gupta, S., Coca, S. G., Chan, L., Melamed, M. L., Brenner, S. K., Hayek, S. S., Sutherland, A., Puri, S., Srivastava, A., Leonberg‐Yoo, A., Shehata, A. M., Flythe, J. E., Rashidi, A., Schenck, E. J., Goyal, N., Hedayati, S. S., Dy, R., Bansal, A., Athavale, A., Nguyen, H. B., … STOP‐COVID Investigators (2021). AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID‐19. Journal of the American Society of Nephrology: JASN, 32(1), 161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed]
- Heesom, L. , Rehnberg, L. , Nasim‐Mohi, M. , Jackson, A. I. R. , Celinski, M. , Dushianthan, A. , Cook, P. , Rivinberg, W. , & Saeed, K. (2020). Procalcitonin as an antibiotic stewardship tool in COVID‐19 patients in the intensive care. Journal of Global Antimicrobial Resistance, 22, 782–784. 10.1016/j.jgar.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , Zhang, L. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395, 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICNARC . (2022). https://www.icnarc.org/our-audit/audits/cmp/reports
- Intensive Care Society and Faculty of Intensive Care Medicine . (2019). Faculty of intensive care medicine [internet]. Guidance for: Prone positioning in adult critical care. Faculty of Intensive Care Medicine 2019. https://www.ics.ac.uk/ICU/Guidance/PDFs/Prone_Position_Guidance_in_Adult_Critical_Care
- Legrand, M. , Bell, S. , Forni, L. , Joannidis, M. , Koyner, J. L. , Liu, K. , & Cantaluppi, V. (2021). Pathophysiology of COVID‐19‐associated acute kidney injury. In. Nature Reviews Nephrology, 17, 751–764. 10.1038/s41581-021-00452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, R. , Ferrari, M. , Nasim‐Mohi, M. , Jackson, A. , Beecham, R. , Veighey, K. , Cusack, R. , Richardson, D. , Grocott, M. , Levett, D. Z. H. , Dushianthan, A. , Gupta, S. , Nixon, J. , Stewart, M. , Dushianthan, A. , Sparkes, D. , Chambers, R. , Nolan, K. , Tanser, S. , … Kipps, C. (2021). Clinical characteristics and outcome of critically ill COVID‐19 patients with acute kidney injury: A single centre cohort study. BMC Nephrology, 22(1), 92. 10.1186/s12882-021-02296-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, A. , Prieto, J. , Mentzakis, E. , Dibas, M. , Mahobia, N. , Baker, P. , Herbert, S. , Smith, T. , Hine, M. , Hall, J. , McClarren, A. , Davidson, M. , Brooks, J. , Fisher, J. , Griffiths, D. , Morgan, H. , Giulietti, C. , Faust, S. N. , & Elkington, P. (2021). Powered respirators are effective, sustainable, and cost‐effective personal protective equipment for SARS‐CoV‐2. Frontiers in Medical Technology, 3. [online first] 10.3389/fmedt.2021.729658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE) . (2020). COVID‐19 rapid guideline: antibiotics for pneumonia in adults in hospital. https://www.nice.org.uk/guidance/ng173/chapter/tests-to-guide-decisions-about-using-antibiotics#tests-to-guide-decisions-about-using-antibiotics [PubMed]
- Schuetz, P. , Beishuizen, A. , Broyles, M. , Ferrer, R. , Gavazzi, G. , Gluck, E. H. , Gonzalez Del Castillo, J. , Jensen, J. U. , Kanizsai, P. L. , Kwa, A. L. H. , Krueger, S. , Luyt, C. E. , Oppert, M. , Plebani, M. , Shlyapnikov, S. A. , Toccafondi, G. , Townsend, J. , Welte, T. , & Saeed, K. (2019). Procalcitonin (PCT)‐guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clinical Chemistry and Laboratory Medicine, 57(9), 1308–1318. 10.1515/cclm-2018-1181 [DOI] [PubMed] [Google Scholar]
- Shahi, A. K. , Ahmed‐Saeed, N. , Taylor, I. , Kiernan, S. , Mahobia, N. , Pelosi, E. , & Saeed, K. (2021). Environmental contamination and personal protective equipment contamination with SARS‐CoV‐2 virus in a real‐life clinical setting. Journal of Infection Prevention, 23, 7–10. 10.1177/17571774211033348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaloganathan, A. A. , Nasim‐Mohi, M. , Brown, M. M. , Abdul, N. , Jackson, A. , Fletcher, S. , Gupta, S. , Grocott, M. P. W. , Dushianthan, A. , Gupta, S. , Nixon, J. , Grocott, M. P. W. , Levett, D. Z. H. , Stewart, M. , Dushianthan, A. , Sparkes, D. , Chambers, R. , Nolan, K. , Tanser, S. , … Kipps, C. (2020). Noninvasive ventilation for COVID‐19‐associated acute hypoxaemic respiratory failure: Experience from a single centre. British Journal of Anaesthesia, 125(4), e368–e371. 10.1016/j.bja.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs, M. (2020). UHS proning team [online]. https://uhs.proning.team
- WHO . (2022). WHO situation report. https://covid19.who.int/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.


