Abstract
Coronavirus disease-19 has had a marked impact on the transplant population and processes of care for transplant centers and organ allocation. Several single-center studies have reported successful utilization of deceased donors with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) tests. Our aims were to characterize testing, organ utilization, and transplant outcomes with donor SARS-CoV-2 status in the United States. We used Scientific Registry of Transplant Recipients data from March 12, 2020 to August 31, 2021 including a custom file with SARS-CoV-2 testing data. There were 35 347 donor specimen SARS-CoV-2 tests, 77.5% upper respiratory samples, 94.6% polymerase chain reaction tests, and 1.2% SARS-CoV-2–positive tests. Donor age, gender, history of hypertension, and diabetes were similar by SARS-CoV-2 status, while positive SARS-CoV-2 donors were more likely African-American, Hispanic, and donors after cardiac death (p-values <.01). Recipient demographic characteristics were similar by donor SARS CoV-2 status. Adjusted donor kidney discard (odds ratio = 2.08, 95% confidence interval [CI] 1.66–2.61) was higher for SARS-CoV-2–positive donors while donor liver (odds ratio = 0.44, 95% CI 0.33–0.60) and heart recovery (odds ratio = 0.44, 95% CI 0.31–0.63) were significantly reduced. Overall post-transplant graft survival for kidney, liver, and heart recipients was comparable by donor SARS-CoV-2 status. Cumulatively, there has been significantly lower utilization of SARS-CoV-2 donors with no evidence of reduced recipient graft survival with variations in practice over time.
KEYWORDS: clinical research/practice, donors and donation: donor evaluation, epidemiology, graft survival, health services and outcomes research, infection and infectious agents, infection and infectious agents—viral: SARS-CoV-2/COVID-19, infectious disease, organ procurement and allocation, organ transplantation in general
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; COVID-19, coronavirus disease of 2019; DBD, donors after brain death; DCD, donors after cardiac death; F-POS, donors with final positive SARS-CoV-2 test; HRSA, Health Resources and Services Administration; HHRI, Hennepin Healthcare Research Institute; OPO, Organ Procurement Organization; OPTN, Organ Procurement and Transplantation Network; P-POS, donors with previous positive SARS-CoV-2 test but final test negative; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAF, standard analytic file; SRTR, Scientific Registry of Transplant Recipients; UNOS, United Network for Organ Sharing
1. INTRODUCTION
The transplant population has been disproportionally affected by the coronavirus disease-19 (COVID-19) pandemic. Transplant candidates and recipients have had higher rates of COVID-19 mortality than the general population and are particularly vulnerable to COVID-19 infection.1, 2, 3, 4, 5 In addition to the direct effects of COVID-19 on the transplant patient population, processes of care for organ donation and allocation of donor organs have also been impacted.6 Early access to testing and logistical impediments had adverse effect on identifying potential deceased donors that may have been affected by SARS-CoV-2. With the exception of lung transplantation, the mechanisms of potential transmission of infection by organ donation have remained theoretical.7, 8, 9 Due to ongoing uncertainties regarding the relative risks of transplantation with donors with prior or active infections, evidence for the safe use of organs from SARS-CoV-2–positive donors has emerged slowly with small case series from individual centers.8 , 10, 11, 12, 13, 14, 15, 16 Although early results appear promising, best practices for utilization of positive SARS-CoV-2 donors remain unclear.7, 8, 9 , 11 , 17, 18, 19
In the current study, our aims were to characterize processes of care and outcomes associated with SARS-CoV-2 donation and transplantation in the United States. We sought to evaluate the incidence and type of SARS-CoV-2 testing over time. In addition, we intended to evaluate the utilization of donor organs with positive SARS-CoV-2 tests and deceased donor and recipient characteristics associated with SARS-CoV-2 status. Finally, we evaluated outcomes of recipients based on presence of donor SARS-CoV-2 testing and variation in procurement of positive SARS-CoV-2 donor organs by Organ Procurement Organizations (OPOs).
2. METHODS
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
In addition to the Standard Analytic Files (SAFs) from the SRTR, we obtained a custom file including documentation of testing for SARS-CoV-2. These data include the source of testing (specimen type), mode of testing and result of SARS-CoV-2 testing. These data were merged by donor identifiers with the SAFs including all deceased donors with organs procured for transplantation and transplant recipient data. These files contain all documented positive and negative test results, which may include multiple tests per donors. We considered donors SARS-CoV-2–positive if any specimens had a positive test, although some may have had subsequent negative tests. However, we also analyzed results based on the most proximate test to the date of donation, which included cases in which donors were initially tested as positive, but subsequent test(s) were negative. For clarity in the results, we designated donors as final SARS-CoV-2–positive (F-POS), which indicates the final test remained positive, and previously positive (P-POS), which indicates that the donor had positive test(s), but the final test was negative. To evaluate trends in specimens tested, we divided the study period into six 3-month periods. Specimen types were available as upper respiratory, lower respiratory, blood or other, which we grouped to upper and lower respiratory and other. Testing method was available as antibody (IgG/IgM/IgA), antigen, nucleic acid detection (e.g., real-time RT-PCR) and other. The vast majority of tests were nucleic acid detection as we further categorized as nucleic acid detection or other.
We used chi-square tests to evaluate the association of categorical variables. We used multivariable logistic models adjusted for donor characteristics to assess the associations of SARS-CoV-2 status with organ recovery and discard. For the model for organ discard, only organs recovered were included. Donors that had a disposition of no authorization obtained or attempted or organs procured for reasons other than transplantation were excluded from the analyses. We used Kaplan–Meier plots and log-rank tests to compare time with overall graft survival (graft loss or death) associated with SARS-CoV-2 donor status censored at last follow-up time. In addition, we used Cox proportional hazard models to evaluate the association of SARS-CoV-2 donor status adjusted for select characteristics including recipient and donor age and primary diagnosis by organ type. Based on the relatively low number of events in the positive SARS-CoV-2 status group we were not able to adjust for other potential confounding factors. All analyses were conducted in SAS (v.9.4). The study was approved by the Cleveland Clinic Institutional Review Board.
3. RESULTS
There were 35 547 total specimen test results available from the infectious disease file. Information regarding the specimens included the source of specimens, testing method, and SARS-CoV-2 status. As depicted in Table 1, specimen results increased over the study period. The majority of specimens derived from upper respiratory sites. However, the proportion of specimens derived from lower respiratory sites increased over the study period (11.5% between March 2020 and May 2020 to 32.1% between June 2021 and August 2021). The primary method of testing was nucleic acid PCR (94.6%), which was consistent over the study period. Positive SARS CoV-2 test results increased over the study period to 2.2% in the latest period and an overall proportion of 1.2%.
TABLE 1.
Characteristics of donor specimens tested (March 2020–August 2021)
| Specimen details | Level | Study quarter n (%) |
Total | |||||
|---|---|---|---|---|---|---|---|---|
| March 12, 2020–May 30, 2020 | June 1, 2020–August 30, 2020 | September 1, 2020–November 30, 2020 | December 1, 2020–February 28, 2021 | March 1, 2021–May 30, 2021 | June 1, 2021–August 31, 2021 | |||
| Specimen type | Lower respiratory | 180 (11.5) | 638 (13.9) | 819 (13.3) | 742 (11.1) | 1908 (22.7) | 2617 (32.1) | 6904 (19.4) |
| Upper respiratory | 1304 (83.4) | 3786 (82.6) | 5241 (84.9) | 5740 (85.8) | 6193 (73.8) | 5280 (64.8) | 27 544 (77.5) | |
| Other | 79 (5.1) | 162 (3.5) | 114 (1.9) | 205 (3.1) | 291 (3.5) | 248 (3.1) | 1099 (3.1) | |
| Test method | Nucleic acid (PCR) | 1480 (94.7) | 4349 (94.8) | 5905 (95.6) | 6268 (93.7) | 7893 (94.1) | 7748 (95.1) | 33 643 (94.6) |
| Othera | 83 (5.3) | 237 (5.2) | 269 (4.4) | 419 (6.3) | 499 (6.0) | 397 (4.9) | 1904 (5.4) | |
| SARS-CoV-2 status | Negative | b (99.5) | 4573 (99.7) | 6154 (99.7) | 6627 (99.1) | 8252 (98.3) | 7962 (97.8) | 35 124 (98.8) |
| Positive | b (0.5) | 13 (0.3) | 20 (0.3) | 60 (0.9) | 140 (1.7) | 183 (2.2) | 423 (1.2) | |
| Total | 1563 | 4586 | 6174 | 6687 | 8392 | 8145 | 35 547 | |
Abbreviations: PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Other includes antibody, antigen, and “other” methods.
Data includes cell counts <10, excluded from reporting specific value for privacy issues.
The disposition of organs that were not recovered, recovered but discarded and transplanted are displayed in Table 2. These data include whether donor organs had a positive SARS-CoV-2 on final result (F-POS), were positive at least once but negative on the final result (previously positive, P-POS) or negative on each test. Data for SARS-CoV-2 status for organs other than kidney, liver, and heart were limited and excluded from the table. These included 263 intestine of which 262 were not recovered and 1 transplanted, 157 double lungs of which 147 were not recovered, 2 discarded and 8 transplanted, 167 single lungs of which 165 were not recovered and 2 transplanted and 251 pancreas with 2 discarded and 16 transplanted. Among all donors, 95.5% had kidneys recovered, and the proportion of recoveries was not statistically different by SARS-CoV-2 status. Among recovered kidneys, discard rates of right kidneys were significantly higher for positive SARS-CoV-2 compared with right kidneys with all negative test(s) (30.2% vs. 21.1%, p < .01) as well as for right kidneys with final test positive (F-POS) compared right kidneys with all negative test(s) (37.7% vs. 21.1%, p < .01). This pattern was consistent for left kidneys with highest discard rates for F-POS SARS-CoV-2 kidneys (30.4%), lower for P-POS donor kidneys (25.1%) and lowest for all negative test(s) (19.8%). Liver recoveries were significantly lower among F-POS donors (47.8%) and P-POS donors (36.1%) compared with livers with all negative test(s) (24.4%, p < .01). There was no significant increase in discard rates among recovered livers associated with SARS-CoV-2 status. Donor heart non-recoveries were significantly higher among F-POS donors (87.0%) and F-POS donors (72.0%) compared with donors with all negative test(s) (65.3%, p < .01).
TABLE 2.
Organ disposition of donors by SARS-CoV-2 status
| Organ (row %)a | SARS-CoV-2 status | Not recovered | Recovered for transplant but not transplanted | Transplanted | Total (n) |
|---|---|---|---|---|---|
| Right kidney | Final test positive (F-POS) | 2 (2.9%) | 26 (37.7%)b | 41 (59.4%) | 69 |
| Previously positive (P-POS)c | 8 (4.0%) | 55 (27.6%)b | 136 (68.3%) | 199 | |
| All test(s) negative | 715 (4.5%) | 3345 (21.1%)b | 11 797 (74.4%) | 15 867 | |
| Left kidney | Final test positive (F-POS) | 3 (4.4%) | 21 (30.4%)b | 45 (65.2%) | 69 |
| Previously positive (P-POS)c | 8 (4.0%) | 50 (25.1%)b | 141 (70.9%) | 199 | |
| All test(s) negative | 727 (4.5%) | 3139 (19.8%)b | 11 992 (75.6%) | 15 858 | |
| Liver | Final test positive (F-POS) | 33 (47.8%) | 4 (5.8%) | 32 (46.4%) | 69 |
| Previously positive (P-POS)c | 70 (36.1%) | 8 (4.1%) | 116 (59.8%) | 194 | |
| All test(s) negative | 3816 (24.4%)c | 1121 (7.2%) | 10 678 (68.4%) | 15 615 | |
| Heart | Final test positive (F-POS) | 60 (87.0%) | 0 (0%) | 9 (13.0%) | 69 |
| Previously positive (P-POS)c | 136 (72.0%) | 0 (0%) | 53 (28.0%) | 189 | |
| All test(s) negative | 9209 (65.3%)c | 47 (0.3%) | 4857 (34.4%) | 14 113 |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sample size for other organs (lung, pancreas, liver segment, intestine, en-bloc kidneys) all <10 for positive SARS-CoV-2 status and excluded from table; dispositions of no authorization requested or obtained and recovered not for transplant excluded from analysis.
p-value <.05 for association of recovery or discard with SARS-CoV-2 status.
P-POS—former positive SARS-CoV-2 test, but final test negative.
There were 19 280 deceased donors in the study period. Of those, 2405 did not have data in the infectious disease file and were excluded from the analyses. Donor characteristics by SARS-CoV-2 status are displayed in Table 3. Donor age, gender, history of hypertension, history of diabetes, high terminal creatinine, cause of death, cytomegalovirus and hepatitis status, and overall kidney donor profile index group were not statistically significantly different by SARS-CoV-2 status. There was a statistically significant association of SARS-CoV-2 status and race/ethnicity and donors after brain and cardiac death. The adjusted odds ratio (AOR) of a donor with positive SARS-CoV-2 status was 1.67 (95% confidence interval [CI] 1.20–2.32) among Black donors and 2.15 (95% CI 1.57–2.93) among Hispanic donors relative to White donors. In addition, positive SARS-CoV-2 status was associated with higher AOR for donors after cardiac death (1.50, 95% CI 1.16–1.94) relative to donors after brain death.
TABLE 3.
Donor characteristics of donors with organs procured for transplantation
| Donor characteristic | Donor CoV-2 status |
Adjusted likelihood of positive CoV-2 status (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|
| Level | Negativea (n = 16 591) | Positive (n = 284) | p-valuea | |||
| Age | 0–17 | 1076 (6.5%) | 19 (6.7%) | .16 | 1.24 (0.67–2.32) | .30 |
| 18–39 | 6193 (37.3%) | 104 (36.6%) | 1.36 (0.87–2.12) | |||
| 40–49 | 3179 (19.2%) | 57 (20.1%) | 1.44 (0.92–2.27) | |||
| 50–59 | 3612 (21.8%) | 74 (26.1%) | 1.60 (1.04–2.47) | |||
| 60+ | 2531 (15.3%) | 30 (10.6%) | Reference | |||
| Gender | Female | 6273 (37.8%) | 101 (35.6%) | .44 | 0.98 (0.76–1.26) | .88 |
| Male | 10 318 (62.2%) | 183 (64.4%) | Reference | |||
| Race/ethnicity | Black | 2565 (15.5%) | 52 (18.3%) | <.001 | 1.67 (1.20–2.32) | <.001 |
| Hispanic | 2353 (14.2%) | 63 (22.2%) | 2.15 (1.57–2.93) | |||
| White | 11 018 (66.4%) | 154 (54.2%) | Reference | |||
| Other | 655 (4.0%) | 15 (5.3%) | 1.83 (1.06–3.16) | |||
| History of hypertension | No | 10 530 (63.5%) | 180 (63.4%) | .98 | Reference | .96 |
| Yes | 6061 (36.5%) | 104 (36.6%) | 0.99 (0.74–1.33) | |||
| History of diabetes | No | 14 391 (86.7%) | 242 (85.2%) | .45 | Reference | .50 |
| Yes | 2200 (13.3%) | 42 (14.8%) | 1.13 (0.79–1.62) | |||
| Donor type | DBD | 12 069 (72.7%) | 184 (64.8%) | .003 | Reference | .002 |
| DCD | 4522 (27.3%) | 100 (35.2%) | 1.50 (1.16–1.94) | |||
| Terminal creatinine >1.5 mg/dl | No | 11 510 (69.4%) | 209 (73.6%) | .13 | Reference | .17 |
| Yes | 5081 (30.6%) | 75 (26.4%) | 0.82 (0.62–1.09) | |||
| Cause of death: stroke | No | 12 511 (75.4%) | 214 (75.4%) | .98 | Reference | .91 |
| Yes | 4080 (24.6%) | 70 (24.7%) | 0.98 (0.73–1.32) | |||
| Donor CMV+ | No | 6294 (37.9%) | 118 (41.6%) | .21 | Reference | .05 |
| Yes | 10 297 (62.1%) | 166 (58.5%) | 0.78 (0.61–1.00) | |||
| Donor hepatitis-C+ | No | 14 828 (89.4%) | 261 (91.9%) | .17 | Reference | .36 |
| Yes | 1763 (10.6%) | 23 (8.1%) | 0.82 (0.53–1.26) | |||
| KDPI (%)a | 0–35 | 1562 (10.4%) | 29 (12.4%) | .31 | Not included in multivariable modela | |
| 36–84 | 7655 (51.0%) | 125 (53.4%) | ||||
| 85+ | 5809 (38.7%) | 80 (34.2%) | ||||
Abbreviation: CI, confidence interval; CMV, cytomegalovirus; KDPI, kidney donor profile index.
Negative includes ‘indeterminate’ and ‘pending’ values; one or more missing variables for KDPI calculation missing and not included; chi-square test p-value.
Type-III p-value for association of donor characteristic with CoV-2 status.
The AOR of organ recovery was not significantly associated with SARS-CoV-2 status for kidneys, but positive donor SARS-CoV-2 livers (AOR = 0.44, 95% CI 0.33–0.60) and hearts (AOR = 0.44, 95% CI 0.31–0.63) were less likely to be recovered, Table 4. Among organs recovered, kidneys with positive SARS-CoV-2 status were significantly more likely to be discarded (AOR = 2.08, 95% CI 1.66–2.61). There was no statistically significant association of SARS-CoV-2 status with liver discard. The number of hearts discarded was too low to estimate the adjusted likelihood of discard with SARS-CoV-2 status. The full models are displayed in Tables S1–S5. In addition, we replicated models limiting the definition of positive SARS-CoV-2 donor organ to the last test result (F-POS). As displayed in Table 5, the statistical significance of each model was consistent, but the specific estimated odds of recovery and discard were different. These results indicated a higher estimated likelihood of discard for kidneys and livers and a lower likelihood of recovery for kidneys, livers and hearts for F-POS donors relative to P-POS donors. Table 6 described the proportion of organs that were recovered and discarded stratified over the study period by SARS-CoV-2 status. As indicated, kidney discard rates among donors with positive SARS-CoV-2 status declined over the study period but were still higher than for SARS-CoV-2-negative donors. Recovery rates for donor livers and hearts were lower for positive SARS-CoV-2–positive donors in the later study period.
TABLE 4.
Multivariable logistic model for odds of donor organ recovery and discard by CoV2-status (any SARS-CoV-2–positive test considered positive)
| Outcome measure | Organ |
||
|---|---|---|---|
| Kidney | Liver | Heart | |
| Adjusted odds ratio for recovery of SARS-CoV-2–positive donorsa | 1.10 (0.68–1.76) | 0.44 (0.33–0.60) | 0.44 (0.31–0.63) |
| Adjusted odds ratio for discard for SARS-CoV-2–positive donorsa | 2.08 (1.66–2.61) | 1.01 (0.55–1.86) | b |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Donors defined as any positive CoV-2 test, full models displayed in Tables S1–S5.
Number of discard events too low to estimate adjusted likelihood.
TABLE 5.
Multivariable logistic model for odds of donor organ recovery and discard by CoV2-status (only final SARS-CoV-2–positive tests [F-POS] considered positive)
| Outcome measure | Organ |
||
|---|---|---|---|
| Kidney | Liver | Heart | |
| Adjusted odds ratio for the recovery of SARS-CoV-2–positive donorsa | 0.66 (0.25–1.71) | 0.40 (0.22–0.71) | 0.16 (0.07–0.36) |
| Adjusted odds ratio for discard for SARS-CoV-2–positive donorsa | 3.33 (2.21–5.02) | 1.55 (0.52–4.59) | b |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Donors defined as final positive SARS-CoV-2 test (F-POS).
Number of discard events too low to estimate adjusted odds ratio.
TABLE 6.
Proportion of organ recovery and discard by study period
| Organ recovery |
|||
|---|---|---|---|
| Organ | SARS-CoV-2 donor status | Study period |
|
| March 2020–November 2020 | December 2020–August 2021 | ||
| Kidney | Negative | 12 730 (95.4%) | 18 092 (95.5%) |
| Positive | 38 (94.7%) | 496 (96.2%) | |
| Liver | Negative | 6280 (77.5%) | 9319 (74.3%)* |
| Positive | 20 (60%) | 242 (60.7%)* | |
| Heart | Negative | 5755 (35.7%) | 8340 (34.4%)* |
| Positive | 18 (44.4%) | 239 (22.6%)* | |
| Organ discard |
|||
|---|---|---|---|
| Organ | SARS-CoV-2 donor status | Study period (n, [% discard]) |
|
| March 2020–November 2020 | December 2020–August 2021 | ||
| Kidney | Negative | 12 143 (19.7%)* | 18 092 (22.6%)* |
| Positive | 36 (44.4%)* | 477 (28.3%)* | |
| Liver | Negative | 4865 (9.2%) | 6922 (9.7%) |
| Positive | 12 (8.3%) | 147 (7.5%) | |
| Heart | Negative | 2054 (0.9%) | 2845 (1.0%) |
| Positive | 8 (0%) | 54 (0%) | |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
p < .05 for chi-square test for the association of proportion of recovery and discard by SARS-CoV-2 status within each era.
Table 7 displays the demographic characteristics of kidney, liver, and heart transplant recipients with donor SARS-CoV-2 status. There was no statistically significant association of recipient age, gender, or race/ethnicity with donor SARS-CoV-2 status for each of the organ transplant groups. Cold ischemia time was significantly longer for positive donor SARS-CoV-2 compared with negative donor SARS-CoV-2 kidney transplants (mean = 19.5 h vs. 18.2 h, p = .006). Cold ischemia time was not statistically significantly different for positive donor SARS-CoV-2 compared with negative donor SARS-CoV-2 liver transplants (mean = 6.2 h vs. 6.1 h, p = .62). There was an increased use of thymoglobulin reported among kidney recipients (71.2% vs. 65.4%, p = .02) with SARS-CoV-2–positive donors but no significant difference in use of any induction medication among liver (p = .77) or heart recipients (p = .83). Table 8 displays the number of transplants by organ and donor SARS-CoV-2 status over time. As indicated the number and proportion of positive donor SARS-CoV-2 status transplants increased within each organ transplant type.
TABLE 7.
Transplant recipient characteristics by donor organ CoV-2 status
| Organ type | Recipient characteristic | Donor CoV-2 status |
p-value | ||
|---|---|---|---|---|---|
| Level | Negativea (n = 16 591) | Positive (n = 284) | |||
| Kidneya | Age | 0–39 | 4673 (20.3%) | 80 (22.7%) | .55 |
| 40–54 | 6654 (28.9%) | 99 (28.1%) | |||
| 55–64 | 6449 (28.0%) | 102 (28.9%) | |||
| 65+ | 5283 (22.9%) | 72 (20.4%) | |||
| Gender | Female | 9097 (39.5%) | 130 (36.8%) | .32 | |
| Male | 13 962 (60.6%) | 223 (63.2%) | |||
| Race/ethnicity | Black | 7587 (28.6%) | 101 (28.6%) | .29 | |
| Hispanic | 4422 (19.2%) | 75 (21.3%) | |||
| White | 8769 (38.0%) | 136 (38.5%) | |||
| Other | 2281 (9.9%) | 41 (11.6%) | |||
| Livera | Age | 0–39 | 1923 (17.7%) | 30 (20.4%) | .50 |
| 40–54 | 2945 (27.1%) | 37(25.2%) | |||
| 55–64 | 3632 (33.4%) | 54 (36.7%) | |||
| 65+ | 2360 (21.7%) | 26 (17.7%) | |||
| Gender | Female | 4050 (37.3%) | 47 (32.0%) | .19 | |
| Male | 6810 (62.7%) | 100 (68.0%) | |||
| Race/ethnicity | Black/otherb | 926 (14.4%) | 19 (12.9%) | .59 | |
| Hispanic | 1827 (16.8%) | 30 (20.4%) | |||
| White | 7467 (68.8%) | 98 (66.7%) | |||
| Hearta | Age | 0–39 | 1270 (26.5%) | 17 (27.4%) | .93 |
| 40–54 | 1116 (23.3%) | 15 (24.2%) | |||
| 55–64 | 1496 (31.3%) | 17 (27.4%) | |||
| 65+ | 903 (18.9%) | 13 (21.0%) | |||
| Gender | Female | 1357 (28.4%) | 17 (27.4%) | .87 | |
| Male | 3428 (71.6%) | 45 (72.6%) | |||
| Race/ethnicity | Black/otherb | 1453 (30.4%) | 19 (30.7%) | .34 | |
| Hispanic | 517 (10.8%) | 11 (17.7%) | |||
| White | 2815 (58.8%) | 32 (51.6%) | |||
Solitary transplants (kidney-pancreas transplants excluded).
Sample size too small to report Black and other race separately for CoV-2-positive donors.
TABLE 8.
Number and proportion of transplants by donor SARS-CoV-2 status
| Study quarter | Organ |
|||||
|---|---|---|---|---|---|---|
| Kidney |
Liver |
Heart |
||||
| SARS-CoV-2 negative | SARS-CoV-2 positive (%) | SARS-CoV-2 negative | SARS-CoV-2–positive (%) | SARS-CoV-2 negative | SARS-CoV-2 positive (%) | |
| March 2020–May 2020 | 1393 | 0 (0%) | 704 | 0 (0%) | 299 | 0 (0%) |
| June 2020–August 2020 | 3687 | 5 (0.1%) | 1752 | 4 (0.2%) | 808 | 3 (0.4%) |
| September 2020–November 2020 | 4323 | 16 (0.4%) | 2045 | 7 (0.3%) | 895 | 5 (0.6%) |
| December 2020–February 2021 | 4058 | 37 (0.9%) | 1913 | 17 (0.9%) | 840 | 5 (0.6%) |
| March 2021–May 2021 | 4908 | 121 (2.4%) | 2252 | 51 (2.2%) | 979 | 23 (2.3%) |
| June 2021–August 2021 | 4690 | 174 (3.6%) | 2194 | 68 (3.0%) | 964 | 26 (2.6%) |
| Total | 23 059 | 353 (1.5%) | 10 860 | 147 (1.3%) | 4785 | 62 (1.3%) |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
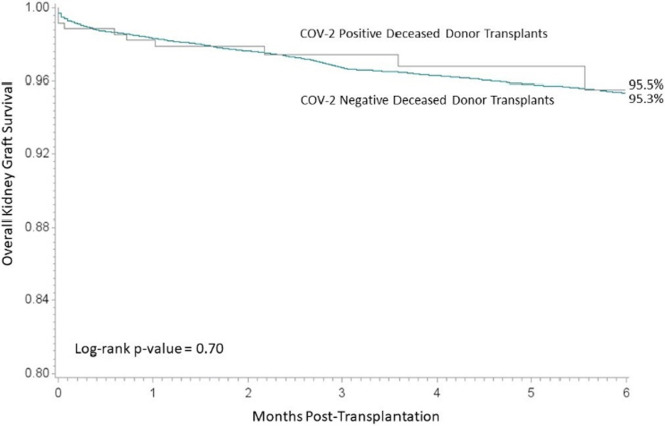
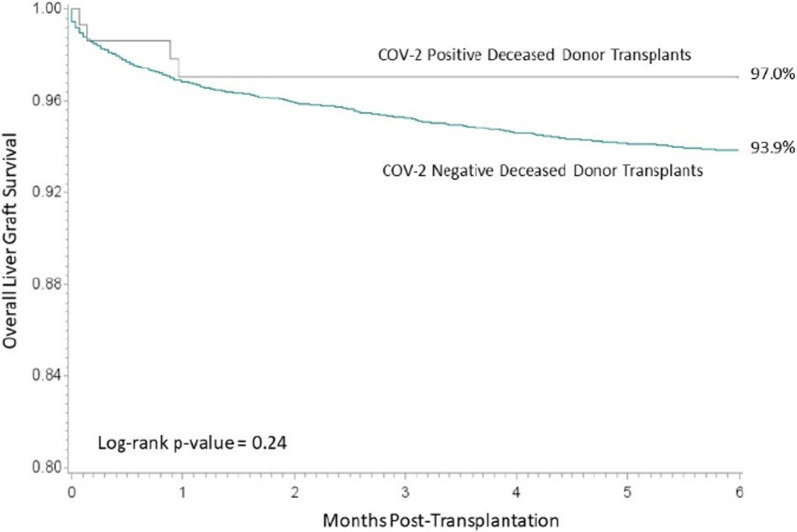
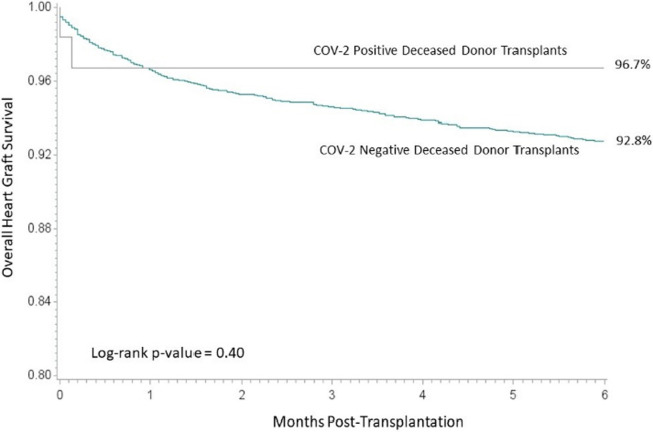
The 6-month overall graft survival for kidney transplant recipients by SARS-CoV-2 status is displayed in Figure 1. The 6-month overall graft survival for kidney transplant recipients was comparable (95.5% for recipients of SARS-CoV-2–positive donor kidneys and 95.3% for recipients of SARS-CoV-2-negative donor kidneys, log-rank p-value = .70). Delayed graft function rates were also similar for both donors after brain death (19.4% vs. 21.1% for SARS-CoV-2–positive and negative, respectively, p = .18) and donors after cardiac death (34.5% vs. 39.3% for SARS-CoV-2–positive and negative, respectively, p = .29). Similarly, liver ( Figure 2) and heart ( Figure 3) overall graft survival was comparable for recipients by donor SARS-CoV-2 status. Graft survival was also similar based on donor organs that were SARS-CoV-2 positive on the final test (F-POS). There were 86 kidney transplant recipients of F-POS SARS-CoV-2 donors with 0 graft losses at 6 months (log-rank p-value = .14), 32 liver recipients with 1 graft loss at 6 months (log-rank p-value = .78), there were not sufficient follow-ups for the nine heart recipients at the end of the study period. Multivariable Cox proportional hazard results, adjusted for donor and recipient age and primary diagnosis, indicated no association of donor SARS-CoV-2 status with overall graft survival. For kidney recipients, the adjusted hazard for graft loss for positive SARS-CoV-2 status donors, relative to negative SARS-CoV-2 donors, was 0.91 (0.49–1.69), for liver recipients, the adjusted hazard for graft loss was 0.58 (0.22–1.56) and heart recipients 0.55 (0.14–2.19).
FIGURE 1.
Overall graft survival for kidney [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
Overall graft survival among liver transplant recipients [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
Overall graft survival among heart transplant recipients [Colour figure can be viewed at wileyonlinelibrary.com]
COVID-causes of death were coded during the study period for transplant recipients. Among kidney transplant recipients, 1.3% (n = 353) had a COVID cause of death from COVID-negative donors with no cases reported for recipients of COVID-positive donors. Similarly for liver recipients, 1.3% (n = 147) had a COVID cause of death from COVID-negative donors with no reported cases among recipients of COVID-positive donors. For heart recipients, 1.3% (n = 62) had a COVID cause of death while no recipients of a COVID-positive donor had a COVID cause of death.
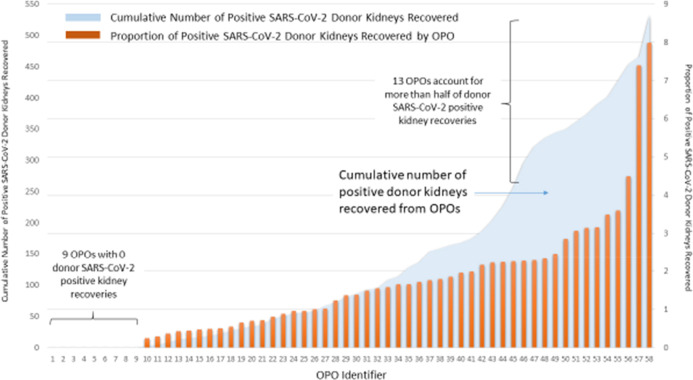
There was wide variation of positive SARS-CoV-2 kidney donors procured for transplantation by OPO, Figure 4. Among the 58 OPOs in the study period, 9 (15.5%) had no SARS-CoV-2–positive donors, the median proportion of positive SARS-CoV-2 donors was 1.4% and the maximum proportion of positive SARS-CoV-2 donors was 8.0%. Also depicted in Figure 4, the cumulative number of positive donors was skewed such that two OPOs had significantly higher proportions of positive donors and over half of the positive donors were from 13 OPOs. Of the 231 kidney transplant centers performing at least one transplant in the study period, the majority 136 (59%) did not transplant any donor kidneys with a prior positive SARS-CoV-2 test. Of those centers that did perform a transplant with a donor kidney with a prior positive SARS-CoV-2–positive test, the median percentage of SARS-CoV-2–positive donor transplants was 2.0% with range from 0.3% to 17.4% of kidney transplant volume.
FIGURE 4.
SARS-CoV-2–positive donor kidneys by Organ Procurement Organization. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
There are several principal findings of our current study characterizing SARS-CoV-2 testing and outcomes in the United States. Processes of testing changed over the course of the COVID-19 pandemic including an increased in testing, greater utilization of lower respiratory samples and an increased incidence of positive SARS-CoV-2 donors. Importantly, SARS-CoV-2–positive donor kidneys had a twofold increase in adjusted discard rates and SARS-CoV-2–positive donor liver and hearts were half as likely to be recovered for transplantation. Finally, recipient outcomes with donors with positive SARS-CoV-2 tests had comparable outcomes as organs with negative SARS-CoV-2 tests. Cumulatively, the study demonstrates that practice patterns changed regarding testing donor organs for SARS-CoV-2, but there has been significantly reduced utilization of these organs for transplant relative to SARS-CoV-2-negative donors. Coupled with early results suggesting similar graft survival among recipients of positive SARS-CoV-2 donor organs, results suggest these organs are an important potential donor source and likely beneficial to patients relative to remaining on the transplant waiting list.
One of the primary findings from the study was that recipient outcomes with use of SARS-CoV-2–positive donor organs are similar to outcomes with negative testing. These data complement prior smaller single-center reports and case series and may provide assurance that, at least short-term outcomes are not compromised by use of these organs.7, 8, 9, 10, 11 , 13 , 20 , 21 In the context of kidney transplantation, the current study indicated no difference in rates of delayed graft function or 6-month graft survival and the characteristics of recipients was similar between negative and positive SARS-CoV-2 status donors. Similarly for liver and transplant recipients, both general characteristics of recipients and 6-month graft survival were similar by SARS-CoV-2 status donors. These results were consistent for the subset of donors with final SARS-CoV-2–positive tests, rather than donors with prior positive tests may have resolved. Although the number of transplants reported from positive SARS-CoV-2 donors was relatively small, there is no evidence in additional risk to allograft survival.
Interestingly, the characteristics of deceased donors with positive and negative SARS-CoV-2 testing was generally similar. These findings provide further assurance that outcomes were not affected by SARS-CoV-2 status and positive SARS-CoV-2 donors were not systematically lower risk for other factors. The exception was that positive SARS-CoV-2 donors were more likely to derive from donors after cardiac death which may reflect a higher incidence of respiratory failure as a cause of death and other processes for identifying and managing donors. In addition, positive SARS-CoV-2 donors were more likely to be of Black and Hispanic race/ethnicity. This may reflect the disproportionate impact COVID-19 has had on these race/ethnic groups including increased hospitalizations and COVID-related deaths, which was also represented in the deceased donor population.5 , 22 , 23 There may also have been different mechanisms of death by donor characteristics or positive SARS-CoV-2 donors may have been proportionally more represented in certain regions of the country with different demographic characteristics.
Importantly, results of the study demonstrate that both recovery and utilization of donors with positive SARS-CoV-2 status were significantly reduced compared with donors with negative SARS-CoV-2 status. For kidneys, most donor organs were recovered at similar rates by SARS-CoV-2 status but had approximately twice the likelihood of discard adjusted for other donor factors. This suggests that donations that may have been beneficial to candidates may have been lost either to logistical challenges or perceived risk associated with positive SARS-CoV-2 status. This perceived risk may be changing over time with greater acceptance in contemporary practice but may illustrate lost opportunities for transplants during the initial phases of the COVID-19 pandemic.24 Similarly, positive SARS-CoV-2 donor livers and hearts were significantly less likely to be recovered. In a context in which transplantation is largely the exclusive option for life-sustaining therapy, these donor organs that were not recovered may also reflect missed transplant opportunities. Recovery and utilization for other organs (including intestine and lung) from donors with positive SARS-CoV-2 test were particularly rare and also indicative of perceived higher risks. Moreover, the loss of potential donors that were not approached associated with SARS-CoV-2 is unknown from these data, but certainly important to understand as an impact to transplant activity. The study also demonstrated that recovery of positive SARS-CoV-2 donor organs was significantly variable by OPO. This may be indicative of variation in initial access to testing, processes to identify and allocate organs with positive SARS-CoV-2 testing and perceived acceptance among local transplant centers.
SARS-CoV-2 testing also significantly changed over the study. There was an increase in overall testing over time and an increase in testing of lower respiratory samples. Testing of the lower respiratory tract was recommended in May 2021 for potential donors of lungs based on the sensitivity of tests from this source.25 The proportion of lower respiratory samples more than doubled over the period and the additional testing may have contributed to the increased positivity rate found in this study.
There are a number of limitations to consider for inferences from the study results. First, there is no detail of the symptoms associated with SARS-CoV-2–positive donors, history of prior infections or whether deaths were directly attributed to COVID-19 among donors. The data also do not include COVID PCR cycle thresholds for donors that would be informative to the analyses. These additional data are important to differentiate the relative infectious status of donors and potential risk among donor organs.26 In addition, testing patterns and modes of testing changed over the study period and sensitivity and specificity of results also likely changed over time, which may affect results over time. For example, donors categorized as SARS-CoV-2–positive based on blood tests likely have different significance than those obtained by RNA sampling. Similarly, the specific timing of positive tests associated with donation may reflect different likelihoods of active infection and risk to recipients. In addition, longer-term recipient outcomes and specific evaluation of applicable complications will be needed to confirm the utility and lack of risk associated with positive SARS-CoV-2–positive donors.
Overall the findings of the current study support that the use of positive SARS-CoV-2 donors for kidney, liver and heart transplantation despite reduced recovery and utilization of these organs during the initial phases of the COVID-19 pandemic. Further studies regarding best practices for utilization of SARS-CoV-2–positive organs, perceptions of risk among transplant candidates and longer-term transplant outcomes are needed to improve transplant opportunities for end-organ disease patients.
Acknowledgments
CONFLICT OF INTEREST
The authors report no conflicts of interest relevant for this study.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data for this study is available from the Scientific Registry of Transplant Recipients.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Table S1
Table S2
Table S3
Table S4
Table S5
REFERENCES
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohan S, King KL, Husain SA, Schold JD. COVID-19-associated mortality among kidney transplant recipients and candidates in the United States. Clin J Am Soc Nephrol. 2021;16(11):1695–1703. doi: 10.2215/CJN.02690221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schold JD, King KL, Husain SA, Poggio ED, Buccini LD, Mohan S. COVID-19 mortality among kidney transplant candidates is strongly associated with social determinants of health. Am J Transplant. 2021;21(7):2563–2572. doi: 10.1111/ajt.16578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li MT, King KL, Husain SA, Schold JD, Mohan S. Deceased donor kidneys utilization and discard rates during COVID-19 pandemic in the United States. Kidney Int Rep. 2021;6(9):2463–2467. doi: 10.1016/j.ekir.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koval CE, Poggio ED, Lin YC, Kerr H, Eltemamy M, Wee A. Early success transplanting kidneys from donors with new SARS-CoV-2 RNA positivity: a report of 10 cases. Am J Transplant. 2021;21(11):3743–3749. doi: 10.1111/ajt.16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molnar MZ, Hall IE, Raghavan D, et al. Kidney transplantation from SARS-CoV-2-positive deceased donor. Am J Transplant. 2021;22:1280–1282. doi: 10.1111/ajt.16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romagnoli R, Gruttadauria S, Tisone G, et al. Liver transplantation from active COVID-19 donors: a lifesaving opportunity worth grasping? Am J Transplant. 2021;21(12):3919–3925. doi: 10.1111/ajt.16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K, Desai NM, Resnick J, et al. Successful kidney transplantation from a deceased donor with severe COVID-19 respiratory illness with undetectable SARS-CoV-2 in donor kidney and aorta. Am J Transplant. 2022;22(5):1501–1503. doi: 10.1111/ajt.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yetmar ZA, Ranganath N, Huebert RC, Rosen CB, Razonable RR, Beam E. Successful liver transplantation from a SARS-CoV-2 positive donor to a positive recipient: potential role of monoclonal antibodies. Transplantation. 2022;106(3):e181–e182. doi: 10.1097/TP.0000000000004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barros N, Ermel A, Mihaylov P, Lacerda M, Fridell J, Kubal C. Deceased donor liver transplantation from a SARS-CoV-2-positive donor to a SARS-CoV-2-positive recipient. Liver Transpl. 2021;27(12):1849–1851. doi: 10.1002/lt.26253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puodziukaite L, Serpytis M, Kundrotaite A, et al. Kidney transplantation from a SARS-CoV-2-positive donor for the recipients with immunity after COVID-19. Transpl Infect Dis. 2021;23(4):e13666. doi: 10.1111/tid.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhand A, Gass A, Nishida S, et al. Successful transplantation of organs from a deceased donor with early SARS-CoV-2 infection. Am J Transplant. 2021;21(11):3804–3805. doi: 10.1111/ajt.16706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21(8):2885–2889. doi: 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceulemans LJ, Van Slambrouck J, De Leyn P, et al. Successful double-lung transplantation from a donor previously infected with SARS-CoV-2. Lancet Respir Med. 2021;9(3):315–318. doi: 10.1016/S2213-2600(20)30524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novysedlak R, Vachtenheim J, Jr, Striz I, Viklicky O, Lischke R, Strizova Z. SARS-CoV-2 viral load assessment in lung transplantation. Physiol Res. 2021;70(S2):S253–S258. doi: 10.33549/physiolres.934760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouphael C, D’Amico G, Ricci K, et al. Successful orthotopic liver transplantation in a patient with a positive SARS-CoV2 test and acute liver failure secondary to acetaminophen overdose. Am J Transplant. 2021;21(3):1312–1316. doi: 10.1111/ajt.16330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saracco M, Romagnoli R, Martini S. Solid non-lung organs from COVID-19 donors in seropositive or naive recipients: where do we stand? Transpl Infect Dis. 2021;24(1):e13761. doi: 10.1111/tid.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayasekera CR, Vikram HR, Rifat Z, et al. Solid organ transplantation from SARS-CoV-2-infected donors to uninfected recipients: a single-center experience. Transplant Direct. 2022;8(2):e1286. doi: 10.1097/TXD.0000000000001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Hoz RM, Mufti AR, Vagefi PA. Short-term liver transplant outcomes from SARS-CoV-2 lower respiratory tract NAT positive donors. Transpl Infect Dis. 2021;24(1):e13757. doi: 10.1111/tid.13757. [DOI] [PubMed] [Google Scholar]
- 22.Chowkwanyun M, Reed AL., Jr Racial health disparities and COVID-19 - caution and context. N Engl J Med. 2020;383:201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 23.Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kute V, Meshram HS, Fleetwood VA, Chauhan S, Lentine KL. Solid organ transplantation in SARS-CoV-2 recovered transplant candidates: a comprehensive review of recent literature. Curr Transplant Rep. 2022;9(2):95–107. doi: 10.1007/s40472-022-00362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar D, Humar A, Keshavjee S, Cypel M. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am J Transplant. 2021;21(7):2623–2624. doi: 10.1111/ajt.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichenberger EM, Kaul DR, Wolfe CR. The pandemic provides a pathway: what we know and what we need to know about using COVID positive donors. Transpl Infect Dis. 2021;23(5):e13727. doi: 10.1111/tid.13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Data Availability Statement
The data for this study is available from the Scientific Registry of Transplant Recipients.