Abstract
Background and objective
Ivermectin is a known anti‐parasitic agent that has been investigated as an antiviral agent against coronavirus disease 2019 (COVID‐19). This study aimed to evaluate the efficacy of ivermectin in mild COVID‐19 patients.
Methods
In this multi‐arm randomized clinical trial conducted between 9 April 2021 and 20 May 2021, a total of 393 patients with reverse transcription‐PCR‐confirmed COVID‐19 infection and mild symptoms were enrolled. Subjects were randomized in a 1:1:1 ratio to receive single‐dose ivermectin (12 mg), double‐dose ivermectin (24 mg) or placebo. The primary outcome was need for hospitalization.
Results
There was no significant difference in the proportion of subjects who required hospitalization between the placebo and single‐dose ivermectin groups (absolute difference in the proportions: −2.3 [95% CI = −8.5, 4.1]) and between the placebo and double‐dose ivermectin groups (absolute difference in the proportions: −3.9 [95% CI = −9.8, 2.2]). The odds of differences in mean change in severity score between single‐dose ivermectin and placebo groups (ORdifference = 1.005 [95% CI: 0.972, 1.040]; p = 0.762) and double‐dose ivermectin and placebo groups (ORdifference = 1.010 [95% CI: 0.974, 1.046]; p = 0.598) were not statistically significant. None of the six adverse events (including mild dermatitis, tachycardia and hypertension) were serious and required extra action.
Conclusion
Single‐dose and double‐dose ivermectin early treatment were not superior to the placebo in preventing progression to hospitalization and improving clinical course in mild COVID‐19.
Keywords: efficacy, ivermectin, mild COVID‐19, randomized clinical trial, SARS‐CoV‐2
Short abstract
We conducted a double‐blinded randomized placebo‐controlled trial including 393 patients with mild coronavirus disease 2019 (COVID‐19) and found that ivermectin, an anti‐parasitic medication with known antiviral properties, was non‐superior to the placebo. Neither a single nor a double dose was better in preventing progression to hospitalization and worsening of the clinical course of COVID‐19 infection.
INTRODUCTION
More than one and a half years from the declaration of the new Coronaviridae species (SARS‐CoV‐2) disease pandemic, coronavirus disease 2019 (COVID‐19), there are major challenges in treating and preventing hospitalization in non‐severe COVID‐19 patients. Currently, monoclonal antibodies (casirivimab and imdevimab), remdesivir and two small‐molecule antiviral tablets (molnupiravir and Paxlovid (Nirmatrelvir/Ritonavir)) have shown efficacy for preventing hospitalization or shortening hospital stay in phase 3 trials of high‐risk patients. 1 , 2 , 3 , 4 , 5 However, monoclonal antibody therapies are generally expensive and must be injected 2 ; molnupiravir and Paxlovid are still unavailable in most countries, 3 , 4 and the antiviral drug, remdesivir, requires injection at designated outpatient facilities and is in short supply in many countries, including Iran. The emergence of new variants and limited vaccination uptake in low‐resource regions highlight the ongoing need for effective, accessible antiviral agents to treat COVID‐19. 6
An important strategy is to repurpose existing drugs with known antiviral activity (e.g., hydroxychloroquine, colchicine, doxycycline, azithromycin, etc.). Discovered in the late 1970s, ivermectin (a dihydro derivative of avermectin) 7 is a well‐established broad‐spectrum anti‐parasitic agent, mainly used against roundworm parasites (i.e., onchocerciasis and strongyloidiasis) and other diseases caused by soil‐transmitted helminths, as well as parasitic skin infections such as scabies. 8 , 9 In vitro studies have shown that ivermectin has antiviral activity against several RNA viruses 10 , 11 , 12 , 13 , 14 through interaction with different protein‐binding sites, which prevents viral replication. 12 , 13 , 15 , 16 Vero cell assays showed that ivermectin has antiviral activity against SARS‐CoV‐2. 17 Ivermectin has been mostly used off‐label in developing countries and even in developed countries.
It is uncertain if ivermectin is effective in real‐world COVID‐19 treatment. The antiviral activity of ivermectin might be attained during the viral replication phase 16 ; therefore, ‘early treatment’ by ivermectin may yield promising results in COVID‐19 patients. 18 Some placebo‐controlled randomized controlled trials (RCTs) on outpatient COVID‐19 subjects 19 , 20 could not find any benefit on the clinical course of infection (i.e., need for hospitalization and resolution of symptoms), although it is proposed that higher than standard doses of ivermectin might yield a faster and better improvement without safety concerns. 21 These studies recommended further large RCTs to assess the efficacy of ivermectin in COVID‐19 treatment.
These studies are heterogenous in terms of the study protocol, sample size, methodology, severity of disease, dosing, control arm and outcomes. 22 Hence, the latest World Health Organization (WHO) recommendation is to not use ivermectin for COVID‐19 patients regardless of disease severity, and to restrict the use to clinical trials until more data are available. 1
In this multi‐arm placebo‐controlled RCT, we investigated the clinical efficacy of single‐dose and double‐dose ivermectin to evaluate the potential benefits of early treatment for mild COVID‐19. Our hypothesis was that double‐dose ivermectin would decrease the need for hospitalization amongst outpatient subjects with COVID‐19 when administered during the first days of infection.
METHODS
Study design and participants
In this multi‐arm parallel‐group, double‐blind RCT, we used stratified random allocation in a 1:1:1 ratio to enrol 393 patients with mild symptomatic COVID‐19, confirmed by a positive reverse transcription (RT)‐PCR test, who visited any of the 14 specialized COVID‐19 outpatient treatment centres affiliated to the Shiraz University of Medical Sciences, Shiraz, Iran, between 9 April 2021 and 20 May 2021. Mild symptomatic COVID‐19 was defined as ambulatory state (score 1 or 2) based on the WHO 9‐point ordinal scale for clinical improvement, ranged from 0 (uninfected; no clinical or virological evidence of infection) to 8 (death). 23
Sample size
When this trial was designed, published data were limited. In Chowdhury and colleagues' trial 24 on comparing the efficacy of ivermectin–doxycycline and hydroxychloroquine–azithromycin treatment regimens on mild COVID‐19 patients, the end‐point hospitalization rates were 0% and 4% (no need for hospitalization of 100% and 96%) amongst the symptomatic participants of the first and second treatment arms, respectively. We calculated a total sample size of 393 mild symptomatic COVID‐19 patients (131 patients in each arm) by assuming that 86% had no need for hospitalization in the placebo group (10% lower than that of active control group in Chowdhury et al.'s study), an anticipated clinical efficacy (absolute difference, effect size) of 10% in reducing hospitalization rate achieved by ivermectin, α = 5% (corresponding with Z 1−α/2 = 1.96), β = 20% (corresponding with Z 1−β = 0.84), enrolment ratio of 1 and with an anticipated 5% drop out rate. The sample size calculation was carried out by the R Shiny graphical user interface (GUI) to the R package ‘multiarm’ (single‐stage multi‐arm clinical trial design for a Bernoulli distributed primary outcome; available from: https://mjgrayling.shinyapps.io/multiarm/).
Inclusion and exclusion criteria
We included patients who had mild symptomatic COVID‐19 confirmed by RT‐PCR test, had symptom onset‐to‐visit interval of less than 48 h, were aged 18–80 years and had oxygen saturation levels of at least 93% in room air. Patients were excluded if they needed hospitalization, needed any other antiviral medications, had severe hepatic disease (e.g., cirrhosis, etc.), severe chronic kidney disease (i.e., stage IV), severe pulmonary disease (e.g., chronic obstructive pulmonary disease), HIV/AIDS, were pregnant or lactating, refused to participate or were participating in another RCT.
Interventions and randomization
Each subject was randomly assigned to the single dose of ivermectin (#4 3 mg tablet, cumulative dose of 12 mg, Stat.; Laboratoire Europhartech, Domes Pharma, France + #4 placebo tablets, at the second day), double‐dose ivermectin (#4 3 mg tablet for 2 days, cumulative dose of 24 mg) or placebo at the day of RT‐PCR‐positive testing (enrolment day) and the next day. At enrolment, demographic data, clinical history (comorbidities, smoking state, oxygen therapy) and baseline symptom scores (i.e., sore throat, dyspnoea, cough, fatigue, headache, chills, feeling hot or feverish, vomiting, confusion, disrupted sense of smell or taste, chest pain, anorexia; binary score for each symptom, yes/no) were recorded.
Performing stratification before random allocation may prevent type I error and improve power for small trials (i.e., less than 400 subjects). 25 Hence, we selected age—one the major variables associated with prognosis—and oxygen saturation level—a severity index. Four strata were generated according to age (<55 and ≥55 years) and oxygen saturation (93%–95% and ≥96%).
Randomization number sequence was generated using the block randomization method (six blocks of three, each contained a unique sequence of A, B and C treatments) in the four strata via PROC PLAN of SAS 9.4(PROC PLAN of SAS 9.4) byan independent unblinded epidemiologist. The investigator and physicians, subjects and the statistician were kept blinded to the allocation sequence. An employee outside the research team entered data into separate datasheets so that the statistician could analyse the data without having access to information about the allocation.
The first follow‐up (day 0) was the next day after a participant consumed the second dose of ivermectin (double‐dose ivermectin arm), first dose of placebo (single‐dose ivermectin arm) or second dose of placebo (placebo arm). At the same time, standard care according to the national guidelines was continued for all subjects (available from: https://treatment.sbmu.ac.ir/index.jsp?pageid=63989&p=1).
Outcome measures
The primary outcome measure was the proportion of subjects who required hospitalization up to 28 days of follow‐up. Secondary outcome measures included the proportion of subjects with resolution of symptoms, required machine ventilation or deceased, as well as time to resolution of symptoms, defined as the first day during the follow‐up period in which the subject reported a severity score of 0. Another secondary outcome was the trend of change in severity scale in the three arms. Symptom scores were individually recoded at days 0, 1, 3, 7, 14, 21 and 28 of follow‐up using subject‐reported outcome checklist. Adverse events were recorded, including the need for additional care, shifting to the other study arm or non‐compliance), events leading to treatment discontinuation and serious events.
Statistical analysis
The CONSORT 2010 flow diagram was used to report the study flow (Figure 1). In case of protocol violation, the results were reported through intention‐to‐treat analysis. The study protocol remained unchanged until the end of the trial for all participants.
FIGURE 1.
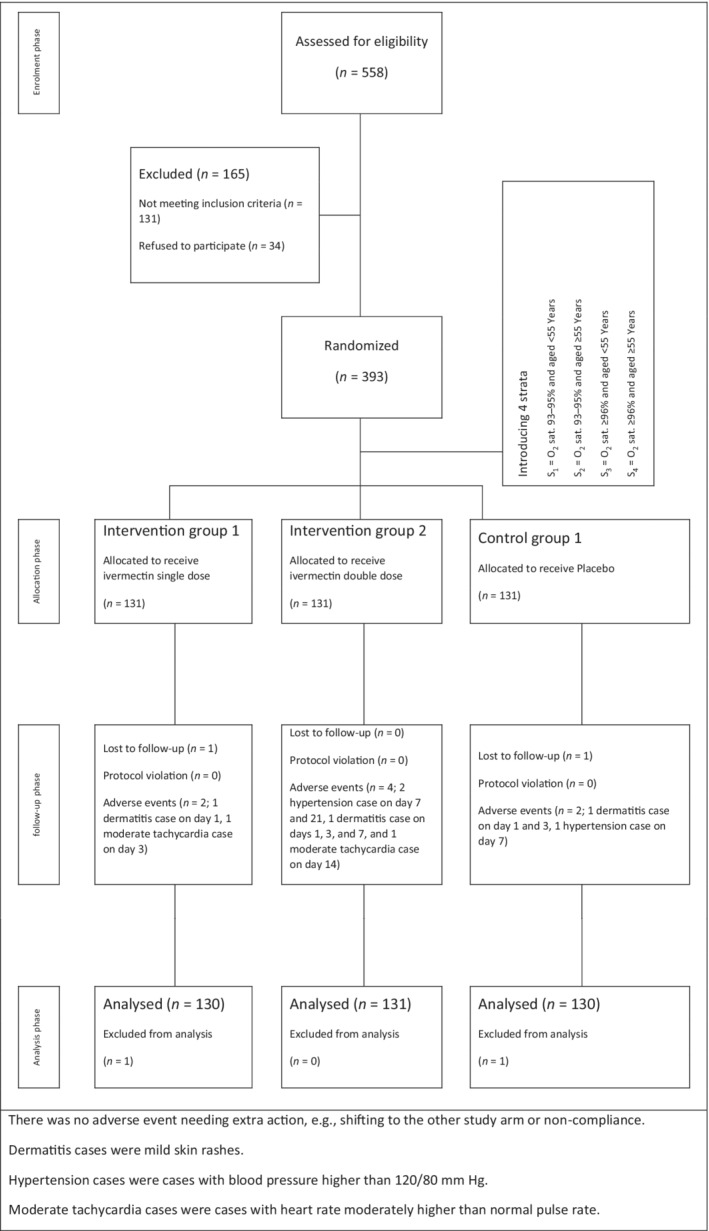
CONSORT flow chart of a randomized clinical trial of single‐dose ivermectin, double‐dose ivermectin and placebo efficacy in mild COVID‐19 subjects
Statistical analysis was carried out using the R programming language (version 4.0.4 for MacOS; PROC PLAN of SAS 9.4). Variables were described using median (interquartile range [IQR]), mean ± SD or 95% CI and frequency (percent), respectively.
The baseline scores of severity scale in the three groups were reported by observed means and least‐squares means (estimated marginal means). Least‐squares means of severity scale in the three arms were calculated using multivariable linear regression using ‘emmeans’ package. We observed that the observed means were inflated by ‘unweighted aggregate symptoms score’.
For each comparative pair (placebo vs. single‐dose or double‐dose ivermectin) in all primary and secondary outcomes, proportions in each group and estimated effect sizes (absolute difference in the proportions or difference is the median) with their precisions (95% CI) around the point estimates were reported (Appendix S1 in the Supporting Information).
Multivariable generalized estimating equation (GEE) models were applied using ‘multgee’ package to compare the effect of ivermectin (single/double dose) with placebo on repeated measurements of WHO's ordinal severity scale in mild COVID‐19 subjects over 30 days of ivermectin trial, which were adjusted for sex, age, smoking state, unweighted aggregate symptoms score and comorbidity (Appendix S2 in the Supporting Information). The significance level was set at p = 0.05.
RESULTS
Study participants
A total of 393 subjects with mild COVID‐19 were enrolled, with 131 receiving placebo, 131 receiving single‐dose ivermectin and 131 receiving double‐dose ivermectin, alongside standard care. Two subjects were excluded from all analyses because they were lost at the first follow‐up day (one in the single‐dose ivermectin group and one in the placebo group). The analysis population included 391 subjects followed up for 4 weeks. Subjects in the three groups were balanced in demographic and baseline clinical characteristics (Table 1). The median age in placebo, single‐dose and double‐dose ivermectin groups were 39.5 (IQR: 17.5), 39.5 (16.5) and 39 (17) years, with a male proportion of 54.6%, 60% and 47.3%, respectively. The median unweighted aggregate symptoms score was similar in the three groups (3 [IQR: 3]), and 78.5%, 84.6% and 74.8% of subjects did not have any known comorbidities at baseline in placebo, single‐dose and double‐dose ivermectin groups, respectively. Cough and fatigue were the most common symptoms in all the three groups. At randomization, the unadjusted and adjusted mean severity scale were similar for all the three groups, specifically 1.81 (SD: 0.39) and 1.77 (95% CI: 1.70, 1.85) in the placebo group, 1.79 (SD: 0.41) and 1.76 (95% CI: 1.68, 1.84) in the single‐dose ivermectin group and 1.83 (SD: 0.38) and 1.79 (95% CI: 1.71, 1.86) in the double‐dose ivermectin group.
TABLE 1.
Demographic and baseline clinical characteristics of mild COVID‐19 subjects
| Variable | Placebo | Ivermectin (S) | Ivermectin (D) |
|---|---|---|---|
| (n = 130) | (n = 130) | (n = 131) | |
| Sex | |||
| Male | 71 (54.6) a | 78 (60) | 62 (47.3) |
| Female | 59 (45.4) | 52 (40) | 69 (52.7) |
| Age, years | 39.5 [17.5] b | 39.5 [16.5] | 39 [17] |
| Smoking | |||
| No | 105 (80.8) | 103 (79.2) | 102 (77.9) |
| Smoker | 11 (8.5) | 12 (9.2) | 16 (12.2) |
| Ex‐smoker | 14 (10.8) | 15 (11.5) | 13 (9.9) |
| Oxygen therapy | |||
| No | 128 (98.5) | 130 (100) | 131 (100) |
| Yes | 2 (1.5) | 0 | 0 |
| Unweighted aggregate symptoms score | 3 [3] | 3 [3] | 3 [3] |
| Oxygen saturation level | |||
| 93%–95% | 19 (14.6) | 21 (16.2) | 19 (14.5) |
| ≥96% | 111 (85.3) | 109 (83.8) | 112 (85.5) |
| Comorbidities | |||
| No | 102 (78.5) | 110 (84.6) | 98 (74.8) |
| Yes | 28 (21.5) | 20 (15.4) | 33 (25.2) |
| Type 2 diabetes | 6 (4.6) | 2 (1.5) | 7 (5.3) |
| Hypertension | 7 (5.4) | 6 (4.6) | 11 (8.4) |
| Cardiovascular disease | 1 (0.8) | 0 | 2 (1.5) |
| Mild chronic kidney disease | 1 (0.8) | 1 (0.8) | 0 |
| Mild liver disease | 0 | 0 | 0 |
| Cancer | 0 | 0 | 1 (0.8) |
| Chronic respiratory disease | 0 | 1 (0.8) | 2 (1.5) |
| Other | 19 (14.6) | 14 (10.8) | 17 (13.0) |
| Symptoms | |||
| Fever (feeling hot or feverish) | 12 (9.2) | 14 (10.8) | 8 (6.1) |
| Headache | 40 (30.8) | 23 (17.7) | 37 (28.2) |
| Chills or shivering | 11 (8.5) | 10 (7.7) | 9 (6.9) |
| Confusion | 20 (15.4) | 21 (16.2) | 23 (17.6) |
| Fatigue, low energy or tiredness | 50 (38.5) | 52 (40) | 61 (46.6) |
| Cough | 62 (47.7) | 60 (46.2) | 56 (42.7) |
| Sore throat | 30 (23.1) | 26 (20) | 23 (17.6) |
| Disrupted sense of smell | 29 (22.3) | 38 (29.2) | 42 (32.1) |
| Disrupted sense of taste | 21 (16.2) | 26 (20) | 32 (24.4) |
| Vomiting | 8 (6.2) | 3 (2.3) | 5 (3.8) |
| Dyspnoea | 15 (11.5) | 17 (13.1) | 16 (12.2) |
| Chest pain | 11 (8.5) | 17 (13.1) | 14 (10.7) |
| Anorexia | 21 (16.2) | 23 (17.7) | 25 (19.1) |
| Ordinal severity scale | |||
| Mean ± SD | 1.81 ± 0.39 | 1.79 ± 0.41 | 1.83 ± 0.38 |
| Least‐squares mean [95% CI] | 1.77 [1.70, 1.85] c | 1.76 [1.68, 1.84] | 1.79 [1.71, 1.86] |
Abbreviations: IQR, interquartile range; ivermectin (S), ivermectin single‐dose; ivermectin (D), ivermectin double‐dose.
Count (percent).
Median [IQR].
Least‐squares means (estimated marginal means) of severity scale in the three arms were calculated using linear regression on full set of independent variables. The observed means were inflated by ‘unweighted aggregate symptoms score’.
Primary outcome
At the end of 28 days of follow‐up, there was no significant difference in the proportion of subjects who required hospitalization between the placebo (8.5%) and single‐dose ivermectin (6.2%) groups (absolute difference in the proportions: −2.3 [95% CI = −8.5, 4.1]) nor between the placebo and double‐dose ivermectin group (4.6%) (absolute difference in the proportions: −3.9 [95% CI = −9.8, 2.2]). One subject in the placebo group was admitted to the intensive care unit (Table 2).
TABLE 2.
Primary and secondary outcomes in ivermectin trial of mild COVID‐19 subjects
| Variable | Placebo | Ivermectin (S) | ∆ [95% CI] a | Ivermectin (D) | ∆ [95% CI] |
|---|---|---|---|---|---|
| (n = 130) | (n = 130) | (n = 131) | |||
| Primary outcome | |||||
| Need for hospitalization | 11 (8.5) b | 8 (6.2) | −2.3 [−8.5, 4.1] | 6 (4.6) | −3.9 [−9.8, 2.2] |
| Adverse events | |||||
| No | 128 (98.5) | 128 (98.5) | ‐ | 127 (96.9) | ‐ |
| Yes | 2 (1.5) | 2 (1.5) | 4 (3.1) | ||
| Follow‐up on day 1 | 1 (0.8) | 1 (0.8) | 1 (0.8) | ||
| Follow‐up on day 3 | 1 (0.8) | 1 (0.8) | 1 (0.8) | ||
| Follow‐up on day 7 | 1 (0.8) | 0 | 2 (1.5) | ||
| Follow‐up on day 14 | 0 | 0 | 1 (0.8) | ||
| Follow‐up on day 21 | 0 | 0 | 1 (0.8) | ||
| Follow‐up on day 28 | 0 | 0 | 0 | ||
| Secondary outcomes | |||||
| Symptoms resolved | |||||
| Follow‐up on day 7 | 77 (59.2) | 79 (60.7) | 1.5 [−10.3, 13.5] | 81 (61.8) | 2.6 [−9.3, 14.5] |
| Follow‐up on day 14 | 99 (76.2) | 95 (73.1) | −3.1 [−13.7, 7.5] | 105 (80.2) | 4.0 [−5.9, 14.1] |
| Follow‐up on day 21 | 108 (83.1) | 116 (89.3) | 6.2 [−2.3, 14.5] | 117 (89.3) | 6.2 [−2.1, 14.5] |
| Follow‐up on day 28 | 117 (90.0) | 121 (93.1) | 3.1 [−3.6, 9.8] | 123 (93.9) | 3.9 [−3.3, 10.1] |
| Time to resolution of symptoms, days | 9 [17.75] c | 9 [18] | 0 [−7, 7] d | 9 [18] | 0 [−7, 7] |
| Need for machine ventilation | 3 (2.3) | 2 (1.5) | −0.8 [−4.1, 2.5] | 1 (0.8) | −1.5 [−4.5, 1.5] |
| Mortality | 0 | 0 | ‐ | 0 | ‐ |
Abbreviations: IQR, interquartile range; ivermectin (S), ivermectin single‐dose; ivermectin (D), ivermectin double‐dose.
Absolute difference in the proportions (∆) [95% CI].
One subject was admitted to the intensive care unit.
Median [IQR].
Absolute difference is the median (∆) [95% CI].
Secondary outcomes
The median time to resolution of symptoms in subjects assigned to single‐dose ivermectin versus placebo was not significantly different (9 (IQR: 18) days vs. 9 (IQR: 17.75) days; absolute difference is the median: 0 [95% CI = −7, 7]), as well as in subjects assigned to double‐dose ivermectin versus placebo (9 (IQR: 18) days vs. 9 (IQR: 17.75) days; absolute difference is the median: 0 [95% CI = −7, 7]).
There were no significant differences in the resolution of symptoms or need for machine ventilation by day 28 between the placebo and single‐dose ivermectin groups (93.1% and 1.5% with single‐dose ivermectin, 90.0% and 2.3% with placebo; absolute difference in the proportions: 3.1 [95% CI = −3.6, 9.8] and −0.8 [95% CI = −4.1, 2.5]) or between the placebo and double‐dose ivermectin groups (93.9% and 0.8% with double‐dose ivermectin, 90.0% and 2.3% with placebo; absolute difference in the proportions: 3.9 [95% CI = −3.3, 10.1] and −1.5 [95% CI = −4.5, 1.5]). Moreover, none of the participants died (Table 2).
Multivariable ordinal GEE models showed that the odds of improving the severity score in the ordinal scale was 1.733 (95% CI: 1.145, 2.022; p < 0.0001) in the placebo group. When assessing the differences in estimated marginal means of change in severity score between the groups, no significant differences were found between the odds of single‐dose ivermectin and placebo groups (ORdifference = 1.005 [95% CI: 0.972, 1.040]; p = 0.762) and double‐dose ivermectin and placebo groups (ORdifference = 1.010 [95% CI: 0.974, 1.046]; p = 0.598) (Table 3 and Figure 2).
TABLE 3.
Summary of multivariable ordinal generalized estimating equation models to assess the difference of three arms in decreasing the WHO's severity scale amongst mild COVID‐19 subjects over 30 days of trial
| Variable | B coefficient (logit scale) | San. SE | San. Z | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Treatment arm (reference: placebo) | ||||||
| Placebo a | 0.160 | 0.012 | 12.886 | 1.733 | 1.145, 2.022 | <0.0001 |
| Ivermectin (single‐dose) b | 0.005 | 0.017 | 0.303 | 1.005 | 0.972, 1.040 | 0.762 |
| Ivermectin (double‐dose) b | 0.010 | 0.018 | 0.527 | 1.010 | 0.974, 1.046 | 0.598 |
| Age, years | −0.017 | 0.005 | −3.02 | 0.984 | 0.974, 0.994 | 0.002 |
| Female sex (reference: male) | 0.267 | 0.134 | 1.99 | 1.306 | 1.004, 1.700 | 0.047 |
| Smoking (reference: non‐smoker) | ||||||
| Ex‐smoker | 0.093 | 0.220 | 0.427 | 1.098 | 0.714, 1.690 | 0.669 |
| Smoker | 0.224 | 0.196 | 1.142 | 1.251 | 0.852, 1.836 | 0.254 |
| Unweighted aggregate symptoms score | −0.202 | 0.031 | −6.478 | 0.817 | 0.768, 0.868 | <0.0001 |
| Comorbidity (reference: negative) | 0.066 | 0.162 | 0.410 | 1.069 | 0.776, 1.469 | 0.682 |
Abbreviations: San., sandwich; WHO, World Health Organization.
Amount of change in severity score per a follow‐up.
Difference in the estimated marginal means of change in severity score per a follow‐up between an active treatment and the placebo.
FIGURE 2.
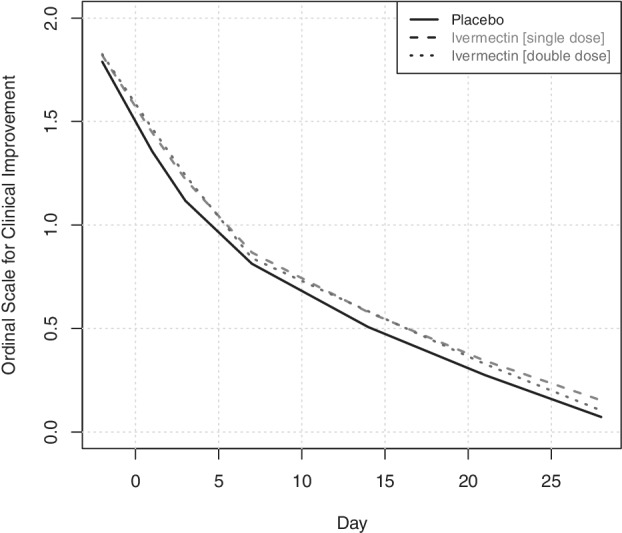
Changes in the World Health Organization's ordinal severity scale amongst ivermectin (single/double dose) and placebo groups of mild COVID‐19 over 30 days of trial
Adverse events
A total of eight subjects (two, two and four in placebo, single‐dose ivermectin and double‐dose ivermectin groups, respectively) developed adverse events. Each group had one subject who developed dermatitis early in the trial (days 1–7). Hypertension developed in one subject of the placebo group at day 7 and in two subjects of the double‐dose ivermectin group at days 7 and 21. Tachycardia developed in one subject of the single‐dose ivermectin group at day 3 and in one subject of the double‐dose ivermectin group at day 14. None of these adverse events required participants to leave the study (Table 2).
DISCUSSION
This multi‐arm parallel‐group double‐blind RCT was designed to investigate the clinical efficacy of early treatment with single‐dose and double‐dose ivermectin in mild COVID‐19 patients. We did not find any significant difference in terms of all primary and secondary outcomes. Although the percent values of patients needing hospitalization or mechanical ventilation or with persistent symptoms were numerically smaller in the ivermectin groups than the placebo, these differences were not clinically significant.
Our findings were consistent with other RCTs that investigated the clinical efficacy of oral ivermectin early treatment of mild COVID‐19 patients. 19 , 20 , 26 While these studies varied in population characteristics, number of subjects, medication protocol and the definition of outcomes, their main findings were universal. No significant difference was found between the active treatment and placebo groups for the median duration of symptoms (4–10 vs. 4–12 days), 19 , 27 resolution of symptoms (82% vs. 79%), 19 worse progression rate (2%–10% vs. 0%–10.5%), 19 , 20 , 26 , 27 need for machine ventilation (0%–1.8% vs. 0%–8.8%), 26 , 28 mortality rate (0% vs. 0%–7%) 26 , 28 and total discharge rate (100% vs. 93%). 26 Ivermectin early treatment was not clinically superior to the placebo for multiple outcomes among mild COVID‐19 patients. These data support the latest WHO conclusions on the pooled data from 16 RCTs 29 and the National Institutes of Health's (NIH) COVID‐19 treatment guidelines, 30 that is, ivermectin is not recommended as standard care for patients with mild COVID‐19. 29 , 30
We recorded two, two and four participants who developed adverse events in the placebo, single‐dose ivermectin and double‐dose ivermectin groups, respectively, none of them severe, which reflects a good safety profile at trialled doses. 27
An important finding of our trial is the lack of clinical benefit from doubling the dose of ivermectin. This was also reported by several similar trials, 19 , 27 , 28 , 29 even with higher than standard doses of ivermectin administered for a longer duration (i.e., 5 consecutive days). 18 , 19 , 27 These studies were heterogenous with regard to definition of outcomes, assessment tools and uncontrolled confounders.
In addition, our study was limited by lack of SARS‐CoV‐2 vaccination data, which might be a potential confounder; however, by 20 May 2021—the last enrolment day of this trial—only ~2.4% of people had been vaccinated against SARS‐CoV‐2 in Iran, 31 with most of them being healthcare staff.
In conclusion, ivermectin early treatment, even using a double dose, was not superior to the placebo in preventing progression to hospitalization and achieving resolution of symptoms in mild COVID‐19.
AUTHOR CONTRIBUTION
Alireza Mirahmadizadeh: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); writing – original draft (equal); writing – review and editing (equal). Ali Semati: Data curation (equal); investigation (equal); project administration (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Alireza Heiran: Formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Mostafa Ebrahimi: Data curation (equal); funding acquisition (equal); project administration (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Abdolrasool Hemmati: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal). Mohammadreza Karimi: Conceptualization (equal); investigation (equal); methodology (equal); project administration (equal); writing – original draft (equal); writing – review and editing (equal). Souzan Basir: Conceptualization (equal); investigation (equal); project administration (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Marjan Zare: Conceptualization (equal); data curation (equal); methodology (equal); project administration (equal); writing – original draft (equal); writing – review and editing (equal). Antonio Charlys da Costa: Conceptualization (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Mohammad Zeinali: Conceptualization (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Maryam Sargolzaee: Conceptualization (equal); investigation (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Owrang Eilami: Conceptualization (equal); data curation (equal); funding acquisition (equal); project administration (equal); resources (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
None declared.
HUMAN ETHICS APPROVAL DECLARATION
This study was conducted in compliance with local regulatory requirements, Good Clinical Practice (GCP) and the Declaration of Helsinki. It was approved by the Ethics Committee of Shiraz University of Medical Sciences (code: IR.SUMS.REC.1399.1202). Each patient signed a written informed consent form before receiving any new treatment and provided permission to publish the results.
Clinical Trial Registration: IRCT20210213050344N1 at https://en.irct.ir
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Kaveh Taghipour for his linguistic editing of this manuscript. We also thank Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for English language editing. The authors are also grateful to Sina Amini, Alireza Rahimi, Ehsan Zare and Kazem Panahi, public health experts at Comprehensive Health Service Centers, and Dr. Narsis Ghahramani and Dr. Tahereh Safari, specialists in Infectious and Tropical Diseases affiliated to Shiraz University of Medical Sciences, Shiraz, Iran, for their cooperation.
Research funding: This work was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (grant number: 99‐7850).
Mirahmadizadeh A, Semati A, Heiran A, Ebrahimi M, Hemmati A, Karimi M, et al. Efficacy of single‐dose and double‐dose ivermectin early treatment in preventing progression to hospitalization in mild COVID‐19: A multi‐arm, parallel‐group randomized, double‐blind, placebo‐controlled trial. Respirology. 2022. 10.1111/resp.14318
Associate Editor: Marcos I. Restrepo; Senior Editor: Paul King
Funding information Shiraz University of Medical Sciences, Grant/Award Number: 99‐7850
DATA AVAILABILITY STATEMENT
The data set generated during the current study is not publicly available due to confidentiality of the patient's information; however, data are available from the corresponding author on reasonable request.
REFERENCES
- 1. World Health Organization . COVID‐19: Clinical Care. Therapeutics and COVID‐19: Living Guideline. [updated 2021 Sep 25; cited 2021 Sep 28]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2
- 2. Ledford H. Antibody therapies could be a bridge to a coronavirus vaccine — but will the world benefit? Nature. 2020;584:333–4. 10.1038/d41586-020-02360-y [DOI] [PubMed] [Google Scholar]
- 3. Willyard C. How antiviral pill molnupiravir shot ahead in the COVID drug hunt. Nature. 2021. Oct 8. 10.1038/d41586-021-02783-1 [DOI] [PubMed] [Google Scholar]
- 4. Ledford H. COVID antiviral pills: what scientists still want to know. Nature. 2021;599:358–9. 10.1038/d41586-021-03074-5 [DOI] [PubMed] [Google Scholar]
- 5. Bloomberg . Remdesivir Averts Hospitalization in Study of High‐Risk Patients. 2021. Sep 22 [cited 2021 Nov 27]. Available from: https://www.bloomberg.com/news/articles/2021-09-22/remdesivir-averts-hospitalization-in-study-of-high-risk-patients
- 6. Mallapaty S. COVID vaccines slash viral spread – but Delta is an unknown. Nature. 2021;596(7870):17–8. 10.1038/d41586-021-02054-z [DOI] [PubMed] [Google Scholar]
- 7. Crump A, Ōmura S. Ivermectin, 'wonder drug' from Japan: the human use perspective. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(2):13–28. 10.2183/pjab.87.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . World Health Organization Model List of Essential Medicines: 21st List 2019. Geneva: World Health Organization; 2019. [cited 2021 Sep 19]. Available from: https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf [Google Scholar]
- 9. Laing R, Gillan V, Devaney E. Ivermectin – old drug, new tricks? Trends Parasitol. 2017;33(6):463–72. 10.1016/j.pt.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Götz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Höper D, et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6:23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lundberg L, Pinkham C, Baer A, Amaya M, Narayanan A, Wagstaff KM, et al. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan equine encephalitis virus replication. Antiviral Res. 2013;100(3):662–72. 10.1016/j.antiviral.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 12. Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1‐4 non‐structural protein 5; protection against all 4 DENV serotypes by the inhibitor ivermectin. Antiviral Res. 2013;99(3):301–6. 10.1016/j.antiviral.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 13. Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β‐mediated nuclear import able to inhibit replication of HIV‐1 and dengue virus. Biochem J. 2012;443(3):851–6. 10.1042/BJ20120150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caly L, Wagstaff KM, Jans DA. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antiviral Res. 2012;95(3):202–6. 10.1016/j.antiviral.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 15. Yang SNY, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. 10.1016/j.antiviral.2020.104760 [DOI] [PubMed] [Google Scholar]
- 16. Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. 2012;67(8):1884–94. 10.1093/jac/dks147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA‐approved drug ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res. 2020;178:104787. 10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, Bazzal AA, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARSCoV‐2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021;13(6):989. 10.3390/v13060989 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. López‐Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID‐19: a randomized clinical trial. JAMA. 2021;325(14):1426–35. 10.1001/jama.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID‐19 (IVERCOR‐COVID19): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):965. 10.1186/s13063-020-04813-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navarro M, Camprubí D, Requena‐Méndez A, Buonfrate D, Giorli G, Kamgno J, et al. Safety of high‐dose ivermectin: a systematic review and metaanalysis. J Antimicrob Chemother. 2020;75(4):827–34. 10.1093/jac/dkz524 [DOI] [PubMed] [Google Scholar]
- 22. Kaur H, Shekhar N, Sharma S, Sarma P, Prakash A, Medhi B. Ivermectin as a potential drug for treatment of COVID‐19: an in‐sync review with clinical and computational attributes. Pharmacol Rep. 2021;73(3):736–49. 10.1007/s43440-020-00195-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . COVID‐19 Therapeutic Trial Synopsis Draft. [updated 2020 Feb 18; cited 2021 Sep 19]. Available from: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis
- 24. Chowdhury A, Shahbaz M, Karim M, Islam J, Dan G, Shuixiang H. A comparative study on ivermectin‐doxycycline and hydroxychloroquine‐azithromycin therapy on COVID‐19 patients. EJMO. 2021;5(1):63–70. [Google Scholar]
- 25. Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19–26. 10.1016/s0895-4356(98)00138-3 [DOI] [PubMed] [Google Scholar]
- 26. Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, Manjhi PK, et al. Evaluation of ivermectin as a potential treatment for mild to moderate COVID‐19: a double‐blind randomized placebo controlled trial in eastern India. J Pharm Pharm Sci. 2021;24:343–50. 10.18433/jpps32105 [DOI] [PubMed] [Google Scholar]
- 27. Buonfrate D, Chesini F, Martini D, Roncaglioni MC, Ojeda Fernandez ML, Alvisi MF, et al. High dose ivermectin for the early treatment of COVID‐19 (COVER study): a randomised, double‐blind, multicentre, phase II, dose‐finding, proof of concept clinical trial. Int J Antimicrob Agents. 2022;59:106516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, et al. Antiviral effect of high‐dose ivermectin in adults with COVID‐19: a proof‐of‐concept randomized trial. EClinicalMedicine. 2021;37:100959. 10.1016/j.eclinm.2021.100959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization . WHO Advises that Ivermectin Only Be Used to Treat COVID‐19 Within Clinical Trials. [updated 2021 Mar 31; cited 2021 Sep 19]. Available from: https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
- 30. Centers for Disease Control and Prevention (CDC) . Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID‐19. [updated 2021 Aug 26; cited 2021 Sep 19]. Available from: https://emergency.cdc.gov/han/2021/han00449.asp
- 31. Google News . Coronavirus (COVID‐19). [updated 2021 Sep 27; cited 2021 Oct 2]. Available from: https://news.google.com/covid19/map?hl=fa&state=7&mid=%2Fm%2F03shp
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data set generated during the current study is not publicly available due to confidentiality of the patient's information; however, data are available from the corresponding author on reasonable request.


