Abstract
Background
The United States (US) data suggest fewer‐than‐expected preterm births in 2020, but no study has examined the impact of exposure to the early COVID‐19 pandemic at different points in gestation on preterm birth.
Objective
Our objective was to determine—among cohorts exposed to the early COVID‐19 pandemic—whether observed counts of overall, early and moderately preterm birth fell outside the expected range.
Methods
We used de‐identified, cross‐sectional, national birth certificate data from 2014 to 2020. We used month and year of birth and gestational age to estimate month of conception for birth. We calculated the count of overall (<37 weeks gestation), early (<33 weeks gestation) and moderately (33 to <37 weeks gestation) preterm birth by month of conception. We employed time series methods to estimate expected counts of preterm birth for exposed conception cohorts and identified cohorts for whom the observed counts of preterm birth fell outside the 95% detection interval of the expected value.
Results
Among the 23,731,146 births in our study, the mean prevalence of preterm birth among monthly conception cohorts was 9.7 per 100 live births. Gestations conceived in July, August or December of 2019—that is exposed to the early COVID‐19 pandemic in the first or third trimester—yielded approximately 3245 fewer moderately preterm and 3627 fewer overall preterm births than the expected values for moderate and overall preterm. Gestations conceived in August and October of 2019—that is exposed to the early COVID‐19 pandemic in the late second to third trimester—produced approximately 498 fewer early preterm births than the expected count for early preterm.
Conclusions
Exposure to the early COVID‐19 pandemic may have promoted longer gestation among close‐to‐term pregnancies, reduced risk of later preterm delivery among gestations exposed in the first trimester or induced selective loss of gestations.
Keywords: COVID‐19 pandemic, pregnancy, premature birth, time series
Synopsis.
Study question
Did preterm births decrease among US conception cohorts exposed to early COVID‐19 disruptions?
What's already known?
US national data suggest lower‐than‐expected counts of preterm birth among live births in March and April of 2020.
What this study adds
We build on prior work by using robust methods to investigate how timing of exposure to the pandemic during gestation impacts birth outcomes. Gestations in the first or third trimester during the early COVID‐19 pandemic yielded fewer‐than‐expected overall and moderately preterm births, while gestations in the late second trimester or third trimester produced fewer‐than‐expected early preterm births. The early pandemic may have promoted longer gestation among close‐to‐term pregnancies, reduced risk of preterm delivery among gestations exposed in the first trimester or induced selective loss.
1. BACKGROUND
In March 2020, the World Health Organization declared the novel coronavirus, SARS‐CoV‐2 and the disease it causes, COVID‐19, a pandemic. Between 19 March and 7 April 2020, 42 United States (US) states issued unprecedented ‘stay‐at‐home’ orders. 1 , 2 These orders, combined with the closing of schools, workplaces and businesses, reportedly reduced the spread of infection, rates of hospitalisation and mortality. 3 At the same time, stay‐at‐home orders, coupled with the fear and uncertainty of a global pandemic, correspond with increased reports of depressive symptoms, anxiety and psychological distress. 4 , 5 For those unable to stay at home, such as foodservice, healthcare and front‐line workers, this time period may have also induced fear for personal safety. 6 , 7 Other substantial environmental and social changes occurred during this period, including reduced mobility, air pollution and in‐person work hours and increased social isolation. 4 , 8 In sum, the early months of the COVID‐19 pandemic—that is March and April of 2020—likely marked a period of an intense population‐level shock.
Prior literature suggests that population‐level shocks—including terrorist attacks, earthquakes and sudden economic contractions—affect gestations in utero in ways that manifest as increased prevalence of adverse birth outcomes including preterm and small for gestational age birth. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Evidence also suggests that population‐level shocks in early gestation are more strongly associated with adverse birth outcomes than shocks in later gestation. 9 , 10
The initial examinations of the impact of the COVID‐19 pandemic on adverse birth outcomes in convenience samples defined by geography and care providers have produced mixed evidence. 17 , 18 National‐level data from the United States, however, suggest lower‐than‐expected values of preterm birth among infants born during March and April of 2020 as well as later in 2020 (i.e. November). 17 , 18 , 19 To our knowledge, however, no existing study has examined the impact of exposure to the population‐level shock of the early COVID‐19 pandemic (i.e. March and April of 2020) at different points in gestation on the outcome of pregnancies. In this study, we provide evidence to fill this gap by using an approach that characterises gestations by conception month (rather than by birth month), and therefore, can assess the timing of in utero exposure to the unprecedented and intense population‐level shock of the early COVID‐19 pandemic. Our objective was to determine—among cohorts conceived from June 2019 to January 2020—whether observed counts of early preterm birth (<33 weeks gestation), moderately preterm birth (33–36 weeks gestation) or overall preterm birth (<37 weeks gestation) fell outside the 95% detection interval of expected counts based on 70 conception cohorts not exposed to the population‐level shock of the early COVID‐19 pandemic.
2. METHODS
2.1. Data and sample
We used de‐identified data from the National Center for Health Statistics (NCHS) birth certificate records from 1 January 2014 to 31 December 2020 (most recent available data), which include all live births in the United States. (n = 26,986,716). 20 Our analytic sample included singleton births to US residents ages 15 to 49 (n = 26,899,970). We excluded records missing length of gestation or birth weight and with implausible combinations of birth weight and gestational age 21 (n = 344,263) and records from the ‘unrevised’ (1989) version of the US Standard Certificate of Live Birth (instead of the ‘revised’ 2003 version) (n = 209,624). We also limited our analysis to singleton births with estimated conception dates between August 2013 and January 2020 (n = 23,731,146) to avoid fixed cohort bias. 22 Including births with conception months prior to August 2013 would, by definition, exclude very preterm births (because birth dates begin in 2014), while including conceptions later than January 2020 would not allow us to observe the entire range of possible gestational ages.
2.2. Exposure
The NCHS All‐County Natality files contain month and year of birth and gestational age in weeks (multiplied by 7 to obtain gestational age in days), which we used to define conception cohorts. Because we lacked exact birth dates, we assigned all births a random day of birth from the set of possible days of birth in that month (e.g. January = 31, February = 28 except in 2016 where February = 29). We then subtracted gestational age in days from the assigned birth date to estimate month of conception.
2.3. Outcomes
Gestational age was based on the NCHS combined (based on both obstetric estimate and date of last menstrual period) estimate of gestational age. We then created a count variable for the overall number of conceptions in each month that resulted in a live birth from August 2013 to January 2020, as well as separate variables for the number of conceptions in each month that resulted in a preterm birth, an early preterm birth or a moderately preterm birth. Overall preterm births were defined as any birth <37 weeks gestation, early preterm births were defined as <33 weeks gestation and moderately preterm births were defined as 33–37 weeks gestation.
2.4. Statistical analysis
Determining whether the count of preterm birth differed from expected values in conception cohorts exposed to the early COVID‐19 pandemic in the United States (i.e. March and April of 2020) requires estimates of preterm birth had the pandemic not occurred. An intuitive approach to devising these ‘counterfactuals’ assumes that the count of preterm births among monthly conception cohorts appears normally and independently distributed over time. If true, the counterfactual value for cohorts conceived from June 2019 to January 2020 (i.e. in gestation from the first through 36th week of pregnancy in March 2020) would equal the mean of counts for cohorts conceived earlier. The researcher could define ‘unexpected’ values as those falling outside a detection interval set a priori.
While the logic of the above approach seems straightforward, the observed count of preterm birth in monthly conception cohorts violates the assumption of independent distribution over time. These monthly time series typically exhibit patterns that can include linear trend, cycles (e.g. seasonality) and the tendency to remain elevated or depressed after high or low values. This ‘autocorrelation’ implies that the mean of prior counts cannot logically serve as the counterfactual for exposed cohorts. Instead, values predicted from autocorrelation would serve as counterfactuals. We, therefore, devised our counterfactuals with Box‐Jenkins methods widely used in engineering and in the natural as well social sciences to detect and model autocorrelation in serial measurements. 23 In our case, these models ‘fit’ autocorrelation and leave monthly residual counts of preterm birth that meet the assumption of normal and independent distribution with an expected value of zero.
Our analyses proceeded through the following steps performed separately for all preterm birth, as well as for early and moderately preterm birth, yielded by monthly conception cohorts in the United States. First, for the 70 cohorts conceived from August 2013 to May 2019, we used Box‐Jenkins methods to model count of preterm births as a function of the count of all births and of autocorrelation. 23 The residuals of these three ‘transfer functions’ meet the assumption of normal and independent distribution with an expected value of zero. Second, we defined the 95% detection interval of the residual series as the product of 1.96 and the residual series' standard deviation. Third, we applied the transfer functions, with parameter values fixed to those estimated in Step 1, to cohorts conceived from June 2019 to January 2020 (i.e. those in gestation from the first through 36th week of pregnancy in March 2020). Fourth, we combined the residuals of from Steps 1 and 2 and graphed them as well as the 95% detection intervals. Fifth, we inferred that among the exposed conception cohorts, those that fell outside the respective detection intervals had yielded unexpectedly high or low incidence of the specified category of preterm birth.
To separate the population stress of the early COVID‐19 pandemic from the potential impact of maternal SARS‐Cov‐2 infection or COVID‐19 illness on in utero gestations, we then repeated the same analysis but excluded births occurring in New York and New Jersey, two states with very high rates of infection, hospitalisation and death due to COVID‐19 at the outset of the pandemic. 24 Much theory 25 predicts, and empirical evidence demonstrates, 26 considerable differences among racialized groups in the risk of preterm birth. We did not set out to review those theories or to extend them to cohorts in gestation during the early pandemic. Hoping to add impetus to such work, we did, however, apply the test procedures described above to preterm births among mothers identified as non‐Hispanic Black (NHB) because they remain at relatively high risk of parturition before 36 completed weeks of gestation. 26
2.5. Ethics approval
This research was approved by the Institutional Review Board at Michigan State University. Data management was performed with SAS 9.4 and time series analyses were conducted with Scientific Computing Associates (River Forest, IL).
3. RESULTS
Over the study period, the mean prevalence of preterm birth among monthly conception cohorts was 9.7 per 100 live births (standard deviation [SD] = 0.4). As shown in Figure S1, preterm birth appeared to drift upward from 2016 to 2018, which coheres with previous reports. 27 The patterns in early and moderately preterm births (Figures S2 and S3) appear qualitatively similar to that of overall preterm. In addition to this pattern, the three series show weak seasonality.
Formal inspection of the autocorrelation function of these series among pre‐COVID months (i.e. 70 conception cohorts, August 2013 to May 2019), identified several patterns (Table 1). These patterns imply a transfer function with an autoregressive parameter at lag 12 months (i.e. AR12) for all three series to control for weak seasonality. In addition, transfer functions for the overall and moderately preterm birth series required an autoregressive parameter at the first lag (i.e. AR1) to control for the tendency for high (or low) values to persist, albeit diminished, into the following month or later. None of the series required differencing (i.e. the ‘I’ in ARIMA) or inclusion of moving average (MA) parameters.
TABLE 1.
Time series results predicting monthly values of selected preterm outcomes in the United States for conception cohorts from August 2013 to May 2019 (i.e. unexposed to COVID‐19 pandemic) as a function of live births and autocorrelation
| Parameter | Lag (months) | Overall preterm | Early preterm | Moderately preterm | |||
|---|---|---|---|---|---|---|---|
| Coef. | SE | Coef. | SE | Coef. | SE | ||
| Constant | ― | 21462.29 | 5157.88 | 1474.86 | 445.96 | 19628.72 | 4646.25 |
| Live Births | 0 | 0.041 | 0.013 | 0.0123 | 0.0015 | 0.0277 | 0.0124 |
| Autoregressive | 1 | 0.523 | 0.116 | ― | ― | 0.519 | 0.116 |
| 12 | 0.897 | 0.088 | 0.544 | 0.112 | 0.885 | 0.087 | |
Figure 1 displays the results of applying the ARIMA transfer function for preterm births in 70 pre‐exposure conception cohorts to all 78 cohorts. Three of the eight conception cohorts exposed to the early COVID‐19 pandemic—conceptions in July, August and December 2019—show fewer than expected overall preterm births. Approximate gestational ages in March 2020 for each of these conception cohorts are as follows: conceived in July 2019, 31 to 39 weeks in March 2020 (for preterm births, 31 to 36 weeks); conceived in August 2019, 27 to 34 weeks in March 2020; and conceived in December 2019, <20 weeks in March 2020. To help the reader interpret the magnitude of this result, the reduction of preterm in these 3 months statistically associated with the early COVID‐19 pandemic, is 4.3% (i.e. 3627 fewer than the 83,666 expected preterm births in these 3 months).
FIGURE 1.
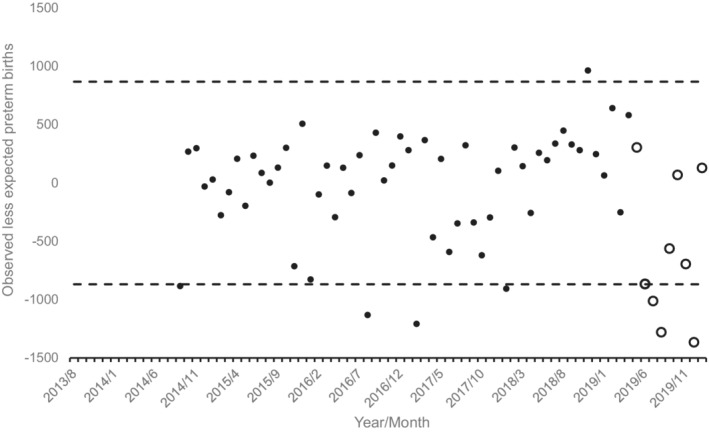
Observed less expected preterm births (i.e. <37 weeks gestation) for 79 monthly conception cohorts in the United States conceived 8/2013 through 1/2020 (13 months lost to modelling). Unfilled circles show cohorts exposed in utero to onset of the COVID‐19 pandemic. Dashed lines indicate 95% detection intervals
As shown in Figure 2, results for early preterm show two detectable ‘negative’ cohorts among those exposed, August and October 2019. Approximate gestational ages in March 2020 for each of these conception cohorts are as follows: conceived in August 2019, 27 to 34 weeks (for early preterm births, 27 to 32 weeks); and conceived in October 2019, 22 to 30 weeks. The magnitude of the decline in these two cohorts is 5.4% (498 fewer than the 9173 expected early preterm births).
FIGURE 2.
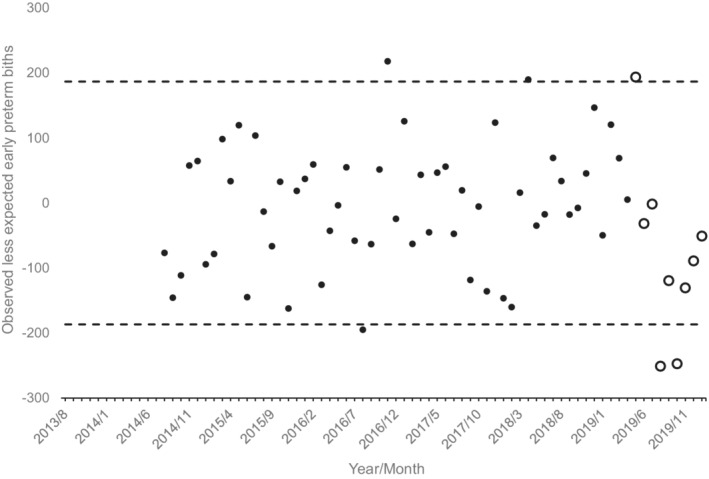
Observed less expected early preterm births (i.e. <33 weeks gestation) for 79 monthly conception cohorts in the United States conceived 8/2013 through 1/2020 (12 months lost to modelling). Unfilled circles show cohorts exposed in utero to onset of the COVID‐19 pandemic. Dashed lines indicate 95% detection intervals
Consistent with the fact that moderately preterm births account for a majority of preterm births (~73% in 2019 27 ), the former show a pattern similar to the latter (Figure 3). Detectably negative cohorts were conceived in July, August and December 2019 and yielded 4.7% or 3245 fewer moderately preterm births that the expected 69,059.
FIGURE 3.
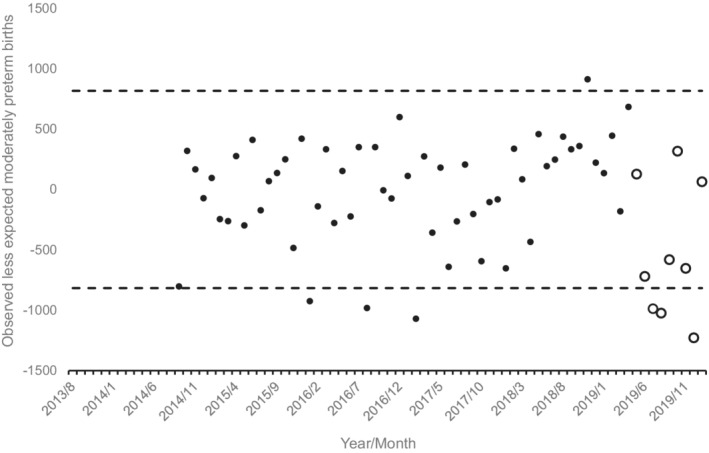
Observed less expected moderately preterm US births (i.e. 33 to 36 weeks gestation) for 79 monthly conception cohorts in the United States conceived 8/2013 through 1/2020 (13 months lost to modelling). Unfilled circles show cohorts exposed in utero to onset of the COVID‐19 pandemic. Dashed lines indicate 95% detection intervals
When we repeated our tests (i.e. preterm birth, early preterm birth and moderately preterm birth) after excluding New York and New Jersey—two states with high SARS‐CoV‐2 infections in March through May 2020—the same cohorts as listed above fell below their expected counts for overall, early and moderately preterm births (Figures S4 through S6). When we repeated our tests among non‐Hispanic Black mothers, we found that among pregnancies exposed to the onset of the pandemic, those conceived in July 2019 fell below the 95% detection interval by yielding 252, or 4.0%, fewer preterm births than the 6326 expected. Of these 252 ‘missing’ births, 246 were moderately preterm.
4. COMMENT
4.1. Principal Findings
Gestations conceived in the United States in July, August or December of 2019—that is exposed to the early COVID‐19 pandemic in the first or third trimester—resulted in fewer overall preterm and moderately preterm births than expected based on 70 previous conception cohorts. In addition, gestations conceived in August and October of 2019—that is exposed to the early COVID‐19 pandemic in the late second or third trimester—resulted in fewer early preterm births than expected.
4.2. Strengths of this study
The use of conception—rather than birth—cohorts allowed us to assess when in gestation foetuses were exposed to the population shock of the early COVID‐19 pandemic. Moreover, whereas most studies in the United States focused on clinically or geographically defined study populations, we use national‐level birth certificate data from 2014 to 2020. Our methodological approach also offers several advantages over other studies; in particular, our interrupted time series design controls for nuanced forms of temporal patterns, an approach which has been shown to reduce potential bias relative to approaches that compare Spring months in 2020 to similar months in prior calendar years. 28
4.3. Limitations of the data
Although our study is one of the first to evaluate whether timing of exposure to the onset of the COVID‐19 pandemic influenced risk of preterm birth among exposed gestations, our analysis precludes a detailed investigation of potential mechanisms. Further, the birth certificate data only included month and year of birth, so we randomly assigned birthdays during the birth month. Measurement error also exists in gestational days, since NCHS only reports completed weeks gestation. Thus, there are two potential sources of error in our estimation of conception month (i.e. birthday and gestational days); we have, however, no reason to believe this error would be differential with respect to conception cohort exposed to the pandemic. At the time of the study, data on births conceived during 2020 and born in 2021 were not available; examining the impact of exposure to later phases of the pandemic on birth outcomes will be an important next step. Our study did not evaluate outcomes of pregnancies that do not end in live birth (e.g. miscarriages/spontaneous loss and fetal deaths), which were unavailable at the time of our study. We were also not able to identify SARS‐CoV‐2 infection or presence of COVID‐19 disease in our study population. 29 , 30 We note that our findings are not necessarily at odds with an increased risk of preterm birth among people with SARS‐CoV‐2 infection. Rather, our findings demonstrate the aggregate impact of exposure to the early COVID‐19 pandemic on pregnancy outcomes and may reflect the sum of risk and protective factors as well as of selective loss.
4.4. Interpretation
Our findings are consistent with a recent NCHS report, demonstrating a 1% absolute decline in overall preterm and small declines in early and late preterm in 2020. 31 Our findings are also consistent with an earlier report—also using US natality data—of below‐expected prevalence of overall preterm among births in March and April of 2020, early (<34 weeks) preterm in March and November of 2020 and late preterm (34–36 weeks) in March to June and November of 2020. 19 Systematic reviews of international literature also suggest that preterm birth may have decreased in high‐income countries, 18 although not worldwide. 17 , 18 On the other hand, US‐based studies examining data from only state or region in the United States have reported mixed findings regarding changes in preterm birth during 2020. 32 , 33 , 34 For example, there was no evidence of changes in preterm birth at 2 Philadelphia hospitals in March to June 2020 or in California from April to July of 2020, but investigators found lower odds of preterm birth in Tennessee in March and April of 2020 compared with previous years. Our study makes an important step forward in our understanding of the population‐level impacts of the pandemic on preterm birth by (1) using monthly conception cohorts to examine whether and how timing of exposure to the early pandemic impacted preterm birth and (2) by using interrupted time‐series modelling to account for time‐related autocorrelation. 28
Importantly, our findings, along with others indicating declines in preterm birth during the early pandemic, are unexpected within the dominant paradigm suggesting that population stress acts a risk factor increasing the likelihood of an adverse pregnancy outcome. 35 , 36 By using conception cohorts to examine timing of exposure to the early pandemic, we sought to shed light on the unexpected finding that preterm birth may have declined during the early pandemic. Our findings, however, suggest that not one but multiple potential mechanisms may have contributed to the lower‐than‐expected prevalence in preterm birth in early 2020. The conception cohorts identified as having lower‐than‐expected counts of overall preterm and moderately preterm would have been at gestational ages of either 27 weeks or later (i.e. exposed in the third trimester, for July and August 2019 conception cohorts) or at less than 20 weeks gestation (i.e. exposed in the first trimester, for December 2019 cohorts) at the beginning of March 2020. The conception cohorts identified as having lower than expected early preterm would have been at gestational ages between 22 to 34 weeks (i.e. late second trimester and third trimester).
Gestations already close to 37 weeks gestation in March and/or April of 2020 may have had an increased probability of being carried to term due to reduction in risk factors for preterm labor such as long in‐person working hours or physical labor. 37 , 38 Changes in clinical practice may also have resulted in fewer moderate or late preterm births during this period. A meta‐analysis of studies from high‐income countries found stronger evidence to support a decline in spontaneous (unlikely influenced by clinical practice around the time of delivery) compared with indicated (more likely influenced by clinical practice around the time of delivery) preterm births, suggesting that clinical practice may not have been a driving factor. 17 However, some studies, including a nationwide study from the Netherlands, find declines in only indicated preterm births. 39 In a study from a single tertiary center in Pennsylvania, Lemon and colleagues examined both spontaneous preterm births and medically indicated preterm births and reported reductions in both types of preterm during the COVID‐19 pandemic, but the declines in spontaneous preterm were limited to white women, those living in more advantaged neighbourhoods, and deliveries at non‐outpatient care facilities, suggesting that the decline in preterm birth was concentrated among more advantaged women. 40 In the current study, we were unable to distinguish between medically indicated and spontaneous preterm birth, but our findings demonstrated that the decline in moderately preterm birth among gestations in the third trimester was also found among mothers identified as non‐Hispanic Black, suggesting that this phenomenon was not limited to white women at the national level in the United States. Inconsistencies in findings across location and setting suggest that drivers of reduction in preterm birth may differ by place, that associations may vary by comparison time period, and that clinical data sets may be prone to selection bias, particularly during a pandemic. These concerns highlight the need for continued work using national data and methods that control for time‐dependent autocorrelation, which may also vary by factors such as race or other sociodemographic characteristics.
Gestations at pre‐ or peri‐viable ages (<20 weeks to 22 weeks) in March of 2020 may also have benefitted from some health‐promoting aspect of the early pandemic that reduced later risk of preterm birth. For example, a 10 ppb increase in nitrogen dioxide (NO2) during gestation is associated with a 5% increase in the odds of preterm birth. 41 From March to May of 2020 in eleven US urban areas, levels of NO2 fell on average by 3.1 ppb, potentially contributing to reduced preterm birth rates. 42 On the other hand, the cohorts exposed to the early COVID‐19 pandemic at <20 to 22 weeks could also have experienced more spontaneous loss or fetal death, resulting in fewer at‐risk pregnancies born preterm. Prior evidence indicates that other population‐level shocks (including COVID‐19 43 ) have resulted in selective loss, particularly of frail male foetuses. 44 , 45 Although meta‐analyses have reported no significant change in stillbirth in high‐income countries associated with the COVID‐19 pandemic, 17 , 18 few of these studies utilise US data. Moreover, pregnancy loss prior to 20 weeks—which is typically poorly documented—has not been examined in relation to COVID‐19. Therefore, the argument of increased selective loss due to the early COVID‐19 pandemic merits further refinement and testing.
Hypothesis‐driven tests of these potential mechanisms—that is changes in clinical practice, reduction in risk factors for preterm birth and selective loss—using national‐level data (e.g. incorporating fetal deaths), time series methods and theory‐driven hypotheses are now necessary to identify whether knowledge gained from declines in preterm birth during the COVID‐19 pandemic can be translated into preventive measures for preterm birth outside the context of a global pandemic.
5. CONCLUSIONS
We found that gestations exposed to the early COVID‐19 pandemic in the first or third trimester had lower‐than‐expected counts of overall and moderately preterm birth, while gestations exposed in the late second or early third trimester had lower‐than‐expected counts of early preterm birth. Although prior evidence suggested a decline in preterm birth during the COVID‐19 pandemic, our approach confirmed that this decline occurred on a national level and among cohorts exposed in both early and late gestation. These findings—which contrast the dominant narrative that stress increases risk of adverse pregnancy outcomes—suggest that the population shock of the early COVID‐19 pandemic may have promoted longer gestation among close‐to‐term pregnancies, reduced risk of later preterm delivery among gestations exposed in the first trimester or induced selective loss of exposed gestations. Future research should discriminate among these mechanisms.
AUTHOR CONTRIBUTIONS
All authors contributed to the study design and theoretical framing of the study, CM and CMB conducted data management and cleaning, TB and RC analyzed the data, all authors contributed to interpretation of results, CM, TB, and RC drafted the manuscript, all authors contributed to writing and editing the manuscript.
CONFLICTS OF INTEREST
We have no conflicts of interest to declare.
Supporting information
Appendix S1
ACKNOWLEDGEMENT
Open access funding enabled and organized by ProjektDEAL.
Margerison CE, Bruckner TA, MacCallum‐Bridges C, Catalano R, Casey JA, Gemmill A. Exposure to the early COVID‐19 pandemic and early, moderate and overall preterm births in the United States: A conception cohort approach. Paediatr Perinat Epidemiol. 2022;00:1‐9. doi: 10.1111/ppe.12894
Funding information
This research was supported in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD103736, PI Bruckner)
Social Media Quote: New US data show unexpected declines in preterm birth among gestations that were in the first or third trimester when the pandemic began in March/April 2020. More research needed to explain why this happened.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the National Center for Health Statistics. Restrictions apply to the availability of these data, which were used under a data use agreement for this study.
REFERENCES
- 1. Moreland A, Herlihy C, Tynan MA, et al. Timing of state and territorial COVID‐19 stay‐at‐home orders and changes in population movement ‐ United States, march 1‐may 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1198‐1203. doi: 10.15585/mmwr.mm6935a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ballotpedia . Status of lockdown and stay‐at‐home orders in response to the coronavirus (COVID‐19) pandemic, 2020. https://ballotpedia.org/Status_of_lockdown_and_stay‐at‐home_orders_in_response_to_the_coronavirus_(COVID‐19)_pandemic_2020. Accessed December 13, 2021.
- 3. Lyu W, Wehby GL. Shelter‐in‐place orders reduced COVID‐19 mortality and reduced the rate of growth in hospitalizations. Health Aff (Millwood). 2020;39(9):1615‐1623. doi: 10.1377/hlthaff.2020.00719 [DOI] [PubMed] [Google Scholar]
- 4. Marroquín B, Vine V, Morgan R. Mental health during the COVID‐19 pandemic: effects of stay‐at‐home policies, social distancing behavior, and social resources. Psychiatry Res. 2020;293:113419. doi: 10.1016/j.psychres.2020.113419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID‐19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55‐64. doi: 10.1016/j.jad.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ripp J, Peccoralo L, Charney D. Attending to the emotional well‐being of the health care workforce in a new York City health system during the COVID‐19 pandemic. Acad Med. 2020;95(8):1136‐1139. doi: 10.1097/ACM.0000000000003414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaitens J, Condon M, Fernandes E, McDiarmid M. COVID‐19 and essential workers: a narrative review of health outcomes and moral injury. Int J Environ Res Public Health. 2021;18(4):1446. doi: 10.3390/ijerph18041446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berman JD, Ebisu K. Changes in U.S. air pollution during the COVID‐19 pandemic. Sci Total Environ. 2020;739:139864. doi: 10.1016/j.scitotenv.2020.139864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torche F. The effect of maternal stress on birth outcomes: exploiting a natural experiment. Demography. 2011;48(4):1473‐1491. doi: 10.1007/s13524-011-0054-z [DOI] [PubMed] [Google Scholar]
- 10. Margerison‐Zilko CE, Li Y, Luo Z. Economic conditions during pregnancy and adverse birth outcomes among singleton live births in the United States, 1990‐2013. Am J Epidemiol. 2017;186(10):1131‐1139. doi: 10.1093/aje/kwx179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Catalano R, Serxner S. The effect of ambient threats to employment on low birth weight. J Health Soc Behav. 1992;33(4):363‐377. [PubMed] [Google Scholar]
- 12. Eskenazi B, Marks AR, Catalano R, Bruckner T, Toniolo PG. Low birthweight in new York City and upstate New York following the events of September 11th. Hum Reprod. 2007;22(11):3013‐3020. [DOI] [PubMed] [Google Scholar]
- 13. Lauderdale DS. Birth outcomes for Arabic‐named women in California before and after September 11. Demography. 2006;43(1):185‐201. [DOI] [PubMed] [Google Scholar]
- 14. Bruckner TA, Catalano R, Ahern J. Male fetal loss in the U.S. following the terrorist attacks of September 11, 2001. BMC public health. 2010;10:273. doi: 10.1186/1471-2458-10-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glynn LM, Wadhwa PD, Dunkel‐Schetter C, Chicz‐DeMet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184(4):637‐642. [DOI] [PubMed] [Google Scholar]
- 16. Margerison‐Zilko CE. Economic contraction and birth outcomes: an integrative review. Hum Reprod update. 2010;16(4):445‐458. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, D'Souza R, Kharrat A, et al. COVID‐19 pandemic and population‐level pregnancy and neonatal outcomes: a living systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2021;100(10):1756‐1770. doi: 10.1111/aogs.14206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9(6):e759‐e772. doi: 10.1016/S2214-109X(21)00079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gemmill A, Casey JA, Catalano R, Karasek D, Margerison CE, Bruckner T. Changes in preterm birth and caesarean deliveries in the United States during the SARS‐CoV‐2 pandemic. Paediatr Perinat Epidemiol. 2021;13. doi: 10.1111/ppe.12811. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Center for Health Statistics . Natality ‐ All County 2014–2020, as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions through the Vital Statistics Cooperative Program. National Center for Health Statistics. [Google Scholar]
- 21. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163‐168. [DOI] [PubMed] [Google Scholar]
- 22. Strand LB, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11:49. doi: 10.1186/1471-2288-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Box G, Jenkins G, Reinsel G, Ljung G. Time Series Analysis: Forecasting and Control. John Wiley & Sons; 2015. [Google Scholar]
- 24. Thompson CN, Baumgartner J, Pichardo C, et al. COVID‐19 outbreak ‐ new York City, February 29‐June 1, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(46):1725‐1729. doi: 10.15585/mmwr.mm6946a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramer MR, Hogue CJ, Dunlop AL, Menon R. Preconceptional stress and racial disparities in preterm birth: an overview. Acta Obstet Gynecol Scand. 2011;29:1307‐1316. doi: 10.1111/j.1600-0412.2011.01136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osterman M, Hamilton B, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2020. Natl Vital Stat Rep. 2021;70(17):1‐50. [PubMed] [Google Scholar]
- 27. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2019. Natl Vital Stat Rep. 2021;70(2):1‐51. [PubMed] [Google Scholar]
- 28. Gemmill A, Casey JA, Margerison CE, Zeitlin J, Catalano R, Bruckner TA. Patterned outcomes, unpatterned counterfactuals, and spurious results: perinatal health outcomes following COVID‐19. Am J Epidemiol. 2022. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Metz TD, Clifton RG, Hughes BL, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID‐19). Obstet Gynecol. 2021;137(4):571‐580. doi: 10.1097/AOG.0000000000004339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karasek D, Baer RJ, McLemore MR, et al. The association of COVID‐19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet reg Health Am. 2021;2:100027. doi: 10.1016/j.lana.2021.100027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamilton BE, Martin JA, Osterman MJK. Births: Provisional Data for 2020. National Center for Health Statistics (U.S.). Division of Vital Statistics; 2021. [PubMed] [Google Scholar]
- 32. Handley SC, Mullin AM, Elovitz MA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS‐CoV‐2 pandemic, march‐June 2020. JAMA. 2021;325(1):87‐89. doi: 10.1001/jama.2020.20991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harvey EM, McNeer E, McDonald MF, et al. Association of Preterm Birth Rate with COVID‐19 statewide stay‐at‐home orders in Tennessee. JAMA Pediatr. 2021;175(6):635‐637. doi: 10.1001/jamapediatrics.2020.6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Main EK, Chang SC, Carpenter AM, et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021;224(2):239‐241. doi: 10.1016/j.ajog.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lima SAM, El Dib RP, Rodrigues MRK, et al. Is the risk of low birth weight or preterm labor greater when maternal stress is experienced during pregnancy? A systematic review and meta‐analysis of cohort studies. PLoS One. 2018;13(7):e0200594. doi: 10.1371/journal.pone.0200594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kramer MS, Lydon J, Seguin L, et al. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169(11):1319‐1326. [DOI] [PubMed] [Google Scholar]
- 37. Cai C, Vandermeer B, Khurana R, et al. The impact of occupational activities during pregnancy on pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2020;222(3):224‐238. doi: 10.1016/j.ajog.2019.08.059 [DOI] [PubMed] [Google Scholar]
- 38. Cai C, Vandermeer B, Khurana R, et al. The impact of occupational shift work and working hours during pregnancy on health outcomes: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2019;221(6):563‐576. doi: 10.1016/j.ajog.2019.06.051 [DOI] [PubMed] [Google Scholar]
- 39. Klumper J, Kazemier BM, Been JV, et al. Association between COVID‐19 lockdown measures and the incidence of iatrogenic versus spontaneous very preterm births in The Netherlands: a retrospective study. BMC Pregnancy Childbirth. 2021;21(1):767. doi: 10.1186/s12884-021-04249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lemon L, Edwards RP, Simhan HN. What is driving the decreased incidence of preterm birth during the coronavirus disease 2019 pandemic? Am J Obstet Gynecol MFM. 2021;3(3):100330. doi: 10.1016/j.ajogmf.2021.100330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ Res. 2018;167:144‐159. doi: 10.1016/j.envres.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 42. Dey T, Tyagi P, Sabath MB, et al. Counterfactual time series analysis of short‐term change in air pollution following the COVID‐19 state of emergency in the United States. Sci Rep. 2021;11(1):23517. doi: 10.1038/s41598-021-02776-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Catalano R, Bruckner T, Casey JA, Gemmill A, Margerison C, Hartig T. Twinning during the pandemic: evidence of selection in utero. Evol Med Public Health. 2021;9(1):374‐382. doi: 10.1093/emph/eoab033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruckner TA, Catalano R. Selection in utero and population health: theory and typology of research. SSM Popul Health. 2018;5:101‐113. doi: 10.1016/j.ssmph.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Catalano R, Goodman J, Margerison‐Zilko C, et al. Timing of birth: parsimony favors strategic over dysregulated parturition. Am J Human Biol. 2015;21:31‐35. doi: 10.1002/ajhb.22737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the National Center for Health Statistics. Restrictions apply to the availability of these data, which were used under a data use agreement for this study.


