Dear Editor,
In the past 2 years, the world has been affected by one of the most widespread and significant public health crises in decades with Coronavirus Disease‐19 (COVID‐19). Several cutaneous reactions have been reported after SARS‐CoV‐2 vaccination. 1 , 2 , 3 , 4 , 5 We describe a unique case of a patient that developed two different autoimmune diseases, systemic lupus erythematosus (SLE) and alopecia areata (AA), after receiving heterologous prime‐boost vaccines. To our knowledge, our case introduces the discussion if the use of different vaccine platforms can modify the patient's immune response.
A 27‐year‐old woman was admitted with bullous, exulcerated and crusty lesions on the face, chest, arms, legs, and oral mucosa accompanied by fever (38°C) and fatigue. The lesions were itchy and painful. She had no family history of autoimmune diseases, and the lesions appeared 3 weeks after receiving the second dose of ChAdOX1 nCoV‐19/AZD1222 (AZ‐FIOCRUZ, Rio de Janeiro, Brazil) vaccine on November 12, 2021. She was previously vaccinated with the first dose of AZD1222 vaccine on July 28, 2021, without intercurrences. Skin examination showed multiple bullous, exulcerated and crusty lesions in oral mucosa, in ears and photoexposed areas (Figure 1A,B), and digital purpuric macules on feet and hands.
FIGURE 1.
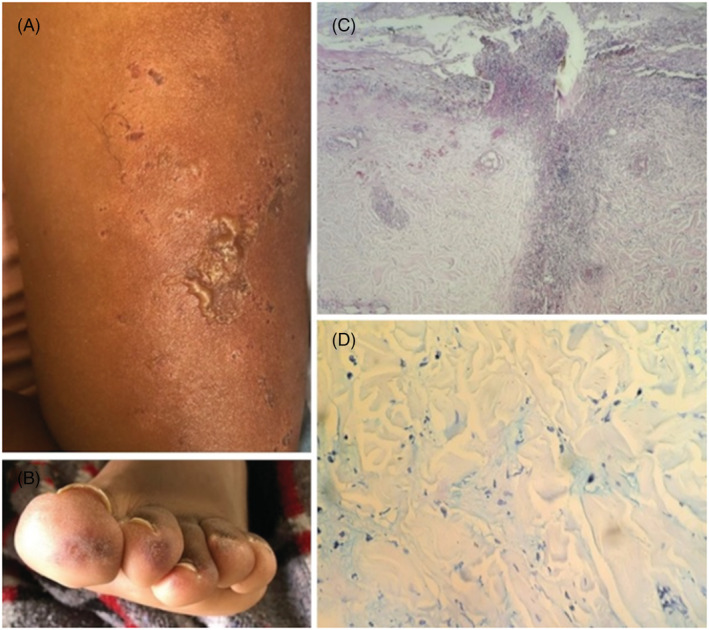
Bullous, exulcerated and crusty lesions on right arm (A). Digital purpuric macules on left foot (B). Spongiosis with an intraepidermal pustule and vacuolar degeneration of the basal layer, moderately dense predominantly superficial and deep perivascular and perianexial mixed infiltrate on H&E examination, magnification at ×10 (C). Immunohistochemical staining for Alcian Blue with mucin deposits in the dermis at ×40 (D)
The patient tested negative for a SARS‐CoV‐2 reverse transcriptase–polymerase chain reaction test. Subsequent investigations revealed anemia with red blood cells 3.27 × 1012/L (normal range [NR], 4.54–5.78), hemoglobin 9.52 g/dl (NR, 13.3–17.2) and hematocrit 29.2% (NR, 38.9–50.9); leukopenia with 2.7 × 109 cells/L (NR, 3.7–9.5 × 109) and lymphopenia at 0.81 × 109 cells/L (NR, 1.0–2.5 × 109), with normal platelet count. The kidney function presented with urine protein/creatinine ratio 1.54 mg/dl (NR, <0.3) and serum creatinine 0.7 mg/dl (NR, 0.60–1.20).
ANA testing was positive at 1:640 (NR, <1:80) dotted and homogeneous mixed pattern (nucleus and metaphase plate), anti‐Sm positive at 70 U/ml (NR, <20), anti‐dsDNA 200 IU/ml (NR, <30), anti‐SS‐A 240 U/ml (NR, <2), and anti‐SS‐B 10 U/ml (NR, <20). Complements C3 and C4 levels were 30 and 2 mg/dl (NR, 90–180 and 16–38), respectively. Anti‐cardiolipin antibodies, beta‐2‐glycoprotein antibodies, and lupus anticoagulant were negative. She had negative blood cultures and negative viral screen for HIV, HBV, and HCV.
Skin biopsy showed spongiosis with an intraepidermal pustule and vacuolar degeneration of the basal layer (Figure 1C). In addition, perivascular, perifollicular and interstitial lymphocytic and neutrophilic infiltration with evident thickening of vessel walls with fibrinoid necrosis and leukocytoclasia were noticed. Immunohistochemical staining with Alcian Blue showed mucin deposits (Figure 1D).
A diagnosis of SLE was established based on the 2019 American College of Rheumatology/European League against Rheumatism classification criteria. 6
The patient was treated with 80 mg/day oral prednisolone and hydroxychloroquine 400 mg/day.
After receiving the booster with the BNT162b2 vaccine (Pfizer‐BioNTech, Philadelphia, PA) on February 15, 2022, the patient noted diffuse alopecia after 15 days and presented to our outpatient clinic with 6 weeks apart (Figure 2A). Trichoscopy showed yellow dots, black dots, dystrophic hair, and white hairs of repilation (Figure 2B). The biopsy displayed a mild lymphocytic infiltrate around the outer follicular sheath without signs of fibrosis (Figure 2C). These findings confirmed the diagnosis of AA.
FIGURE 2.
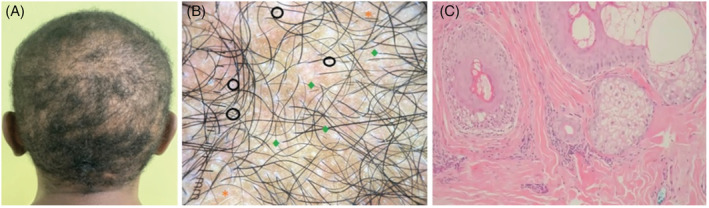
Generalized alopecia areata involving the temporo‐parietal, occipital, and vertex areas (A). Trichoscopic examination showing black dots (black circle), broken hairs (green rhombus), and white hairs of repilation (orange asterisk) (B). Mild lymphocytic infiltrate around the outer follicular sheath with no signs of fibrosis on H&E examination, magnification at ×10 (C)
Our patient showed an initial presentation of SLE and also hair loss immediately after SARS‐CoV‐2 vaccination, suggesting a pathophysiological association among them. Although there is no conclusive evidence to prove a causal relationship, the concept that no drug is completely harmless can also be applied to vaccines, which certainly play an important role in improving human health, but could be potential triggers for autoimmune diseases. 2 , 7 , 8
The close temporal context, the normalization of the laboratory parameters related to SLE and the absence of other trigger factors support the possibility that the BNT162b2 vaccination caused AA in our case. However, we cannot fully exclude SLE playing a synergistic role in the pathogenesis of AA.
We evaluated the causality of the “Adverse Events Following Immunization” after COVID‐19 vaccination based on the WHO guidelines. 9
Vojdani et al. 10 identified several cross‐reactive interactions with SARS‐CoV‐2 spike protein that may lead to specific autoimmune patterns. SARS‐CoV‐2 vaccines are generally deemed safe, but concerns have been raised about developing an auto‐immune response in individuals undergoing vaccination with the production of antibodies to SARS‐CoV‐2 spike glycoproteins.
Nevertheless, the possibility of skin manifestations or worsening of auto‐immune diseases should not discourage vaccination, as the benefits outweigh the risks. We are just at the beginning of understanding about the efficacy and toxicity of the novel SARS‐CoV‐2 vaccines.
AUTHOR CONTRIBUTIONS
Shirley Braga Lima Gamonal: Conceptualization and design of the study; article draft and revision; final approval of the version to be submitted. Nathália Couri Vieira Marques: Conceptualization and design of the study; article draft and revision; final approval of the version to be submitted. Heitor Motta Bini Pereira: Conceptualization and design of the study; article draft and revision; final approval of the version to be submitted. Aloisio Carlos Couri Gamonal: Conceptualization and design of the study; article draft and revision; final approval of the version to be submitted.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENT
The patient gave written consent for the use of her photographs and her clinical history.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zavala‐Miranda MF, González‐Ibarra SG, Pérez‐Arias AA, Uribe‐Uribe NO, Mejia‐Vilet JM. New‐onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID‐19 vaccination. Kidney Int. 2021;100(6):1340‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watad A, De Marco G, Mahajna H, et al. Immune‐mediated disease flares or new‐onset disease in 27 subjects following mRNA/DNA SARS‐CoV‐2 vaccination. Vaccines. 2021;9(5):435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zagaria O, Villani A, Ruggiero A, Potestio L, Fabbrocini G, Gallo L. New‐onset lichen planus arising after COVID‐19 vaccination. Dermatol Ther. 2022;35(5):e15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Potestio L, Napolitano M, Bennardo L, Fabbrocini G, Patruno C. Atopic dermatitis exacerbation after Covid‐19 vaccination in dupilumab‐treated patients. J Eur Acad Dermatol Venereol. 2022;36(6):e409‐e411. [DOI] [PubMed] [Google Scholar]
- 5. Kreuter A, Licciardi‐Fernandez MJ, Burmann SN, Burkert B, Oellig F, Michalowitz AL. Induction and exacerbation of subacute cutaneous lupus erythematosus following mRNA‐based or adenoviral vector‐based SARS‐CoV‐2 vaccination. Clin Exp Dermatol. 2022;47(1):161‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aringer M, Costenbader K, Daikh D, et al. 2019 European league against rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151‐1159. [DOI] [PubMed] [Google Scholar]
- 7. Gallo G, Mastorino L, Tonella L, Ribero S, Quaglino P. Alopecia areata after COVID‐19 vaccination. Clin Exp Vaccine Res. 2022;11(1):129‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scollan ME, Breneman A, Kinariwalla N, et al. Alopecia areata after SARS‐CoV‐2 vaccination. JAAD Case Rep. 2022;20:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Global Manual on Surveillance of Adverse Events Following Immunization, 2016 update; 2014. https://apps.who.int/iris/handle/10665/206144
- 10. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS‐CoV‐2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11:617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


