Abstract
Background
Hemostatic disturbances with coronavirus disease 2019 (COVID‐19) can predispose to tricuspid and right heart thrombi in very rare instances.
Aim
We describe a 29‐year‐old female patient without a previous cause of thrombosis who developed large tricuspid valve thrombus (TVT) and moderate‐to‐severe tricuspid regurgitation (TR) during the course of COVID‐19 infection.
Materials and Methods
Persistant fever and tachycardia with thrombocytopenia and high d‐dimer increased the index of suspicion. The diagnosis was made by bedside transthoracic echocardiography (TTE) and cardiac magnetic resonance (CMR). Surgery was performed for thrombectomy and tricuspid valve replacement with a tissue valve.
Discussion and conclusion
Detection of TVT in COVID‐19 patients on the basis of high index of suspicion, bedside TTE and noninvasive CMR helps early surgical treatment and subsequent reduction of mortality and hospital stay.
Keywords: cardiac magnetic resonance, COVID‐19, hypercoagulation, thrombus, tricuspid valve
Abbreviations
- CMR
cardiac magnetic resonance
- COVID‐19
coronavirus disease 2019
- HRCT
high‐resolution computed tomography
- PCR
polymerase chain reaction
- RA
right atrium
- RV
right ventricle
- TR
tricuspid regurgitation
- TTE
transthoracic echocardiography
- TVT
tricuspid valve thrombus
1. INTRODUCTION
More than 2 years after the appearance of coronavirus disease 2019 (COVID‐19), the relevant research is still ongoing. COVID‐19 can lead to hemostatic disorders and thrombotic diseases due to exaggerated inflammatory process, decreased tissue oxygenation, and endothelial dysfunction. 1
Intracardiac thrombi, including tricuspid valve thrombus (TVT), are very rare life‐threatening complications of COVID‐19 which indicate a high index of suspicion to provide early proper management. 2 , 3 Herein, we report a surgically treated patient with TVT associated with moderate‐to‐severe tricuspid regurgitation (TR) during the course of COVID‐19 pneumonia.
2. CASE REPORT
A 29‐year‐old female patient presented with a 5‐day history of fever, palpitations, and dyspnea. There was no history of chronic illness, trauma, surgery, or intravenous drug abuse. The patient had a positive polymerase chain reaction (PCR) test for COVID‐19 followed by home treatment and partial clinical improvement before re‐occurence of fever and shortness of breath.
On admission, she had a fever (temperature of 38°C), sinus tachycardia, tachypnea, normal blood pressure, and oxygen saturation of 94% in room air. The laboratory investigations (Table 1) revealed a total leukocytic count of 12,000/μl (lymphocyte count: 15%) and C‐reactive protein (CRP) of 67.4 mg/L, with a positive PCR test.
Table 1.
Laboratory hematologic results during the hospital stay of the patient and at the first postoperative follow‐up visit
| Test | Reference range | At admission | Before surgery | After surgery | At discharge | At follow‐up |
|---|---|---|---|---|---|---|
| (Jan 26, 2022) | (Feb 8, 2022) | (Feb 15, 2022) | (Feb 26, 2022) | (Mar 13, 2022) | ||
| Total leukocytic count (×103) | 4–11 | 12 | 14.27 | 10.6 | 6.8 | 6.5 |
| Red blood cell (×106) | 4–5.5 | 3.95 | 4.2 | 4.2 | 4.15 | 4.35 |
| Hemoglobin (g/dL) | 12–16 | 10 | 11.65 | 11.1 | 10.7 | 11.8 |
| Hematocrite (%) | 36–46 | 31.9 | 36.5 | 33.6 | 32.6 | 35.7 |
| Platelet count (×103) | 150–400 | 175 | 144 | 231 | 275 | 272 |
| Lymphocyte (%) | 25–45 | 15 | 19 | 30 | 26 | 35 |
| Neutrophil (%) | 45–75 | 80 | 78 | 60 | 65 | 55 |
| CRP (mg/L) | <6 | 67.4 | 24 | 12 | 6 | 4 |
| d‐dimer (mg/L) | 0.19–0.52 | 1.40 | 2.30 | 0.68 | 0.50 | 0.46 |
Abbreviation: CRP, C‐reactive protein.
2.1. Diagnostic workup
During the hospital stay, the patient underwent diagnostic and therapeutic workups as appropriate (Figure 1). High‐resolution computed tomography (HRCT) revealed multiple peripheral patchy ground‐glass opacities at both lung fields associated with pneumonic consolidation (Figure 2). The patient received the standard treatment of COVID‐19 pneumonia with no need for mechanical ventilation.
Figure 1.
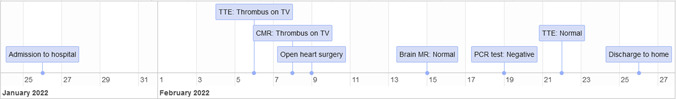
Timline of in‐hospital diagnostic and therapeutic workup. CMR, cardiac magnetic resonance; MR, magnetic resonance; PCR, polymerase chain reaction; TTE, transthoracic echocardiography; TV, tricuspid valve.
Figure 2.
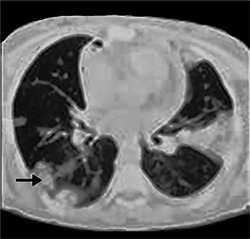
Computed tomography of the chest shows consolidation and peripheral ground‐glass lung opacities (arrow).
Persistent fever and tachycardia with elevated d‐dimer and thrombocytopenia indicated bedside transthoracic echocardiography (TTE) which revealed large mobile tricuspid valve mass, mostly thrombus, measuring 33 × 17 mm and protruding into the right atrium (RA) and right ventricle (RV), with moderate‐to‐severe TR and moderate pulmonary hypertension. Furthermore, cardiac magnetic resonance (CMR) showed a TVT of 28 × 21 × 11 mm oscillating into the right ventricle with preserved ventricular function (Figure 3). Blood culture showed no growth.
Figure 3.
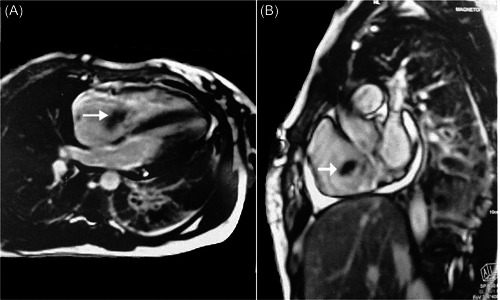
Cardiac magnetic resonance shows tricuspid valve thrombus (white arrows).
2.2. Treatment
To avoid thromboembolism, the patient underwent cardiac surgery through median sternotomy. Intraoperatively, a mass attached to the septal leaflet was identified with no other masses in RA and RV (Figure 4). Beating heart removal of the mass followed by tricuspid valve replacement with tissue valve #31. Tissue culture showed no growth.
Figure 4.
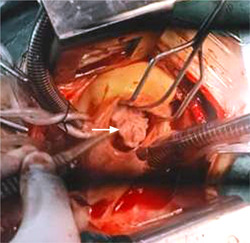
Intraoperative view of tricuspid valve thrombus attached to septal leaflet (white arrow).
2.3. Outcome
The postoperative course was uneventful except for delirium and agitation on the third postoperative day that improved on the medical treatment with the absence of any lesion in brain MR study. The patient was discharged home 17 days after surgery with a negative PCR test, and she continued on a regimen of antibiotic therapy and oral anticoagulants. On follow‐up visits, chest X‐ray, TTE, and laboratory investigations were normal with the improved general condition.
3. DISCUSSION
Thromboembolic complications are reported in 27% of COVID‐19 patients 1 with challenging diagnosis due to the similarity of its clinical manifestations and laboratory findings with the usual features of COVID‐19 infection. Thus, a high index of clinical suspicion is recommended in addition to emergency pulmonary angiography or echocardiography in suspected patients. 4 Our report describes a rare case of large TVT in COVID‐19 female patients during the third wave of the pandemic. One previous report described a large thrombus‐in‐transit through the tricuspid valve into the RV in adult males, 2 and another report described a large TVT extended to the RA and RV and attached to the tip of a central venous catheter in a child. 3
Our patient had unremarkable risk factors for thrombosis before COVID‐19 infection. The clinical, laboratory, and imaging workups to distinguish the thrombotic disease from COVID‐19 infection are challenging due to: (1) similar presentation of dyspnea, chest pain, tachypnea, and tachycardia in patients with thrombosis or COVID‐19; (2) routine use of prophylactic anticoagulants in all COVID‐19 patients; (3) usual elevation of d‐dimer and pro‐inflammatory markers in both conditions; and (4) limited mobility of COVID‐19 patients which may delay the proper imaging study to identify thrombotic diseases.
In our case, TVT was initially detected on bedside TTE; however, TTE could not differentiate thrombus from vegetation thus CMR was performed for further evaluation. CMR has the ability to determine the acuity of a thrombus and to differentiate it from cardiac tumors or other true cardiac lesions. 5 Moreover, CMR has a specific role in COVID‐19 patients to determine cardiovascular complications because of its high accuracy in the evaluation of the myocardial structure, function, tissue characterization, and perfusion. 6
The decision for surgery in our case aimed to avoid subsequent complications of a large mobile thrombus (>2cm) associated with moderate‐to‐severe TR. The treatment of TVT includes medical therapy with anticoagulants or fibrinolytic, surgery, and percutaneous directed retrieval. Each modality has its benefits and risks. 7 Therefore, the treatment should be decided on an individual basis depending on the size and dynamics of the thrombus, surgical fitness, ventricular function, and hemodynamic stability. 3 , 8
4. CONCLUSION
COVID‐19 is a predisposing factor to thromboembolic events. Excluding intracardiac thromboembolism is essential as part of the COVID‐19 workup. A bedside TTE and CMR help accurate assessment of clinically suspected TVT. Early surgical removal is efficient.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Case reports are waived from our ethical committee approval. The study conforms to the recognized standards of the Declaration of Helsinki.
Kamal YA, Al‐Elwany SE, Elsayed MM. Large tricuspid valve thrombus complicating COVID‐19 pneumonia. J Card Surg. 2022;1‐4. 10.1111/jocs.16761
REFERENCES
- 1. Klok, FA , Kruip, MJHA , van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan HMW, Khan MR, Munir A, Moughrabieh A, Changezi HU. A giant right‐heart thrombus‐in‐transit in a patient with COVID‐19 pneumonia. Am J Case Rep. 2020;21:e927380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bigdelian H, Sedighi M, Sabri MR, et al. Right atrial thrombus in a COVID‐19 child treated through cardiac surgery. Front Cardiovasc Med. 2020;7:579522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Konstantinides SV, Meyer G, Becattini C, et al. ESC scientific document group: 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543‐603. [DOI] [PubMed] [Google Scholar]
- 5. Johnson EM, Gage KL, Feuerlein S, Jeong D. Cardiac magnetic resonance for the evaluation of suspected cardiac thrombus: conventional and emerging techniques. J Vis Exp. 2019;148:1‐7. [DOI] [PubMed] [Google Scholar]
- 6. Clark DE, Aggarwal SK, Phillips NJ, Soslow JH, Dendy JM, Hughes SG. Cardiac magnetic resonance in the evaluation of COVID‐19. Card Fail Rev. 2022;8:e09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gunduz Y, Ucar A, Vatan MB, Keser N. Tricuspid valve thrombus causing acute pulmonary embolism. BMJ Case Rep. 2013;2013:bcr2012006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arumairaj AJ, Boma N, Mushiyev S, Morcos M, Habtes I. Infected right ventricle thrombus as a cause of persistent sepsis. Cureus. 2020;12(10):e10751. [DOI] [PMC free article] [PubMed] [Google Scholar]


