Abstract
Background
ICU admission due to COVID‐19 may result in cognitive and physical impairment. We investigated the long‐term cognitive and physical status of Danish ICU patients with COVID‐19.
Methods
We included all patients with COVID‐19 admitted to Danish ICUs between March 10 and May 19, 2020. Patients were the contacted prospectively at 6 and 12 months for follow‐up. Our primary outcomes were cognitive function and frailty at 6 and 12 months after ICU admission, estimated by the Mini Montreal Cognitive Assessment, and the Clinical Frailty Scale. Secondary outcomes were 6‐ and 12‐month mortality, health‐related quality of life (HRQoL) assessed by EQ‐5D‐5L, functional status (Barthel activities of daily living and Lawton–Brody instrumental activities of daily living), and fatigue (Fatigue Assessment Scale). The study had no information on pre‐ICU admission status for the participants.
Results
A total of 326 patients were included. The 6‐ and 12‐month mortality was 37% and 38%, respectively. Among the 204 six‐month survivors, 105 (51%) participated in the 6‐month follow‐up; among the 202 twelve‐month survivors, 95 (47%) participated in the 12‐month follow‐up. At 6 months, cognitive scores indicated impairment for 26% (95% confidence interval [CI], 11.4–12.4) and at 12 months for 17% (95% CI, 12.0–12.8) of participants. Frailty was indicated in 20% (95% CI, 3.4–3.9) at 6 months, and for 18% (95% CI, 3.3–3.8) at 12 months. Fatigue was reported by 52% at 6 months, and by 47% at 12 months. For HRQoL, moderate, severe, or extreme health problems were reported by 28% at 6 months, and by 25% at 12 months.
Conclusion
Long‐term cognitive, functional impairment was found in up to one in four of patients surviving intensive care for COVID‐19. Fatigue was present in nearly half the survivors at both 6 and 12 months. However, pre‐ICU admission status of the patients was unknown.
Keywords: cognitive, covid‐19, fatigue, follow‐up, frailty, functional, intensive care
Editorial Comment
In this prospective study of 326 individuals admitted to the ICU with COVID‐19 in Denmark, 20%–25% of individuals screened positive for cognitive impairment and frailty at 6 and 12 months. Fatique was common and health‐related quality of life was reduced among responders. It should be noticed that a baseline status was not available prior to ICU admission and participation among survivors was roughly 50%. The results suggest that there is a high degree of reduced functional status and quality of life following ICU admission for COVID‐19.
1. INTRODUCTION
In December 2019, a new type of coronavirus, the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) emerged in Wuhan, China, and started a pandemic. 1 The clinical manifestations of the human virus infection was called coronavirus disease‐19 (COVID‐19) and symptoms range from asymptomatic or mild symptoms to acute respiratory distress syndrome (ARDS) and multiorgan failure. 2
The first patients with severe COVID‐19 were admitted to intensive care units (ICU) in Denmark in March 2020. 3 During this initial stage of the COVID‐19 pandemic, in spring 2020, the Danish ICU COVID‐19 database was established to monitor critically ill patients suffering from COVID‐19. 4
Previous studies have shown that survivors of critical illness treated in the ICU may experience a decline in cognitive and functional status, which can last for a long time after discharge. 5 , 6 , 7 This often includes an experience of fatigue and loss of health‐related quality of life (HRQoL). 8
We, therefore, anticipated that ICU patients with COVID‐19 would experience similar cognitive and functional impairments after discharge. 2 , 6 Consequently, the aim of the present study was to investigate cognitive and functional impairments, fatigue, and HRQoL in Danish ICU survivors of COVID‐19 at 6 and 12 months after ICU admission.
2. METHODS
2.1. Study design
This was a nationwide, population‐based, prospective cohort study investigating the 6‐ and 12‐month long‐term outcomes of all patients admitted to a Danish ICU between March 10 to May 19, 2020 and retrospectively registered in the Danish ICU COVID‐19 database. 4 The reporting of the study follows the STROBE guideline for reporting observational studies.
2.2. Ethics
The Danish Patient Safety Authority approved the establishment of the Danish COVID‐19 ICU database and waived consent from the individual patients due to the retrospective nature of the database (ref. no. 31‐1521‐293). 4
The Danish Data Protection Agency approved this study (REG‐135‐2020). National Ethics Committee waived consent (J.nr. 20‐000013), because no ethical approval for this type of study is warranted in Denmark. The responsible investigator of the database granted permission to use the information from the database in this study. Furthermore, the heads of each ICU with surviving patients at 6 months were contacted to gain acceptance to contact their individual patients.
2.3. The Danish ICU COVID‐19 database
All patients with a positive polymerase chain reaction test for SARS‐CoV‐2 either prior to or during ICU admission were registered in the Danish COVID‐19 ICU database. Data registered included baseline characteristics, comorbidities, time on ventilator, vasopressor used, renal replacement therapy (CRRT), length of ICU and hospital stay, and mortality (Appendix S1).
2.4. Patients and enrolment in the follow‐up study
After acceptance from the participating ICUs, all their surviving patients were contacted by a secure online digital mailbox, e‐Boks, providing information about their registration in the nationwide database during their admission to the ICU with COVID‐19, and an invitation to participate in the follow‐up study. If the patients had no access to e‐Boks, contact was attempted by telephone. To optimize the opportunity to participate in the study, patients who did not reply within 2 weeks after the invitation by e‐Boks were given a final attempt by telephone.
All participants provided oral and written informed consent to the follow‐up study upon first telephone contact.
2.5. Outcomes
The primary outcomes were cognitive function and frailty status at 6 and 12 months after ICU admission estimated by the Mini Montreal Cognitive Assessment (MiniMoCA) and Clinical Frailty Scale (CFS). Secondary outcomes were 6‐ and 12‐month mortality, self‐rated HRQoL estimated by EuroQol (EQ)‐5D‐5L, functional status by Barthel activities of daily living (ADL) score, and the Lawton–Brody instrumental activities of daily living (IADL) score, and fatigue by Fatigue Assessment Scale (FAS). Furthermore, the association between ventilator days and CFS, and MiniMoCA, respectively, were explored. 9 , 10
2.6. Data collection
Participants were interviewed by telephone at 6 and 12 (±2) months after admission to the ICU. The interviews lasted approximately 20 min and were performed by the same interviewer (S.W.). The order of the questionnaires was: MiniMoCA, EQ‐5D‐5L, ADL, IADL, FAS, and CFS. All instruments were validated to be used in telephone interviews.
MiniMoCA version 2.1 is a validated questionnaire for assessing cognitive function. It consists of five cognitive domains; attention, verbal learning and memory, executive function/language, and orientation, covered by four subtests. The total score range from 0 to 15, and scores of 11 or above are considered normal. 11 A modified nonvalidated Danish translation has recently been made available and was used in this study. 11 , 12
EQ‐5D‐5L is a validated self‐reporting HRQoL questionnaire exploring five domains: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. The participants are asked to rate each domain into five levels, ranging from 1 indicating no problems to 5 indicating extreme problems. Furthermore, on the EQ‐VAS, the participants mark their current overall health status on that particular day on a Visual Analog Scale (VAS) between best and worst imaginable health (0–100). 13 We used the Danish validated version for the study.
Barthel ADL describes the participants' function within 10 items of ADL. The total score ranges from 0 to 20, a high score indicates high level of independence. 14 A validated Danish translation was used in the study. 15
Lawton–Brody IADL measures more complex activities necessary for living an independent life in one's own housing and consists of eight domains for women and five domains for men. Scores range from 0 to 8 for women and 0 to 5 for men; the higher scores the better function. 16 A Danish version was used. 17
The FAS describes the participant's level of fatigue by 10 statements reflecting physical and mental fatigue. 18 Each question is answered with scores 1 to 5 indicating how often the statement occurs. Total score ranges from 10 to 50, scores of 22 or higher indicate fatigue, and scores above 34 indicate extreme fatigue. 19 A Danish validated version was used in this study.
CFS is a 9‐level scale indicating the level of frailty. Based on the overall functional status and the medical history of the patient, the interviewer places the participant in one of nine categories: very fit, well, managing well, vulnerable, mildly frail, moderately frail, severely frail, very severely frail, and terminally ill (Scores 1–9). 20 Scores of 5 and above indicates frailty. 21 , 22 , 23 A case‐based validated Danish translation of version 1.2 was used. 24
Furthermore, baseline characteristics, days on ventilator, use of vasopressors and renal replacement therapy, ICU and hospital length of stay were obtained from the Danish ICU COVID‐19 database.
2.7. Statistics
No sample size calculation was performed because of the descriptive and hypothesis‐generating nature of the study. Data was described with mean and standard deviation (SD) for normally distributed data and median and interquartile range (IQR) for nonparametric data, or proportions if relevant. A p value less than .05 was considered statistically significant. Confidence intervals of the proportion were calculated using the binomial proportion. For correlation analysis, we used the Pearson correlation analyses for normally distributed data measured on continuous scales. The Spearman rank order correlation was applied for ordinal data and nonparametric distributed data. The association between outcomes and predictive variables was performed by linear regression.
Statistical analyses were made in R, version 3.6.3.
3. RESULTS
Twenty‐nine Danish ICUs registered data on 326 patients in the Danish ICU COVID‐19 database. 4 Two hundred four patients were alive 6 months after ICU admission, corresponding to a mortality of 37%. At 12 months follow‐up the mortality rate was 38%.
Twenty‐three ICUs had surviving patients at 6‐month follow‐up. Twenty‐one ICUs granted permission to contact patients regarding the follow‐up. One hundred eighty‐nine patients received an invitation at 6 months, of whom 105 participated in the 6‐month follow‐up interview, and 95 participated in the 12‐month follow‐up interview. In total, 110 patients participated in one of the two follow‐up interviews, among whom 90 participated in both (Figure 1). Patients from all five Danish regions participated (Table 1).
FIGURE 1.
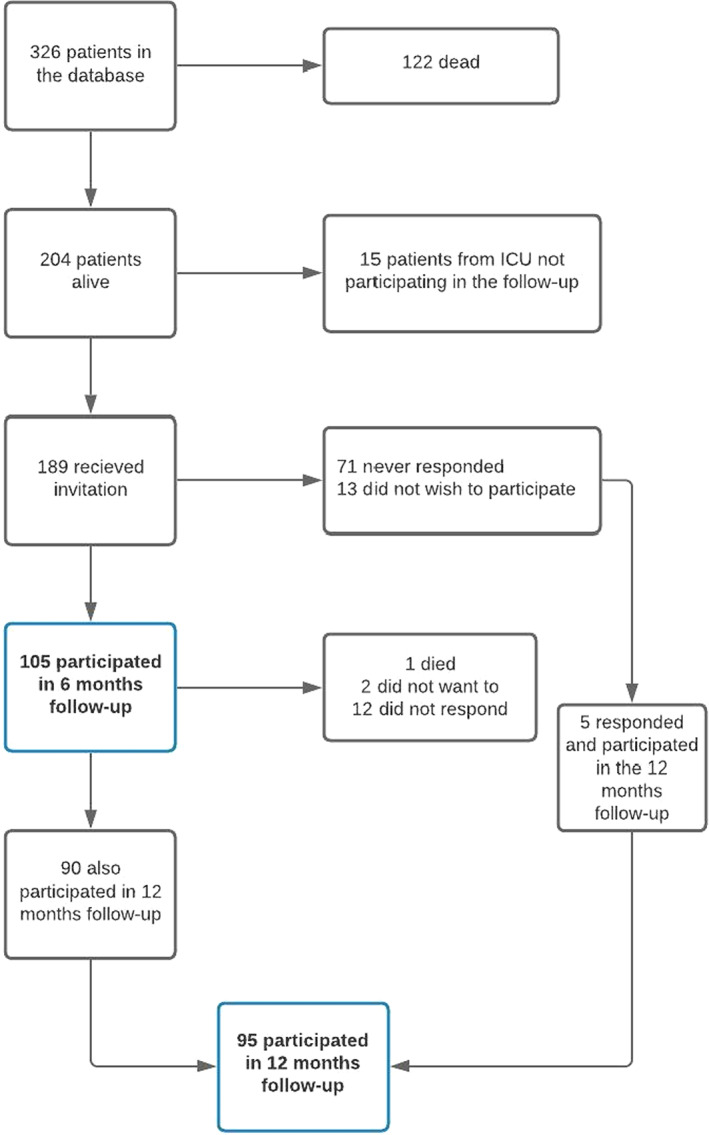
Participant flow diagram
TABLE 1.
Characteristics
| Interviewed n = 110 | Not interviewed n = 94 | |
|---|---|---|
| Age, median (range) | 67 (25–86) | 62 (23–90) |
| Male, n (%) | 77 (70%) | 64 (68%) |
| Invasive mechanical ventilation, n (%) | 88 (80%) | 72 (77%) |
| Ventilator days median (IQR) | 9.5 (4–17) | 11.5 (3.3–18) |
| Renal replacement therapy, n (%) | 15 (14%) | 17 (18%) |
| ICU length of stay—days, median (IQR) | 13.5 (8–21) | 14 (7.3–23) |
| Comorbidity (any), n (%) | 73 (66%) | 59 (63%) |
| Hypertension | 54 (49%) | 41 (44%) |
| Ischaemic heart disease | 13 (12%) | 11 (12%) |
| Heart failure | 3 (3%) | 4 (4%) |
| Chronic pulmonary disease | 16 (15%) | 14 (15%) |
| Chronic kidney disease | 13 (12%) | 5 (5%) |
| Liver cirrhosis | 0 | 0 |
| Diabetes | 22 (20%) | 17 (18%) |
| Active cancer | 2 (2%) | 3 (3%) |
| Hematological malignancy | 4 (4%) | 2 (2%) |
| Immunosuppressed | 8 (7%) | 8 (9%) |
| Region, n | ||
| Capital region | 39 | 41 |
| Zealand region | 21 | 4 |
| Northern region | 10 | 10 |
| Central region | 25 | 17 |
| Southern region | 15 | 22 |
Baseline characteristics for the interviewed versus noninterviewed participants were generally similar, except for the median age being higher for the interviewed participants (67 vs. 62 years). The proportion of participants needing mechanical ventilation was similar in the two populations (80% vs. 77%). However, the noninterviewed participants had a longer time on the ventilator (11.5 vs. 9.5 days) and they tended to have a higher use of dialysis treatment (18% vs. 14%) (Table 1).
The cognitive function measured by MiniMoCA had a median score of 13 (IQR: 10–14) at 6 months and 13 (IQR: 11–14) at 12 months (Table 2). Twenty‐six percent (n = 27) had cognitive scores indicating impaired cognitive function (MiniMoCA < 11) at 6 months, and 17% (n = 16) at 12 months (Appendix S2). Two participants could not cooperate to the investigation at the 6‐ and 12‐month interviews due to impaired hearing.
TABLE 2.
Outcomes
| 6 months [median (IQR)] 105 participants | 12 months [median (IQR)] 95 participants | |
|---|---|---|
| MiniMoCA | 13 (10–14) | 13 (11–14) |
| Clinical Frailty Score | 3 (3–4) | 3 (3–4) |
| EQ‐5D‐5L | ||
| Q1 (Mobility) | 1 (1–2) | 1 (1–2) |
| Q2 (Self‐care) | 1 (1–1) | 1 (1–1) |
| Q3 (Usual activities) | 1 (1–2) | 1 (1–2) |
| Q4 (Pain/discomfort) | 2 (1–3) | 1 (1–2) |
| Q5 (Anxiety/depression) | 1 (1–2) | 1 (1–1) |
| EQ‐VAS | 70 (50–80) | 70 (51–80) |
| Barthel ADL | 20 (20–20) | 20 (20–20) |
| Lawton–Brody IADL | ||
| Female | 8 (7–8) | 8 (7–8) |
| Male | 5 (4–5) | 5 (4–5) |
| Fatigue Assessment Scale | 24 (14–37) | 23 (15–33) |
Abbreviations: Barthel ADL, Barthel activities of daily living; CFS, Clinical Frailty Score; FAS, Fatigue Assessment Scale; IADL, instrumental activities of daily living; MiniMoCA, Mini Montreal Cognitive Assessment.
Pearson correlation analysis showed a low correlation between cognitive function and time on ventilator at 6 and 12 months (ρ = −.03 and −.06, respectively). In multiple linear regression analysis, we found no association between cognitive function and time on ventilator (Appendix S5).
The participants presented a clinical frailty median score of 3 (IQR: 3–4) at both 6‐ and 12‐month interviews (Table 2). Twenty percent (n = 21) were scored as frail (>4) at 6 months, and 18% (n = 17) at 12 months (Appendix S3). The Pearson correlation analysis showed a low correlation between CFS and time on ventilator at both 6 and 12 months (ρ = .28 and .17, respectively). We found a significant association between CFS and ventilator time in the multiple linear regression analysis at 6 months (Figure 2). However, this association was not found at 12‐month follow‐up (Appendix S5).
FIGURE 2.
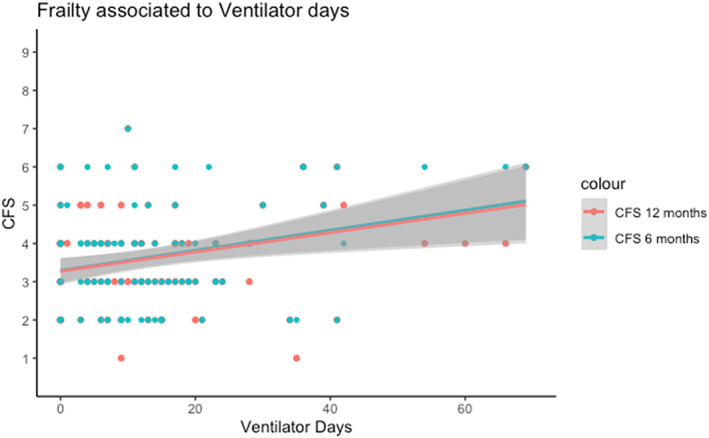
Ventilator days and Clinical Frailty Score (CFS) analyzed in a linear regression model with 95% confidence intervals. Multiple linear regression analysis showed a significant association between frailty and ventilator days (p = .02) at 6 months, but not at 12 months (p = .12)
According to the EQ‐5D‐5L, the participants generally presented a high HRQoL in all five domains (Appendix S4). The median for self‐reported EQ‐VAS was 70 (IQR: 50–80) at 6 months, and 70 (IQR: 51–80) at 12 months (Table 2). EQ‐VAS was reported below 50 by 28% (n = 29) at 6 months and by 25% (n = 24) at 12 months.
Generally, the participants had high scores in Barthel ADL and in Lawton–Brody IADL questionnaires, indicating the highest level of independence at both 6 and 12 months. Median score for Barthel ADL was 20 (IQR: 20–20) at 6 and 12 months (Table 2). Lawton–Brody IADL scores were reported gender specific, and with median 8 (IQR: 7–8) and 5 (IQR: 4–5) for females and males, respectively, at both 6 and 12 months (Table 2).
At 6 months, 52% (n = 47) of the participants had FAS scores indicating fatigue. Median score was 24 (IQR: 14–37). At 12 months 47% (n = 45) scored as fatigue, and median score was 23 (IQR: 15–33) (Table 2). Younger participants reported fatigue more frequently (Figure 3). Spearman correlation analysis showed a moderate correlation between age and FAS at 6 months (ρ = −.32) and at12 months (ρ = −.23). We found no significant difference between gender (Appendix S6).
FIGURE 3.
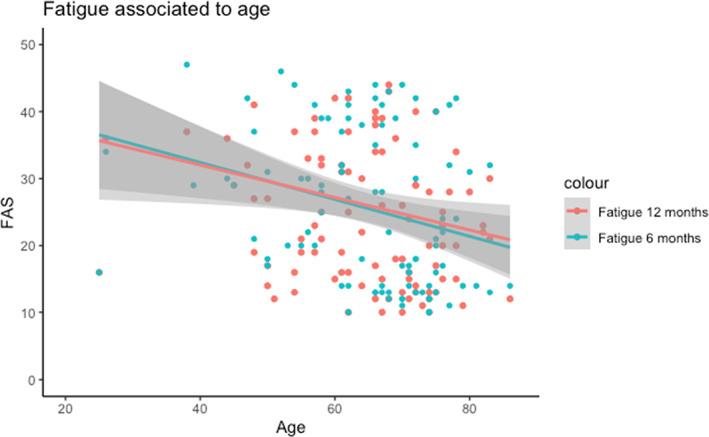
Age and Fatigue Assessment Scale analyzed in a linear regression model with 95% confidence intervals. Multiple regression analysis showed that fatigue was negatively associated with age (p = .006) at 6 months and at 12 months (p = .05)
4. DISCUSSION
We present the results of a nationwide, population‐based follow‐up study of Danish ICU patients with COVID‐19. At 6 and 12 months after ICU admission, the survivors showed cognitive impairments at both follow‐up time points, frailty in about one‐fifth of participants at both time points. Participants presented a high level of independence and HRQoL at both time points. Nearly, half the participants reported fatigue at both 6 and 12 months after their illness.
More than one in three of Danish COVID‐19 patients admitted to ICU treatment had died within 1 year. Similar mortality has been reported in other and larger COVID‐19 ICU populations. 25 , 26 Furthermore, the mortality for unselected ICU populations, 27 and particularly for mechanically ventilated patients with ARDS, is within the same range. 28
One in four of the participants had cognitive impairments 6 months after ICU admission, and one in six at 12‐month follow‐up. Similar studies using MiniMoCA have found a higher prevelance of cognitive impairments. A Belgian follow‐up study, found that 47% of COVID‐19 ICU survivors had cognitive impairments, this was however estimated at 3 months. 29
Karnatovskaia et al found an association between time on ventilator and cognitive impairment, 10 which we could not confirm. Generally, a challenge of ICU follow‐up studies is that the preadmission cognitive and functional status is rarely known. Consequently, we do not know if the presented impairments are related to the COVID‐19 critical illness or not.
Twenty percent of the participants were scored frail at 6 months, and 18% at 12 months. Previous studies have reported a high association between frailty and 30‐day mortality. 22 , 27 Since we do not have preadmission frailty status, there is a risk of the frailest patients dying within the first 6 months after ICU admission. Thus, our data might be biased showing less frailty than could be the case at an earlier time point. At 6 months we found that the longer time on the ventilator was associated with increased frailty. We could not retrieve these findings significant at 12 months. As we have relatively few patients in our study, these findings should be interpreted with caution. In addition, causality is challenged because we do not have the preadmission frailty status of the patients.
The participants reported high scores for ADL and IADL indicating the highest level of independency. This is dissimilar to other studies that have found a decline in ADL and IADL post‐ICU, both short‐ and long‐term. 30 , 31 It is interesting that the physical functional status was overall good, especially when we consider that 26% have cognitive impairments and 20% are frail at 6 months. This might suggest that MiniMoCA and CFS are more sensitive tools in the follow‐up context. The questions in ADL and IADL questionaries are subjective, whereas the MiniMoCA is a more objective measure, and the CFS is an investigator‐operated scale, synthesizing the information gathered during the interview. Another aspect is that 70% of our population is male, and as the Lawton–Brody IADL discriminates between genders and does not score the male's performance in the domains: laundry, food preparation, and housekeeping, some information regarding advanced skills is lost.
A major reported consequence after COVID‐19 is short and long‐term fatigue. 32 We found that approximately 50% of the participants reported fatigue at 6‐ and 12‐months follow‐up. Our findings are in accordance with other COVID‐19 follow‐up studies investigating ICU and ward patients, 33 , 34 , 35 , 36 which suggests that COVID‐19 is associated with excessive fatigue. Unlike our study, a previous study found more females reporting fatigue than males. 37 We found a higher proportion of fatigue among the younger patients, and these findings are in accordance with a previous study of a mixed, non‐COVID ICU population. 37 One explanation for this could be the different preadmission status for participants of different ages. The older patient might have a lower preadmission functional status than the younger patient and might even have experienced some degrees of fatigue prior to the critical illness. The younger participants may also still have an energy‐consuming job, and the expectations of normal living might be higher than for the retired participants. A Dutch study investigated the ability to return to work after ICU treatment of COVID‐19 and found 58% reported problems 1 year after. 36 Unfortunately, we do not have data on the resumption of work.
Overall, we found no major improvements in the functional status of the participants between 6‐ and 12‐month follow‐up. One explanation could be the participant's ICU length of stay. Studies have shown that critical illness, ICU treatment, and long hospital stays leave the patients in a frailer state, 38 which might impact the recovery time. As COVID‐19 survivors have a very long ICU length of stay, 3 , 4 it could be expected that their recovery in cognitive and functional domains will be a long process.
Follow‐up studies rarely include all participants, and a concern in general is that nonresponders could be either more or less impaired than the responders. Despite our efforts, 66 patients never responded to the invitation to join the follow‐up study, and we do not know why. A Danish study exploring the characteristics of nonresponders in a follow‐up program after intensive care found the nonresponders to have a higher age, a longer length of ICU stay, and more likely to be living alone. 39 Except for differences in time in ventilator and renal replacement treatment, general baseline characteristics in our study were similar between the interviewed versus not interviewed participants. We did not find a relevant difference in age nor length of ICU stay between interviewed and noninterviewed. Unfortunately, we do not know the civil status of the participants and cannot explore this issue further.
This study represents the long‐term impairments after COVID‐19 during the first wave. Since therapeutic strategies have changed during the different waves of the pandemic, further studies are needed to investigate and compare the long‐term impairments in COVID‐19 patients from other waves, to reveal the long‐term effects of the different treatments.
The strengths of our study are the broad representation of participants from all five Danish regions. Also, the baseline characteristics of the interviewed versus noninterviewed are similar. Both indicate that this study might be representable of the total Danish COVID ICU population. Furthermore, we have data from two time points making it possible to explore the dynamics in status over time. Another strength is that the same interviewer (S.W.) carried out all the interviews, which minimizes the interrater variability of the data.
This study has several limitations. First, we have a relatively small sample size, which weakens our results. However, we invited the majority of patients who were still alive from the database. Second, the proportion of lost‐to‐follow‐up is relatively large for this study and it increases the risk of attrition bias. Finally, the lack of information on preadmission cognitive and functional status for the participants is a limitation.
5. CONCLUSION
In this follow‐up study of all Danish ICU patients with COVID 19 in the initial phase of the pandemic, we found cognitive impairments in 26% and 17%, at 6 and 12 months, respectively. We found frailty in 20% and 18% of the participants, at 6 and 12 months, respectively. There was an association between time on ventilator and frailty at the 6‐month follow‐up. Furthermore, fatigue was a challenge for approximately 50% the survivors at both time points, especially in the younger participants. Based on retrospectively registered data in the Danish ICU COVID‐19 database, no information on preadmission cognitive and functional status on patients is available. In accordance with our data, there seem to be no changes in the impairments between 6‐ and 12‐month follow‐up.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1: Supporting Information
Weihe S, Mortensen CB, Haase N, et al. Long‐term cognitive and functional status in Danish ICU patients with COVID‐19. Acta Anaesthesiol Scand. 2022;66(8):978‐986. doi: 10.1111/aas.14108
REFERENCES
- 1. WHO . WHO announces COVID‐19 outbreak a pandemic. 2020. Accessed February 20, 2021. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic
- 2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324:782‐793. [DOI] [PubMed] [Google Scholar]
- 3. Haase N. Dansk Intensiv COVID‐19 rapport. 2021. Accessed August 8, 2021. www.cric.nu/danish-icu-covid-19-report/
- 4. Haase N, Plovsing R, Christensen S, et al. Characteristics, interventions and longer‐term outcomes of COVID‐19 ICU patients in Denmark—a nationwide, observational study. Acta Anaesthesiol Scand. 2021;65:68‐75. [DOI] [PubMed] [Google Scholar]
- 5. Patel MB, Morandi A, Pandharipande PP. What's new in post‐ICU cognitive impairment? Intensive Care Med. 2015;41:708‐711. [DOI] [PubMed] [Google Scholar]
- 6. Rooney S, Webster A, Paul L. Systematic review of changes and recovery in physical function and fitness after severe acute respiratory syndrome‐related coronavirus infection: implications for COVID‐19 rehabilitation. Phys Ther. 2020;100:1717‐1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson‐LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50‐56. [DOI] [PubMed] [Google Scholar]
- 8. Bagshaw SM, Stelfox HT, Johnson JA, et al. Long‐term association between frailty and health‐related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015;43:973‐982. [DOI] [PubMed] [Google Scholar]
- 9. Estrup S, Kjer CKW, Vilhelmsen F, Poulsen LM, Gøgenur I, Mathiesen O. Cognitive function 3 and 12 months after ICU discharge‐a prospective cohort study. Crit Care Med. 2018;46:e1121‐e1127. [DOI] [PubMed] [Google Scholar]
- 10. Karnatovskaia LV, Schulte PJ, Philbrick KL, et al. Psychocognitive sequelae of critical illness and correlation with 3 months follow up. J Crit Care. 2019;52:166‐171. [DOI] [PubMed] [Google Scholar]
- 11. Nasreddine Z. Mini Montreal Cognitive Assessment (MiniMoCA) Version 2.1. 2019. Accessed January 25, 2021. https://mocatest.org/remote-moca-testing/.
- 12. Kjaer M‐BN, Meyhoff TS, Madsen MB, et al. Long‐term patient‐important outcomes after septic shock: a protocol for 1‐year follow‐up of the CLASSIC trial. Acta Anaesthesiol Scand. 2020;64:410‐416. [DOI] [PubMed] [Google Scholar]
- 13. van Reenen MJ. EQ‐5D‐5L User guide Basic information on how to use the EQ‐5D‐5L instrument. 2015. https://euroqol.org/publications/user-guides/.
- 14. Green CR, Mohs RC, Schmeidler J, Aryan M, Davis KL. Functional decline in Alzheimer's disease: a longitudinal study. J Am Geriatr Soc. 1993;41:654‐661. [DOI] [PubMed] [Google Scholar]
- 15. Lauritsen J, Maribo T. Barthel‐20 indeks, dansk standardoversættelse. 2007. Accessed January 28, 2021. https://www.etf.dk/uploads/uploads/public/documents/Redskaber/barthel-20_indeks.pdf
- 16. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179‐186. [PubMed] [Google Scholar]
- 17. Poulsen LM, Estrup S, Mortensen CB, Andersen‐Ranberg NC . Delirium in intensive care. Curr Anesthesiol Rep. 2021;11(4):516‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hendriks C, Drent M, Elfferich M, De Vries J. The fatigue assessment scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med. 2018;24:495‐503. [DOI] [PubMed] [Google Scholar]
- 19. WASOG . Fatigue Assessment Scale (FAS). 2020. Accessed February 7, 2021. https://www.wasog.org/educational-material/fatigue-assessment-scale.html
- 20. Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23:210‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flaatten H, de Lange DW, Morandi A, et al. The impact of frailty on ICU and 30‐day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med. 2017;43:1820‐1828. [DOI] [PubMed] [Google Scholar]
- 22. Fernando SM, McIsaac DI, Rochwerg B, et al. Frailty and invasive mechanical ventilation: association with outcomes, extubation failure, and tracheostomy. Intensive Care Med. 2019;45:1742‐1752. [DOI] [PubMed] [Google Scholar]
- 23. Guidet B, de Lange DW, Boumendil A, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46:57‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sk N, Fournaise A, Lauridsen J, et al. Cross‐sectoral inter‐rater reliability of the clinical frailty scale—a Danish translation and validation study. BMC Geriatr. 2020;20:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. COVID‐ICU Group . Clinical characteristics and day‐90 outcomes of 4244 critically ill adults with COVID‐19: a prospective cohort study. Intensive Care Med. 2021;47:60‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia. 2020;75:1340‐1349. [DOI] [PubMed] [Google Scholar]
- 27. de Geer L, Fredrikson M, Tibblin AO. Frailty predicts 30‐day mortality in intensive care patients: a prospective prediction study. Eur J Anaesthesiol. 2020;37:1058‐1065. [DOI] [PubMed] [Google Scholar]
- 28. Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end‐expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335‐1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rousseau A‐F, Minguet P, Colson C, et al. Post‐intensive care syndrome after a critical COVID‐19: cohort study from a Belgian follow‐up clinic. Ann Intensive Care. 2021;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geense W, Zegers M, Dieperink P, Vermeulen H, van der Hoeven J, van den Boogaard M. Changes in frailty among ICU survivors and associated factors: results of a one‐year prospective cohort study using the Dutch clinical frailty scale. J Crit Care. 2020;55:184‐193. [DOI] [PubMed] [Google Scholar]
- 31. da Silveira LTY, da Silva JM, Soler JMP, Sun CYL, Tanaka C, Fu C. Assessing functional status after intensive care unit stay: the Barthel Index and the Katz Index. Int J Qual Heal Care. 2018;30:265‐270. [DOI] [PubMed] [Google Scholar]
- 32. Haas JS, Teixeira C, Cabral CR, et al. Factors influencing physical functional status in intensive care unit survivors two years after discharge. BMC Anesthesiol. 2013;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sundhedsstyrelsen . Senfølger ved COVID‐19. 2021. Accessed September 1, 2021. https://www.sst.dk/-/media/Udgivelser/2021/Corona/Senfølger/Anbefalinger-for-senfoelger-efter-covid-19.ashx?la=da&hash=77E0083548DBED190E3919EF9B71111409ED608A.
- 34. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93:1013‐1022. [DOI] [PubMed] [Google Scholar]
- 35. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27:601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang C, Huang L, Wang Y, et al. 6‐Month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heesakkers H, van der Hoeven JG, Corsten S, et al. Clinical outcomes among patients with 1‐year survival following intensive care unit treatment for COVID‐19. JAMA. 2022;327:559‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Engberg I, Segerstedt J, Waller G, Wennberg P, Eliasson M. Fatigue in the general population‐ associations to age, sex, socioeconomic status, physical activity, sitting time and self‐rated health: the northern Sweden MONICA study 2014. BMC Public Health. 2017;17:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kjaer MN, Mortensen CB, Hjortrup PB, Rygård SL, Andersen I, Perner A. Factors associated with non‐response at health‐related quality of life follow‐up in a septic shock trial. Acta Anaesthesiol Scand. 2018;62:357‐366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


